Abstract
McCune-Albright syndrome (MAS) is a rare multisystem disorder characterized by the triad of polyostotic fibrous dysplasia (PFD), endocrine disorders and café-au-lait skin pigmentation. Ninety percent of MAS patients have FD lesions in the craniofacial area, resulting in significant orofacial deformity, dental disorders, bone pain and compromised oral health. Maxillo-mandibular FD is also associated with dental developmental disorders, malocclusion, and high caries index. There is limited data on the outcomes of dental treatments in maxillo-mandibular FD/MAS patients, because clinicians and researchers have limited access to patients, and there are concerns that dental surgery may activate quiescent jaw FD lesions to grow aggressively. This report highlights current perspectives on dental management issues associated with maxillo-mandibular FD within the context of MAS.
Keywords: Fibrous dysplasia, McCune-Albright syndrome, dental anomalies, gene mutation, oral health
INTRODUCTION
Fibrous dysplasia (FD) is a skeletal disorder characterized by replacement of normal bone and marrow by fibrous tissue, leading to fracture, deformity, and pain (Figure 1) 1. FD may affect a single bone (monostotic FD) or multiple bones (polyostotic FD), and may occur in association with café-au-lait skin pigmentation and hyperfunctioning endocrinopathies, including precocious puberty, hyperthyroidism, hypercortisolism, growth hormone excess, and fibroblast growth factor (FGF)23-mediated hypophosphatemia. When FD occurs in combination with one or more extra-skeletal features, it is termed McCune-Albright syndrome (MAS) 2–4. MAS is relatively rare with an estimated prevalence ranging from 1/100,000 to 1/1,000,000, however FD is more common, accounting for approximately 7% of all benign bone tumors 4, 5.
FIGURE 1. Images of a 12-year-old girl with fibrous dysplasia/McCune-Albright syndrome (FD/MAS).

A) Photograph showing café-au-lait pigmentation on the posterior trunk (black arrow), and expansion in FD of the left femur leading to deformity and limb-length discrepancy. Shortening of the left lower extremity has resulted in an altered stance with pelvic obliquity (white arrowhead). B) Technetium-99 bone scintigraphy shows patchy areas of increased radiotracer uptake in FD in the skull, humeri, radii, femurs and fibulas (black arrows). FD in the proximal femurs has led to bilateral coxa varus (“shepherd’s crook” deformities) (black arrowheads). C) A characteristic facial café-au-lait macule is limited to one side without crossing the midline (‘respecting the midline’) and exhibits typical ragged “coast of Maine” borders. D). Radiographs show bilateral femoral FD and “shepherd’s crook” deformities (white arrowhead) that is more severe on left.
ETIOLOGY AND PATHOGENESIS
MAS arises from somatic activating mutations in GNAS gene, which encodes the Gsα subunit of the heterotrimeric G protein complex6, 7. This results in constitutive activation of adenylyl cyclase and overproduction of 3′,5′-cyclic adenosine monophosphate (cAMP) 7, 8. The severity of the disease phenotype depends on when the mutation occurs during embryogenesis, and the locations where mutated progeny cells subsequently migrate. If the mutation occurs during formation of the inner cell mass, all three germ cell layers will be affected and the individual will develop MAS. Mutations occurring later in embryogenesis will have a more limited phenotype, such as isolated FD, or endocrinopathies without associated bone disease.
At the cellular level, FD is considered a disease of the skeletal stem cell/osteoblastic lineage in which excess cAMP impairs the ability of bone skeletal stem cells to differentiate to mature functioning osteoblasts 9. In normal bone, remodeling is a coordinated cycle of sequential osteoclastic bone resorption followed by osteoblastic bone matrix deposition. In FD bone, remodeling is altered by replacement of normal bone and hematopoietic marrow by abnormal osteogenic tissue and bone trabeculae. The increased bone resorption and fibrous tissue deposition in FD/MAS is associated with increased secretion of interleukin (IL)-6 and RANKL, which contain a cAMP response elements in their promoters 10, 11, 12. Thus, the abnormal patterns of bone formation and resorption that characterize FD/MAS lesions are both related to mutation of Gsα and overproduction of cAMP.
