Abstract
Background
The decline of photosynthesis in plants under low sink demand is well known. Previous studies focused on the relationship between stomatal conductance (g s) and net photosynthetic rate (P n). These studies investigated the effect of changes in Photosystem II (PSII) function on the P n decline under low sink demand. However, little is known about its effects on different limiting steps of electron transport chain in PSII under this condition.
Methodology/Principal Finding
Two-month-old bean plants were processed by removing pods and flowers (low sink demand). On the 1st day after low sink demand treatment, a decline of P n was accompanied by a decrease in g s and internal-to-ambient CO2 concentration ratio (C i/C a). From the 3rd to 9th day, P n and g s declined continuously while C i/C a ratio remained stable in the treatment. Moreover, these values were lower than that of control. Wk (a parameter reflecting the damage to oxygen evolving complex of the donor side of PSII) values in the treatment were significantly higher than their corresponding control values. However, RCQA (a parameter reflecting the number of active RCs per excited cross-section of PSII) values in the treatment were significantly lower than control from the 5th day. From the 11th to 21st day, P n and g s of the treatment continued to decline and were lower than control. This was accompanied by a decrease of RCQA, and an increase of Wk. Furthermore, the quantum yield parameters φ Po, φ Eo and ψ Eo in the treatment were lower than in control; however, C i/C a values in the treatment gradually increased and were significantly higher than control on the 21st day.
Conclusions
Stomatal limitation during the early stage, whereas a combination of stomatal and non-stomatal limitation during the middle stage might be responsible for the reduction of P n under low sink demand. Non-stomatal limitation during the late stages after the removal of the sink of roots and pods may also cause P n reduction. The non-stomatal limitation was associated with the inhibition of PSII electron transport chain. Our data suggests that the donor side of PSII was the most sensitive to low sink demand followed by the reaction center of PSII. The acceptor side of PSII may be the least sensitive.
Introduction
The hypothesis for the end-product inhibition of photosynthesis was proposed over a century ago [1]. According to this concept, owing to low sink demand, the accumulation of assimilates in source leaves may be responsible for the reduction of the photosynthetic rate. This reduction is accomplished by inhibiting the activities of the related metabolic enzymes as a direct feedback, which is also called feed forward [2], [3]. Several studies focused on investigating the photosynthetic response of plants to sink-source manipulation. These studies attempted to understand the mechanism of change in photosynthesis in many perennial woody species or annual herbaceous plants under low sink demand [4]–[12].
Studies on ‘accumulation of assimilates’ in source leaves showed some evidence supporting the hypothesis of end-product inhibition of photosynthesis [13], [14]. However, other studies, including our study on peaches and apples did not show a relationship between assimilate accumulation and the reduction of net photosynthetic rate (P n) under low sink demand [4], [6]–[8], [11], [15]. The mechanism by which low sink demand affects photosynthesis is still unclear.
In general, a decrease in P n is accompanied by partial stomatal closure of source leaves under low sink demand [16]. Hence, down-regulated stomatal conductance (g s) was suggested as the initial response to low sink manipulation [4], [6]–[8], [11].The reduction of intercellular CO2 concentrations (C i) caused by lower g s restricts the rate of gas exchange via stomata, resulting in stomatal limitation of photosynthesis [17]. Furthermore, the structure and function of photosystem II (PSII) is inhibited or damaged, leading to a non-stomatal limitation of photosynthesis [6], [18]. Therefore, stomatal limitation of photosynthesis takes place mostly under low PAR (photosynthetic active radiation) or during the early period while the non-stomatal limitations occur under high PAR (i.e. around noon) or during the late period under low sink demand [19].
Inhibition of PSII on the electron donor side causes a decrease in chlorophyll fluorescence, whereas inhibition on the electron acceptor side increases it [20], [21]. Thus, chlorophyll fluorescence may be used to detect changes in the photosynthetic apparatus in vivo [21]–[23]. Since the non-destructive measurements can be done with a high resolution of 10 µs, Strasser et al. developed a method for the analysis of increase in kinetics of fast fluorescence [24]–[27]. All oxygenic photosynthetic materials investigated so far have shown a polyphasic fluorescence rise consisting of a sequence of phases, denoted as O, J, I, and P (OJIP test), where O stands for the minimum fluorescence level, P is for the peak, and J and I are intermediate levels between O and P levels [22], [23], [28]. The OJIP-test is a powerful tool for the in vivo investigation of the structural property and photosynthetic activity of PSII. This includes the activity of donor side (i.e. oxygen evolving complex, OEC), reaction center (i.e. reaction center chlorophyll of PSII, P680) and acceptor side (i.e. pheophytin (Phe), plastoquinone (QA and QB)), for light absorption, energy trapping, and electron transport [23]–[32]. Hence, Chlorophyll a fluorescence technique helps understand the mechanism of down-regulation of P n after the sink demand is reduced. Low sink demand and non-stomatal limitation of P n cause a decrease in the fraction of light energy used in electron transport [6]. However, the limiting steps (donor acceptor side, reaction center or acceptor side) of electron transport chain are not well understood. Moreover, the response process under low sink demand is dynamic. Previous studies mainly focused on the diurnal variations in photosynthesis during the early period, or a few days (at most one week) after source-sink manipulation [6]–[8], [11], [19]. Therefore, studying the mechanism of long term effects of low sink demand on P n would provide new insights into this process.
In the present study, we examined the short and long term response of photosynthesis to low sink demand in bean (Vicia faba L.) plants. We used photosynthetic gas exchange and the OJIP-test technique to study the limiting steps of photosynthetic electron transport (including donor side, reaction center, and acceptor side of PSII). The objective of this study was to understand the underlying relationship between net photosynthetic rate and the electron transport chain of PSII under reduced sink demand in higher plants.
