Abstract
An increasing body of evidence suggests that dysregulation of iron metabolism contributes to age-related pathologies. We have previously observed increased hepatic iron with aging, and that environmental heat stress stimulates a further increase in iron and oxidative liver injury in old rats. The purpose of this study was to determine a mechanism for the increase in hepatic iron in old rats after heat stress. Young (6 mo) and old (24 mo) Fischer 344 rats were exposed to two heating bouts separated by 24 h. Livers were harvested after the second heat stress, and protein levels of the iron import protein, transferrin receptor-1 (TFR1), and the iron export protein, ferroportin (Fpn) were determined by immunoblot. In the nonheated condition, old rats had lower TFR1 expression, and higher Fpn expression. After heat stress, TFR1 declined in the old rats, and iron chelation studies demonstrated that this decline was dependent on a hyperthermia-induced increase in iron. TFR1 did not change in the young rats after heat stress. Since TFR1 is inversely regulated by iron, our results suggest that the increase in intracellular iron with aging and heat stress lower TFR1 expression. Fpn expression increased in both age groups after heat stress, but this response was delayed in old rats. This delay in the induction of an iron exporter suggests a mechanism for the increase in hepatic iron and oxidative injury after heat stress in aged organisms.
Keywords: TFR1, ferroportin, hyperthermia, deferoxamine, aging, DMT1
Introduction
Heat stress represents a multifaceted environmental challenge that includes perturbations to homeostasis at all levels of inquiry. In addition to cardiovascular [1, 2], and nervous [3] system adjustments, heat stress elicits subcellular damage to internal organs such as the liver [4]. With aging, these changes are exacerbated, which likely contributes to the increased incidence of morbidity and mortality observed in elderly populations after heat waves [5, 6]. In animal models, aging is associated with enhanced subcellular injury after heat stress, which includes damage to mitochondria and lysosomes [7, 8], as well as an increase in intracellular iron [9]. Iron can participate in the generation of reactive species that can lead to oxidative damage, and we have shown that iron plays a role in oxidative liver injury in old rats after heat stress [9].
The liver is an important organ for physiologic iron homeostasis. The liver plays a critical role in the sensing and regulation of total body iron status as the major site of expression of the iron-regulatory hormone, hepcidin. In addition, together with the spleen, the liver participates in the constitutive turnover of iron from transferrin and effete red blood cells. Specifically, Kupffer cells (the resident macrophages of the liver) phagocytose senescent red blood cells, degrade the hemoglobin, and then release the iron liberated from heme into the circulation, bound to transferrin. Aging results in iron accumulation in hepatocytes and Kupffer cells of the liver [9], as well as in other organs such as the kidney [10], brain [10, 11], and skeletal muscle [12, 13]. The mechanisms for the iron retention with aging are unclear, but might involve enhanced iron uptake, or reduced iron export in these tissues, potentially resulting from dysregulation of iron transport proteins.
The major mechanism of iron import into cells is through receptor-mediated endocytosis of the plasma iron transport protein, transferrin (Tf), which is mediated primarily by transferrin receptor-1 (TFR1). After endocytosis, the iron is liberated from Tf in the acidic lysosomal compartment [14]. Transferrin can then be recycled back to the circulation via exocytosis, while the iron is distributed to appropriate intracellular compartments by divalent metal ion transporter-1 (DMT1) [15]. The major form of control of TFR1 expression in the liver is through iron regulatory protein-1 (IRP1), which binds to the iron responsive element (IRE) in the 3′region of TFR1 mRNA [14]. When cellular iron is low, IRP1 binds to the IRE of TFR1 and stabilizes the mRNA, leading to enhanced translation of TFR1. When the cell is iron-replete, IRP1 forms complexes with iron-sulfur clusters that confer aconitase activity and it no longer binds IREs. Thus, under iron replete or iron overload conditions, TFR1 mRNA stability is decreased, which results in a decrease in TFR1 protein. TFR1 is down-regulated by iron, but upregulated by inflammation [16, 17], with the latter being an adaptive response that is presumed to limit the availability of circulating iron to pathogens.
