Abstract
Objective
We investigated the prognostic clinicopathologic factors associated with overall survival (os) and progression-free survival (pfs) in the once-daily continuous administration of first-line sunitinib in a consecutive cohort of Turkish patients with metastatic renal cell carcinoma (rcc).
Methods
The study enrolled 77 Turkish patients with metastatic rcc who received sunitinib in a continuous once-daily dosing regimen between April 2006 and April 2011. Univariate analyses were performed using the log-rank test.
Results
Median follow-up was 18.5 months. In univariate analyses, poor pfs and os were associated with 4 of the 5 factors in the Memorial Sloan–Kettering Cancer Center (mskcc) score: Eastern Cooperative Oncology Group performance status of 2 or higher, low hemoglobin, high corrected serum calcium, and high lactate dehydrogenase. In addition to those factors, hypoalbuminemia, more than 2 metastatic sites, liver metastasis, non–clear cell histology, and the presence of sarcomatoid features on pathology were also associated with poor pfs; and male sex, hypoalbuminemia, prior radiotherapy, more than 2 metastatic sites, lung metastasis, nuclear grade of 3 or 4 for the primary tumour, and the presence of sarcomatoid features were also associated with poorer os. The application of the mskcc model distinctly separated the pfs and os curves (p < 0.001).
Conclusions
Our study identified prognostic factors for pfs and os with the use sunitinib as first-line metastatic rcc therapy and confirmed that the mskcc model still appears to be valid for predicting survival in metastatic rcc in the era of molecular targeted therapy.
Keywords: Metastatic renal cell carcinoma, prognostic factors, sarcomatoid features, first-line therapy, vascular endothelial growth factor, vegf antagonist
1. INTRODUCTION
Metastatic renal cell carcinoma (rcc) has historically been resistant to chemotherapy and hormonal therapy. Immunotherapy has been used as a first-line treatment in advanced or metastatic rcc with limited success. However, the treatment of metastatic rcc has changed dramatically with the availability of new treatment options. Molecular research into the pathogenesis of rcc has provided valuable information about the altered signalling pathways in rcc, including that of vascular endothelial growth factor (vegf) and its receptor1. Molecularly targeted therapies were specifically developed to target these signal transduction pathways. Clinical trials have demonstrated a survival benefit for targeted agents, particularly in clear cell rcc patients2. However, metastatic rcc progresses in all patients, resulting in a critical need to determine patient risk and to optimize treatment.
Sunitinib is an orally administered, multi-targeted receptor tyrosine kinase inhibitor3,4. A prospective randomized phase iii clinical trial of systemic treatment in untreated metastatic rcc patients has demonstrated the superiority of sunitinib over interferon with respect to objective response rate, progression-free survival (pfs), and overall survival (os)2,5. An acceptable safety profile for sunitinib was also shown. In the first Turkish study conducted in metastatic rcc patients, we found that response rates and tolerability with continuous once-daily administration of sunitinib were comparable to those observed in earlier randomized studies6. The results of previous studies also demonstrated that sunitinib is widely used in the first- or second-line settings in metastatic rcc in Turkey.
The natural history of metastatic rcc is quite variable. The role of factors that predict outcomes is therefore an important consideration in the evaluation and development of new treatment strategies. In this era of vegf-targeted therapies, new prognostic variables are required for clinical trial design, patient counselling, and risk-directed therapy. Currently, the most widely used prognostic model comes from the Memorial Sloan–Kettering Cancer Center (mskcc). The model developed at mskcc classifies risk in rcc patients as favourable, intermediate, or poor according to the number of factors that predict survival7. Although the mskcc model was independently validated by investigators at the Cleveland Clinic8 and used for the study and interpretation of cytokine and targeted drug therapies, it is important to note that the prognostic risk profiles were derived during the era of immunotherapy and were limited to a population of patients eligible for participation in immunotherapy-based clinical trials. In addition, a question commonly asked by clinicians is whether rcc patients in clinical trials are representative of the rcc patients seen in ordinary clinical practice. Many patients with rcc, particularly those with poorer prognoses, do not meet trial inclusion criteria. Thus, new prognostic profiles with updated survival data are needed to reflect the current treatment paradigm for patients with metastatic rcc.
The aim of the present study was therefore to investigate the prognostic clinicopathologic factors associated with os and pfs in the once-daily continuous administration of first-line sunitinib delivered using an institutional treatment protocol in a consecutive cohort of Turkish patients with metastatic rcc.