Materials and Methods
Plant Materials
The experiment was carried out during the month of October and November in 2012 at the Institute of Botany, Chinese Academy of Sciences, in Beijing, China. Seeds of ‘Daqingshan’ fava bean of uniform size were sown in plastic pots (15 cm diameter) containing plant peat moss and garden soil (1∶1, v/v). Routine commercial bean production irrigation and pest control methods were applied. The plants were grown in a greenhouse and cultivated as a single shoot under natural sunlight conditions at 30–60% relative humidity, a temperature of 18–32°C, and noon maximum photosynthetically active radiation (PAR) of about 1000 µmol photons m−2 s−1 in greenhouse.
Treatments
Two-month-old bean plants of uniform size with eight mature compound leaves and five pods were topped (Oct. 17), and were divided into two groups. Six days later (beginning at 1800 h, Oct. 23), one group was subjected to low sink demand (LS) manipulation. This was done by girdling the base of the shoot (a horizontal 5-mm-wide band) with a razor blade in order to block the transport of assimilates from source leaves to the root while allowing water flow via xylem. In addition, pods and flowers were removed completely to minimize sink demand. For the control group, a longitudinal girdling of the same area as the horizontal girdling band was applied to the same part of the plants, in order to minimize the possible effect of physical injury [6], [11]. Five biological replicates were taken for all experiments.
Measurement of Photosynthetic Gas Exchange Parameters
Terminal leaflets of the third fully expanded compound leaves were sampled from plants subject to a fixed PAR of 1100 µmol photons m−2s−1, a flow rate of 500 µmol s−2, and a 6 cm2 leaf area. These leaflet samples were used to measure gas exchange parameters including P n, g s, C i and ambient CO2 concentration (C a) using a portable photosynthesis system Li-6400 (Li-Cor Inc., Lincoln, NE). The measurements of photosynthetic gas exchange were carried out between 0800 h and 1600 h on October 24, one day after initiating sink-source manipulation. The measurements were recorded only one time at 1300 h (photosynthetically active radiation at noon is usually the highest during a day) from the day 3 to 21 (the end of the experiment) at an interval of 2 to 5 days.
Chlorophyll a Fluorescence Kinetics Transient Analysis (OJIP-test)
The OJIP-test parameters were measured according to Luo et al. on the same leaves for which gas exchange estimations were done [33]. The measurements were carried out by using a Handy-Plant Efficiency Analyzer (Hansatech Instruments, King’s Lynn, Norfolk, UK) on leaves after dark adaptation for more than 15 min [34], [35]. The transients were induced by red light of about 3000 µmol m−2 s−1 provided by an array of six light emitting diodes (peak wavelength, 650 nm). The fluorescence signals were recorded from 10 µs to 1 s. The data acquisition rate was 10 µs for the first 2 ms and 1 ms each thereafter. The fluorescence signal at 50 µs was considered to be the level; therefore it was a true F o. The following data from the original measurements were used: F m: maximal fluorescence intensity; F k : fluorescence intensity at 300 µs [required for calculation of the initial slope (M o) of the relative variable fluorescence (V) kinetics and Wk]; F j : the fluorescence intensity at 2 ms (the J-step); F i : the fluorescence intensity at 30 ms (I-step) [26], [36]. The derived parameters were as follows: the parameter Wk on the donor side of PSII was assumed to represents the damage to oxygen evolving complex (OEC), Wk = (F k−F o)/(F j−F o); the parameter RCQA was assumed to represents the density of QA-reducing reaction centers (RCs), calculated as the number of active PSII RCs per cross section (CS) at t = tm: RCQA = RC/CSm = φ Po×(V j/M o)×(ABS/CSm), here, ABS represents the total photon flux absorbed by the PSII antenna pigments. According to the energy flux theory proposed by Strasser et al., the total ABS is partially trapped by PSII RCs, and the fraction of ABS used for reduce QA is labeled as TR. However, the electron transport flux from QA to QB is labeled as ET [24]; then the yield indices or flux ratios can be derived. The parameter φ Po can be considered as the maximum quantum yield of primary photochemistry, calculated as the ratio of TR/ABS at t = 0: φ Po = TRo/ABS = 1−F o/F m; the parameter ψ Eo was assumed to the probability that a trapped exciton moves an electron into the electron transport chain beyond QA −, ψ Eo = ET o/TR o = (F m−F j)/(F m−F o); the parameter φ Eo can be considered as the quantum yield of the electron transport flux from QA to QB (at t = 0), φ Eo = ET o/ABS = (F m−F j)/F m. All these parameters are shown in Table S1.
Statistical Analyses
Data were processed with SPSS 13.0 for Windows, and each value of the means and standard errors in the figures represents five replicates. Differences were considered significantly at a probability level of P<0.05 by a t - test.
