Abstract
Breast cancer is heterogenous, with variable expression of the estrogen receptor (er), progesterone receptor (pr), and human epidermal growth factor receptor 2 (her2). Overexpression of her2 is generally considered a negative prognostic feature, but whether outcomes for her2-positive early breast cancer remain different from those for other subtypes in the era of trastuzumab-based adjuvant therapy is unknown.
Methods
Using a retrospective chart review, we compared overall survival (os) and relapse-free survival (rfs) in 3 groups of patients with early-stage breast cancer: er-positive or pr-positive (or both) and her2-negative [“hormone receptor–positive” (hr+)]; her2-positive (her2+); and er-negative, pr-negative, and her2-negative [“triple-negative” (tn)].
Results
In the 503 charts analyzed (332 hr+, 94 her2+, 77 tn), the 5-year os and rfs were, respectively, 94.2% and 87.2% for hr+ patients, 88.6% and 74.9% for her2+ patients, and 85.4% and 76.2% for tn patients. On multivariate analysis, the os for the her2+ subtype was similar to that for the hr+ subtype (hazard ratio:1.07; 95% confidence interval: 0.31 to 3.67 with hr+ as reference), but os was significantly worse for tn patients than for hr+ patients (hazard ratio: 4.37; 95% confidence interval: 1.56 to 12.24). In her2+ patients, the 5-year os and rfs trended better for patients with er+ or pr+ disease than for patients with er-negative and pr-negative disease (5-year os: 92.1% vs. 86.9%; 5-year rfs: 79.8% vs. 71.4%). Of her2+ patients, just 80.9% received trastuzumab, including 33.3% of her2+ patients with sub-centimetre tumours.
Conclusions
In the trastuzumab era, patients with her2+ and hr+ early breast cancer have similar outcomes, while tn patients experience a significantly worse os than either of the foregoing groups. Outcomes for her2+ patients may differ by er and pr status. Trastuzumab was underutilized in this cohort.
Keywords: her2-positive breast cancer, survival, trastuzumab, adjuvant
1. INTRODUCTION
Breast cancer is a heterogeneous disease. Gene expression studies have identified five distinct molecular subtypes of breast cancer: luminal A, luminal B, human epidermal growth factor receptor 2 (her2)–enriched, basal-like, and normal breast-like1. Other subtypes (such as claudin-low) continue to be defined2. The use of gene expression profiling is currently limited in many clinical settings, and so histopathologic markers such as estrogen receptor (er), progesterone receptor (pr), and her2 are used as surrogates for the molecular subtypes, which divide breast tumours into distinct phenotypes with distinct outcomes.
Studies comparing outcomes between the different subtypes have shown that her2 overexpression, which is found in 15%–20% of human breast cancers3, is associated with increased risk of locoregional recurrence4–9 and increased breast cancer mortality9–10. Overexpression of her2 is thus generally considered to be a negative prognostic feature with an increased adjusted risk of breast cancer mortality that is approximately doubled11. However, the development of agents specifically targeting her2 has transformed the management of patients with these tumours. One of the first of these targeted agents was trastuzumab (Herceptin: Genentech, San Francisco, CA, U.S.A.), a monoclonal humanized antibody that binds the extracellular domain of her2. That binding interferes with the signal transduction cascade initiated by her2 overexpression and possibly stimulates an immune response to tumour cells overexpressing the receptor12.
Trastuzumab in combination with cytotoxic chemotherapy improves clinical outcomes in not only metastatic13–16 but also early node-positive and node-negative17–19her2-positive (her2+) invasive breast cancers. It has been approved by Health Canada for the adjuvant treatment her2+ breast cancer since 200620.
Since the advent of adjuvant trastuzumab as the standard of care for her2+ tumours, few studies have compared outcomes in early breast cancer by molecular subtype (or histopathologic features). In a study of stage ii and iii breast cancer treated with modern-era systemic therapy, including trastuzumab for most patients with her2+ disease (86%), her2 overexpression was associated with a risk of locoregional recurrence that was significantly lower than that observed for her2-negative (her2–) tumours4. Thus, it is hypothesized that the use of adjuvant trastuzumab has improved outcomes in her2+ disease to reach similarity with outcomes in her2– disease.
