Abstract
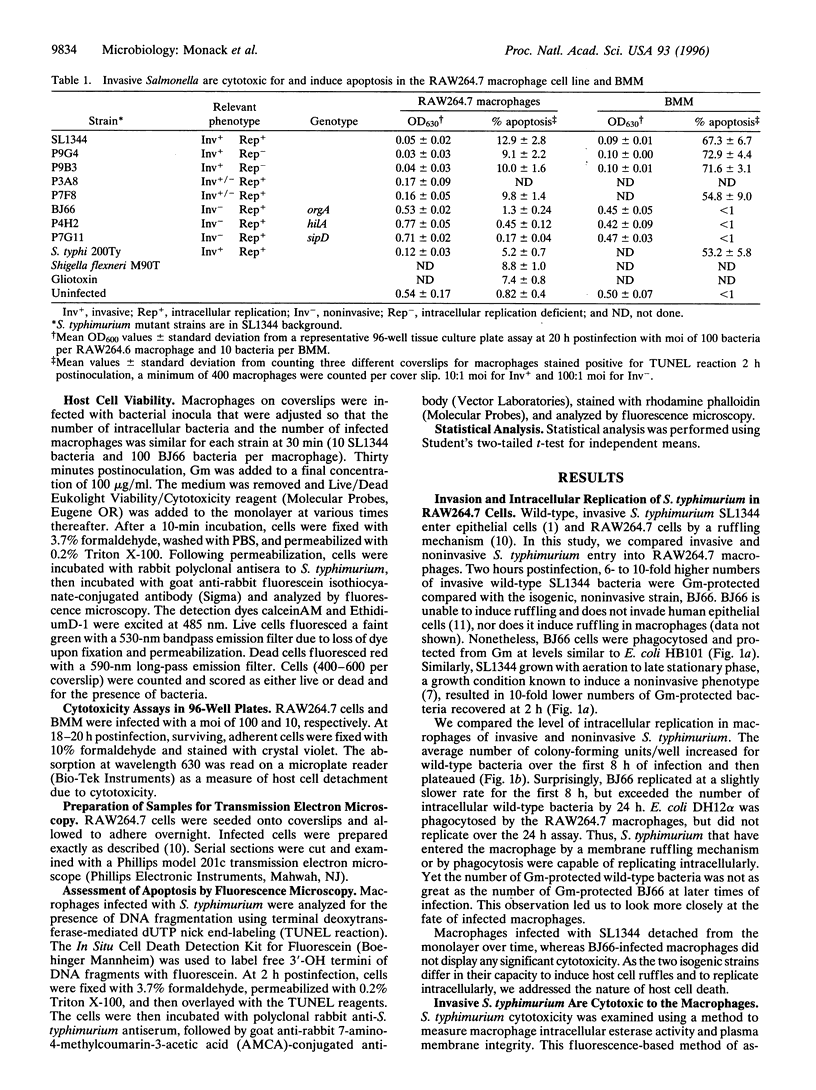
Invasive Salmonella typhimurium induces dramatic cytoskeletal changes on the membrane surface of mammalian epithelial cells and RAW264.7 macrophages as part of its entry mechanism. Noninvasive S. typhimurium strains are unable to induce this membrane ruffling. Invasive S. typhimurium strains invade RAW264.7 macrophages in 2 h with 7- to 10-fold higher levels than noninvasive strains. Invasive S. typhimurium and Salmonella typhi, independent of their ability to replicate intracellularly, are cytotoxic to RAW264.7 macrophages and, to a greater degree, to murine bone marrow-derived macrophages. Here, we show that the macrophage cytotoxicity mediated by invasive Salmonella is apoptosis, as shown by nuclear morphology, cytoplasmic vacuolization, and host cell DNA fragmentation. S. typhimurium that enter cells causing ruffles but are mutant for subsequent intracellular replication also initiate host cell apoptosis. Mutant S. typhimurium that are incapable of inducing host cell membrane ruffling fail to induce apoptosis. The activation state of the macrophage plays a significant role in the response of macrophages to Salmonella invasion, perhaps indicating that the signal or receptor for initiating programmed cell death is upregulated in activated macrophages. The ability of Salmonella to promote apoptosis may be important for the initiation of infection, bacterial survival, and escape of the host immune response.