Results
Gas Exchange Parameters
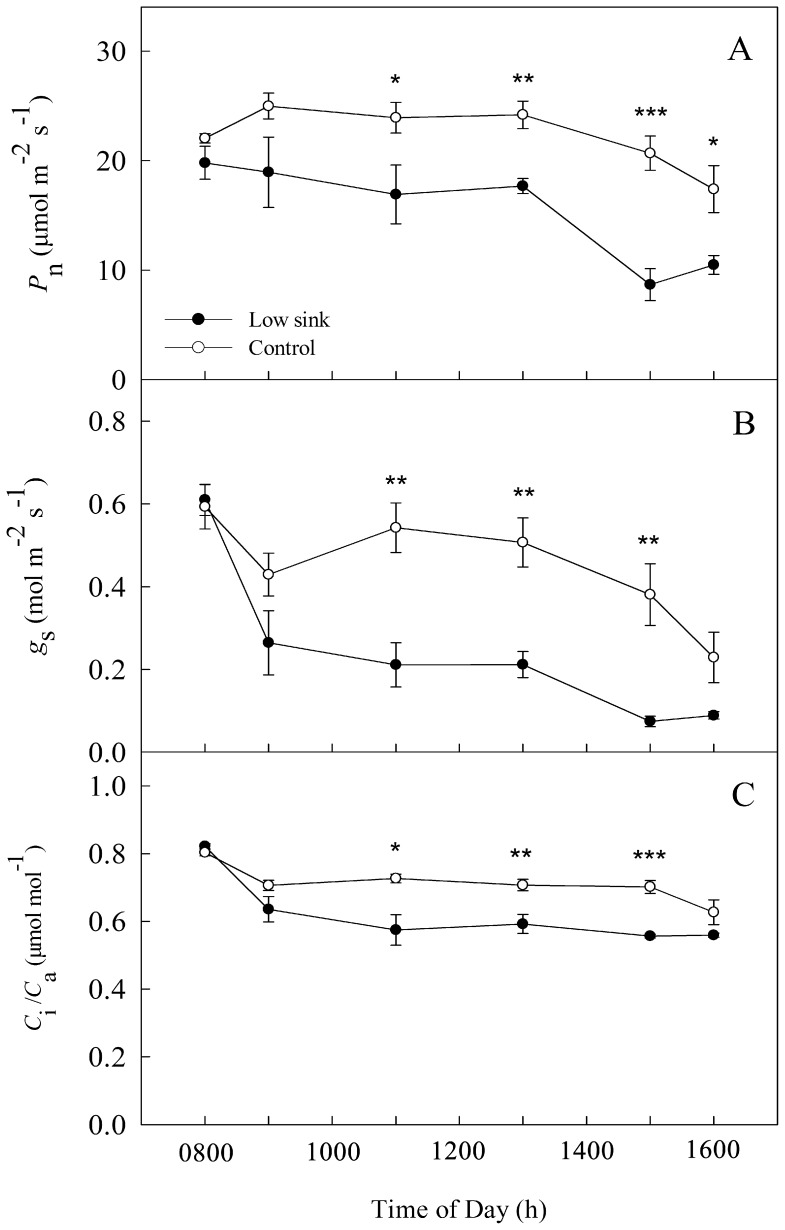
For investigating the changes in gas exchange parameters under low sink conditions, we removed roots and pods of fava bean seedlings at 1800 h, Oct. 23 of 2012. At 0800 and 0900 h on the 1st day after the low sink demand (LS) there were no differences on P n, g s and C i/C a (internal-to-ambient CO2 concentrations ratio) between LS and the control. However, P n, g s and C i/C a were significantly lower in the LS plants as compared with control from 1100 h to 1600 h (Fig. 1).
Figure 1. Diurnal variation in gas exchange parameters, including net photosynthesis rate (P n), stomatal conductance (g s) and internal-to-ambient CO2 concentration ratio (C i/C a) in bean source leaves in response to low sink demand on the 1st day after removing the sink of roots and pods.
Each value represents the mean of five replicates, and error bars represent ± S.E. The asterisks *, ** and *** indicate significant difference at P<0.05, 0.01 and 0.001 between the control and low sink, respectively.
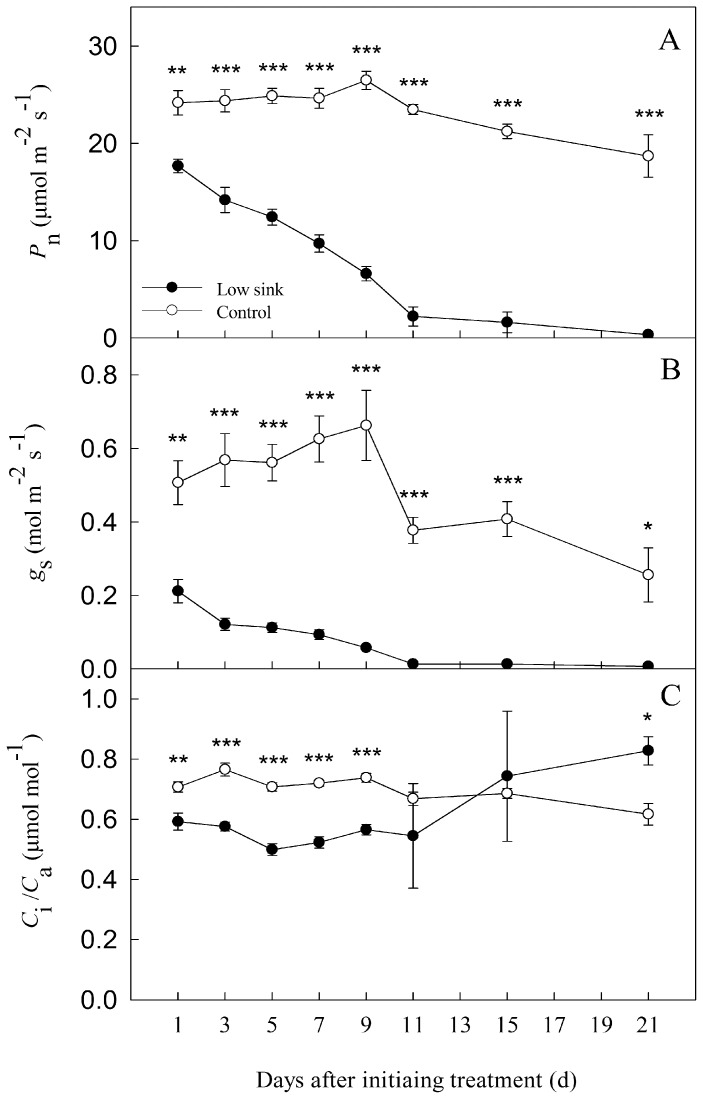
As shown in Fig. 2, from the 3rd to 9th day after removing sink demand of roots and pods, P n, g s and C i/C a at 1300 h in LS plants were significantly lower than their controls. Moreover, P n and g s gradually decreased in the plants under low sink demand while they remained relatively stable in the control plants.
Figure 2. The response of gas exchange parameters including net photosynthesis rate (P n), stomatal conductance (g s) and internal-to-ambient CO2 concentration ratio (C i/C a) in bean source leaves to the treatment of low sink demand at 1300 h from the 1st to 21st day after removing the sink of roots and pods.