The present study was designed to retrospectively compare treatments and outcomes in patients with early-stage breast cancer diagnosed during 2005–2006, based on the 3 molecular subtypes: er-positive (er+) or pr+ (or both) and her2– [“hormone receptor positive” (hr+)]; her2+; and er-negative (er–), pr-negative (pr–), and her2– [“triple negative” (tn)]. The primary outcome measure was relapse-free survival (rfs) at 5 years, and the secondary outcome was 5-year overall survival (os).
2. METHODS
2.1. Study Design
We conducted a retrospective chart review for all patients with a diagnosis of stage i–iii invasive breast cancer seen during 2005–2006 at the Odette Cancer Centre (Sunnybrook Health Sciences Centre, Toronto, Ontario). From among the 871 patient records identified, 503 met the inclusion criteria: female patient, 18 years of age or older, seen by a medical oncologist at the cancer centre between January 1, 2005, and December 31, 2006, for a new diagnosis of stage i–iii invasive breast cancer. Exclusion criteria included unavailable er, pr, and her2 status for the primary tumour; male sex; a diagnosis of ductal carcinoma in situ; metastatic disease at the time of diagnosis; a concurrent malignancy (a non-breast cancer); and one-time consultation with no followup data. The study was approved by the Sunnybrook Health Sciences Centre Research Ethics Board (project identification number 158-2010).
2.2. Data Collection
We collected data on patient demographics, tumour characteristics, cancer stage at diagnosis, specifics of treatment (dates of surgery, hormonal therapy, radiation, and chemotherapy), recurrence, date and location of recurrence, and date of death. For locally advanced breast cancer, the tumour size was determined from magnetic resonance imaging, clinical measurement, mammographic measurement, or surgical pathology (in order of descending priority); nodal status was denoted as “Nx.”
2.3. Classification of Groups
Patients were categorized into 3 groups: hr+, her2+, and tn. Tumour er and pr status was determined on the basis of immunohistochemistry, with staining of 1% or more of tumour cell nuclei being considered positive21. Immunohistochemistry was also used to determine her2 status, with a polyclonal antibody (TAB250 and CB11) being raised against the her2/neu oncoprotein. Test results of 0 to 1+ were considered negative, and 3+ was considered positive. Fluorescence in situ hybridization was performed for all equivocal (2+) immunohistochemistry results; a ratio of 2.0 or higher for her2 gene signal to chromosome 17 signal was considered positive, in accordance with prospective randomized clinical trials of adjuvant trastuzumab17–19 and with eligibility criteria for funding for adjuvant trastuzumab by Cancer Care Ontario22.
2.4. Endpoints
The primary and secondary endpoints of this study were 5-year rfs and os respectively. The rfs was calculated from date of diagnosis to date of first relapse or death from any cause. “Date of diagnosis” was defined as the date of first definitive treatment, including surgical or medical therapy. Survival times were censored to the date of last contact for subjects who were lost to follow-up. The os was calculated as duration from cancer diagnosis to date of death from any cause.
2.5. Statistical Analysis
For the three subtypes, baseline characteristics (such as age) were compared using an analysis of variance test for continuous variables; a Fisher exact test was used for categorical variables (such as sex and tumour stage). The Kaplan–Meier product limit method was used to estimate os and rfs. The logit transformation was used to estimate 95% confidence intervals (cis) for the percentage of patients surviving at a particular time. Age-adjusted logistic regressions were used to derive odds ratios and 95% cis. Cox proportional hazards models were used to derive adjusted (for age, stage, histologic grade, chemotherapy treatment, and lymph node status) hazard ratios (hrs) and 95% cis for os and rfs by disease subtype.