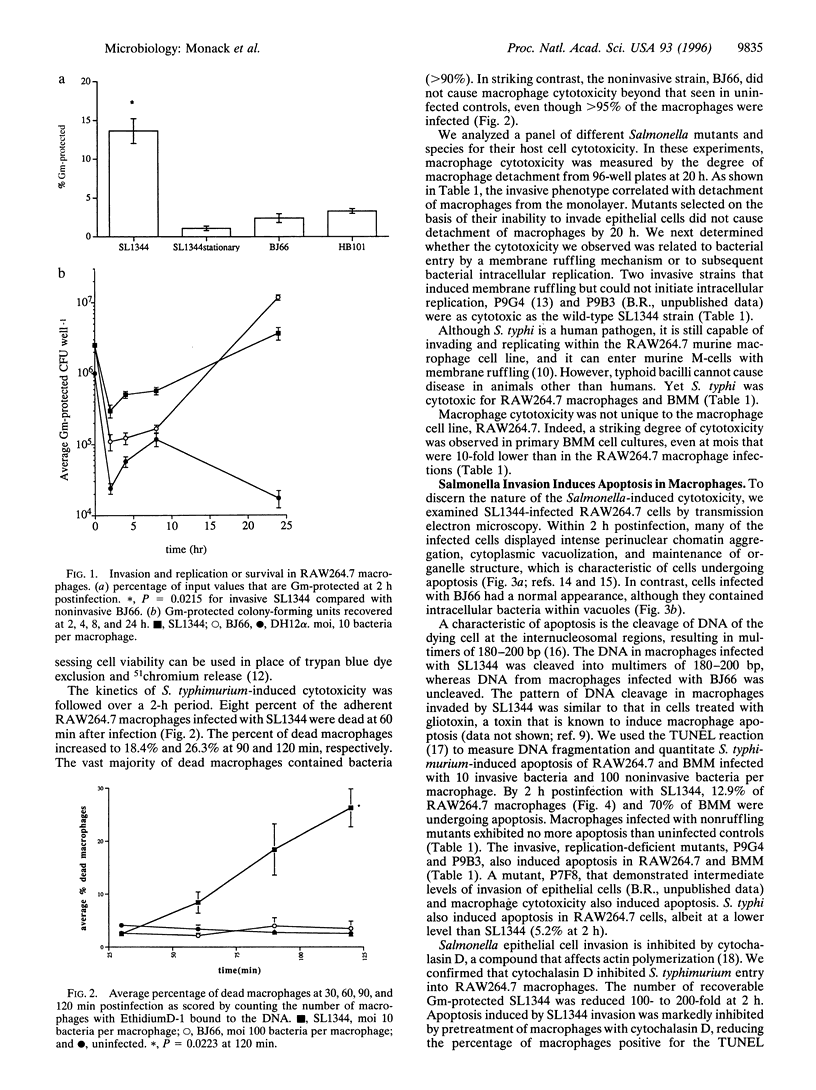
Full text
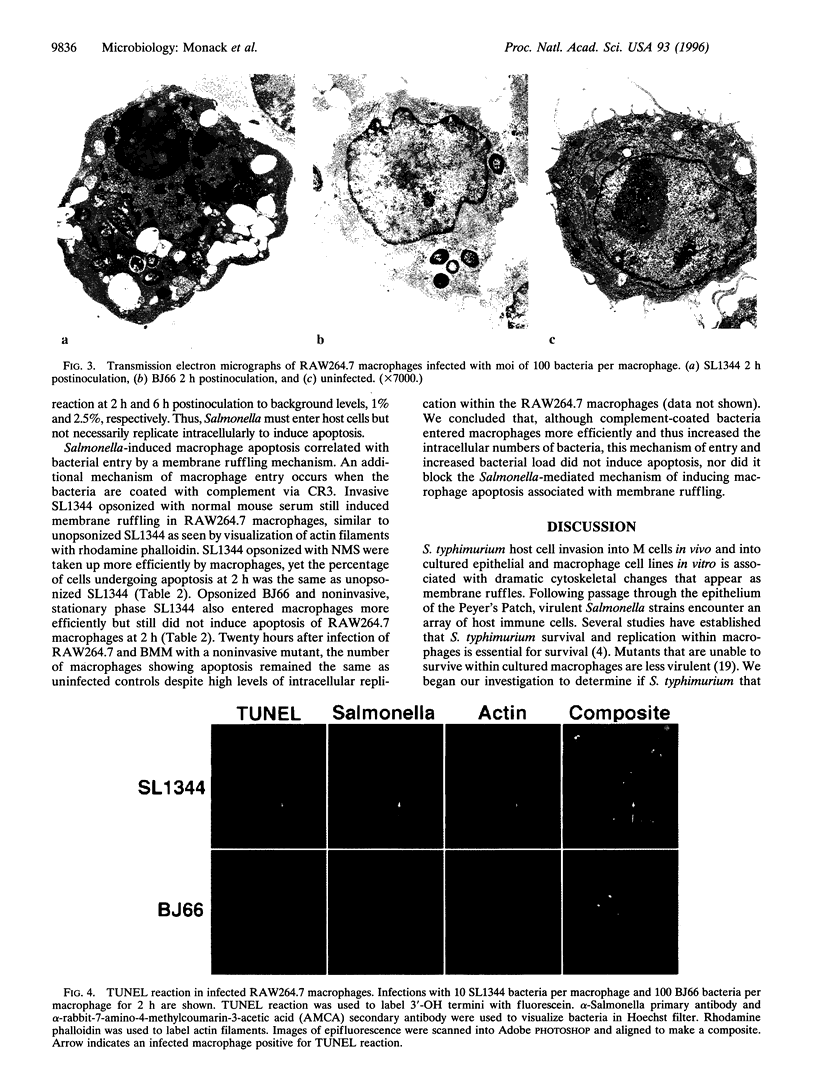
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai T., Hiromatsu K., Nishimura H., Kimura Y., Kobayashi N., Ishida H., Nimura Y., Yoshikai Y. Endogenous interleukin 10 prevents apoptosis in macrophages during Salmonella infection. Biochem Biophys Res Commun. 1995 Aug 15;213(2):600–607. doi: 10.1006/bbrc.1995.2174. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Ashany D., Song X., Lacy E., Nikolic-Zugic J., Friedman S. M., Elkon K. B. Th1 CD4+ lymphocytes delete activated macrophages through the Fas/APO-1 antigen pathway. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):11225–11229. doi: 10.1073/pnas.92.24.11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J. B., Galán J. E., Falkow S. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell. 1993 Jun 4;73(5):903–920. doi: 10.1016/0092-8674(93)90270-z. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989 Jan;57(1):1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zychlinsky A. Apoptosis induced by bacterial pathogens. Microb Pathog. 1994 Oct;17(4):203–212. doi: 10.1006/mpat.1994.1066. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Comparison of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988 Aug;70(8):1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- Francis C. L., Ryan T. A., Jones B. D., Smith S. J., Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993 Aug 12;364(6438):639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- Francis C. L., Starnbach M. N., Falkow S. Morphological and cytoskeletal changes in epithelial cells occur immediately upon interaction with Salmonella typhimurium grown under low-oxygen conditions. Mol Microbiol. 1992 Nov;6(21):3077–3087. doi: 10.1111/j.1365-2958.1992.tb01765.x. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán C. A., Domann E., Rohde M., Bruder D., Darji A., Weiss S., Wehland J., Chakraborty T., Timmis K. N. Apoptosis of mouse dendritic cells is triggered by listeriolysin, the major virulence determinant of Listeria monocytogenes. Mol Microbiol. 1996 Apr;20(1):119–126. doi: 10.1111/j.1365-2958.1996.tb02494.x. [DOI] [PubMed] [Google Scholar]