Each value represents the mean of five replicates, and error bars represent ± S.E. The asterisks *, ** and *** indicate significant difference at P<0.05, 0.01 and 0.001 between the control and treatment, respectively.
From the 11th to 21st day, P n values at 1300 h in LS plants were still significantly lower than control, accompanied by lower g s. Moreover, P n in the plants under low sink demand continuously declined, and g s values were almost zero. During this period, C i/C a in the LS plants gradually rose, and reached the highest value on 21st day; moreover, this was higher than in the control (Fig. 2).
Chlorophyll a Fluorescence Parameters
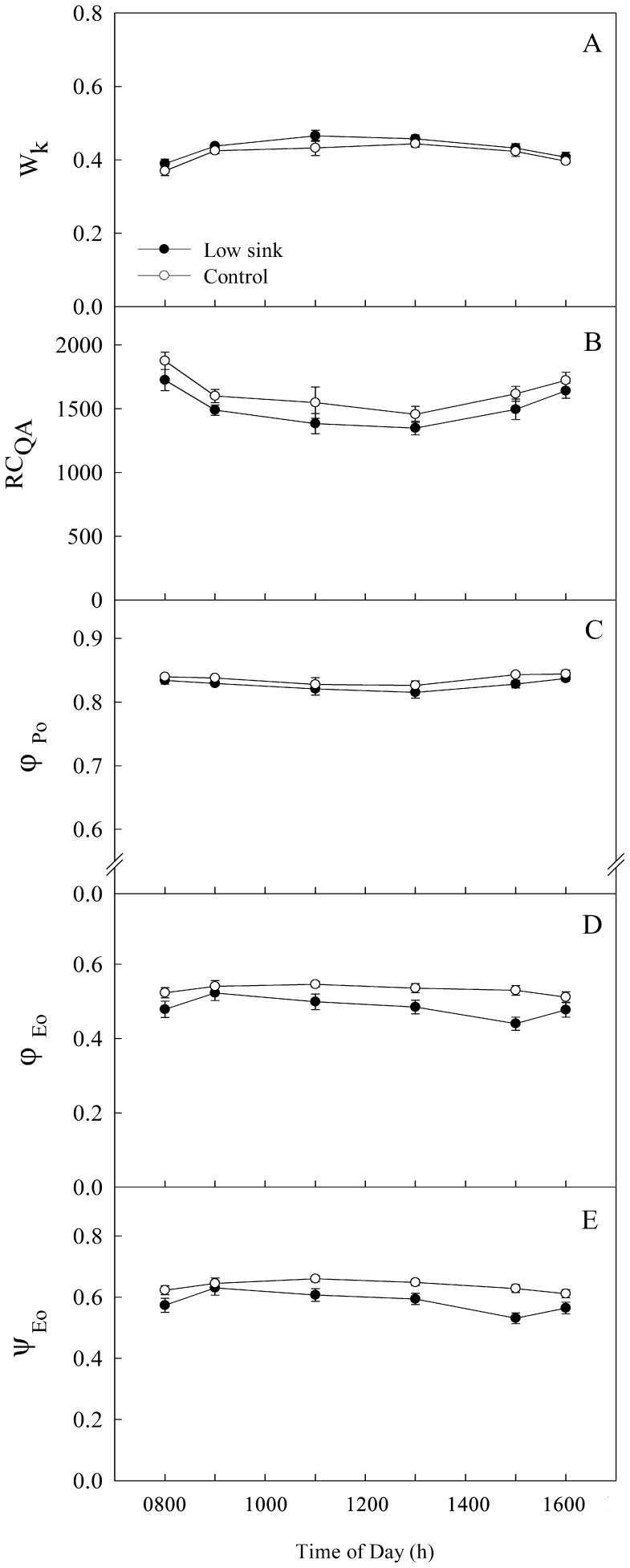
OJIP test was conducted in order to explore the relationship between P n decline and electron transport chain of PSII under LS treatment. We conducted the OJIP test at 0800 and 0900 h on the 1st day after sink demand of roots and pods were removed. OJIP-test parameters Wk, RCQA, φ Po, φ Eo and ψ Eo remained relatively stable throughout the day in both control and LS plants on the first day after the sink demand of roots and pods was removed. Moreover, there were no significant differences in these parameters between LS and the control (Fig. 3).
Figure 3. The change in parameters including Wk, RCQA, φ Po, φ Eo and ψ Eo involved in electron transport chain of PSII in bean (V. faba L.) source leaves on the 1st day after removing the sink of roots and pods.
Each value represents the mean of five replicates, and error bars represent ±S.E. The asterisks *, ** and *** indicate significant difference at P<0.05, 0.01 and 0.001 between the control and treatment, respectively.