3. RESULTS
3.1. Sample Characteristics
Table i presents the baseline characteristics of study subjects by tumour subtype. Of the 503 patients, 332 (66.0%) had hr+ disease, 94 (18.7%) had her2+ disease, and 77 (15.3%) had tn disease. Of the 94 her2+ patients, 39 (41.5%) had her2+ and er+ or pr+ tumours and 55 (58.5%) had her2+, er–, and pr–tumours.
TABLE I.
Baseline characteristics by tumour subtype
| Variable |
Tumour subtypes
|
p Value | ||
|---|---|---|---|---|
| er+ or pr+ or both, and her2– | her2+ | er–, pr–, and her2– | ||
| Patients [n (%)] | 332 (66.0) | 94 (18.7)a | 77 (15.3) | |
| Mean age (years) | 56.3±12.4 | 53.0±12.1 | 49.5±11.4 | <0.001 |
| Tumour stage [n (%)]b | ||||
| i | 155 (46.7) | 30 (31.9) | 20 (26.0) | 0.001 |
| ii | 121 (36.5) | 36 (38.3) | 38 (49.4) | |
| iii | 56 (16.9) | 28 (29.8) | 19 (24.7) | |
| Cancer type [n (%)] | ||||
| Ductal | 273 (82.2) | 92 (97.9) | 67 (87.0) | <0.001c |
| Lobular | 41 (12.4) | 1 (1.1) | 1 (1.3) | |
| Mixed | 3 (0.9) | 0 (0.0) | 0 (0.0) | |
| Other | 15 (4.5) | 1 (1.1) | 9 (11.7) | |
| Histologic grade [n (%)] | ||||
| Well differentiated | 89 (27.6) | 3 (3.3) | 3 (4.0) | <0.001 |
| Moderately differentiated | 178 (55.1) | 37 (41.1) | 16 (21.1) | |
| Poorly differentiated | 56 (17.3) | 50 (55.6) | 57 (75.0) | |
| Tumour size [n (%)] | ||||
| <1 cm | 49 (14.8) | 15 (16.0) | 5 (6.5) | 0.001 |
| 1–2 cm | 151 (45.5) | 31 (33.0) | 20 (26.0) | |
| 2.1–5 cm | 100 (30.1) | 39 (41.5) | 39 (50.7) | |
| >5 cm | 32 (9.6) | 9 (9.8) | 13 (16.9) | |
| Lymph node status [n (%)] | ||||
| Positive | 116 (36.4) | 39 (42.4) | 22 (29.0) | 0.197 |
| Negative | 203 (63.6) | 53 (57.6) | 54 (71.1) | |
| Surgery [n (%)] | ||||
| Lumpectomy | 190 (58.3) | 49 (52.1) | 45 (59.2) | 0.530 |
| Mastectomy | 136 (41.7) | 45 (47.9) | 31 (40.8) | |
| Chemotherapy [n (%)] | ||||
| Any chemotherapy | 142 (42.8) | 81 (86.2) | 68 (88.3) | |
| Anthracycline only | 68 (47.9) | 33 (40.7) | 22 (32.4) | 0.128c |
| Taxane only | 1 (0.7) | 2 (2.5) | 0 (0.0) | |
| Anthracycline plus taxane | 70 (49.3) | 42 (51.9) | 45 (66.2) | |
| Other | 3 (2.1) | 4 (4.9) | 1 (1.5) | |
| Radiation therapy [n (%)] | 250 (75.3) | 75 (79.8) | 69 (89.6) | 0.021 |
| Hormone therapy [n (%)] | ||||
| Tamoxifen | 84 (29.0) | 2 (6.1) | 0 (0.0) | <0.001c |
| ai | 121 (41.7) | 26 (78.8) | 0 (0.0) | |
| Tamoxifen then ai | 59 (20.3) | 1 (3.0) | 0 (0.0) | |
| ai then tamoxifen | 24 (8.3) | 4 (12.1) | 0 (0.0) | |
| Other | 2 (0.7) | 0 (0.0) | 0 (0.0) | |
| Trastuzumab [n (%)] | 0 (0.0) | 76 (80.9) | 0 (0.0) | <0.001c |
Of 94 her2+ patients, 39 (41.5%) had er+ or pr+ tumours, and 55 (58.5%) had er–, pr–tumours.
