Abstract
Context
The Edmonton Symptom Assessment System (ESAS) is a brief, widely adopted multidimensional questionnaire to evaluate patient-reported symptoms.
Objectives
To develop a Korean version of the ESAS (K-ESAS) and to perform a psychometric analysis in Korean patients with advanced cancer.
Methods
We tested the K-ESAS in two pilot studies with 15 patients each. We assessed internal consistency, test-retest reliability, and concurrent validity in 163 Korean patients, who completed the K-ESAS along with the Korean versions of the M. D. Anderson Symptom Inventory (K-MDASI) and the Hospital Anxiety and Depression Scale (K-HADS) twice. Thirty-eight patients completed the questionnaires again seven days later to assess responsiveness.
Results
K-ESAS scores had good internal consistency, with a Cronbach’s alpha coefficient of 0.88, indicating that no questions had undue influence on the score. Pearson correlation coefficients for K-ESAS symptom scores between baseline and after 2–4 hours ranged from 0.72 (95% confidence interval [CI] 0.64, 0.79) to 0.87 (95% CI 0.82, 0.90), indicating strong test-retest reliability. For concurrent validity, Pearson correlation coefficients between K-ESAS symptom scores and corresponding K-MDASI symptom scores ranged from 0.70 (95% CI 0.62, 0.77) to 0.83 (95% CI 0.77, 0.87), indicating good concurrent validity. For the K-HADS, concurrent validity was good for anxiety (r = 0.73, 95% CI 0.65, 0.79) but moderate for depression (r = 0.4, 95% CI 0.26, 0.52). For responsiveness, changes in K-ESAS scores after seven days were moderately correlated with changes in K-MDASI scores but weakly correlated with changes in K-HADS scores.
Conclusion
The K-ESAS is a valid and reliable tool for measuring multidimensional symptoms in Korean cancer patients.
Keywords: advanced cancer, Edmonton Symptom Assessment System, ESAS, Korea
Introduction
Patients with advanced cancer often suffer from multiple moderate to severe symptoms, including physical and psychosocial distress.1–2 The prevalence of multiple symptoms in this patient population has been reported to range from 55% to 84%, with a median of 11 symptoms per patient (range 1–27).3
The Edmonton Symptom Assessment System (ESAS) is a 10-item, multidimensional assessment tool that was designed to obtain patient-reported symptom ratings. It uses a 0–10 score range to measure distress associated with both physiologic and psychological symptoms. The ESAS has been translated and validated in multiple languages but not Korean. 4–9 Thus, we developed a Korean version of the ESAS (K-ESAS) using pilot studies, and performed a psychometric analysis to validate the assessment tool for use in Korean patients with cancer.
Methods
Patient Recruitment
To test and validate the K-ESAS, we recruited patients with advanced cancer (locally advanced, relapsed or refractory, or metastatic disease) who were undergoing treatment from nine oncology clinics at university hospitals or from a veteran’s hospital in South Korea between May 7 and August 21, 2012. To be eligible, patients needed to be at least 20 years old and able to understand written Korean. Patients with symptomatic brain metastasis, uncontrolled psychiatric disease, and cognitive dysfunction, including dementia or delirium, were not eligible to participate. Patient demographics were collected upon enrollment; patients completed initial questionnaires on the same day. This study was reviewed and approved by the institutional review boards of each Korean institution as well as by The University of Texas M. D. Anderson Cancer Center Institutional Review Board. All patients provided written informed consent; the informed consent form was approved by each institutional review board.
Instruments
K-ESAS
The ESAS uses numerical scales ranging from 0 to 10 to assess the average intensity of the following symptoms experienced during the previous 24 hours (10 indicates highest intensity): pain, fatigue, nausea, depression, anxiety, drowsiness, loss of appetite, decreased well-being, dyspnea, and sleep disturbance.4, 10, 11
To validate the Korean version of the ESAS that we developed in pilot studies (see below), we compared patient responses to K-ESAS questions with their responses to similar questions on Korean versions of the M. D. Anderson Symptom Inventory (K-MDASI) and the Hospital Anxiety and Depression Scale (K-HADS).