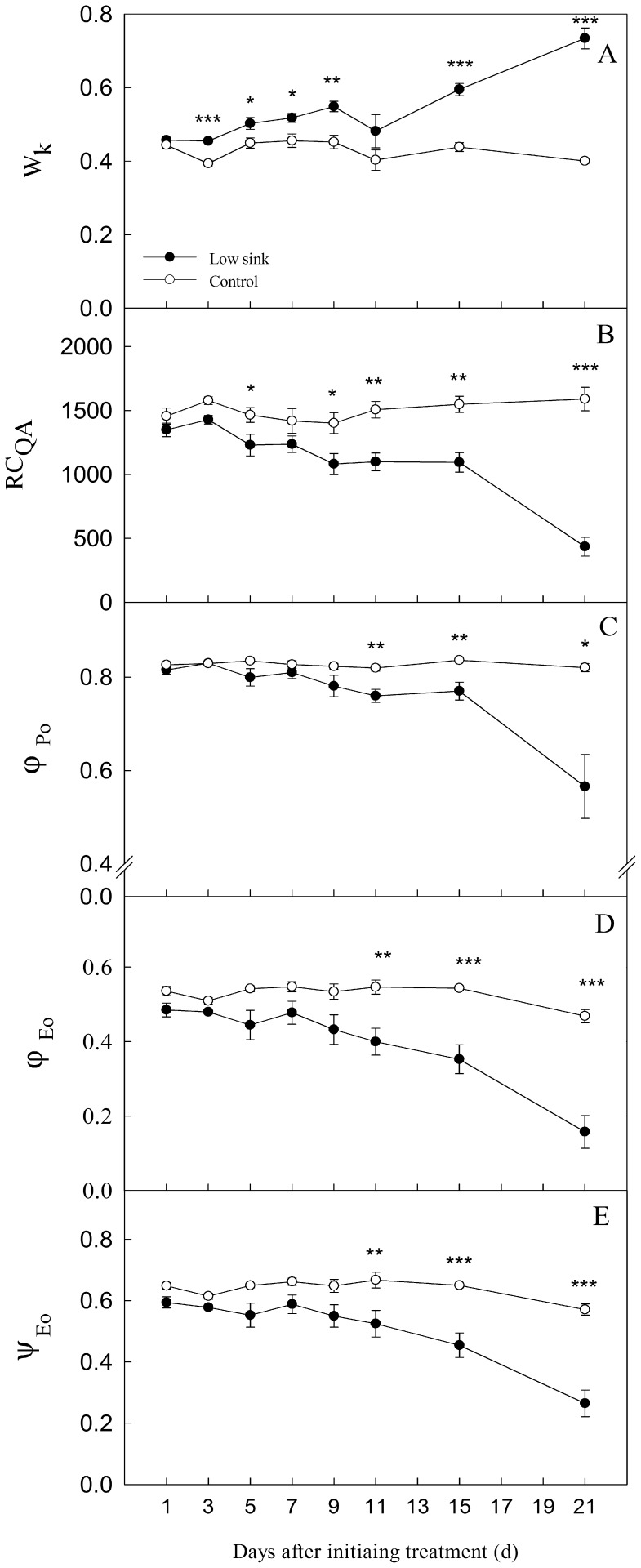
From the 3rd day through the end of the experiment, Wk, RCQA, φ Po, φ Eo and ψ Eo remained relatively stable in control plants. Wk increased, but RCQA, φ Po, φ Eo and ψ Eo decreased progressively in LS plants (Fig. 4). However, there were differences in the sensibility of these parameters in response to low sink demand of plants.
Figure 4. The change in parameters including Wk, RCQA, φ Po, φ Eo and ψ Eo involved in electron transport chain of PSII in bean (V. faba L.) source leaves at 1300 h from the 1st to 21st day after removing the sink of roots and pods.
Each value represents the mean of five replicates, and error bars represent ± S.E. The asterisks *, ** and *** indicate significant difference at P<0.05, 0.01 and 0.001 between the control and treatment, respectively.
The Wk was sensitive to low sink demand in plants. From the 3rd day after removing the sink of roots and pods, Wk values in LS plants were significantly higher than those in control (Fig. 4A). Moreover, the Wk of the last two measurements in LS plants on the 15th and 21st days after removing the sink demand increased sharply, resulting in about 35% and 83% higher values than control, respectively.
The RCQA values in LS plants significantly differed from those in control from the 5th day (Fig. 4B), then progressively decreased until the 15th day after the sink was removed. A sharp decrease in RCQA in LS plants was observed, but it was only 27% of the control value at the end of the experiment.
The parameters φ Po, φ Eo and ψ Eo of PSII showed a significant response to low sink was found. The treatment of low sink demand resulted in a significant decrease from the 11th day (Fig. 4C, D, E). In LS plants, the values for φ Po, φ Eo and ψ Eo were about 69%, 33% and 46% of control values at the end of the experiment, respectively.
Correlation of Different Parameters of Gas Exchange and Chlorophyll a Fluorescence under Low Sink Demand
Under low sink demand, P n was positively correlated with g s, RCQA, φ Po, φ Eo and ψ Eo and negatively correlated with C i/C a and Wk during the experimental period (Table 1). g s was positively correlated with RCQA, φ Po, φ Eo and ψ Eo and negatively correlated with Wk; however, g s had no significant correlation with C i/C a. C i/C a was positively correlated with Wk and negatively correlated with RCQA, φ Po, φ Eo and ψ Eo. Wk was negatively correlated with RCQA, φ Po, φ Eo and ψ Eo. There were positive correlations among RCQA, φ Po, φ Eo and ψ Eo.
Table 1. Correlation among different parameters of gas exchange and chlorophyll a fluorescence under low sink demand.
| P n | g s | C i/C a | Wk | RCQA | φ Po | φ Eo | ψ Eo | |
| P n | 1 | |||||||
| g s | 0.944** | 1 | ||||||
| C i/C a | −0.334** | −0.161 | 1 | |||||
| Wk | −0.717** | −0.609** | 0.531** | 1 | ||||
| RCQA | 0.721** | 0.623** | −0.447** | −0.909** | 1 | |||
| φ Po | 0.610** | 0.504** | −0.498** | −0.804** | 0.862** | 1 | ||
| φ Eo | 0.652** | 0.555** | −0.555** | −0.797** | 0.821** | 0.889** | 1 | |
| ψ Eo | 0.613** | 0.519** | −0.561** | −0.766** | 0.757** | 0.844** | 0.989** | 1 |
The asterisks **indicate a significant correlation at P<0.01.
Discussion
The reduction of P n in higher plants may result from stomatal or non-stomatal limitations or due to a combination of both [37]. When stomatal limitation of P n occurs, Ci (C i/C a) decreases in parallel with a decrease in g s. On the other hand, when non-stomatal limitation of P n occurs, C i (C i/C a) increases or remains stable in parallel with decreased g s [37], [38]. Simultaneous measurement of chlorophyll a fluorescence extends this analysis, providing a means to determine the states of electron transport and energy partitioning in the photosynthesis apparatus including PSII and PSI [37], [39]. The P n of plants under low sink demand declined significantly during the 1st day after removing the sink of roots and pods. This was accompanied by a decrease in g s and C i/C a (Fig. 1). These results are similar to Setters’ results on the soybean plants [10]. Moreover, there were no significant differences in chlorophyll a parameters of PSII between LS and the control (Fig. 2). At this stage, there was a typical stomatal limitation of P n.