CLINICAL PRESENTATIONS
Clinical signs and symptoms of the skeletal, endocrine and cutaneous components of MAS may present at varying stages of development. Café-au-lait skin hyperpigmentation in MAS is often observed at birth. It has characteristic irregular borders analogous to the ‘coast of Maine’, unlike the smooth borders of the hyperpigmentation in neurofibromatosis, analogous to the ‘coast of California’. MAS associated café-au-lait hyperpigmentation is typically described as “respecting” the midline, either by terminating at or reflecting around the midline (Figure 1). This distribution pattern reflects patterns of early embryonic cell migration. There is a wide clinical spectrum of FD severity, depending on the amount and areas of the affected skeleton (Figure 1). As FD patients grow older, features of FD in the appendicular skeleton may manifest with fracture, limp or bone pain, which in small children may manifest as complaints of tiredness and easy fatigability 4. Also, long bones affected by FD are prone to fracture and progressive deformities such as coxa vara of the proximal femur, commonly termed the “shepherd’s crook” deformity (Figure 1). Interestingly, facial asymmetry may be the first sign of craniofacial FD, and it may be associated with hearing and more rarely vision loss 13. Precocious puberty is common in girls, and occurs due to spontaneous development of functioning ovarian cysts. This presents as episodic periods of estrogen excess, early breast development, growth acceleration, and vaginal bleeding. Precocious puberty is less common in boys, however they frequently present with macro-orchidism and testicular ultrasound abnormalities consistent with Leydig and Sertoli cell hyperplasia (Figure 2)14. Hyperthyroidism typically develops during infancy or early childhood, and may be diagnosed by abnormalities seen on thyroid function tests or radiographic abnormalities on thyroid ultrasound (Figure 2) 15. Patients with growth hormone excess from MAS-associated pituitary disease (Figure 2) initially present with growth acceleration. If untreated, growth hormone excess causes craniofacial expansion and optic neuropathy, so early diagnosis and treatment is a critical component of management 14, 16.
FIGURE 2. Radiographic images of endocrine organs in McCune-Albright syndrome.

A) Testicular ultrasound of a patient with McCune-Albright syndrome (MAS) shows a heterogeneous, mixed cystic and solid lesion characteristic of Leydig and Sertoli cell hyperplasia (white arrows). B) Thyroid ultrasound in a patient with MAS-associated hyperthyroidism demonstrates characteristic heterogeneity with a cystic, “Swiss cheese”-like appearance (white arrow). C) Magnetic resonance imaging of the brain in a patient with MAS-associated growth hormone excess shows a large, dumbbell-shaped growth hormone- and prolactin-secreting pituitary macroadenoma (white arrows); the bright spot (white arrowhead) indicates the posterior pituitary.
DIAGNOSIS
FD/MAS is a clinical diagnosis based on a combination of clinical, biochemical, and radiographic findings. Mutation analysis of FD tissue is problematic due to the mosaic nature of the disease, and is rarely helpful for clinical management. All patients suspected of having FD/MAS should undergo screening for thyroid and pituitary involvement that include thyroid function tests, thyroid ultrasound, and insulin growth factor (IGF)-1 level. Although testicular ultrasound is a helpful diagnostic tool in boys, routine pelvic ultrasound is rarely informative in girls without clinical signs of precocious puberty due to the episodic nature of ovarian cysts. It is essential to monitor growth velocity in children, because all of the endocrine manifestations of MAS can cause growth alterations. In children ages 5 years and older, technetium (99mTc-methylene diphosphonate) bone scintigraphy is useful to identify areas of FD, which can be further evaluated with plain radiographs (Figure 1) 17, 18. The radiographic appearance of FD is affected by both location and disease activity. During childhood, appendicular and craniofacial FD demonstrates a homogeneous ‘ground glass’ appearance. The disease activity typically declines during adulthood, at which point FD may adopt a more sclerotic, heterogeneous appearance.
This is illustrated in Figure 3, where panoramic radiographs and computed tomography (CT) of the jaws demonstrate ground glass trabeculation that also includes mixed radiolucent/radio-opaque lesions and thinning of the cortical margin 19. FD in the axial skeleton (including the spine, ribs, and pelvis) may be difficult to visualize on plain films, so bone scintigraphy is useful to detect lesions in these areas17. Histologically, FD demonstrates unique site-specific features 1, 20. In the cranial bones, it displays a dense, sclerotic trabecular bone pattern with an interconnected network similar to the pattern observed in Paget’s disease. In the jaws, the pattern is characterized by the presence of significant amounts of sclerotic bone, however, bone trabeculae are discontinuous, and in the axial/appendicular bones, they display a ‘Chinese character pattern’ with the fibrous tissue predominating over the abnormal bone trabeculae (Figure 4) 1. The lamellation pattern seen in normal bone is conspicuously absent in the de novo bone formed within areas of FD. Rather FD exhibits a pattern consistent with woven bone.