K-MDASI
The MDASI uses a scale ranging from 0 to 10, with 0 being “not at all” and 10 being “as bad as you can imagine,” to assess the highest intensity experienced of each of 13 symptoms during the previous 24 hours: pain, fatigue, disturbed sleep, feeling of being distressed, shortness of breath, memory disruption, lack of appetite, drowsiness, dry mouth, vomiting, and numbness.12 The MDASI also includes questions to assess how much the symptoms interfered with six aspects of the patient’s life during the previous 24 hours: general activity, mood, walking ability, normal work (including housework and work outside the home), relationships with others, and enjoyment of life. The same 0–10 scale is used for these questions, with 0 indicating “does not interfere” and 10 indicating “completely interferes.” 12 The validity and reliability of the K-MDASI have been established previously.13
K-HADS
We used a validated Korean translation of the HADS (K-HADS) to assess symptoms of depression and anxiety in our study.14 This 14-item questionnaire (seven questions relating to depression and seven relating to anxiety) assesses relative symptom frequency over the past week, and each question response is based on a four-point scale ranging from 0 (not at all) to 3 (very often). A higher score indicates a greater likelihood of depression or anxiety. Scores of 8–10 indicate mild symptoms; 11–15, moderate symptoms; and 16 or higher, severe symptoms.15
Translation and Pilot Studies
An expert panel was assembled, comprising seven physicians directly involved in the care of patients with cancer (J.H.K., S.-H.N., S.K., Y.S.H., K.H.L., S.W.S., and S.Y.Y.). Each member of the panel translated the ESAS into Korean independently and panel members then compared translations to reach a consensus for a single version of the translation (pT). Back-translations of pT were done by two independent bilingual native English speakers who were not part of the panel, and these back-translations were then compared with the original ESAS to assess inconsistencies. The translation and back-translation process was repeated until the back-translated ESAS was identical to the original ESAS.
This version of the K-ESAS (T1) was tested in a pilot study with 15 patients from Kangdong Sacred Heart Hospital and VHS Medical Center, who were recruited as described above. These patients completed the K-ESAS (T1), along with the K-MDASI, and K-HADS; the responses they provided on the K-ESAS (T1) were compared with their responses to similar questions on the K-MDASI and K-HADS to assess the feasibility of the K-ESAS (T1). We then administered a survey exploring whether the patients preferred to complete the K-ESAS (T1) alone or with the assistance of a nurse, the clarity of the instructions, appropriateness of the order of items, ease of completion, and any additional suggestions, including additional symptoms to include in the scale.16
On the basis of feedback gathered in the first pilot study, the first Korean translation of the ESAS (T1) was modified by the expert panel. The modified version of K-ESAS (T2) was then tested in a second pilot study with 15 new patients recruited from Kangdong Sacred Heart Hospital, VHS Medical Center, and Good Samaritan Hospital, as described above. The same survey was used in the second pilot study as in the first pilot study to assess patients’ opinions of the K-ESAS (T2). No additional modifications were made as a result of this study; the panel decided to use the T2 version of the K-ESAS in the main validation study.
Validation Study
Patients who were not involved in the pilot studies completed the K-ESAS, the K-MDASI, and the K-HADS twice, completing the second set of questionnaires 2–4 hours after the first to test internal consistency and test-retest reliability. Patients whose pain scores on the K-ESAS were four or higher were asked to complete the assessments a third time seven (±1) days later to test the responsiveness of the K-ESAS to changes in symptoms.
Statistical Analysis
Sample Size Calculation
We assumed that 152 evaluable patients in the main validation study would provide 80% power to prove internal consistency (Cronbach’s alpha >0.65, assuming a true internal consistency of at least 0.75) using a one-sided Cronbach’s alpha test at a significance level of 2.5%. A sample size of 152 evaluable patients also was assumed to provide 79% power to show test-retest reliability (correlation coefficient >0.50, assuming a true reliability of at least 0.65) using a two-sided test of Pearson correlation at a significance level of P < 0.05. Assuming a 15% dropout rate, we estimated that at least 179 patients would need to be enrolled to obtain a minimum sample size of 152 patients.
Psychometric Analysis
We determined the concurrent validity of the K-ESAS by calculating the Spearman correlation between K-ESAS and K-MDASI scores for pain, fatigue, nausea, drowsiness, loss of appetite, dyspnea, and sleep disturbance, and between K-ESAS and K-HADS scores for depression and anxiety.