The chlorophyll a fluorescence transient analysis (OJIP-test) is a powerful tool in photosynthesis research to probe the PSII reactions, which may express the state of donor side, reaction center and acceptor side of PSII [31], [40], [41]. Any treatment or stress condition, which affects the donor side capacity will make the K-step apparent in the OJIP-test [31]. Therefore, the K-step can be used as an indicator of injury to the donor side. In general, the ratio Wk was used to show the changes in the amplitude in the K step; the higher the Wk values, the larger was the damage to the PSII donor side [31], [42]–[46]. In the OJIP-test, RCQA is assumed to show the density of QA-reducing PSII reaction center; thus lower the RCQA values, the larger is the damage to PSII reaction center [41], [47]. Interestingly, from the 3rd day to the 9th day, as compared with control, P n of the LS plants continued to decline, accompanied by a decrease of g s; however, C i/C a remained relatively stable. Furthermore, during this period, Wk values in the LS plants were significantly higher while RCQA values were lower than in control (Fig. 4). Therefore, P n decline during this period was not mainly due to stomatal limitation, but also because of non-stomatal limitation. The change of Wk is the earliest among the OJIP parameters suggesting that the donor side might be the most sensitive to the treatment of low sink demand. Similarly, a decline in RCQA under LS was observed from the 5th day, indicating that the low sink demand may also result in the decrease in density of QA-reducing PSII reaction centers during the middle period. In summary, the donor side of PSII was the first to be damaged, followed by damage to the reaction center.
From the 11th to the 21st day of LS treatment, P n declined significantly, accompanied by a decrease in g s and RCQA; however, there was an increase in Wk and C i/C a. In addition, other PSII parameters such as φ Po, φ Eo and ψ Eo in LS plants became lower than those in control. This was probably due to the damage to the acceptor side of PSII. The lower the ψ Eo and φ Eo values, the greater is the damage to PSII [48]–[52]. The acceptor side of PSII was much less influenced compared with the donor side and the reaction center under low sink demand. It can be speculated that when the acceptor side of PSII was damaged, the P n decline was due to non-stomatal limitation. Moreover, throughout the LS treatment, P n was positively correlated with g s, RCQA, φ Po, φ Eo and ψ Eo while it was negatively correlated with C i/C a and Wk (Table 1). This suggests that non-stomatal limitation may contribute to the P n reduction. Although previous results from peach trees [6], young apple trees [7], [53] and dahlia [11] showed that the decline in P n in the late stages of low sink demand were because of non-stomatal limitation, these reports did not analyze the components of the PSII electron transport chain.
Interestingly, the increase in C i/C a during the 15th to the 21st day after LS treatment is dramatically different from other parameters (Fig. 2). This increase is attributed to changes in ABA levels [37], starch accumulation [54], and leaf temperature [18], [55], etc. These factors probably interact with each other, or one of them may play a main role, or have different roles during different periods after the removal of sink from the plants.
Conclusions
The reduction in P n in source leaves of bean plants under low sink demand is not only caused by stomatal limitation during the early stages, but also by non-stomatal limitation during the middle and later stages after the removal of the sink of roots and pods. This inhibition of P n decrease by non-stomatal limitation under low sink demand was associated with the inhibition of the PSII electron transport chain. Our results suggest that the donor side of PSII is the most sensitive to low sink demand, followed by the reaction center of PSII. The acceptor side of PSII was found to be the least sensitive to low sink demand. The reduction of the acceptor side ability of PSII occurred during the late period.
Supporting Information
Summary of parameters, formulae and their description using data extracted from chlorophyll a fluorescence transient (OJIP-test).
(DOCX)
Funding Statement
This work is supported by grants from the National Natural Science Foundation of China (Grant number 31270718). No additional external funding was received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Boussingault JB (1868) Agronomie, chimie agricole et physiologie. 2nd ed. Paris: Mallet Bachelier. pp. 236–312.