FIGURE 3. Images of an adult woman with McCune-Albright syndrome and craniofacial fibrous dysplasia.
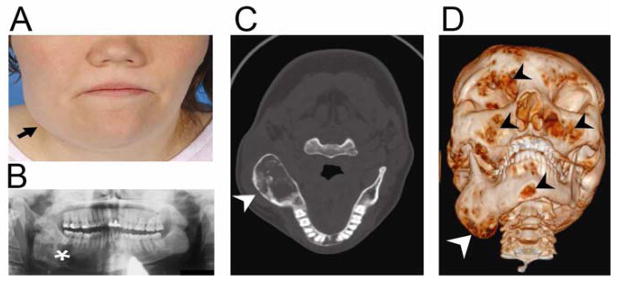
A) Facial asymmetry resulting from expansion of a fibrous dysplasia (FD) lesion in the right mandible. B) Panoramic radiograph shows a mixed radiolucent/radio-opaque pattern characteristic of FD. C) Computed tomography (CT) of the head shows expansion of FD in the right mandible (arrow). D) Three dimensional volume rendering of CT images shows expansion of FD in the right mandible (white arrow), as well as multiple smaller areas of FD (black arrowheads).
FIGURE 4. Histological features of fibrous dysplasia.
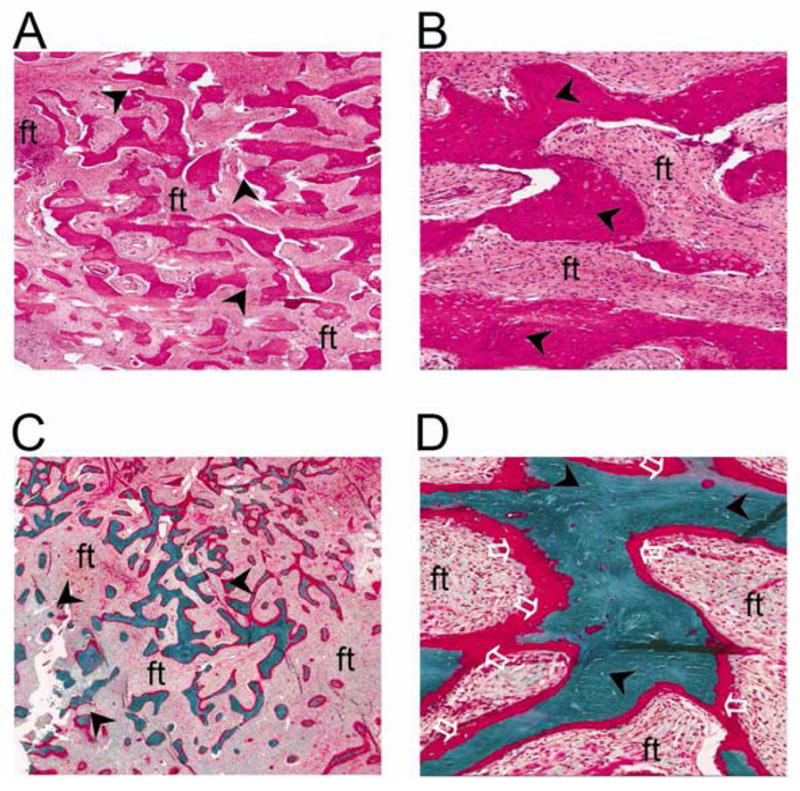
Hematoxylin/eosin stained sections of maxillary fibrous dysplasia [FD] (A, low power and B high power) demonstrate haphazard trabecular bone matrix (‘Chinese character’ pattern, black arrowheads) within a fibrous stroma. Goldner’s trichrome stained sections from calvarial FD (C, low power and D, high power) demonstrate similar haphazard bone matrix deposition (back arrowheads) and unmineralized regions (white clear arrows) that indicate osteomalacia [ft = fibrous tissue].