Responsiveness (the ability of the questionnaire to detect changes in symptoms), which was assessed in patients whose pain score was four or higher on the baseline K-ESAS assessment, was determined by comparing the differences between K-ESAS symptom scores at baseline and K-ESAS symptom scores at follow-up (7 ± 1 days later) with differences between K-MDASI scores or K-HADS scores (for depression and anxiety) at baseline and follow-up. Differences between K-ESAS symptom scores at baseline and follow-up were compared among patients using a two-sided Wilcoxon signed-rank test; differences were considered significant at P < 0.05.
Results
Pilot Studies
Table 1 summarizes the patient characteristics. Patients who had less than a high school education expressed confusion with the Korean translation of the term “well-being;” the Korean word used was “an-nyung,” which also is used in everyday greetings. Thus, we decided to change the word “an-nyung” to a longer phrase that more explicitly describes “well-being” as it pertains to the body and mind. No additional suggestions were gleaned from the first pilot study.
Table 1.
Patient Demographic and Clinical Characteristics
| First Pilot Study (N=15) | Second Pilot Study (N=15) | Main Validation Study (N=163) | |
|---|---|---|---|
|
| |||
| Characteristic | No. (%) | No. (%) | No. (%) |
| Mean age, years (95% CI) | 60.1 (57.3, 62.9) | 65.3 (60.9, 69.6) | 60 (58.1, 61.5) |
| Sex | |||
| Female | 2 (13) | 4 (27) | 40 (25) |
| Male | 13 (87) | 11 (73) | 123 (75) |
| Education level | |||
| Less than high school | 3 (20) | 7 (47) | 51 (31) |
| High school | 6 (40) | 6 (40) | 70 (43) |
| More than high school | 6 (40) | 1 (7) | 39 (24) |
| No response | 0 | 0 | 3 (2) |
| ECOG Performance Status | |||
| 0 | 2 (13) | 2 (13) | 9 (6) |
| 1 | 11 (74) | 11 (74) | 130 (80) |
| 2 | 2 (13) | 2 (13) | 19 (12) |
| 3 | 0 | 0 | 4 (2) |
| 4 | 0 | 0 | 1 (1) |
| Disease status | |||
| Metastatic | 12 (80) | 8 (53) | 125 (77) |
| Local relapse | 0 | 0 | 11 (7) |
| Locally advanced | 1 (7) | 6 (40) | 21 (13) |
| Advanced (hematologic) | 2 (13) | 1 (7) | 6 (4) |
| Cancer diagnosis | |||
| Gastrointestinal tract cancer | 11 (73) | 4 (27) | 60 (37) |
| Thoracic malignancy | 0 | 5 (33) | 28 (17) |
| Head and neck cancer | 1 (7) | 1 (7) | 18 (11) |
| Hepatobiliary cancer | - | 2 (13) | 17 (10) |
| Genitourinary/gynecologic cancer | 1 (7) | 1 (7) | 14 (9) |
| Hematologic cancer | 2 (13) | 2 (13) | 12 (7) |
| Breast cancer | - | - | 6 (4) |
| Other | - | - | 8 (5) |
| Patient type | |||
| Inpatient | 2 (13) | 15 (100) | 152 (93) |
| Outpatient | 13 (87) | 11 (7) | |
| Current treatment | |||
| Chemotherapy | 15 (100) | 11 (73) | 116 (71) |
| Radiation | 0 | 1 (7) | 12 (7) |
| Chemoradiation | 0 | - | 6 (4) |
| Other | 0 | 3 (20) | 29 (18) |
| Purpose of current treatment | |||
| Curative | 3 (20) | 2 (13) | 9 (6) |
| Palliative | 12 (80) | 13 (87) | 154 (94) |
CI = confidence interval, ECOG = Eastern Cooperative Oncology Group.