2. METHODS
2.1. Patient Population
Our study enrolled 77 patients with histologically verified metastatic rcc who were treated with sunitinib between April 2006 and April 2011. Patient data were collected from a consecutive prospective patient series at the Institute of Oncology, Istanbul University, Istanbul, Turkey. Demographics and clinicopathologic characteristics—including age, sex, rcc histologic subtype, Eastern Cooperative Oncology Group (ecog) performance status (ps), sites of metastasis, laboratory findings, and patient survival—were recorded from charts. In all rcc patients, the diagnosis was based on a histologic analysis of specimens obtained by radical nephrectomy or ultrasonography-guided needle biopsy. All available slides were retrieved and reviewed by a single expert genitourinary pathologist (IK).
Patients were included in the study independent of histologic subtype. Patients with an ecog ps of 4 and those with severe concomitant medical illnesses were excluded. All patients received sunitinib as first-line systemic treatment on an outpatient basis. Sunitinib was administered at a once-daily dose of 37.5 mg. Treatment cycles were repeated every 4 weeks without interruption between cycles unless participants experienced disease progression or severe toxicity. A dose reduction of sunitinib (to 25 mg) was allowed depending on the type and severity of adverse events. Treatment was discontinued in patients with progression or severe toxicity after dose reduction. Thyroid dysfunction and arterial hypertension were managed with appropriate medication without dose reduction.
Each patient was classified according to the mskcc risk scoring system at the beginning of the treatment period7. Computed tomography or magnetic resonance imaging was performed at baseline and every third treatment cycle to assess clinical response according to the Response Evaluation Criteria in Solid Tumors, version 1.09.
The local institutional review board approved the study, and all patients provided written informed consent before participating. The study met the requirements of the Declaration of Helsinki.
2.2. Statistical Analysis
The prognostic variables investigated in our analysis were based on a general review of pretreatment features. The os was calculated from the date of the first dose of sunitinib to either the date of death, the date of the final follow-up visit, or the study end date. The pfs was calculated from the date of the first dose of sunitinib to the date of death from any cause or to disease progression as defined by the Response Evaluation Criteria in Solid Tumors.
Survival curves were estimated using the Kaplan–Meier method, and the log-rank test was used to compare curves. All baseline factors were examined as binary variables. Each variable was investigated using a univariate analysis for os and pfs. Multivariate analysis was not performed because of the limited sample size and the low number of outcome events. All p values represent 2-sided tests of statistical significance, with p < 0.05 being considered statistically significant. The SPSS software application (version 16.0: SPSS, Chicago, IL, U.S.A.) was used for the statistical analyses.
3. RESULTS
3.1. Clinical Features
The median patient age was 58 years (range: 26–80 years), and the 77 patients included 50 men (65%) and 27 women (35%). The diagnosis in 68 patients (88%) was clear cell rcc; the others were diagnosed with papillary (n = 8) or chromophobe (n = 1) rcc. According to the mskcc r isk scoring system, 19% of the patients were classed as favourable-risk (n = 15); 53%, as intermediate-risk (n = 41); and 27% as poor-risk (n = 21). Most of the patients (n = 68, 88%) had already undergone nephrectomy. Metastasectomy, including lung and retroperitoneal areas, had been performed in 17 patients (22%). The most frequent sites of metastasis were lung (49 patients, 64%), lymph nodes (36 patients, 47%), bone (31 patients, 40%), liver (15 patients, 19%), and brain (8 patients, 10%).
3.2. Patient Outcome
The median treatment duration was 10 months (range: 2–42 months), with 26% of patients achieving a confirmed partial response; 3%, a complete response; and 48%, stable disease. The remaining 23% of patients experienced disease progression. At the time of the present analysis, 42 patients had developed disease progression, and 28 patients had died.
3.2.1. Survival Analyses
Median follow-up was 18.5 months (range: 2–43 months). At study end, 47 patients (64%) were alive, and 2 patients had been lost to follow-up. Estimated median pfs and os were 13 months (95% confidence interval: 5.6 to 20.4 months) and 25 months (95% confidence interval: 18.7 to 31.2 months) respectively.