The export of iron from cells is crucial for the maintenance of appropriate intracellular iron concentrations, as well as to provide iron to the bone marrow for erythropoiesis. To release iron, cells can secrete the iron storage molecule, ferritin [18], or release iron via the iron exporter, ferroportin (Fpn). Currently, Fpn is the only protein known to mediate export of iron from cells such as macrophages, enterocytes, and hepatocytes [19 - 22]. In some models, there is an association between low Fpn expression and iron retention in the liver [23, 24]. Moreover, conditional knockout of ferroportin in 10-12 day old mice results in accumulation of iron in hepatocytes and Kupffer cells [22]. These studies suggest a strong role for Fpn in hepatic iron export. Like TFR1, Fpn responds to extracellular stressors. Specifically, Fpn mRNA is upregulated with endoplasmic reticulum stress [25] and iron administration [26]. Disparate results have been obtained with regard to hepatic Fpn protein levels after inflammation, with one study finding a decrease [16] and another study finding an increase [17] at similar times after an injection of turpentine.
Aging is associated with low-grade inflammation [27], as well as tissue iron retention. Since heat stress further increases hepatic iron in old rats [9], the purpose of this study was to characterize the expression of proteins involved in the uptake and export of iron in these animals. Given that increased levels of TFR1 mRNA and protein are associated with increased iron influx after hepatic ischemia-reperfusion [28], and that heat stress is associated with hypoxia in the liver [29], it is possible that the increase in nonheme iron after heat stress in old rats is due to enhanced iron influx through TFR1. To our knowledge, the effects of age on iron transport proteins in the liver have not been investigated, and little is known about the response of these proteins to a physiological stressor such as hyperthermia. Since iron can mediate oxidative damage in old rats in response to heat stress [9], an understanding of the proteins that are involved in iron regulation is important.
In this investigation, we show a novel response in the livers of aged rats to environmental heat stress, which is characterized by downregulation of TFR1, and delayed and attenuated upregulation of Fpn. Studies using the iron chelator, deferoxamine (DFO) demonstrated that the downregulation of TFR1 was dependent on an increase in intracellular iron. Compared to young rats, the upregulation of Fpn was delayed in old rats, which may contribute to the transient accumulation of hepatic iron in this age group.
Materials and methods
Animal experiments
Chronic iron administration
Archived liver samples from male Sprague-Dawley rats (200-250g; ~2 months of age) from a previous study [30] were utilized to determine the effects of chronic iron administration on hepatic iron regulatory proteins. Animals were housed in polyethylene cages, fed a standard rat diet, and allowed water ad libitum. Iron dextran was administered by i.p. injection every two weeks for six months. In the first four injections, rats were given 225 mg/kg iron dextran; for subsequent injections, 450 mg/kg was administered per rat. Control animals received injections of an equivalent amount of dextran at similar times. The animals were cared for according to the criteria from the National Research Council, and the protocol was approved by the Animal Research Committee of the John Cochrane Veterans Administration Medical Center. Animals were euthanized under deep anesthesia and livers were immediately frozen in liquid nitrogen.
Aging and heat stress experiments
Fischer 344 rats (young, 6 mo; old, 24 mo) were obtained from the National Institute on Aging. All animal protocols described below were approved by the University of Iowa Institutional Animal Care and Use Committee. Rats were housed in polyethylene cages in the Animal Care Facility, and maintained on a rodent diet with water ad libitum. Young and old rats were exposed to a two-heat stress protocol; this protocol has been described in detail previously [7, 8, 9]. Briefly, core temperature (Tc) was measured with a colonic thermistor probe inserted 6-7 cm into the distal colon. The probe was attached to a YSI temperature monitor and Tc was monitored continuously. On day 1, Tc was elevated from ~37°C to 41.0°C over a period of 1 h at a rate of 0.06°C/min, and then maintained at this temperature for an additional 30 min. At the same time on day 2, animals were exposed to an identical heat stress. As a control for the heat stress protocol, nonheated rats were fitted with a thermistor probe and maintained at room temperature (~21.0°C) on both days at similar times as the heat stress experiments. This heat stress model mimics what elderly humans would experience during a heat wave (i.e. repeated exposures to high ambient temperature conditions). This model elicits cellular dysfunction, which includes an increase in hepatic nonheme iron and oxidative stress [9, 31], but does not cause mortality or heat stroke in either age group. Preliminary experiments in the laboratory established that a single heating bout did not result in changes in hepatic iron or oxidative stress in young or old rats; therefore, the two-heat stress protocol was utilized in the present study.