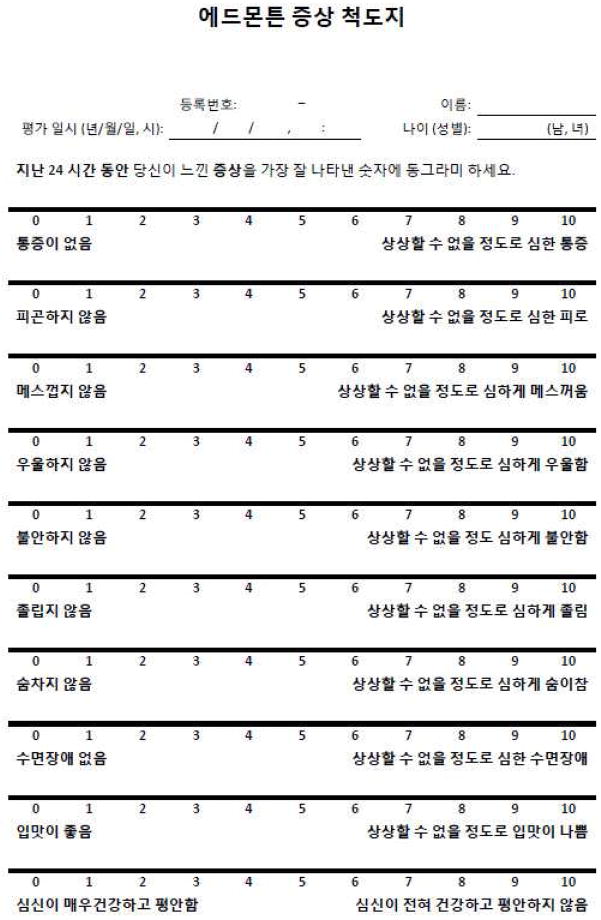
In the second pilot study, only one patient stated that the instructions were not clear; the others reported no problems with the second version of the K-ESAS (T2), which was ultimately used for further psychometric analysis (Fig. 1).
Fig. 1.
Validated Korean version of the Edmonton Symptom Assessment System.
In both pilot studies, some patients indicated that they found it difficult to express symptom severity in numbers; however, after the research nurse offered further explanation, they understood and were able to complete the questionnaires.
We also asked patients to comment on the order of questions, the wording, and any additional symptoms. All patients reported no difficulties in terms of the order and wording except for well-being. One patient in the first pilot study suggested sweating as an additional symptom, and another patient in the second pilot study suggested dizziness as an additional symptom.
Psychometric Analysis of the K-ESAS
During the enrollment period for the main validation study, 177 of 197 (90%) patients who were asked agreed to join the study. Among those enrolled, one patient withdrew consent before the survey and another developed delirium and could not participate. Nine patients were excluded from the study because they did not have advanced disease. Thus, a total of 166 patients completed the first survey. However, three were excluded from the analysis: one patient lost his completed survey and the other two did not have time to complete the second survey on the same day. Thus, 163 patients were included in the final analysis of internal consistency, test-retest reliability, and concurrent validity. Forty-six of these patients also were asked to repeat the surveys a week later to assess responsiveness, but only 38 were able to do so.
Patient Characteristics
Demographic and clinical data for patients who participated in the main validation study are shown in Table 1. Median baseline nausea K-MDASI scores were higher among outpatients (median score = 2) than inpatients (median score = 0); however, no other baseline symptom scores for the K-ESAS, K-MDASI, or K-HADS differed between any patient subgroups.
Internal Consistency and Test-Retest Reliability
All K-ESAS scores had good internal consistency, with a Cronbach’s alpha coefficient of 0.88. The internal consistency remained high after removal of individual symptom scores, with Cronbach’s alpha coefficients ranging from 0.86 to 0.88, indicating that no individual question had undue influence on the total K-ESAS score (Table 2).
Table 2.
Internal Consistency of the K-ESAS Among 163 Patients
| K-ESAS Item | Symptom Prevalence (%) | Mean (SD) K-ESAS Score | Cronbach Alpha If Removed |
|---|---|---|---|
| Pain | 67.5 | 2.4 (2.4) | 0.88 |
| Fatigue | 83.4 | 3.2 (2.5) | 0.86 |
| Nausea | 54.6 | 2.1 (2.6) | 0.87 |
| Depression | 63.8 | 2.3 (2.5) | 0.86 |
| Anxiety | 63.2 | 2.2 (2.4) | 0.86 |
| Drowsiness | 79.1 | 3.1 (2.5) | 0.87 |
| Loss of appetite | 61.3 | 3.8 (3.1) | 0.87 |
| Feeling of well-being | 66.3 | 3.8 (2.7) | 0.86 |
| Dyspnea | 78.5 | 2.0 (2.2) | 0.88 |
| Sleep disturbance | 86.5 | 2.8 (2.8) | 0.87 |
K-ESAS = Korean version of the Edmonton Symptom Assessment System; SD = standard deviation.
Table 3 shows test-retest reliability of the K-ESAS, which was measured within 2–4 hours of the initial questionnaire. Pearson correlation coefficients ranged from 0.72 (95% confidence interval [CI] 0.64, 0.79) to 0.87 (95% CI 0.82, 0.90), indicating strong test-retest reliability.