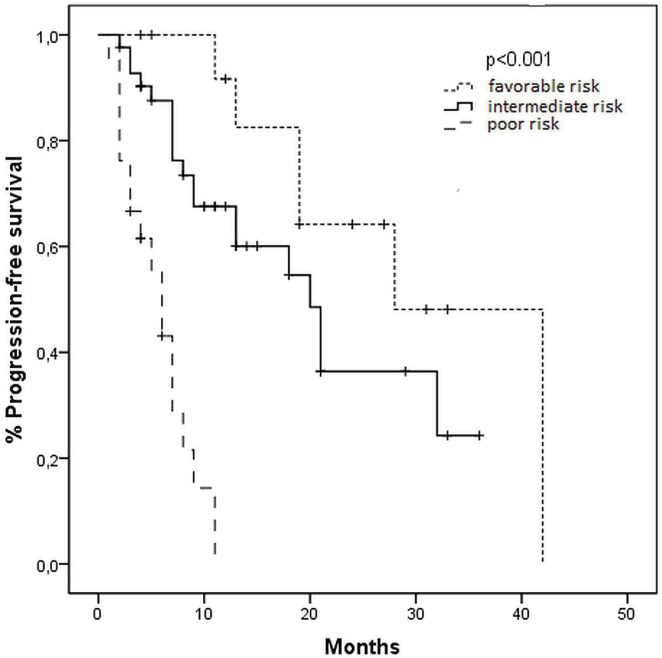
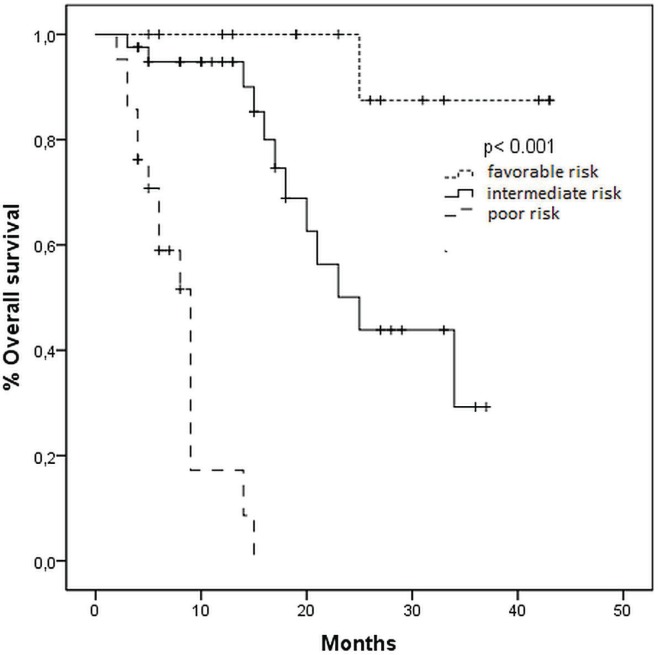
Median pfs stratified by mskcc risk status was 28, 21, and 6 months for favourable-, intermediate-, and poor-risk patients respectively (p < 0.001, Figure 1). Median os time was not reached for the favourable-risk group, 25 months for the intermediate-risk group, and 9 months for the poor-risk group (p < 0.001, Figure 2).
FIGURE 1.
Kaplan–Meier curves for progression-free survival in patients treated with sunitinib, by risk group (Memorial Sloan–Kettering Cancer Center criteria).
FIGURE 2.
Kaplan–Meier curves for overall survival in patients treated with sunitinib, by risk group (Memorial Sloan–Kettering Cancer Center criteria).
3.2.2. Univariate Analysis of Risk Factors for PFS and OS
Table i presents all 21 covariates, with their univariate analyses. In the mskcc score, 4 of the 5 factors—ecog ps of 2 or greater, low hemoglobin, high corrected serum calcium, and high lactate dehydrogenase (ldh)—were associated with poor pfs and os. In addition to those factors, hypoalbuminemia, more than 2 metastatic sites, liver metastasis, non–clear cell histology, and presence of sarcomatoid features on pathology were also associated with poor pfs. Nuclear grade, prior radiotherapy, and lung metastasis did not appear to have an impact on pfs. The pfs was also unaffected by sex, age, platelet count, neutrophil count, creatinine, time from diagnosis to current treatment, nephrectomy, and bone or brain metastasis. Male sex, hypoalbuminemia, prior radiotherapy, more than 2 metastatic sites, lung metastasis, nuclear grade 3 or 4 of the primary tumour, and presence of sarcomatoid features were also associated with poorer os. Nephrectomy and non–clear cell histology had a borderline significant impact on os. Notably, no other factors were associated with differences in os.