Because of rounding, percentages may not add to exactly 100%.
By Fisher exact test because of small sample sizes.
er = estrogen receptor, positive (+) or negative (–); pr = progesterone receptor, positive (+) or negative (–); her2 = human epidermal growth factor receptor 2, positive (+) or negative (–); ai = aromatase inhibitor.
3.2. Association Between Subtype and Other Prognostic Indicators
We observed a significant difference between the three breast cancer subtypes in the overall distribution of patient age (p < 0.001), tumour stage (p < 0.001), histologic grade (p < 0.001), and tumour size (p = 0.001, Table i). The tn patients were younger (p < 0.001) and had larger (p = 0.001), higher-stage (p = 0.001), and higher–histologic grade tumours at diagnosis (p < 0.001). They more frequently received adjuvant radiation therapy (p = 0.021).
3.3. Survival
The median follow-up period was 62 months (range: 17 days–85 months). The 5-year os for all subjects was 92.0% (95% ci: 89.0% to 94.2%), and the rfs was 83.2% (95% ci: 79.4% to 86.3%). Table ii presents 5-year os and rfs by tumour subtype, er and pr status, and her2 status.
TABLE II.
Five-year overall survival and relapse-free survival by tumour subtype and receptor status
| Tumour variable |
Survival type [% (95% ci)]
|
|
|---|---|---|
| Overall | Relapse-free | |
| Subtype | ||
| er+ or pr+ or both, and her2– | 94.2 (90.8 to 96.4) | 87.2 (82.8 to 90.5) |
| her2+ | 88.6 (77.4 to 94.4) | 74.9 (64.1 to 82.9) |
| er–, pr–, and her2– | 85.4 (74.3 to 91.9) | 76.2 (64.4 to 84.5) |
| er and pr status | ||
| er+ and pr+ | 94.1 (90.9 to 96.2) | 86.4 (82.2 to 89.6) |
| er– and pr– | 85.9 (78.0 to 91.2) | 74.1 (65.1 to 81.1) |
| her2 status | ||
| Positive | 88.6 (77.4 to 94.4) | 74.9 (64.1 to 82.9) |
| Negative | 92.6 (89.3 to 94.9) | 85.1 (81.0 to 88.3) |
| Overall | 92.0 (89.0 to 94.2) | 83.2 (79.4 to 86.3) |
er = estrogen receptor, positive (+) or negative (–); pr = progesterone receptor, positive (+) or negative (–); her2 = human epidermal growth factor receptor 2, positive (+) or negative (–).
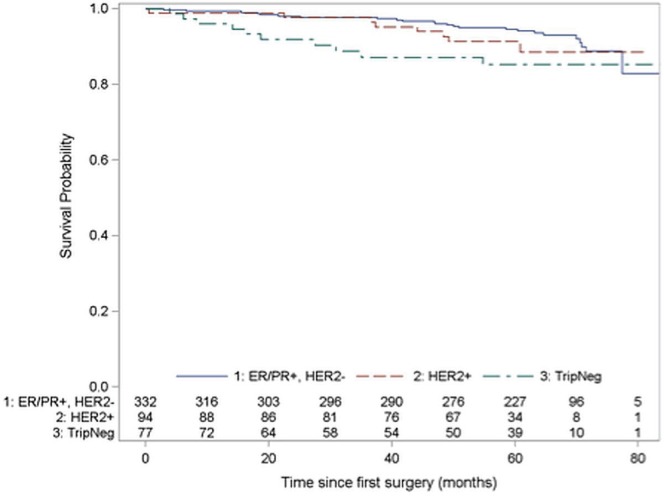
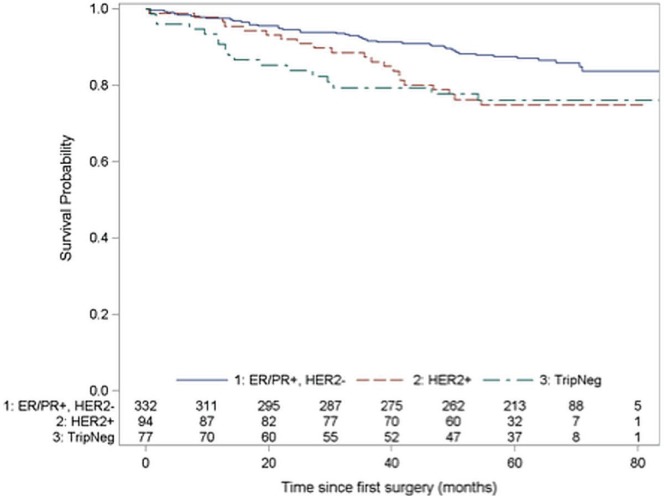
The 5-year os was 94.2% (95% ci: 90.8% to 96.4%) for hr+ patients, 88.6% (95% ci: 77.4% to 94.4%) for her2+ patients, and 85.4% (95% ci: 74.3% to 91.9%) for tn patients. Figure 1 shows the associated Kaplan–Meier curves. The 5-year rfs was 87.2% (95% ci: 82.8% to 90.5%) for hr+ patients, 74.9% (95% ci: 64.1% to 82.9%) for her2+ patients, and 76.2% (95% ci: 64.4% to 84.5%) for tn patients (Figure 2).