Table 3.
Test-Retest Reliability (after 2–4 hours) of the K-ESAS Among 163 Patients
| K-ESAS Item | Mean Test Score (95% CI) | Mean Retest Score (95% CI) | Mean Difference (95% CI) | Pearson Correlation Coefficient (95% CI) |
|---|---|---|---|---|
| Pain | 2.35 (1.98, 2.72) | 2.38 (2.02, 2.74) | 0.66 (0.48, 0.84) | 0.81 (0.75, 0.86) |
| Fatigue | 3.20 (2.81, 3.59) | 3.29 (2.88, 3.71) | 0.93 (0.71, 1.14) | 0.80 (0.74, 0.85) |
| Nausea | 2.10 (1.69, 2.51) | 2.42 (2.00, 2.83) | 0.70 (0.51, 0.89) | 0.84 (0.78, 0.88) |
| Depression | 2.33 (1.95, 2.72) | 2.44 (2.04, 2.84) | 0.63 (0.46, 0.79) | 0.86 (0.81, 0.89) |
| Anxiety | 2.21 (1.84, 2.59) | 2.44 (2.05, 2.83) | 0.79 (0.60, 0.98) | 0.82 (0.77, 0.87) |
| Drowsiness | 3.06 (2.67, 3.44) | 3.10 (2.70, 3.51) | 0.87 (0.69, 1.06) | 0.82 (0.76, 0.86) |
| Loss of appetite | 3.84 (3.36, 4.32) | 3.64 (3.19, 4.09)a | 0.77 (0.55, 0.98) | 0.87 (0.82, 0.90) |
| Feeling of well-being | 3.79 (3.37, 4.20) | 3.77 (3.34, 4.20) | 1.06 (0.79, 1.32) | 0.72 (0.64, 0.79) |
| Dyspnea | 2.02 (1.67, 2.36) | 2.21 (1.85, 2.56) | 0.62 (0.44, 0.80) | 0.86 (0.81, 0.90) |
| Sleep disturbance | 2.80 (2.37, 3.23) | 2.78 (2.35, 3.21) | 0.63 (0.44, 0.83) | 0.84 (0.79, 0.88) |
K-ESAS = Korean version of the Edmonton Symptom Assessment System; CI = confidence interval.
n = 162.
Concurrent Validity
Symptom scores on the K-ESAS were strongly correlated with matched symptom scores on the K-MDASI, with Pearson correlation coefficients ranging from 0.70 (95% CI 0.62, 0.77) to 0.83 (95% CI 0.77, 0.87) (Table 4). Anxiety scores on the K-ESAS were strongly correlated with the corresponding scores on the K-HADS (r = 0.73, 95% CI 0.65, 0.79); however, K-ESAS depression scores were only moderately correlated with depression scores on the K-HADS (r = 0.4, 95% CI 0.26, 0.52). Concurrent validity of the score for well-being on the K-ESAS was not tested because no corresponding question is given on the K-MDASI or K-HADS.
Table 4.
Concurrent Validity of K-ESAS Symptom Scores, as Measured by Their Correlation With Corresponding K-MDASI or K-HADS Symptom Scores at Baseline (n = 163)
| K-ESAS Item | Pearson Correlation Coefficient (95% CI)a
|
|
|---|---|---|
| K-MDASI | K-HADS | |
| Pain | 0.80 (0.74, 0.85) | |
| Fatigue | 0.74 (0.66, 0.80) | |
| Nausea | 0.83 (0.77, 0.87) | |
| Depression | 0.40 (0.26, 0.52) | |
| Anxiety | 0.73 (0.65, 0.79) | |
| Drowsiness | 0.70 (0.62, 0.77) | |
| Loss of appetite | 0.74 (0.66, 0.80) | |
| Feeling of well-being | – | – |
| Dyspnea | 0.75 (0.67, 0.81) | |
| Sleep disturbance | 0.74 (0.66, 0.80) | |
K-ESAS = Korean version of the Edmonton Symptom Assessment System; K-MDASI = Korean version of the M. D. Anderson Symptom Inventory; K-HADS = Korean version of the Hospital Anxiety and Depression Scale; CI = confidence interval.
P < 0.0001.