Liver samples were harvested immediately (0 h), and at 2 and 24 hours after the second heat stress in separate groups of animals. These time points were chosen to coincide with previous studies showing an early increase iron and oxidative stress, followed by a return of these measures to nonheated values at 24 h [9, 31]. Rats were euthanized under deep anesthesia and the liver was snap-frozen in liquid nitrogen.
Iron administration in young Fischer 344 rats
Because old rats have higher concentrations of hepatic iron than young rats, iron was administered to young rats (6 mo) in order to normalize hepatic iron concentrations (HIC) between age groups. A separate group of young rats was administered a single dose of iron dextran (15 mg/kg - i.p.) seven days prior to the first day of the heating protocol. This dose of iron effectively doubled hepatic iron contents in the young rats, resulting in HIC that were similar to the HIC in old rats (Supplemental Tables 1 and 2). Administration of iron by this means also mimicked the cellular localization of iron in the old rats [9]. Control rats were given a single dose of dextran in sterile 0.9% NaCl. Control and iron-treated rats were subsequently divided into heated (0 h) and nonheated groups. The 0-h time point was chosen due to the elevation in liver iron and oxidative injury observed in old rats at this time point [9, 31].
Iron chelation in old Fischer 344 rats
To decrease liver iron in old rats (24 mo), a separate group of old animals was treated with the iron chelator, deferoxamine (DFO – Sigma Aldrich, St. Louis, MO). Rats were injected i.p. with 200 mg/kg DFO dissolved in sterile 0.9% NaCl every 12 h (8am and 8pm) beginning three days before the first heating bout. On day 4 (first heating bout), rats were injected 2 h before the heating, then again at 8pm. On day 5, rats were again injected 2 h prior to heating, then euthanized immediately (0 h) after the second heating bout. Control rats were given injections of sterile 0.9% NaCl at similar times as the DFO rats. Control and DFO rats were subdivided into nonheated and heated (0 h) groups. In both iron manipulated groups, liver collection was performed as described above.
Nonheme iron quantitation
Hepatic iron content was determined according to the method of Torrance and Bothwell as described previously [9, 32]. Values for these sets of animals have been reported previously [9] and are repeated in Supplemental Tables 1, 2 and 3 to assist with interpretation of results.
Immunoblotting
Frozen liver samples were homogenized using RIPA buffer (50 mM Tris, 150 mM NaCl, 0.25% sodium deoxycholate, 1% triton-X, 1 mM EDTA, 1 mM Na3VO4), with a protease/phosphatase inhibitor cocktail (HALT #78440, Thermo Scientific, Rockford, IL). Samples (50 μg) from whole-liver homogenates were loaded into freshly poured 12% polyacrylamide gels and run at 100V for 90 min. Proteins were transferred to a nitrocellulose membrane, then blocked for 30-60 min in 5% milk in tween-tris buffered saline (TTBS). Membranes were incubated overnight at 4°C with primary antibody, then washed with TTBS and incubated for 1 h in the appropriate secondary antibody. Anti-TFR1 was purchased from Invitrogen (136800), and used at a 1:1000 dilution, with goat anti-mouse antibody (sc-2031, Santa Cruz Biotechnologies, Santa Cruz, CA) at a 1:4000 dilution. Anti-DMT1 was purchased from Santa Cruz (sc-30926) and used at a 1:250 dilution with donkey anti-goat antibody (sc-2020, Santa Cruz) at a 1:1000 dilution. Anti-Fpn (Ref [33]) was used at a 1:2000 dilution with goat anti-rabbit antibody (sc-2030, Santa Cruz) at a 1:2000 dilution. Membranes were washed with TTBS, exposed with chemiluminescent reagent (Thermo Scientific, Rockford, IL), then photographed using the ChemiDoc XRS+ imager with Image Lab Software (Bio Rad, Hercules, CA).