TABLE I.
Univariate analyses of survival by clinicopathologic and laboratory covariates
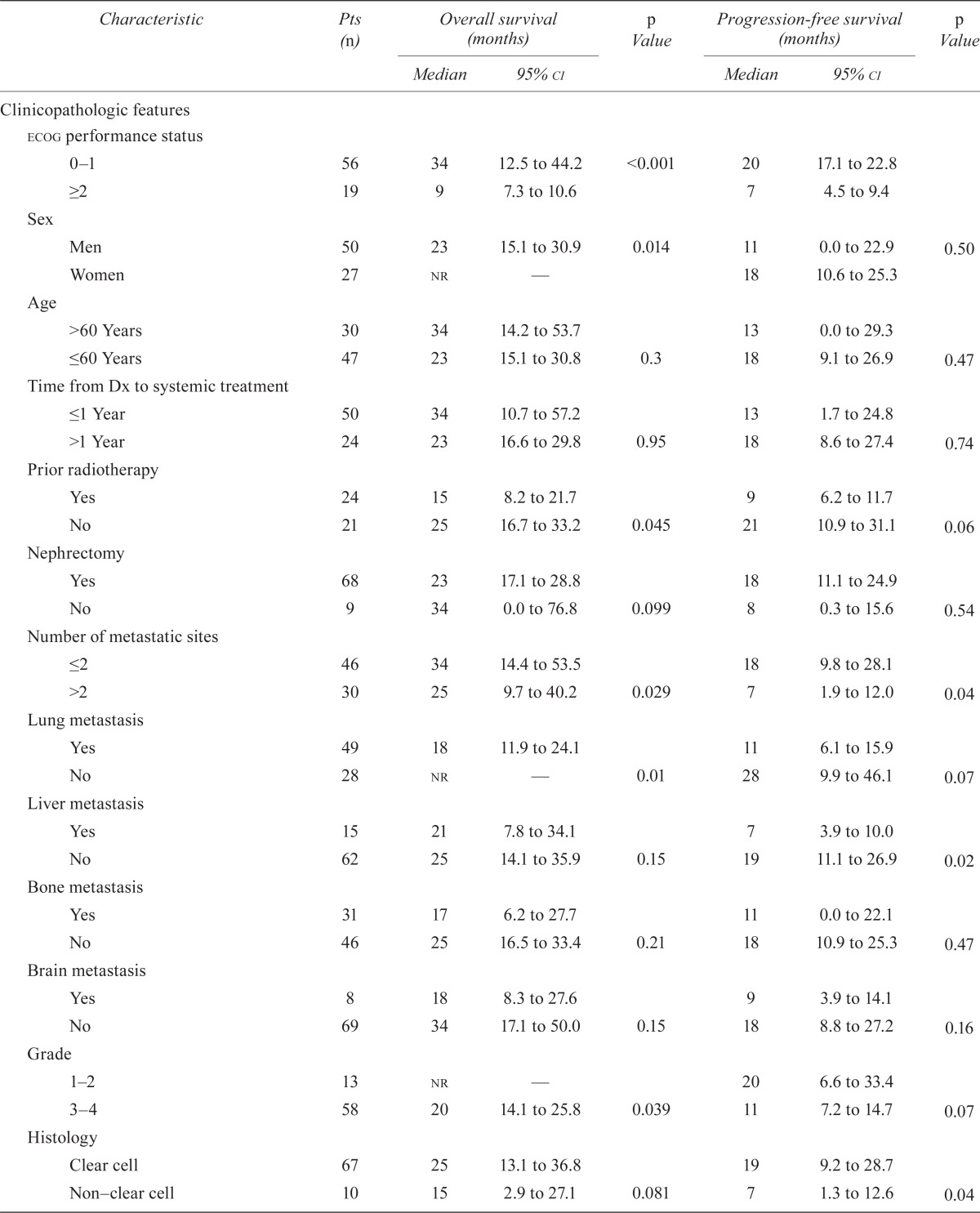
| Characteristic | Pts (n) |
Overall survival (months)
|
p Value |
Progression-free survival (months)
|
p Value | ||
|---|---|---|---|---|---|---|---|
| Median | 95% ci | Median | 95% ci | ||||
| Clinicopathologic features | |||||||
| ecog performance status | |||||||
| 0–1 | 56 | 34 | 12.5 to 44.2 | <0.001 | 20 | 17.1 to 22.8 | |
| ≥2 | 19 | 9 | 7.3 to 10.6 | 7 | 4.5 to 9.4 | ||
| Sex | |||||||
| Men | 50 | 23 | 15.1 to 30.9 | 0.014 | 11 | 0.0 to 22.9 | 0.50 |
| Women | 27 | nr | — | 18 | 10.6 to 25.3 | ||
| Age | |||||||
| >60 Years | 30 | 34 | 14.2 to 53.7 | 13 | 0.0 to 29.3 | ||
| ≤60 Years | 47 | 23 | 15.1 to 30.8 | 0.3 | 18 | 9.1 to 26.9 | 0.47 |
| Time from Dx to systemic treatment | |||||||
| ≤1 Year | 50 | 34 | 10.7 to 57.2 | 13 | 1.7 to 24.8 | ||
| >1 Year | 24 | 23 | 16.6 to 29.8 | 0.95 | 18 | 8.6 to 27.4 | 0.74 |
| Prior radiotherapy | |||||||
| Yes | 24 | 15 | 8.2 to 21.7 | 9 | 6.2 to 11.7 | ||
| No | 21 | 25 | 16.7 to 33.2 | 0.045 | 21 | 10.9 to 31.1 | 0.06 |
| Nephrectomy | |||||||
| Yes | 68 | 23 | 17.1 to 28.8 | 18 | 11.1 to 24.9 | ||
| No | 9 | 34 | 0.0 to 76.8 | 0.099 | 8 | 0.3 to 15.6 | 0.54 |
| Number of metastatic sites | |||||||
| ≤2 | 46 | 34 | 14.4 to 53.5 | 18 | 9.8 to 28.1 | ||
| >2 | 30 | 25 | 9.7 to 40.2 | 0.029 | 7 | 1.9 to 12.0 | 0.04 |
| Lung metastasis | |||||||
| Yes | 49 | 18 | 11.9 to 24.1 | 11 | 6.1 to 15.9 | ||
| No | 28 | nr | — | 0.01 | 28 | 9.9 to 46.1 | 0.07 |
| Liver metastasis | |||||||
| Yes | 15 | 21 | 7.8 to 34.1 | 7 | 3.9 to 10.0 | ||
| No | 62 | 25 | 14.1 to 35.9 | 0.15 | 19 | 11.1 to 26.9 | 0.02 |
| Bone metastasis | |||||||
| Yes | 31 | 17 | 6.2 to 27.7 | 11 | 0.0 to 22.1 | ||
| No | 46 | 25 | 16.5 to 33.4 | 0.21 | 18 | 10.9 to 25.3 | 0.47 |
| Brain metastasis | |||||||
| Yes | 8 | 18 | 8.3 to 27.6 | 9 | 3.9 to 14.1 | ||
| No | 69 | 34 | 17.1 to 50.0 | 0.15 | 18 | 8.8 to 27.2 | 0.16 |
| Grade | |||||||
| 1–2 | 13 | nr | — | 20 | 6.6 to 33.4 | ||