Responsiveness
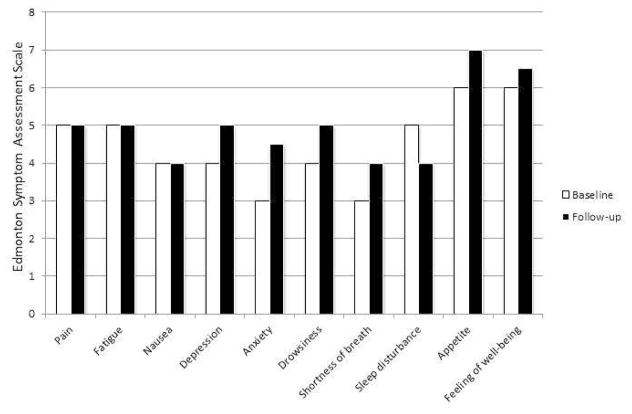
Differences in K-ESAS scores were moderately correlated with differences in K-MDASI scores; however, the correlation between differences in K-ESAS scores and differences in K-HADS scores was not significant. Results of the Wilcoxon signed-rank test indicated that only the fatigue scores on the K-ESAS changed significantly after seven days (P = 0.017); however, the median fatigue score at baseline and follow-up was the same (Table 5, Fig. 2).
Table 5.
Responsiveness of K-ESAS Symptom Scores After Seven Days, as Measured by the Correlation Between Changes in K-ESAS Scores and Changes in Corresponding K-MDASI or K-HADS Symptom Scores (n = 38)
| K-ESAS Item | Pearson Correlation Coefficient (95% CI)
|
P-value | |
|---|---|---|---|
| K-MDASI | K-HADS | ||
| Pain | 0.42 (0.11, 0.65) | 0.0044 | |
| Fatigue | 0.42 (0.12, 0.65) | 0.0037 | |
| Nausea | 0.57 (0.30, 0.75) | <0.0001 | |
| Depression | 0.05 (−0.28, 0.36) | 0.3886 | |
| Anxiety | 0.25 (−0.08, 0.53) | 0.0645 | |
| Drowsiness | 0.47 (0.17, 0.68) | 0.0013 | |
| Loss of appetite | 0.54 (0.26, 0.73) | 0.0008 | |
| Feeling of Well-being | – | – | 0.0062 |
| Dyspnea | 0.49 (0.19, 0.69) | 0.0002 | |
| Sleep disturbance | 0.40 (0.09, 0.63) | 0.0044 | |
K-ESAS = Korean version of the Edmonton Symptom Assessment System; K-MDASI = Korean version of the M. D. Anderson Symptom Inventory; K-HADS = Korean version of the Hospital Anxiety and Depression Scale; CI = confidence interval.
Fig. 2.
Median changes in the K-ESAS scores after 7 (±1) days (n = 38).
Discussion
We have developed a validated Korean version of the ESAS for use in Korean patients with advanced cancer undergoing treatment at an oncology clinic. The K-ESAS showed good internal consistency, test-retest reliability, and concurrent validity.
The ESAS was originally developed and applied in the palliative care setting.10 However, it has since even been used in various other cancer and non-cancer populations.17, 18 In our study, we tested the K-ESAS in a population of Korean patients seen at oncology clinics. Chang et al.19 also used oncology outpatients to validate the English version of the ESAS, and, as in our study, their results indicated good internal consistency and reliability. K-ESAS symptom scores in our study population were mild to moderate (median scores ranged from 2.0 to 3.8), and the prevalence of symptoms as assessed in the K-ESAS ranged from 54.6% to 86.5%, which was similar to the prevalence of symptoms reported by Chang et al. Taken together, our findings suggest that the ESAS is a reasonable tool for multidimensional symptom assessment in routine oncology practice.
We used the K-MDASI to validate patient responses to seven physical K-ESAS items. Both tools assess symptoms during the same time frame, the previous 24 hours, but the K-MDASI is meant to assess the worst symptoms whereas the K-ESAS assesses average symptoms during that time period. However, the strong correlation between the scores indicated good concurrent validity for the K-ESAS.
In contrast, the correlation between the K-HADS and the K-ESAS, particularly for depression, was relatively low. These results are consistent with those of Vignaroli et al.,20 who found that the ESAS was only moderately correlated with the HADS (r = 0.4), and those of Pantilat et al.,21 who found that the ESAS was not well correlated with a 15-item geriatric depression scale (r = 0.34). Conversely, Bagha et al. found that the ESAS depression score was relatively highly correlated with the Patient Health Questionnaire-9 (r = 0.72, P < 0.0001).22 The low correlation noted in our study and others may be explained by the fact that the ESAS assesses symptoms only during the previous 24 hours, whereas many other depression screening tools, including the HADS, assess symptoms over 1–2 weeks. Further study with larger sample sizes may be needed to assess the concurrent validity of depression scores in the ESAS or the K-ESAS.