- 2. Mason TG, Maskell EJ (1928) Studies on the transport of carbohydrates in the cotton plant. I. A study of diurnal variation in the carbohydrates of leaf, bark, and wood, and of the effects of ringing. Annals of Botany 42: 189–253. [Google Scholar]
- 3. Neales TF, Incoll LD (1968) Control of leaf photosynthesis rate by level of assimilate concentration in leaf–a review of hypothesis. Botanical Review 34: 107–125. [Google Scholar]
- 4. Cheng JS, Fan PG, Liang ZC, Wang YQ, Niu N, et al. (2009) Accumulation of end products in source leaves affects photosynthetic rate in peach via alteration of stomatal conductance and photosynthetic efficiency. Journal of the American Society for Horticultural Science 134: 667–676. [Google Scholar]
- 5. DaMatta FM, Cunha RL, Antunes WC, Martins SCV, Araujo WL, et al. (2008) In field-grown coffee trees source-sink manipulation alters photosynthetic rates, independently of carbon metabolism, via alterations in stomatal function. New Phytologist 178: 348–357. [DOI] [PubMed] [Google Scholar]
- 6. Duan W, Fan PG, Wang LJ, Li WD, Yan ST, et al. (2008) Photosynthetic response to low sink demand after fruit removal in relation to photoinhibition and photoprotection in peach trees. Tree Physiology 28: 123–132. [DOI] [PubMed] [Google Scholar]
- 7. Fan PG, Li LS, Duan W, Li WD, Li SH (2010) Photosynthesis of young apple trees in response to low sink demand under different air temperatures. Tree Physiology 30: 313–325. [DOI] [PubMed] [Google Scholar]
- 8. Li WD, Duan W, Fan PG, Yan ST, Li SH (2007) Photosynthesis in response to sink-source activity and in relation to end products and activities of metabolic enzymes in peach trees. Tree Physiology 27: 1307–1318. [DOI] [PubMed] [Google Scholar]
- 9. Nebauer SG, Renau-Morata B, Guardiola JL, Molina RV (2011) Photosynthesis down-regulation precedes carbohydrate accumulation under sink limitation in Citrus. Tree Physiology 31: 169–177. [DOI] [PubMed] [Google Scholar]
- 10. Setter TL, Brun WA, Brenner ML (1980) Stomatal closure and photosynthetic inhibition in soybean leaves induced by petiole girdling and pod removal. Plant Physiology 65: 884–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yan ST, Li XD, Li WD, Fan PG, Duan W, et al. (2011) Photosynthesis and chlorophyll fluorescence response to low sink demand of tubers and roots in Dahlia pinnata source leaves. Biologia Plantarum 55: 83–89. [Google Scholar]
- 12. Zhao HM, Zheng HB (2009) Research advance on physiological changes respond to alteration of source-sink relationship in soybean. Soybean Science 28: 736–739. [Google Scholar]
- 13. Nii N (1997) Changes of starch and sorbitol in leaves before and after removal of fruits from peach trees. Annals of Botany 79: 139–144. [Google Scholar]
- 14. Nii N, Yamaguchi K, Nishimura M (1995) Effects of Fruiting on Amylase Activity and Ribulose Bisphosphate Carboxylase-Oxygenase Content in Peach Leaves. Journal of the Japanese Society for Horticultural Science 64: 267–273. [Google Scholar]
- 15. Urban L, Alphonsout L (2007) Girdling decreases photosynthetic electron fluxes and induces sustained photoprotection in mango leaves. Tree Physiology 27: 345–352. [DOI] [PubMed] [Google Scholar]
- 16. Tan CS, Buttery BR (1986) Photosynthesis, stomatal conductance, and leaf water potential in response to temperature and light in peach. Hortscience 21: 1180–1182. [Google Scholar]
- 17. Cheng YH, Arakawa O, Kasai M, Sawada S (2008) Analysis of reduced photosynthesis in the apple leaf under sink-limited conditions due to girdling. Journal of the Japanese Society for Horticultural Science 77: 115–121. [Google Scholar]
- 18. Li WD, Li SH, Yang SH, Yang JM, Zheng XB, et al. (2005) Photosynthesis in response to sink-source manipulations during different phenological stages of fruit development in peach trees: regulation by stomatal aperture and leaf temperature. Journal of Horticultural Science & Biotechnology 80: 481–487. [Google Scholar]
- 19. Wu BH, Huang HQ, Fan PG, Li SH, Liu GJ (2008) Photosynthetic responses to sink-source manipulation in five peach cultivars varying in maturity date. Journal of the American Society for Horticultural Science 133: 278–283. [Google Scholar]
- 20.Govindjee, Amesz J, Fork DC, editors (1986) Light emission by plants and bacteria. Orlando: Academic Press, Inc. 638 p. [Google Scholar]
- 21.Papageorgiou GC, Govindjee editors (2004) Chlorophyll a fluorescence: A signature of photosynthesis. Dordrecht: Springer. 818 p. [Google Scholar]
- 22. Govindjee (1995) Sixty-three years since Kautsky: chlorophyll a fluorescence. Australian Journal of Plant Physiology 22: 131–160. [Google Scholar]
- 23. Stirbet A (2011) Govindjee (2011) On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: basics and applications of the OJIP fluorescence transient. Journal of Photochemistry and Photobiology B: Biology 104: 236–257. [DOI] [PubMed] [Google Scholar]
- 24. Strasser RJ, Srivastava A (1995) Govindjee (1995) Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochemistry and Photobiology 61: 32–42. [Google Scholar]
- 25.Strasser RJ, Govindjee (1992) The Fo and the OJIP fluorescence rise in higher plants and algae. In: Argyroudi-Akoyunoglou JH, editor. Regulation of Chloroplast Biogenesis. New York: Plenum Press. pp. 423–426.
- 26.Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee, editors. Chlorophyll a fluorescence: A signature of photosynthesis. Dordrecht: Springer. pp. 321–362.
- 27.Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P, editors. Probing Photosynthesis: Mechanism, Regulation and Adaptation. London: Taylor & Francis Publishers. pp. 445–483.
- 28. Stirbet A (2012) Govindjee (2012) Chlorophyll a fluorescence induction: a personal perspective of the thermal phase, the J-I-P rise. Photosynthesis Research 113: 15–61. [DOI] [PubMed] [Google Scholar]
- 29. Lin ZH, Chen LS, Chen RB, Zhang FZ, Jiang HX, et al. (2009) CO2 assimilation, ribulose-1,5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport probed by the JIP-test, of tea leaves in response to phosphorus supply. BMC Plant Biology 9: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Srivastava A, Guisse B, Greppin H, Strasser R (1997) Regulation of antenna structure and electron transport in photosystem II of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP. Biochimica et Biophysica Acta-Bioenergetics 1320: 95–106. [Google Scholar]
- 31. Strasser BJ (1997) Donor side capacity of Photosystem II probed by chlorophyll a fluorescence transients. Photosynthesis Research 52: 147–155. [Google Scholar]
- 32.Strasser RJ, Srivastava A, Tsimilli-Michael M (1999) Screening the vitality and photosynthetic activity of plants by fluorescent transient. In: Behl R, Punia M, Lather B, editors. Crop improvement for food security. India: SSARM. pp. 72–115.