After staining for the protein of interest, membranes were stained with Ponceau staining solution to confirm equal loading and transfer. In initial experiments, we observed that both β-actin and GAPDH increased significantly with age. Moreover, there was a trend for an increase in GAPDH in the old group immediately (0h) following heat stress (data not shown). Therefore, band densities of the protein of interest were normalized to a consistent band on the Ponceau-stained membrane (~40 kDa) and further normalized to the young nonheated group, which was given a value of 1. Image Lab Software was used to quantify the density of protein bands. To minimize analytic variability, every comparison (i.e., nonheated to heated, and young versus old at each time point) was run head-to-head on the same gel. For example, to compare the young and old nonheated groups, seven samples from each group were run on the same gel. This process was repeated until all comparisons were made. A similar process was used in the iron manipulation studies. Values in each graph represent the values determined in this manner, and statistics were performed on these numbers.
Statistics
Results were analyzed by two-factor ANOVA to determine the effects of age and heat stress, and to determine significant differences in the iron manipulation and heat stress studies. Where appropriate, Tukey’s post hoc test was used to determine significant differences between groups. A p-value of less than 0.05 was considered statistically significant. The SPSS program (IBM) was utilized for statistical purposes.
Results
Hepatic iron concentrations in young (6 mo) and old (24 mo) rats
In old rats, HIC was two-fold higher compared to young rats in the control, nonheated condition, and at all times after heat stress (p<0.05; Table S1). Immediately (0 h) after heat stress, there was a further increase in nonheme iron in the old rats only (Table S1). Nonheme iron did not change significantly in the young groups after heat stress. Iron administration to young rats significantly increased HIC to levels similar to old rats (Table S2). Treatment of old rats with DFO significantly reduced HIC, but did not lower HIC to concentrations observed in young rats (Table S3). The limited ability of DFO to decrease HIC has been reported elsewhere [34].
Effects of aging and iron manipulation on iron regulatory proteins
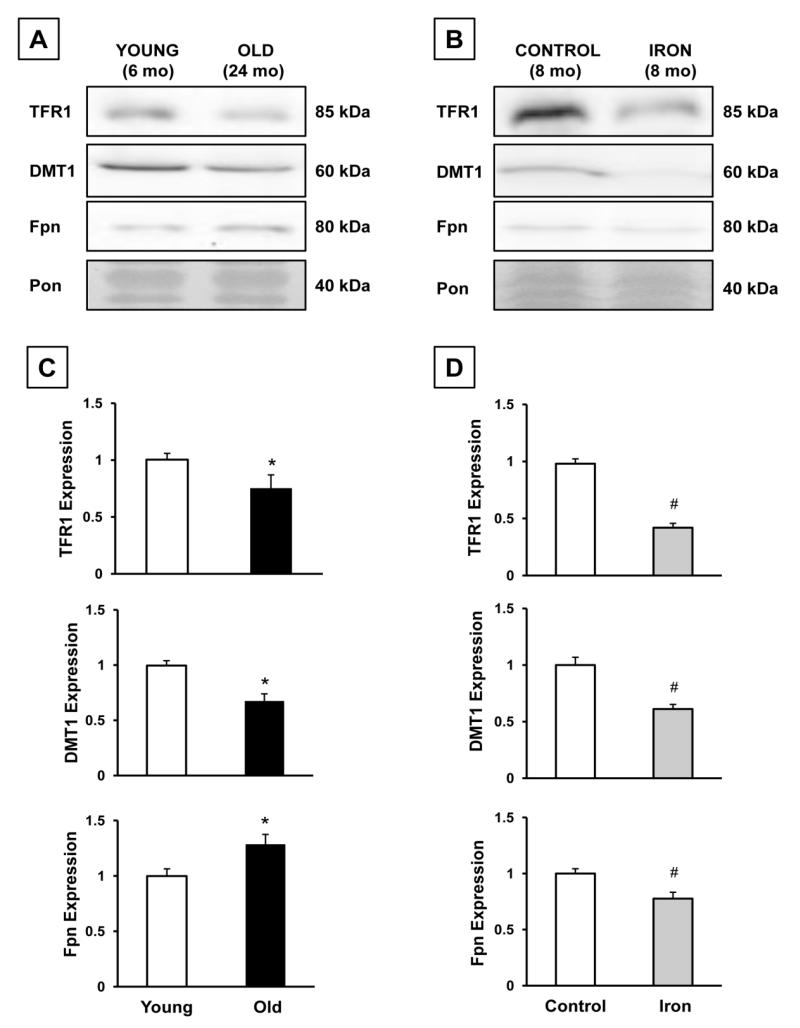
Since aging was associated with an increase in HIC, we considered the possibility that any changes we observed in the expression of the iron regulatory proteins might be due to an increase in hepatic iron content. In order to determine the effects of increased hepatic iron content on the iron transporters, we assessed the levels of these proteins in archived samples from a previous experiment in which rats (~2 mo) were chronically iron-loaded over a six-month period [30], and compared them to young (6 mo) and old (24 mo) rats. Chronic iron administration in resulted in a nearly 60-fold increase in HIC versus control (10,706 vs. 189 μg/g, p<0.001). In both aged rats (24 mo) and chronically iron-treated rats (8 mo), there was a significant decrease in the levels of TFR1 and DMT1 (p<0.05). Whereas aging was associated with an increase in ferroportin protein, ferroportin protein levels were lower in chronically iron-administered rats compared to controls (Fig. 1).