MEDICAL MANAGEMENT
The multi-organ clinical pattern makes dental and medical management of patients with FD/MAS complex and challenging. Therefore, coordination of comprehensive care involving interactions between dentist, endocrinologist, primary care provider, orthopedic surgeon, physical therapist and social workers is essential. The endocrine features of FD/MAS are treated either by surgical removal of affected glands or by medications directed at hormonal inhibition or blockade 21. However, there is still no satisfactory treatment to alter disease progression of FD. Surgical treatment of FD involves orthopedic hardware to stabilize bones and mediate deformity. Discouragingly, these existing methods of surgical treatments still have unsatisfactory outcomes13, 22, 23.
In the craniofacial region, surgery is indicated when the lesion causes loss of function of vital structures such as optic nerves, or for cosmetic recontouring to improve appearance. Screening for and treatment of FGF23-mediated hypophosphatemia is a critical component of FD management, as phosphate wasting may worsen bone pain and place patients at higher risk for fracture and deformity. Bisphosphonates have been moderately effective in relieving FD-related bone pain, but are ineffective in altering the disease course17, 24. The bisphosphonate dosing regimen in FD/MAS is similar to that of Paget’s disease because the drug is cycled ‘on and off’ so patients are often off bisphosphonate for several months until pain recurs4. FD/MAS patients do not routinely undergo treatment for skin hyperpigmentation (Figure 1), although there has been one report of successful laser therapy for a facial café-au-lait macule25.
ORAL AND DENTAL IMPLICATIONS
Approximately 90% of patients with FD have lesions in the craniofacial bones including the maxilla and mandible 13, 21, 26. FD can cause massive expansion of the craniofacial complex, severe malocclusion and facial disfigurement (Figure 3) 13, 19, 20. FD lesions can grow rapidly, leading to bone expansion and displacement of adjacent structures such as the orbit and teeth 13, 20, 27, 28. The metabolic dysfunctions and disordered bone architecture in FD/MAS can potentially affect tooth development and eruption 29, 30. Although FD is a disease of the skeletal stem cell/osteoblastic lineage in which excess cAMP impairs the ability of the stem cell to differentiate to mature functioning osteoblasts 9, it is unclear whether excess cAMP affects the developing tooth, either directly or indirectly.
There is clinical concern that FD/MAS can disrupt the coordinated sequential events involved in replacement of primary dentition with permanent dentition, but it is also unclear if the presence of FD in the jaws has any effects on tooth development and function. FD is associated with dental disorders such as enamel hypoplasia, dentin dysplasia, taurodontic pulp, odontoma, tooth displacement, malocclusion, and high caries index 20. These anomalies are seen in approximately 28% of patients with craniofacial FD, and have significant impact on their oral health status and management. Pain is common in craniofacial FD, affecting approximately 40% of patients 17, 31. Rapid growth of jaw FD may also be associated with pathological lesions such as aneurysmal bone cysts, or more rarely malignant transformation to osteosarcoma or other forms of sarcoma 20, 21, 32–36. Taurodontism, a condition characterized by enlargement of pulp chamber, is often associated with presence of endocrine disorders in FD/MAS20, suggesting that FD, endocrinopathies, or a combination of these may impact tooth development in FD/MAS patients.
DENTAL MANAGEMENT ISSUES
Dental management of FD/MAS is medically complex because treatment of dental disorders must be balanced with multiple factors, including skeletal disease burden, endocrine disorders, multiple medications and general debility. Due to the presence of often-complex medical comorbidities, the dental aspects of FD/MAS are frequently overlooked, and dental needs are often underserved. Furthermore, the variable clinical, radiological and histological presentations of FD, as well as apparent risk of malignant transformation, cause some dental practitioners to delay or avoid dental surgical procedures in FD 32, 34–36. Also, some dental healthcare providers may also feel uneasy about treating FD/MAS patients because of previous subjective reports that dental surgery might exacerbate jaw FD, transforming a quiescent lesion to an aggressively growing lesion32, 33, 40. There are also concerns regarding bisphosphonate-associated osteonecrosis of the jaw (ONJ) 29, 30, 32, 34, 35, 37. However, ONJ is rare in FD/MAS, possibly due to the relatively lower bisphosphonate dosing regimens compared to doses used in cancer patients, or perhaps due to the relatively hypervascular nature of FD. While bisphosphonate doses used in FD/MAS are higher than those used in osteoporosis therapy, risk of ONJ does not appear to be greater than that seen in osteoporosis patients.