| 3–4 | 58 | 20 | 14.1 to 25.8 | 0.039 | 11 | 7.2 to 14.7 | 0.07 |
| Histology | |||||||
| Clear cell | 67 | 25 | 13.1 to 36.8 | 19 | 9.2 to 28.7 | ||
| Non–clear cell | 10 | 15 | 2.9 to 27.1 | 0.081 | 7 | 1.3 to 12.6 | 0.04 |
| Sarcomatoid | |||||||
| Without | 56 | nr | — | 20 | 12.6 to 27.3 | ||
| With | 21 | 9 | 7.2 to 10.8 | <0.001 | 7 | 4.1 to 9.8 | 0.01 |
| mskcc risk group | |||||||
| Favourable | 15 | nr | — | 28 | 9.9 to 46.0 | ||
| İntermediate | 41 | 25 | 21.2 to 28.7 | <0.001 | 21 | 12.1 to 29.9 | 0.01 |
| Poor | 21 | 9 | 7.4 to 10.5 | 6 | 4.1 to 7.8 | ||
| Laboratory data | |||||||
| Hemoglobina | |||||||
| Normal | 51 | nr | — | 19 | 11.1 to 26.9 | ||
| Anemia | 26 | 23 | 5.7 to 40.2 | 0.015 | 7 | 4.2 to 9.7 | 0.006 |
| Corrected calciumb | |||||||
| >10 mg/dL | 12 | 9 | 2.6 to 15.4 | 7 | 6.1 to 7.9 | ||
| ≤10 mg/dL | 63 | 34 | 19.5 to 48.5 | 0.006 | 19 | 10.6 to 27.3 | 0.03 |
| Lactate dehydrogenasec | |||||||
| ≥1.5x×uln | 7 | 9 | 4.8 to 13.2 | 7 | 0.0 to 19.8 | ||
| <1.5× uln | 68 | 25 | 18.8 to 31.2 | 0.006 | 19 | 10.6 to 27.4 | 0.01 |
| Neutrophil countd | |||||||
| ≥ uln | 23 | 15 | 7.9 to 22.0 | 9 | 3.6 to 14.3 | ||
| < uln | 51 | 34 | 19.6 to 48.4 | 0.16 | 19 | 10.6 to 27.4 | 0.30 |
| Platelet counte | |||||||
| ≥ uln | 19 | 23 | 0.0 to 52.6 | 19 | 5.5 to 32.5 | ||
| < uln | 56 | 25 | 17.8 to 32.1 | 0.3 | 13 | 4.9 to 21.1 | 0.50 |
| Creatinine | |||||||
| ≥1.5 mg/dL | 11 | 17 | 13.9 to 20.1 | 13 | 0.4 to 25.5 | ||
| <1.5 mg/dL | 62 | 25 | 14.1 to 35.8 | 0.36 | 19 | 8.2 to 29.7 | 0.12 |
| Albumin | |||||||
| ≥4 g/dL | 31 | 34 | 19.8 to 48.2 | 19 | 7.4 to 30.5 | ||
| <4 g/dL | 17 | 14 | 4.7 to 23.2 | 0.016 | 8 | 4.8 to 11.2 | 0.05 |
Lower limits of reference range: men, 13.0 g/dL; women, 11.5 g/dL.
Reference range was 8.3–10.7 mg/dL. Corrected calcium = measured total Ca (mg/dL) + 0.8 [4.0 – serum albumin (g/dL)].
Upper limit of reference range: 450 U/L.
Upper limit of reference range: 7.7×103/mm3.
Upper limit of reference range: 450×103/mm3.