Figure 1.
Comparison of the effects of aging and chronic iron loading on hepatic iron regulatory proteins. A: representative immunoblots of liver samples from young (6 mo) and old (24 mo) rats probed for TFR1, DMT1, and Fpn. Ponceau-stained membranes (Pon) are the bottom blot. B: representative immunoblots from control, and chronically iron-loaded rats (8 mo) probed for TFR1, DMT1, and Fpn. Ponceau-stained membranes (Pon) are the bottom blot. C: Quantitation of band densities shown in panel A. Results were normalized to the density of the Ponceau stain and further normalized to the young group. Results are representative of 7-9 young and old rats. Results are expressed as means + SEM. * Significant (p<0.05) effect of aging. D: Quantitation of band densities shown in panel B. Results were normalized to the density of the Ponceau stain and further normalized to the control group. Results are representative of five control and five chronically iron-loaded rats. Results are expressed as means + SEM. # Significant effect of iron-administration.
TFR1 expression
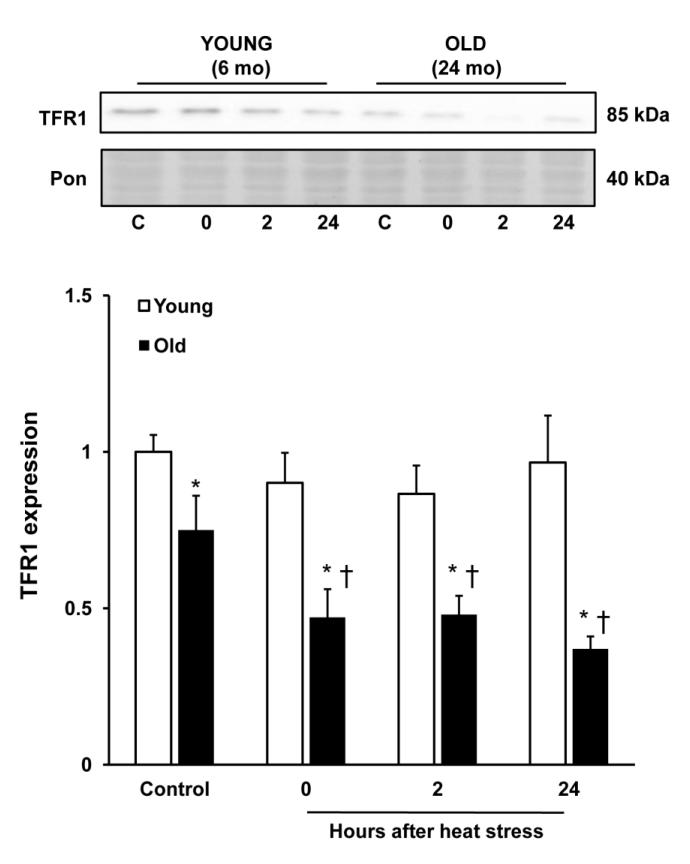
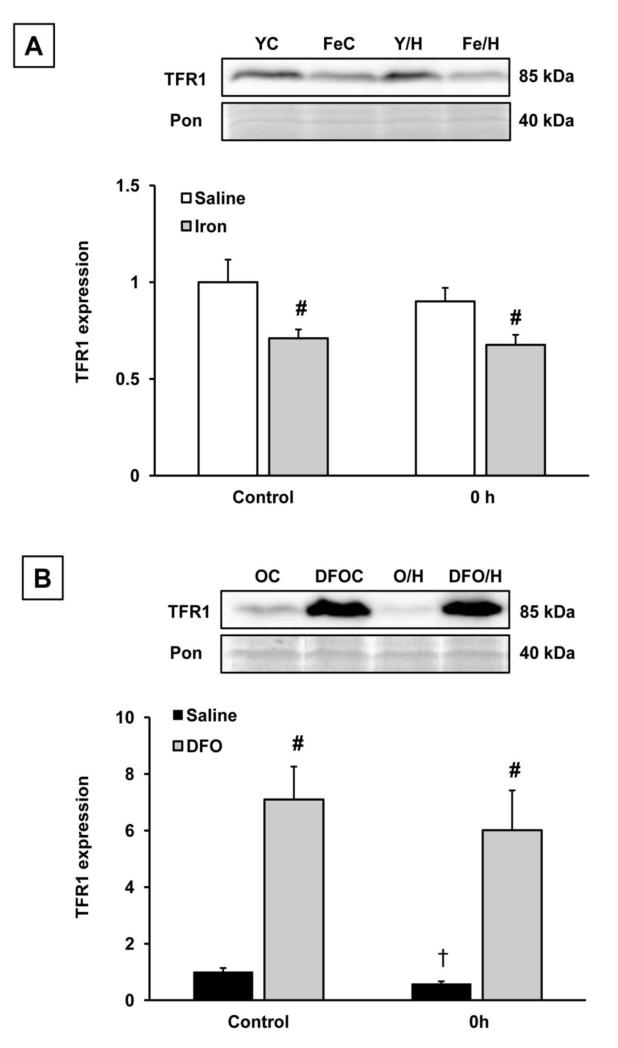
Aging was associated with significant downregulation of hepatic TFR1, and after heat stress, hepatic levels of TFR1 protein were further decreased in old rats at all time points (Fig. 2). In contrast, levels of TFR1 were unaffected by heat stress in the young groups at all time points (Fig. 2). While there was no effect of heat stress on TFR1 in young (6 mo) rats, a single dose of iron dextran lowered TFR1 expression by approximately 30% in these animals (Fig. 3A). There was not a combined effect of heat stress and iron in young rats (Fig. 3A). In old rats given DFO, TFR1 levels were seven-fold higher compared to saline-treated animals under nonheated conditions, and remained high following heat stress (Fig. 3B).
Figure 2.