Due to the severity of dental malocclusion and high caries index in patients with maxilla-mandibular FD20, more frequent recalls may be required for scaling and root planning to control dental plaque accumulation. The use of electric toothbrush and application of topical fluoride may be helpful to control dental caries. When caries extends to the pulp chamber in a tooth with a taurodontic pulp, root canal therapy may be challenging. This is an indication for prompt referral to an endodontist for management. Severe malocclusion may also require orthodontic intervention. When orthodontic therapy is clinically indicated, the timing should be carefully coordinated among the dental healthcare providers. Literature reports on outcomes of orthodontic therapies in FD/MAS are still unclear. One theory is that orthodontic tooth movement tends to be rapid in FD jaw. Our experience is that orthodontic therapy takes much longer than in normal patient population; and relapse is more common because teeth tend to return to their original position after removal of orthodontic appliances20, 38. It may be advisable to delay orthodontic therapy till after the age of skeletal maturity based on individual patients’ needs and outcomes of orthodontic evaluation. The advantage is that FD disease activity tends to decrease after skeletal maturity, which is reflected in a decline in bone turnover markers, a decrease in the number of mutated lesional skeletal stem/progenitor cells, and a tendency of FD histology to improve over time17, 39. While root canal therapy in a taurodontic tooth and orthodontic tooth movement in FD-related malocclusion are some clinical challenges faced by FD/MAS patients, it is still unclear if other dental anomalies would pose any treatment challenges. Disorders of the liver, heart, and spleen have been associated with MAS40–42, so it is imperative to consult with the patient’s physician on medical stability for dental therapy before embarking on extensive dental surgery.
Unfortunately, there is limited data on effectiveness and outcomes of dental therapies in maxillo-mandibular FD/MAS patients because clinicians and researchers have access to very few and small patient pools20, 37. To address the healthcare needs of FD/MAS patient populations, the National Institutes of Health (NIH) Office of Rare Diseases Research (ORDR), the National Institute of Dental and Craniofacial Research (NIDCR), and the Fibrous Dysplasia Foundation organized an International meeting in October 2010 in Bethesda, MD to determine best clinical practice and future research on FD/MAS. One clear outcome was that clinical and basic scientists must share research data and tissue samples to foster new research that will expand our understanding of FD/MAS and improve dental and medical treatment outcomes 43.
CONCLUSION
The broad clinical spectrum of FD/MAS makes dental care challenging and medically complex. Therefore, healthcare providers often refer FD/MAS patients to dentists equipped with special patient care facilities. Literature reports so far indicate that routine dental care can be safely and successfully carried out in FD/MAS patients with minimal complications38, 44. However, there are still several unanswered questions: Is healing delayed in FD bone after tooth extraction? Does dental surgery aggravate FD lesion? Are FD/MAS patients more prone to endodontic therapy, since the variable radiographic FD patterns in the alveolar region may mimic pulpally induced periapical lesions? Is orthodontic therapy unusually protracted in FD/MAS patents? Are FD/MAS patients easily prone to orthodontic treatment relapse due to poor quality of FD bone with teeth moving away from where they were orthodontically positioned? These are areas that additional research can address to expand our understanding of the outcomes of dental extractions, dental implants, root canal therapy and orthodontic therapy in FD/MAS patients with maxillo-mandibular FD.
Acknowledgments
This work was supported in part by National Institutes of Health/United States Department of Health and Human Services (NIH/DHHS) Bethesda MD grant R21DE022826 from the National Institute of Dental and Craniofacial Research (NIDCR) and grant K22CA169089 from the National Cancer Institute (NCI) [SOA]; the Division of Intramural Research NIDCR, a part of the Intramural Research Program of NIH/DHHS [MTC and AMB]; and the Bone Health Program at Children’s National Medical Center, Washington DC [AMB].