Pts = patients; ci = confidence interval; ecog = Eastern Cooperative Oncology Group performance status; nr = not reported; Dx = diagnosis; mskcc = Memorial Sloan–Kettering Cancer Center; uln = upper limit of normal.
4. DISCUSSION
In the present analysis, we assessed prognostic factors affecting os and pfs in a series of 77 rcc patients treated with continuous daily sunitinib. In contrast with studies that used anti-vegf treatments, our study cohort was homogenous, and all patients received sunitinib as first-line treatment10–12. Additionally, patients were enrolled into the study consecutively, accurately reflecting current clinical practice in advanced rcc. Median pfs (13 months) and os (25 months), and the objective response rate (29%) in our patient cohort were found to be comparable with the results of previously reported randomized studies (11 and 26 months, 4 months, and 31% respectively)2.
Therapies targeting vegf have created a new environment for clinical trial development and patient care involving patients with metastatic rcc. Prognostic and predictive models are required to adequately stratify patients in clinical trials, to provide relevant clinical information to patients receiving therapy, and to facilitate risk-directed treatment selection in clinical practice13. For example, sunitinib or bevacizumab plus interferon alfa have been cited as the preferred treatment options for metastatic rcc patients with favourable- or intermediate-risk features14. In contrast, temsirolimus has been recommended as a preferred treatment option for rcc patients with poor-risk features, because of a large phase iii trial that was directed primarily to that patient population15. A biologic surrogate marker that predicts a favourable response to a targeted agent is preferred before a patient receives that agent. Currently, validated markers do not exist, although certain positive associations have recently been published16–18. Until such markers are prospectively validated, patient selection will rely on the baseline clinicopathologic characteristics of candidates for targeted therapies.