Heat stress is associated with downregulation of TFR1 in old rats. Immunoblot analysis of TFR1 after heat stress in young (6 mo) and old (24 mo) rats. Liver samples from young and old rats were analyzed for TFR1 protein expression in the nonheated condition, and at the indicated times after heat stress. TFR1 expression was normalized to Ponceau staining, and ratios were further normalized to the young, nonheated group. * Significant effect of aging; † significant effect of heat stress within an age group (p<0.05). Results are expressed as means + SEM; n=7-9 young and old rats under nonheated conditions, and at each time point after heat stress.
Figure 3.
Effects of iron manipulation on TFR1 protein levels in young and old rats. A: Young (6 mo) rats were treated with a single dose of iron-dextran (15 mg/kg) five days prior to the first heat stress. Samples were analyzed from saline-treated and iron-treated young rats in the nonheated condition, and at 0h after the second heat stress. Density of the TFR1 band was normalized to the Ponceau band, and values were further normalized to the young controls. YC: young control; FeC: young nonheated, iron-loaded; Y/H: young heated (0 h); Fe/H: young iron-loaded, heated (0 h) B: Old (24 mo) rats were treated with DFO (200 mg/kg every 12h) beginning at day 3 before the first heat stress. Samples were analyzed from saline-treated and DFO-treated old rats in the nonheated condition, and at 0 h after the second heat stress. Density of the TFR1 band was normalized to the Ponceau stain, and then further normalized to the old controls. OC: old control; DFOC: old nonheated, deferoxamine-treated; O/H: old heated (0 h); DFO/H: old deferoxamine-treated, heated (0 h). # Significant effect of iron manipulation; † significant effect of heat stress within an age group (p<0.05). Results are presented as means + SEM; n=5-6 animals in each condition.