Footnotes
DISCLOSURES
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Riminucci M, Liu B, Corsi A, Shenker A, Spiegel AM, Robey PG, et al. The histopathology of fibrous dysplasia of bone in patients with activating mutations of the Gs alpha gene: site-specific patterns and recurrent histological hallmarks. J Pathol. 1999;187(2):249–58. doi: 10.1002/(SICI)1096-9896(199901)187:2<249::AID-PATH222>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 2.McCune D. Osteitis fiborsa cystica: the case of a nine-year-old girl who also exhibits precocious puberty, multiple pigmentation of the skin and hyperthyroidism. Am J Dis Child. 1936;52:743–4. [Google Scholar]
- 3.Albright FBA, Hampton AO, Smith P. Syndrome characterized by osteitis fibrosa disseminata, areas of pigmentation and endocrine dysfunction with precocious puberty in females. NEngl J Med. 1937;216:727–46. [Google Scholar]
- 4.Dumitrescu CE, Collins MT. McCune-Albright syndrome. Orphanet J Rare Dis. 2008;3:12. doi: 10.1186/1750-1172-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorfman HD, Czerniak B. Fibroosseous Lesions. In: Dorfman HD, Czerniak B, editors. Bone Tumors. St Louis, MO: Mosby; 1998. pp. 441–91. [Google Scholar]
- 6.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome [see comments] N Engl J Med. 1991;325(24):1688–95. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 7.Schwindinger WF, Francomano CA, Levine MA. Identification of a mutation in the gene encoding the alpha subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome. Proc Natl Acad Sci U S A. 1992;89(11):5152–6. doi: 10.1073/pnas.89.11.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shenker A, Weinstein LS, Sweet DE, Spiegel AM. An activating Gs alpha mutation is present in fibrous dysplasia of bone in the McCune-Albright syndrome. J Clin Endocrinol Metab. 1994;79(3):750–5. doi: 10.1210/jcem.79.3.8077356. [DOI] [PubMed] [Google Scholar]
- 9.Riminucci M, Fisher LW, Shenker A, Spiegel AM, Bianco P, Gehron Robey P. Fibrous dysplasia of bone in the McCune-Albright syndrome: abnormalities in bone formation [see comments] Am J Pathol. 1997;151(6):1587–600. [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto T, Ozono K, Kasayama S, Yoh K, Hiroshima K, Takagi M, et al. Increased IL-6-production by cells isolated from the fibrous bone dysplasia tissues in patients with McCune-Albright syndrome. J Clin Invest. 1996 Jul 1;98(1):30–5. doi: 10.1172/JCI118773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riminucci M, Kuznetsov SA, Cherman N, Corsi A, Bianco P, Gehron Robey P. Osteoclastogenesis in fibrous dysplasia of bone: in situ and in vitro analysis of IL-6 expression. Bone. 2003 Sep;33(3):434–42. doi: 10.1016/s8756-3282(03)00064-4. [DOI] [PubMed] [Google Scholar]
- 12.Piersanti S, Remoli C, Saggio I, Funari A, Michienzi S, Sacchetti B, et al. Transfer, analysis, and reversion of the fibrous dysplasia cellular phenotype in human skeletal progenitors. J Bone Miner Res. 2010 May;25(5):1103–16. doi: 10.1359/jbmr.091036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JS, FitzGibbon E, Butman JA, Dufresne CR, Kushner H, Wientroub S, et al. Normal vision despite narrowing of the optic canal in fibrous dysplasia. The New England journal of medicine. 2002 Nov 21;347(21):1670–6. doi: 10.1056/NEJMoa020742. [DOI] [PubMed] [Google Scholar]
- 14.Boyce AM, Chong WH, Yao J, Gafni RI, Kelly MH, Chamberlain CE, et al. Denosumab treatment for fibrous dysplasia. J Bone Miner Res. 2012 Jul;27(7):1462–70. doi: 10.1002/jbmr.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celi FS, Coppotelli G, Chidakel A, Kelly M, Brillante BA, Shawker T, et al. The role of type 1 and type 2 5′-deiodinase in the pathophysiology of the 3,5,3′-triiodothyronine toxicosis of McCune-Albright syndrome. The Journal of clinical endocrinology and metabolism. 2008 Jun;93(6):2383–9. doi: 10.1210/jc.2007-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akintoye SO, Chebli C, Booher S, Feuillan P, Kushner H, Leroith D, et al. Characterization of gsp-mediated growth hormone excess in the context of McCune-Albright syndrome. The Journal of clinical endocrinology and metabolism. 2002 Nov;87(11):5104–12. doi: 10.1210/jc.2001-012022. [DOI] [PubMed] [Google Scholar]