- Hensel M., Shea J. E., Gleeson C., Jones M. D., Dalton E., Holden D. W. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995 Jul 21;269(5222):400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- Hermant D., Ménard R., Arricau N., Parsot C., Popoff M. Y. Functional conservation of the Salmonella and Shigella effectors of entry into epithelial cells. Mol Microbiol. 1995 Aug;17(4):781–789. doi: 10.1111/j.1365-2958.1995.mmi_17040781.x. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Jones B. D., Falkow S. Identification and characterization of a Salmonella typhimurium oxygen-regulated gene required for bacterial internalization. Infect Immun. 1994 Sep;62(9):3745–3752. doi: 10.1128/iai.62.9.3745-3752.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. D., Falkow S. Salmonellosis: host immune responses and bacterial virulence determinants. Annu Rev Immunol. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- Jones B. D., Ghori N., Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J Exp Med. 1994 Jul 1;180(1):15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. D., Paterson H. F., Hall A., Falkow S. Salmonella typhimurium induces membrane ruffling by a growth factor-receptor-independent mechanism. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10390–10394. doi: 10.1073/pnas.90.21.10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert L. E., Paulnock D. M. Differential induction of activation markers in macrophage cell lines by interferon-gamma. Cell Immunol. 1989 May;120(2):401–418. doi: 10.1016/0008-8749(89)90208-6. [DOI] [PubMed] [Google Scholar]
- Lee C. A., Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren S. W., Stojiljkovic I., Heffron F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1996 Apr 30;93(9):4197–4201. doi: 10.1073/pnas.93.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascopella L., Raupach B., Ghori N., Monack D., Falkow S., Small P. L. Host restriction phenotypes of Salmonella typhi and Salmonella gallinarum. Infect Immun. 1995 Nov;63(11):4329–4335. doi: 10.1128/iai.63.11.4329-4335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninger J. M., Mak T. W. Signal transduction, mitotic catastrophes, and death in T-cell development. Immunol Rev. 1994 Dec;142:231–272. doi: 10.1111/j.1600-065x.1994.tb00892.x. [DOI] [PubMed] [Google Scholar]