FIGURE 1.
Kaplan–Meier curves for overall survival by receptor group status. Log-rank chi-square for differences in survival across strata: χ2 (df: 2) = 5.31, p = 0.070. er = estrogen receptor; pr = progesterone receptor; her2 = human epidermal growth factor receptor 2; TripNeg = triple-negative (er–, pr–, and her2-negative).
FIGURE 2.
Kaplan–Meier curves for relapse-free survival by receptor group status. Log-rank chi-square for differences in survival across strata: χ2 (df: 2) = 9.82, p = 0.007. er = estrogen receptor; pr = progesterone receptor; her2 = human epidermal growth factor receptor 2; TripNeg = triple negative (er–, pr–, and her2-negative).
Among patients with er+ or pr+ status, the 5-year os was 94.1% (95% ci: 90.9% to 96.2%), and the 5-year rfs was 86.4% (95% ci: 82.2% to 89.6%). Those survival rates were significantly higher than the rates for patients with er– and pr– disease, whose 5-year os was 85.9% (95% ci: 78.0% to 91.2%), and whose 5-year rfs was 74.1% (95% ci: 65.1% to 81.1%). For patients with her2+ tumours, the 5-year os was 88.6% (95% ci: 77.4% to 94.4%), and the rfs was 74.9% (95% ci: 64.1% to 82.9%). Among her2– patients, the 5-year os was 92.6% (95% ci: 89.3% to 94.9%), and the 5-year rfs was 85.1% (95% ci: 81.0% to 88.3%).
3.4. Adjuvant Systemic Therapies
Of the 503 patients, 291 (57.8%) received adjuvant chemotherapy. This treatment was used more frequently in patients with her2+ (86.2%) and tn (88.3%) disease; it was used in only 42.8% of hr+ patients (Table i). Most her2+ patients received anthracycline-based adjuvant chemotherapy, with 33 patients (40.7%) receiving anthracycline only, and 42 patients (51.9%) receiving anthracycline–taxane combination chemotherapy. Among the her2+ patients, 76 (80.9%) received trastuzumab. Only 5 patients (33.3%) with sub-centimetre tumours received this therapy.
For her2+ patients who received trastuzumab, the 5-year rfs and os were 75% and 89% respectively; for those who did not, the survival percentages were 76% and 88% respectively.
On multivariable analysis adjusted for age, stage, histologic grade, chemotherapy treatment, and lymph node status, the tn tumour subtype was associated with a risk of mortality that was significantly increased compared with that for the hr+ subtype, which served as the reference group (hr: 4.37; 95% ci: 1.56 to 12.24); patients with the her2+ subtype had an outcome similar to that in patients with the hr+ subtype (hr: 1.07; 95% ci: 0.31 to 3.67; Table iii).