Some patients in our study, especially those with less than a high school education, found it difficult to express the severity of symptoms as numbers and needed additional explanation from the research nurse. Other studies also revealed a similar observation. 23,24 Further studies are needed to examine factors that may hinder a patient’s ability to use a numeric scale, to help improve patient-reported outcome measures.
In contrast to the study by Watanabe et al., 25 patients in our study did not report difficulties with the order and contents of the K-ESAS. Watanabe et al. compared the revised version of the ESAS (r-ESAS) to the original ESAS. Patients in this study expressed minimal differences related to the order of questions and the anchor. There were no differences in psychometric properties, but patients preferred completing the r-ESAS. Therefore, for the purpose of research, both versions of the ESAS have similar validity and reliability. This might be explained by differences in culture or clinical settings.
Our study is partly limited by the small number of patients (38 of 163) who were able to provide follow-up data. Furthermore, a follow-up period of seven days may be too short to detect any change in symptoms. This may partially explain the low responsiveness of the K-ESAS in this study. Further research is necessary to document follow-up data in a larger sample size and for a longer follow-up period.
Our results indicate that the K-ESAS is a reliable tool for the assessment of multiple symptoms in Korean patients with advanced cancer. Our translation of the ESAS into a new language and validation of the translated ESAS in a clinical setting demonstrates the wide applicability of the tool, allowing comparison of patients’ distress across multiple settings and in multiple countries.26 The short time needed to complete the K-ESAS allows clinicians to easily assess symptom changes over time, especially in a graphic display (Fig. 1). As the field of symptom and toxicity evaluation moves toward patient-reported outcomes,27 the K-ESAS provides a simple and reliable way of assessing both disease burden and the effects of treatment.
Acknowledgments
Eduardo Bruera is supported in part by National Institutes of Health grants RO1NRO10162-01A1, RO1CA122292-01, and RO1CA124481-01. The University of Texas M. D. Anderson Cancer Center is supported in part by a Cancer Center Support Grant (CA016672) from the National Institutes of Health.
All authors would like to express appreciation for the research nurses and the patients who were involved in the study at the participating hospitals.
Footnotes
Disclosures
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Curtis EB, Krech R, Walsh TD. Common symptoms in patients with advanced cancer. J Palliat Care. 1991;7:25–29. [PubMed] [Google Scholar]
- 2.Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007;37:16–21. doi: 10.1093/jncimonographs/lgm005. [DOI] [PubMed] [Google Scholar]
- 3.Walsh D, Donnelly S, Rybicki L. The symptoms of advanced cancer: relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer. 2000;8:175–179. doi: 10.1007/s005200050281. [DOI] [PubMed] [Google Scholar]
- 4.Carvajal A, Centeno C, Watson R, Bruera E. A comprehensive study of psychometric properties of the Edmonton Symptom Assessment System (ESAS) in Spanish advanced cancer patients. Eur J Cancer. 2011;47:1863–1872. doi: 10.1016/j.ejca.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Stiel S, Matthes ME, Bertram L, et al. [Validation of the new version of the minimal documentation system (MIDOS) for patients in palliative careý: the German version of the Edmonton Symptom Assessment Scale (ESAS)] [in German] Schmerz. 2010;24:596–604. doi: 10.1007/s00482-010-0972-5. [DOI] [PubMed] [Google Scholar]
- 6.Yeşilbalkan OU, Ozkutuk N, Karadakovan A, Turgut T, Kazgan Validity and reliability of the Edmonton Symptom Assessment Scale in Turkish cancer patients. Turk J Cancer. 2008;38:62–67. [Google Scholar]
- 7.Chinda M, Jaturapatporn D, Kirshen AJ, Udomsubpayakul U. Reliability and validity of a Thai version of the Edmonton Symptom Assessment Scale (ESAS-Thai) J Pain Symptom Manage. 2011;42:954–960. doi: 10.1016/j.jpainsymman.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Pautex S, Berger A, Chatelain C, Herrmann F, Zulian GB. Symptom assessment in elderly cancer patients receiving palliative care. Crit Rev Oncol Hematol. 2003;47:281–286. doi: 10.1016/s1040-8428(03)00043-x. [DOI] [PubMed] [Google Scholar]