One of the most widely used predictive models for patients with metastatic rcc is the mskcc system developed by Motzer et al., which categorizes patients into favourable-, intermediate-, and poor-risk groups according to number of adverse factors, a time from diagnosis to start of systemic therapy of less than 1 year, elevated serum ldh, high corrected serum calcium, anemia, and low performance status. In 2005, Mekhail et al.8 suggested several modifications to the “2002 Motzer score” variables, such as the addition of prior exposure to radiotherapy and of variables indicating the presence of nodal, hepatic, and lung metastases. In 2009, a large retrospective study by Heng et al.10 of prognostic factors for os in patients receiving vegf-targeted agents devised and internally validated a model that replicates the Motzer methodology and relies on 4 of the 5 Motzer criteria (hemoglobin, corrected calcium, Karnofsky ps, and time from diagnosis to treatment) in addition to neutrophil and platelet counts. Other recent data from Patil et al.13 were prospectively obtained from patients treated with first-line sunitinib or interferon alfa in a phase iii clinical trial. For sunitinib, a multivariate analysis of pfs identified 5 independent predictors: serum ldh, presence of 2 or more metastatic sites, no prior nephrectomy, ecog ps, and baseline platelet count. By contrast, a multivariate analysis of os identified serum ldh, corrected serum calcium, time from diagnosis to treatment, hemoglobin, ecog ps, and presence of bone metastasis as predictors.
In our study, laboratory parameters (hypoalbuminemia, anemia, hypercalcemia, and high ldh), histopathologic data (high nuclear grade, non–clear cell histology, and presence of sarcomatoid features on pathology), more than 2 metastatic sites, and poor ecog ps were associated with adverse outcomes. Karnofsky ps, anemia, ldh elevation, and hypercalcemia are factors that have previously been reported in the mskcc criteria and that might reflect any or all of increased tumour burden, aggressive tumour biology, or paraneoplastic processes8,19,20. Furthermore, neutrophilia and thrombocytosis might be markers of inflammation related to the overproduction of cytokines resulting from increased tumour burden or aggressive tumour biology21,22. However, our study did not reveal any association between those variables and survival.
The prognostic role of metastasis localization was also evident. Outcomes were improved in metastatic rcc patients with pulmonary metastasis, but pfs was inferior in patients with hepatic metastasis. Bone and brain metastasis had no effect on prognosis. Factors that are traditionally included—such as time from diagnosis to current treatment and nephrectomy status—were not found to be informative in the present study. Nearly all the patients included in our study had already had a nephrectomy. The median os and pfs of the entire patient cohort (25 and 13 months respectively)—and of the patient strata with favourable (os: not reached; pfs: 28 months), intermediate (os: 25 months; pfs: 21 months), and poor prognosis (os: 9 months; pfs: 6 months)—are longer than those seen in earlier studies from the era of immunotherapy2,7.
In the present study, sarcomatoid differentiation was associated with short and inferior pfs and os. Metastatic rcc with sarcomatoid differentiation is also associated with poor outcomes after chemotherapy or immunotherapy. In a recent study that included patients who had sarcomatoid rcc, the clinical response to vegf-targeted agents compared favourably with responses to chemotherapy or immunotherapy in earlier studies23. The same study also suggested that outcomes with anti-vegf therapy were better in patients with underlying clear cell histology than in those with non–clear cell sarcomatoid metastatic rcc. Further validation of the suggestion that sarcomatoid differentiation is a negative prognostic indicator for os is required.
One limitation of our study is that the sample was relatively small and came from a single oncology centre in Turkey, which limits the generalizability of the results. In addition, the sample was drawn from a heterogeneous population: just 9 patients had non–clear cell carcinoma. This study also did not address or investigate the prognostic value of molecular markers for rcc. Prospective investigation of clinical and molecular features in a large number of patients with rcc will be required. Future studies performing similar evaluations of patients with metastatic rcc in other countries are needed.
5. CONCLUSIONS
The mskcc prognostic factors appear to retain their validity for predicting survival in metastatic rcc in the era of molecular targeted therapy. Continued progress in the identification of patient-specific prognostic factors for metastatic rcc will require further advances in the knowledge of tumourspecific biology.
6. ACKNOWLEDGMENTS
We thank all the patients and their families, and the investigators from the various sites and their staff members, for their participation in this study.
7. CONFLICT OF INTEREST DISCLOSURES
The authors declare that they have no financial conflicts of interest.