TABLE III.
Multivariate analysisa of the association between tumour subtype and overall survival or relapse-free survival
| Tumour variable |
Overall survival
|
Relapse-free survival | ||
|---|---|---|---|---|
| hr | 95%ci | hr | 95% ci | |
| Subtype | ||||
| er+ or pr+ or both, and her2– | Reference | Reference | ||
| her2+ | 1.34 | 0.52 to 3.48 | 1.61 | 0.88 to 2.95 |
| er–, pr–, and her2– | 2.95 | 1.13 to 7.72 | 1.86 | 0.91 to 3.80 |
| er and pr status | ||||
| er+ and pr+ | 0.42 | 0.19 to 0.91 | 0.58 | 0.33 to 1.00 |
| er– and pr– | Reference | Reference | ||
| her2 status | ||||
| Positive | 0.91 | 0.38 to 2.18 | 1.30 | 0.76 to 2.25 |
| Negative | Reference | Reference | ||
Controlled for age, tumour stage, tumour grade, adjuvant chemotherapy, and positive lymph node status.
er = estrogen receptor, positive (+) or negative (–); pr = progesterone receptor, positive (+) or negative (–); her2 = human epidermal growth factor receptor 2, positive (+) or negative (–)
4. DISCUSSION
The treatment of breast cancer has progressed significantly since the early 1990s. One significant advance was the advent of adjuvant trastuzumab for patients with early-stage breast cancer. Our study is one of the first to examine the relative outcomes for the subtypes of breast cancer since adjuvant trastuzumab became the standard of care for Canadian patients with her2+ early-stage breast cancer. Our results indicate that the 5-year os and rfs for the entire cohort were excellent, but variable by receptor status (a surrogate for molecular subtype).
At baseline, we found a distribution of breast cancer subtypes similar to that reported by other immunohistochemical studies23–26. We also observed that both her2+ and tn tumours were more prevalent among younger women and more frequently exhibited pathologic characteristics associated with more aggressive tumour behaviour. As such, we found that outcomes in both of the latter subtype groups were worse than those in the hr+ (luminal) subtype group.
In our study, 18.7% of breast cancers overexpressed her2, which is similar to the incidence of 18%–20% reported in the literature3. On multivariate analysis, patients with her2+ tumours had rates of os and rfs similar to those in hr+ patients, with a hr of 1.07. Previous studies in which her2+ patients did not receive trastuzumab showed worse outcomes for those patients relative to hr+ patients. For example, Dawood et al.25 showed that the 5-year os was 94% for luminal A (defined as er+ or pr+, her2–, grade 1–2), 85% for luminal B (defined as er+ or pr+, her2+; or er+ or pr+, her2– and high grade), and 80% for her2-type (defined as er–, pr–, and her2+). They also demonstrated that the 5-year rfs was 93% for luminal A, 82% for luminal B, and 78% for her2-type. In a multivariate model with luminal A tumours as reference, the hr for breast cancer death was 1.90 for luminal B and 1.36 for her2-type25. Thus, our study suggests although her2-overexpressing breast cancer is associated with poor prognosis relative to hr+ breast cancer, integration of appropriate systemic chemotherapy together with trastuzumab may mitigate the risk and improve outcomes in patients with this subtype of breast cancer.
Trastuzumab was underutilized for the her2+ patients in our study, being used in only 76 of the 94 in this group (80.9%). Our results are similar to those observed by Noonan et al.27, who, in a study of early-stage her2+ breast cancer in Newfoundland and Labrador, found that only 76% of patients with her2+ breast cancer received adjuvant trastuzumab. Of the 18 her2+ patients in our study who did not receive adjuvant trastuzumab, 10 (55.5%) had subcentimetre tumours (presumably the reason that adjuvant trastuzumab was omitted), and 3 (16.7%) declined systemic treatment. No reason was provided for the omission of trastuzumab in the 5 remaining patients (27.8%).