- 17.Collins MT, Kushner H, Reynolds JC, Chebli C, Kelly MH, Gupta A, et al. An instrument to measure skeletal burden and predict functional outcome in fibrous dysplasia of bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2005 Feb;20(2):219–26. doi: 10.1359/JBMR.041111. [DOI] [PubMed] [Google Scholar]
- 18.Hart ES, Kelly MH, Brillante B, Chen CC, Ziran N, Lee JS, et al. Onset, progression, and plateau of skeletal lesions in fibrous dysplasia and the relationship to functional outcome. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007 Sep;22(9):1468–74. doi: 10.1359/jbmr.070511. [DOI] [PubMed] [Google Scholar]
- 19.Akintoye SO, Otis LL, Atkinson JC, Brahim J, Kushner H, Robey PG, et al. Analyses of variable panoramic radiographic characteristics of maxillo-mandibular fibrous dysplasia in McCune-Albright syndrome. Oral diseases. 2004 Jan;10(1):36–43. doi: 10.1046/j.1354-523x.2003.00971.x. [DOI] [PubMed] [Google Scholar]
- 20.Akintoye SO, Lee JS, Feimster T, Booher S, Brahim J, Kingman A, et al. Dental characteristics of fibrous dysplasia and McCune-Albright syndrome. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2003 Sep;96(3):275–82. doi: 10.1016/s1079-2104(03)00225-7. [DOI] [PubMed] [Google Scholar]
- 21.Collins MT. Spectrum and natural history of fibrous dysplasia of bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2006 Dec;21( Suppl 2):P99–P104. doi: 10.1359/jbmr.06s219. [DOI] [PubMed] [Google Scholar]
- 22.Leet AI, Chebli C, Kushner H, Chen CC, Kelly MH, Brillante BA, et al. Fracture incidence in polyostotic fibrous dysplasia and the McCune-Albright syndrome. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2004 Apr;19(4):571–7. doi: 10.1359/JBMR.0301262. [DOI] [PubMed] [Google Scholar]
- 23.Stanton RP, Ippolito E, Springfield D, Lindaman L, Wientroub S, Leet A. The surgical management of fibrous dysplasia of bone. Orphanet J Rare Dis. 2012 May 24;7( Suppl 1):S1. doi: 10.1186/1750-1172-7-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plotkin H, Rauch F, Zeitlin L, Munns C, Travers R, Glorieux FH. Effect of pamidronate treatment in children with polyostotic fibrous dysplasia of bone. J Clin Endocrinol Metab. 2003 Oct;88(10):4569–75. doi: 10.1210/jc.2003-030050. [DOI] [PubMed] [Google Scholar]
- 25.Ozawa T, Tateishi C, Shirakawa M, Murakami E, Ishii M, Harada T. Long-term follow-up of a case of cheek hyperpigmentation associated with McCune-Albright syndrome treated with Q-switched ruby laser. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al] 2011 Feb;37(2):263–6. doi: 10.1111/j.1524-4725.2010.01864.x. [DOI] [PubMed] [Google Scholar]
- 26.Valentini V, Cassoni A, Marianetti TM, Terenzi V, Fadda MT, Iannetti G. Craniomaxillofacial fibrous dysplasia: conservative treatment or radical surgery? A retrospective study on 68 patients. Plastic and reconstructive surgery. 2009 Feb;123(2):653–60. doi: 10.1097/PRS.0b013e318196bbbe. [DOI] [PubMed] [Google Scholar]
- 27.Ricalde P, Horswell BB. Craniofacial fibrous dysplasia of the fronto-orbital region: a case series and literature review. J Oral Maxillofac Surg. 2001;59(2):157–67. doi: 10.1053/joms.2001.20487. discussion 67–8. [DOI] [PubMed] [Google Scholar]
- 28.Horgan MA, Delashaw JB, Dailey RA. Bilateral proptosis: an unusual presentation of fibrous dysplasia. Br J Neurosurg. 1999;13(3):335–7. doi: 10.1080/02688699943817. [DOI] [PubMed] [Google Scholar]
- 29.Catena DL, Glick GL. Monostotic fibrous dysplasia with dental anomalies. Report of a case. Oral Surg Oral Med Oral Pathol. 1971;32(1):136–40. doi: 10.1016/0030-4220(71)90259-3. [DOI] [PubMed] [Google Scholar]