8. REFERENCES
- 1.Motzer RJ. New perspectives on the treatment of metastatic renal cell carcinoma: an introduction and historical overview. Oncologist. 2011;16(suppl 2):1–3. doi: 10.1634/theoncologist.2011-S2-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 3.Mendel DB, Laird AD, Xin X, et al. In vivo antitumour activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–37. [PubMed] [Google Scholar]
- 4.Hutson TE, Figlin RA. Novel therapeutics for metastatic renal cell carcinoma. Cancer. 2009;115(suppl):2361–7. doi: 10.1002/cncr.24235. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–90. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yildiz I, Sen F, Basaran M, et al. Response rates and adverse effects of continuous once-daily sunitinib in patients with advanced renal cell carcinoma: a single-center study in Turkey. Jpn J Clin Oncol. 2011;41:1380–7. doi: 10.1093/jjco/hyr151. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–96. doi: 10.1200/JCO.20.1.289. [DOI] [PubMed] [Google Scholar]
- 8.Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan–Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol. 2005;23:832–41. doi: 10.1200/JCO.2005.05.179. [DOI] [PubMed] [Google Scholar]
- 9.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 10.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–9. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 11.Choueiri TK, Garcia JA, Elson P, et al. Clinical factors associated with outcome in patients with metastatic clear-cell renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. Cancer. 2007;110:543–50. doi: 10.1002/cncr.22827. [DOI] [PubMed] [Google Scholar]
- 12.Yuasa T, Tsuchiya N, Urakami S, et al. Clinical efficacy and prognostic factors for overall survival in Japanese patients with metastatic renal cell cancer treated with sunitinib. BJU Int. 2012;109:1349–54. doi: 10.1111/j.1464-410X.2011.10534.x. [DOI] [PubMed] [Google Scholar]
- 13.Patil S, Figlin RA, Hutson TE, et al. Prognostic factors for progression-free and overall survival with sunitinib targeted therapy and with cytokine as first-line therapy in patients with metastatic renal cell carcinoma. Ann Oncol. 2011;22:295–300. doi: 10.1093/annonc/mdq342. [DOI] [PubMed] [Google Scholar]
- 14.Molina AM, Motzer RJ. Current algorithms and prognostic factors in the treatment of metastatic renal cell carcinoma. Clin Genitourin Cancer. 2008;6(suppl 1):S7–13. doi: 10.3816/CGC.2008.s.002. [DOI] [PubMed] [Google Scholar]
- 15.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 16.Choueiri TK, Vaziri SA, Jaeger E, et al. Von Hippel–Lindau gene status and response to vascular endothelial growth factor targeted therapy for metastatic clear cell renal cell carcinoma. J Urol. 2008;180:860–5. doi: 10.1016/j.juro.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Anglesio MS, George J, Kulbe H, et al. il6–stat3–hif signaling and therapeutic response to the angiogenesis inhibitor sunitinib in ovarian clear cell cancer. Clin Cancer Res. 2011;17:2538–48. doi: 10.1158/1078-0432.CCR-10-3314. [DOI] [PubMed] [Google Scholar]
- 18.Tran HT, Liu Y, Zurita AJ, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol. 2012;13:827–37. doi: 10.1016/S1470-2045(12)70241-3. [DOI] [PubMed] [Google Scholar]
- 19.Kim HL, Belldegrun AS, Freitas DG, et al. Paraneoplastic signs and symptoms of renal cell carcinoma: implications for prognosis. J Urol. 2003;170:1742–6. doi: 10.1097/01.ju.0000092764.81308.6a. [DOI] [PubMed] [Google Scholar]
- 20.Sandhu DP, Munson KW, Harrop JS, Hopton MR, Ratcliffe WA. Humoral hypercalcaemia in renal carcinoma due to parathyroid hormone related protein. Br J Urol. 1993;72:848–50. doi: 10.1111/j.1464-410X.1993.tb16285.x. [DOI] [PubMed] [Google Scholar]
- 21.Donskov F, von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J Clin Oncol. 2006;24:1997–2005. doi: 10.1200/JCO.2005.03.9594. [DOI] [PubMed] [Google Scholar]
- 22.Suppiah R, Shaheen PE, Elson P, et al. Thrombocytosis as a prognostic factor for survival in patients with metastatic renal cell carcinoma. Cancer. 2006;107:1793–800. doi: 10.1002/cncr.22237. [DOI] [PubMed] [Google Scholar]
- 23.Golshayan AR, George S, Heng DY, et al. Metastatic sarcomatoid renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. J Clin Oncol. 2009;27:235–41. doi: 10.1200/JCO.2008.18.0000. [DOI] [PubMed] [Google Scholar]