- 9.Moro C, Brunelli C, Miccinesi G, et al. Edmonton Symptom Assessment Scale: Italian validation in two palliative care settings. Support Care Cancer. 2006;14:30–37. doi: 10.1007/s00520-005-0834-3. [DOI] [PubMed] [Google Scholar]
- 10.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 11.Garyali A, Palmer JL, Yennurajalingam S, et al. Errors in symptom intensity self-assessment by patients receiving outpatient palliative care. J Palliat Care. 2006;9:1059–1065. doi: 10.1089/jpm.2006.9.1059. [DOI] [PubMed] [Google Scholar]
- 12.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M. D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Yun YH, Mendoza TR, Kang IO, et al. Validation study of the Korean version of the M. D Anderson Symptom Inventory. J Pain Symptom Manage. 2006;31:345–352. doi: 10.1016/j.jpainsymman.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Oh SM, Min KJ, Park DB. A study on the standardization of the Hospital Anxiety and Depression Scale for Koreans: a comparison of normal, depressed and anxious groups. J Korean Neuropsych Assoc. 1999;38:289–296. [Google Scholar]
- 15.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe S, Nekolaichuk C, Beaumont C, Mawani A. The Edmonton Symptom Assessment System--what do patients think? Support Care Cancer. 2009;17:675–683. doi: 10.1007/s00520-008-0522-1. [DOI] [PubMed] [Google Scholar]
- 17.Miyasaki JM, Long J, Mancini D, et al. Palliative care for advanced Parkinson disease: an interdisciplinary clinic and new scale, the ESAS-PD. Parkinsonism Relat Disord. 2012;18(Suppl 3):S6–9. doi: 10.1016/j.parkreldis.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Davison SN, Jhangri GS, Johnson JA. Longitudinal validation of a modified Edmonton Symptom Assessment System (ESAS) in haemodialysis patients. Nephrol Dial Transplant. 2006;21:3189–3195. doi: 10.1093/ndt/gfl380. [DOI] [PubMed] [Google Scholar]
- 19.Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88:2164–2171. doi: 10.1002/(sici)1097-0142(20000501)88:9<2164::aid-cncr24>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Vignaroli E, Pace EA, Willey J, et al. The Edmonton Symptom Assessment System as a screening tool for depression and anxiety. J Palliat Med. 2006;9:296–303. doi: 10.1089/jpm.2006.9.296. [DOI] [PubMed] [Google Scholar]
- 21.Pantilat SZ, O’Riordan DL, Dibble SL, Landefeld CS. An assessment of the screening performance of a single-item measure of depression from the Edmonton Symptom Assessment Scale among chronically ill hospitalized patients. J Pain Symptom Manage. 2012;43:866–873. doi: 10.1016/j.jpainsymman.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagha SM, Macedo A, Jacks LM, et al. The utility of the Edmonton Symptom Assessment System in screening for anxiety and depression. Eur J Cancer Care (Engl) 2013;22:60–69. doi: 10.1111/j.1365-2354.2012.01369.x. [DOI] [PubMed] [Google Scholar]
- 23.Selby D, Cascella A, Gardiner K, et al. A single set of numerical cutpoints to define moderate and severe symptoms for the Edmonton Symptom Assessment System. J Pain Symptom Manage. 2010;39:241–249. doi: 10.1016/j.jpainsymman.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Gill A, Daines P, Selby D. What do symptom scores mean: observations on discrepancies when defining symptoms using words and numbers. Eur J Oncol Nurs. 2010;14:435–438. doi: 10.1016/j.ejon.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe SM, Nekolaichuk C, Beaumount C, et al. A multicenter study comparing two numerical version of the Edmonton Symptom Assessment System in palliative care patients. J Pain Symptom Manage. 2011;41:456–466. doi: 10.1016/j.jpainsymman.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Cummings G, Biondo PD, Campbell D, et al. Can the global uptake of palliative care innovations be improved? Insights from a bibliometric analysis of the Edmonton Symptom Assessment System. Palliat Med. 2011;25:71–82. doi: 10.1177/0269216310381449. [DOI] [PubMed] [Google Scholar]
- 27.Basch E, Abernethy AP, Mullins CD, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30:4249–4255. doi: 10.1200/JCO.2012.42.5967. [DOI] [PubMed] [Google Scholar]