- 30.Wannfors K, Lindskog S, Olander KJ, Hammarstrom L. Fibrous dysplasia of bone and concomitant dysplastic changes in the dentin. Oral Surg Oral Med Oral Pathol. 1985;59(4):394–8. doi: 10.1016/0030-4220(85)90065-9. [DOI] [PubMed] [Google Scholar]
- 31.Kelly MH, Brillante B, Collins MT. Pain in fibrous dysplasia of bone: age-related changes and the anatomical distribution of skeletal lesions. Osteoporos Int. 2008 Jan;19(1):57–63. doi: 10.1007/s00198-007-0425-x. [DOI] [PubMed] [Google Scholar]
- 32.Sadeghi SM, Hosseini SN. Spontaneous conversion of fibrous dysplasia into osteosarcoma. The Journal of craniofacial surgery. 2011 May;22(3):959–61. doi: 10.1097/SCS.0b013e31820fe2bd. [DOI] [PubMed] [Google Scholar]
- 33.Pfeiffer J, Kayser G, Boedeker CC, Ridder GJ. Posttraumatic reactive fibrous bone neoformation of the anterior skull base mimicking osteosarcoma. Skull Base. 2008 Sep;18(5):345–51. doi: 10.1055/s-0028-1086058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diah E, Morris DE, Lo LJ, Chen YR. Cyst degeneration in craniofacial fibrous dysplasia: clinical presentation and management. Journal of neurosurgery. 2007 Sep;107(3):504–8. doi: 10.3171/JNS-07/09/0504. [DOI] [PubMed] [Google Scholar]
- 35.Kaushik S, Smoker WR, Frable WJ. Malignant transformation of fibrous dysplasia into chondroblastic osteosarcoma. Skeletal Radiol. 2002 Feb;31(2):103–6. doi: 10.1007/s002560100436. [DOI] [PubMed] [Google Scholar]
- 36.de Araujo PI, Soares VY, Queiroz AL, dos Santos AM, Nascimento LA. Sarcomatous transformation in the McCune-Albright syndrome. Oral and maxillofacial surgery. 2012 Jun;16(2):217–20. doi: 10.1007/s10006-011-0286-5. [DOI] [PubMed] [Google Scholar]
- 37.Esposito SJ, Gabriel L, Smith JD, Zins JE. Fibrous dysplasia: a case report. Compend Contin Educ Dent. 1995;16(7):652, 4–6, 8–9. quiz 60. [PubMed] [Google Scholar]
- 38.Lee JS, FitzGibbon EJ, Chen YR, Kim HJ, Lustig LR, Akintoye SO, et al. Clinical guidelines for the management of craniofacial fibrous dysplasia. Orphanet J Rare Dis. 2012 May 24;7( Suppl 1):S2. doi: 10.1186/1750-1172-7-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riminucci M, Robey PG, Saggio I, Bianco P. Skeletal progenitors and the GNAS gene: fibrous dysplasia of bone read through stem cells. J Mol Endocrinol. 2010 Dec;45(6):355–64. doi: 10.1677/JME-10-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boston BA, Mandel S, LaFranchi S, Bliziotes M. Activating mutation in the stimulatory guanine nucleotide-binding protein in an infant with Cushing’s syndrome and nodular adrenal hyperplasia. J Clin Endocrinol Metab. 1994;79(3):890–3. doi: 10.1210/jcem.79.3.8077378. [DOI] [PubMed] [Google Scholar]
- 41.DeMarco L, Stratakis CA, Boson WL, Jakbovitz O, Carson E, Andrade LM, et al. Sporadic cardiac myxomas and tumors from patients with Carney complex are not associated with activating mutations of the Gs alpha gene. Hum Genet. 1996;98(2):185–8. doi: 10.1007/s004390050187. [DOI] [PubMed] [Google Scholar]
- 42.Shenker A, Weinstein LS, Moran A, Pescovitz OH, Charest NJ, Boney CM, et al. Severe endocrine and nonendocrine manifestations of the McCune-Albright syndrome associated with activating mutations of stimulatory G protein GS. J Pediatr. 1993;123(4):509–18. doi: 10.1016/s0022-3476(05)80943-6. [DOI] [PubMed] [Google Scholar]
- 43.NIDCR/ORDR/NIH. [Accessed November 15, 2011];International Meeting on Fibrous Dysplasia of Bone/McCune-Albright Syndrome: Best Clinical Practice and Future Research organized by National Institute of Dental and Craniofacial Research (NIDCR) and Office of Rare Diseases Research (ORDR)/National Institutes of Health. 2010 cited 2011. Available from: http://rarediseases.info.nih.gov/ScientificConferences.aspx?PageID=5&ID=1064.
- 44.Yeow VK, Chen YR. Orthognathic surgery in craniomaxillofacial fibrous dysplasia. J Craniofac Surg. 1999 Mar;10(2):155–9. doi: 10.1097/00001665-199903000-00012. [DOI] [PubMed] [Google Scholar]