DMT1 expression
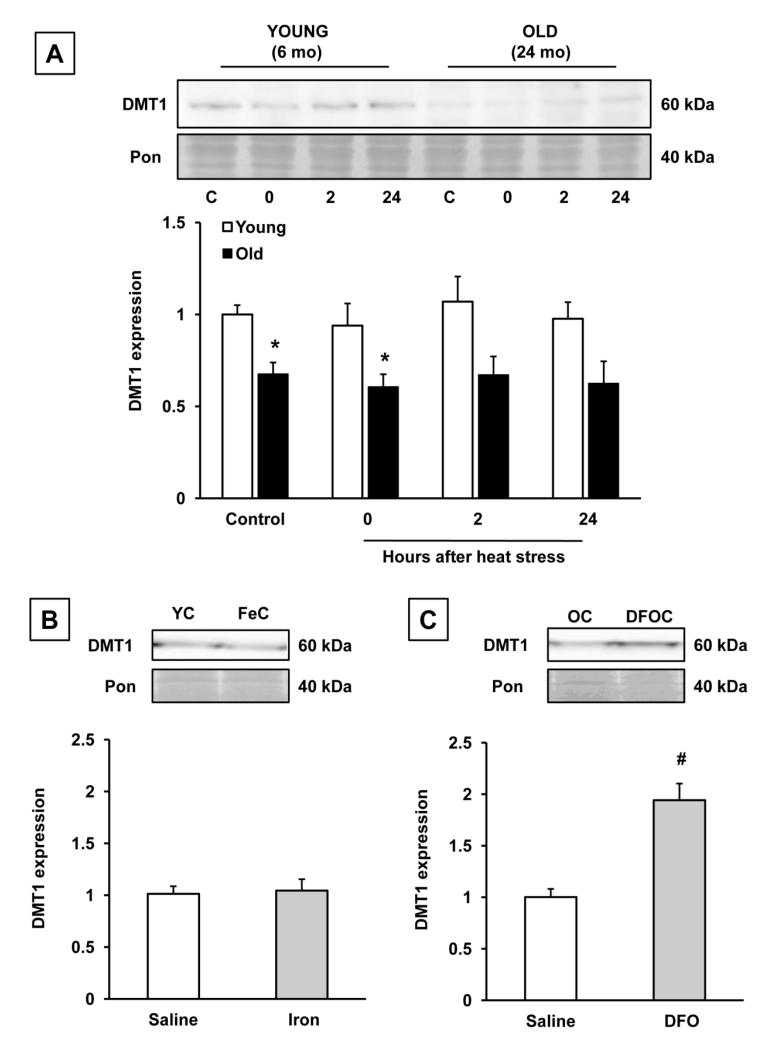
Divalent metal ion transporter-1 (DMT1) is an iron regulatory protein that mediates uptake of iron by intestinal epithelial cells from the gut lumen [35, 36]. DMT1 is also involved in intracellular iron trafficking [37], and its expression is regulated by iron in a similar manner as TFR1 [38]. Due to the potential role of DMT1 in iron handling, we were interested in characterizing its expression in our model. Similarly to TFR1, DMT1 expression was 30% lower in old (24 mo), compared to young (6 mo) rats. Heat stress had no significant effects on the expression of DMT1 in either age group (Fig. 4A). While DMT1 expression did not change in young rats seven days after a single injection of iron dextran (Fig. 4B), treatment of old rats with DFO upregulated the expression of DMT1 approximately two-fold (Fig. 4C).
Figure 4.
Effects of aging, heat stress, and iron manipulation on DMT1 protein expression in the liver. A: Time course evaluation of DMT1 expression after heat stress in young (6 mo) and old (24 mo) rats. * Significant (p<0.05) effect of age; n= 7-9 young and old rats in the nonheated condition and at each time point after heat stress. B: DMT1 expression in saline-treated and iron-treated young rats; n=5-6 rats in the saline and iron-loaded condition. YC: young, saline-treated, nonheated; FeC: young nonheated, iron-loaded. C: Effects of DFO treatment on DMT1 expression in old, nonheated rats; n= 5-6 old rats in the saline-treated and DFO-treated groups, # significant effect of DFO treatment in old rats (p<0.05). OC: old saline-treated, nonheated; DFOC: old nonheated, deferoxamine-treated. Results are expressed as means + SEM.