- 33. Luo HB, Ma L, Xi HF (2011) Photosynthetic Responses to Heat Treatments at Different Temperatures and following Recovery in Grapevine (Vitis amurensis L.) Leaves. PLoS ONE 6: e23033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schansker G, Toth SZ, Strasser RJ (2006) Dark recovery of the Chl a fluorescence transient (OJIP) after light adaptation: The qT-component of non-photochemical quenching is related to an activated photosystem I acceptor side. Biochimica Et Biophysica Acta-Bioenergetics 1757: 787–797. [DOI] [PubMed] [Google Scholar]
- 35. Schansker G, Toth SZ, Strasser RJ (2005) Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochimica Et Biophysica Acta-Bioenergetics 1706: 250–261. [DOI] [PubMed] [Google Scholar]
- 36.Strasser BJ, Strasser RJ (1995) Measuring fast fluorescence transients to address environmental questions: the JIP test. In: Mathis P, editor. Photosynthesis: From Light to Biosphere. The Netherlands: Kluwer Academic. pp. 977–980.
- 37. Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annual Review of Plant Physiology 33: 317–345. [Google Scholar]
- 38. Downton WJS, Berry JA (1982) Chlorophyll fluorescence at high temperature. Biochimica Et Biophysica Acta 679: 474–478. [Google Scholar]
- 39. Long SP, Bernacchi CJ (2003) Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of Experimental Botany 54: 2393–2401. [DOI] [PubMed] [Google Scholar]
- 40. Chen LS, Cheng LL (2010) The acceptor side of photosystem II is damaged more severely than the donor side of photosystem II in ‘Honeycrisp’ apple leaves with zonal chlorosis. Acta Physiologiae Plantarum 32: 253–261. [Google Scholar]
- 41. Yan K, Chen P, Shao H, Zhao S, Zhang L, et al. (2012) Responses of Photosynthesis and Photosystem II to Higher Temperature and Salt Stress in Sorghum. Journal of Agronomy and Crop Science 198: 218–226. [Google Scholar]
- 42. Guha A, Sengupta D, Reddy AR (2013) Polyphasic chlorophyll a fluorescence kinetics and leaf protein analyses to track dynamics of photosynthetic performance in mulberry during progressive drought. Journal of Photochemistry and Photobiology B: Biology 119: 71–83. [DOI] [PubMed] [Google Scholar]
- 43. Kyselakova H, Prokopova J, Naus J, Novak O, Navratil M, et al. (2011) Photosynthetic alterations of pea leaves infected systemically by pea enation mosaic virus: A coordinated decrease in efficiencies of CO2 assimilation and photosystem II photochemistry. Plant Physiology and Biochemistry 49: 1279–1289. [DOI] [PubMed] [Google Scholar]
- 44. Venkatesh J, Upadhyaya CP, Yu JW, Hemavathi A, Kim DH, et al. (2012) Chlorophyll a fluorescence transient analysis of transgenic potato overexpressing D-galacturonic acid reductase gene for salinity stress tolerance. Horticulture Environment and Biotechnology 53: 320–328. [Google Scholar]
- 45. Wang SZ, Zhang DY, Pan XL (2012) Effects of arsenic on growth and photosystem II (PSII) activity of Microcystis aeruginosa . Ecotoxicology and Environmental Safety 84: 104–111. [DOI] [PubMed] [Google Scholar]
- 46. Zhang ZS, Li G, Gao HY, Zhang LT, Yang C, et al. (2012) Characterization of Photosynthetic Performance during Senescence in Stay-Green and Quick-Leaf-Senescence Zea mays L. Inbred Lines. Plos One 7: e42936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yan K, Chen P, Shao H, Zhang L, Xu G (2011) Effects of Short-Term High Temperature on Photosynthesis and Photosystem II Performance in Sorghum. Journal of Agronomy and Crop Science 197: 400–408. [Google Scholar]
- 48. Chen H-X, Li P-M, Gao H-Y (2006) Alleviation of photoinhibition by calcium supplement in salt-treated Rumex leaves. Physiologia Plantarum 129: 386–396. [Google Scholar]
- 49. Ni LX, Acharya K, Hao XY, Li SY, Li Y, et al. (2012) Effects of Artemisinin on Photosystem II Performance of Microcystis aeruginosa by In Vivo Chlorophyll Fluorescence. Bulletin of Environmental Contamination and Toxicology 89: 1165–1169. [DOI] [PubMed] [Google Scholar]
- 50. Xiang MM, Chen SG, Wang LS, Dong ZY, Huang JH, et al. (2013) Effect of vulculic acid produced by Nimbya alternantherae on the photosynthetic apparatus of Alternanthera philoxeroides. Plant Physiology and Biochemistry 65: 81–88. [DOI] [PubMed] [Google Scholar]
- 51. Albert KR, Mikkelsen TN, Ro-Poulsen H (2005) Effects of ambient versus reduced UV-B radiation on high arctic Salix arctica assessed by measurements and calculations of chlorophyll a fluorescence parameters from fluorescence transients. Physiologia Plantarum 124: 208–226. [Google Scholar]
- 52. Li PM, Cheng LL, Gao HY, Jiang CD, Peng T (2009) Heterogeneous behavior of PSII in soybean (Glycine max) leaves with identical PSII photochemistry efficiency under different high temperature treatments. Journal of Plant Physiology 166: 1607–1615. [DOI] [PubMed] [Google Scholar]
- 53. Wünsche JN, Greer DH, Laing WA, Palmer JW (2005) Physiological and biochemical leaf and tree responses to crop load in apple. Tree Physiology 25: 1253–1263. [DOI] [PubMed] [Google Scholar]
- 54. Paul MJ, Pellny TK (2003) Carbon metabolite feedback regulation of leaf photosynthesis and development. Journal of Experimental Botany 54: 539–547. [DOI] [PubMed] [Google Scholar]
- 55. Li SH, Genard M, Bussi C, Huguet JG, Habib R, et al. (2001) Fruit quality and leaf photosynthesis in response to microenvironment modification around individual fruit by covering the fruit with plastic in nectarine and peach trees. Journal of Horticultural Science & Biotechnology 76: 61–69. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of parameters, formulae and their description using data extracted from chlorophyll a fluorescence transient (OJIP-test).
(DOCX)