- Saito S., Shinomiya H., Nakano M. Protein phosphorylation in murine peritoneal macrophages induced by infection with Salmonella species. Infect Immun. 1994 May;62(5):1551–1556. doi: 10.1128/iai.62.5.1551-1556.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari M., Beidler D. R., Dixit V. M. CrmA-inhibitable cleavage of the 70-kDa protein component of the U1 small nuclear ribonucleoprotein during Fas- and tumor necrosis factor-induced apoptosis. J Biol Chem. 1995 Aug 11;270(32):18738–18741. doi: 10.1074/jbc.270.32.18738. [DOI] [PubMed] [Google Scholar]
- Waring P., Eichner R. D., Müllbacher A., Sjaarda A. Gliotoxin induces apoptosis in macrophages unrelated to its antiphagocytic properties. J Biol Chem. 1988 Dec 5;263(34):18493–18499. [PubMed] [Google Scholar]
- Warren M. K., Vogel S. N. Bone marrow-derived macrophages: development and regulation of differentiation markers by colony-stimulating factor and interferons. J Immunol. 1985 Feb;134(2):982–989. [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Zhang F., zur Hausen A., Hoffmann R., Grewe M., Decker K. Rat liver macrophages express the 55 kDa tumor necrosis factor receptor: modulation by interferon-gamma, lipopolysaccharide and tumor necrosis factor-alpha. Biol Chem Hoppe Seyler. 1994 Apr;375(4):249–254. doi: 10.1515/bchm3.1994.375.4.249. [DOI] [PubMed] [Google Scholar]
- Zheng L. M., Zychlinsky A., Liu C. C., Ojcius D. M., Young J. D. Extracellular ATP as a trigger for apoptosis or programmed cell death. J Cell Biol. 1991 Jan;112(2):279–288. doi: 10.1083/jcb.112.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychlinsky A., Fitting C., Cavaillon J. M., Sansonetti P. J. Interleukin 1 is released by murine macrophages during apoptosis induced by Shigella flexneri. J Clin Invest. 1994 Sep;94(3):1328–1332. doi: 10.1172/JCI117452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychlinsky A., Kenny B., Ménard R., Prévost M. C., Holland I. B., Sansonetti P. J. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol Microbiol. 1994 Feb;11(4):619–627. doi: 10.1111/j.1365-2958.1994.tb00341.x. [DOI] [PubMed] [Google Scholar]
- Zychlinsky A., Prevost M. C., Sansonetti P. J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992 Jul 9;358(6382):167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]