In our study, 15 her+ patients had sub-centimetre tumours. Of those patients, only 5 (33.3%) received adjuvant trastuzumab. Of the 15, 13 had node-negative disease. Only 3 of the her2+ patients with sub-centimetre, node-negative disease (23.1%) received adjuvant trastuzumab. Both of the patients with sub-centimetre, node-positive her2+ tumours received adjuvant trastuzumab (100%). Patients with sub-centimetre, node-negative her2+ tumours who received trastuzumab had rates of 5-year rfs and os that were similar to rates in patients who did not receive trastuzumab (88.9% vs. 100% and 100% vs. 100%). However, that finding is limited by the small number of patients included in this analysis (n = 13, with 10 patients receiving trastuzumab, and 3 not receiving it).
Our study also highlights that her2+ disease in itself represents a heterogeneous group. Analysis of the her2+ group revealed that outcomes differed by er and pr status. The 5-year os and rfs rates trended better in patients with er+ or pr+ disease than in patients with er– and pr– disease [5-year os: 92.1% (95% ci: 70.4% to 98.1%) vs. 86.9% (95% ci: 73.0% to 93.9%); 5-year rfs: 79.8% (95% ci: 62.0% to 89.8) vs. 71.4% (95% ci: 56.4% to 82.1%)]. That finding is consistent with other studies that have shown a heterogenous biology for her2+ tumours. Carey et al.26 previously showed that patients with her2+, er− tumours were particularly prone to early and frequent relapse and experienced particularly poor survival. That knowledge may become useful for the selection of patients who need more aggressive treatment.
Our study has several potential limitations, including its retrospective design and the relatively small number of patients with certain tumour subtypes, which limited the power for statistical comparisons between receptor subgroups. Accordingly, our comparison of survival outcomes may not have reached statistical significance. Because of the small number of her2+ patients, our analysis combined patients who were her2+ and hr+ (that is, luminal B) with patients who were her2+ and er– and pr– into a single subgroup; however, as shown both in our study and in others26, those two groups are likely have different outcomes. Finally, classification based on er, pr, and her2 status is only an approximation for the underlying molecular breast cancer subtypes; however, because cost and technical issues have typically rendered gene expression profiling impractical as a routine diagnostic tool, the use of standard histopathologic surrogates—as in our study—is likely more relevant for practicing clinicians at this time.
Our study is one of the first to examine outcomes by breast cancer subtype since the introduction of adjuvant trastuzumab for her2+ tumours. Strengths of the study include its collection of information about adjuvant treatment, the inclusion of patients with her2+ sub-centimetre tumours, and its perspective on use of trastuzumab in a population-based setting in Canada. The study provides insights into the patient population selected for treatment, ability to deliver therapy, and breast cancer outcomes in a non-trial setting.
5. CONCLUSIONS
In the era of her2-targeted therapy, patients with her2+ tumours experience outcomes similar to those for her2–, hr+ breast cancer. By contrast, her2+, hr– breast cancer may represent a subtype with a particularly high risk of recurrence and mortality. Future studies need to focus on the development of improved adjuvant therapies for the her2+, hr– and the tn breast cancer subtypes, which are associated with the worst prognosis.
6. CONFLICT OF INTEREST DISCLOSURES
The present study was an unsponsored and unfunded research project.
7. REFERENCES
- 1.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Prat A, Parker JS, Karginova O, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, Mc-Guire WL. Human breast cancer: correlation of relapse and survival with amplification of the her-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 4.Panoff JE, Hurley J, Takita C, et al. Risk of locoregional recurrence by receptor status in breast cancer patients receiving modern systemic therapy and post-mastectomy radiation. Breast Cancer Res Treat. 2011;128:899–906. doi: 10.1007/s10549-011-1495-1. [DOI] [PubMed] [Google Scholar]
- 5.Gabos Z, Thoms J, Ghosh S, et al. The association between biological subtype and locoregional recurrence in newly diagnosed breast cancer. Breast Cancer Res Treat. 2010;124:187–94. doi: 10.1007/s10549-010-1135-1. [DOI] [PubMed] [Google Scholar]
- 6.Chen XS, Ma CD, Wu JY, et al. Molecular subtype approximated by quantitative estrogen receptor, progesterone receptor and her2 can predict the prognosis of breast cancer. Tumori. 2010;96:103–10. doi: 10.1177/030089161009600117. [DOI] [PubMed] [Google Scholar]
- 7.Arvold ND, Taghian AG, Niemierko A, et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. 2011;29:3885–91. doi: 10.1200/JCO.2011.36.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–91. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 9.Cancello G, Maisonneuve P, Rotmensz N, et al. Prognosis in women with small (T1mic,T1a,T1b) node-negative operable breast cancer by immunohistochemically selected subtypes. Breast Cancer Res Treat. 2011;127:713–20. doi: 10.1007/s10549-011-1465-7. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien KM, Cole SR, Tse CK, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16:6100–10. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haque R, Ahmed SA, Inzhakova G, et al. Impact of breast cancer subtypes and treatment on survival: an analysis spanning two decades. Cancer Epidemiol Biomarkers Prev. 2012;21:1848–55. doi: 10.1158/1055-9965.EPI-12-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldenberg MM. Trastuzumab, a recombinant dna-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clin Ther. 1999;21:309–18. doi: 10.1016/S0149-2918(00)88288-0. [DOI] [PubMed] [Google Scholar]
- 13.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-her2 monoclonal antibody in women who have her2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 14.Mass RD, Press MF, Anderson S, et al. Evaluation of clinical outcomes according to her2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin Breast Cancer. 2005;6:240–6. doi: 10.3816/CBC.2005.n.026. [DOI] [PubMed] [Google Scholar]
- 15.Slamon DJ, Leyland–Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against her2 for metastatic breast cancer that overexpresses her2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 16.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of her2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–26. doi: 10.1200/JCO.20.3.719. [DOI] [PubMed] [Google Scholar]
- 17.Piccart–Gebhart MJ, Procter M, Leyland–Jones B, et al. Herceptin Adjuvant (hera) Trial Study Team. Trastuzumab after adjuvant chemotherapy in her2-positive breast cancer. N Engl J Med. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 18.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable her2-positive breast cancer. N Engl J Med. 2005;353:1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 19.Slamon D, Eiermann W, Robert N, et al. on behalf of the Breast Cancer International Research Group Adjuvant trastuzumab in her2-positive breast cancer. N Engl J Med. 2011;365:1273–83. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann–La Roche Limited . Herceptin (Trastuzumab for Infusion) [product monograph] Mississauga, ON: Hoffmann–La Roche Limited; 2012. [Google Scholar]
- 21.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95. doi: 10.1200/JCO.2009.25.6529. [Erratum in: J Clin Oncol 2010;28:3543] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer Care Ontario (cco) Adjuvant to Metastatic Trastuzumab for her2/ neu-Overexpressing Breast Cancer [eligibility form] Toronto, ON: CCO; 2013. [Google Scholar]
- 23.Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on er/pr and her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7:4–13. doi: 10.3121/cmr.2008.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Casar JM, Martín A, García C, et al. Characterization of breast cancer subtypes by quantitative assessment of biological parameters: relationship with clinicopathological characteristics, biological features and prognosis. Eur J Obstet Gynecol Reprod Biol. 2008;141:147–52. doi: 10.1016/j.ejogrb.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Dawood S, Hu R, Homes MD, et al. Defining breast cancer prognosis based on molecular phenotypes: results from a large cohort study. Breast Cancer Res Treat. 2011;126:185–92. doi: 10.1007/s10549-010-1113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 27.Noonan KL, McCarthy J, Powell E, Laing K, Edwards S, McCrate F. A population-based analysis of patients with early-stage her2-positive breast cancer in Newfoundland and Labrador. Oncol Exch. 2012;11:14–19. [Google Scholar]