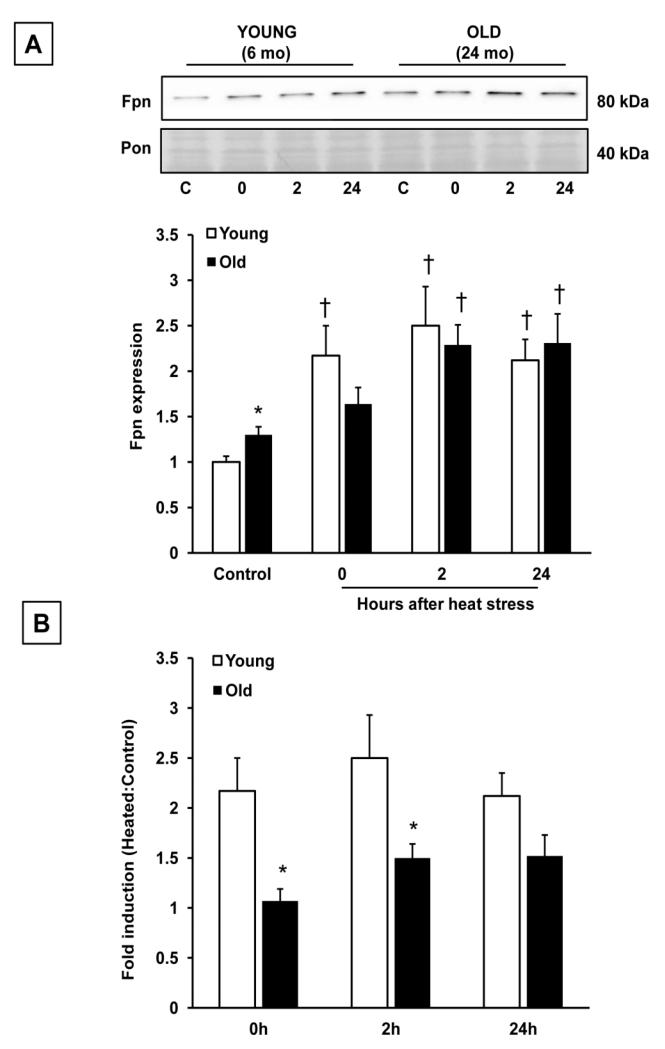
Ferroportin expression
Under nonheated conditions, the livers of old (24 mo) rats had approximately 30% higher levels of Fpn protein than the young (6 mo) rats (Fig. 5A). Fpn levels increased in both age groups after hyperthermia, but there were significant differences in the rapidity and magnitude of this response. In the young rats, Fpn was increased immediately following heat stress and remained high for 24 h thereafter. In the old animals, the increase in Fpn was delayed, with levels not increasing significantly until 2 h after heating. Even at 2 hrs, the increase in Fpn was blunted compared to the young animals, as demonstrated by the lower fold-induction of Fpn after heat stress in old rats (Fig. 5B). Levels of Fpn protein were not altered by short-term treatment with either iron-dextran (young rats) or DFO (old rats; Fig S1).
Figure 5.
Heat stress increases hepatic ferroportin levels in the livers of young and old rats. A: Liver samples from young (6 mo) and old (24 mo) rats were analyzed for Fpn protein expression in the nonheated condition, and at the indicated times after heat stress. Fpn expression was normalized to Ponceau staining, and ratios were further normalized to the young control. B: Fold induction of Fpn after heat stress in young and old rats at 0, 2 and 24 h after heating. Densitometry values from each heated time point were normalized to nonheated values within each age group. Results are expressed as means + SEM; n=7-9 young and old rats under nonheated conditions, and at each time point after heat stress. * Significant effect of aging; † significant effect of heat stress within an age group (p<0.05).
Discussion
Aging is associated with iron accumulation in several organs [9 - 13]. Since iron is implicated in the pathology of age-related conditions [39, 40], it is important to further develop an understanding of how iron regulation is altered in old animals under control and stressed conditions. Here, we show changes in iron regulatory proteins with aging in vivo that are similar to changes induced by chronic iron treatment. Few studies have examined the effects of a physiologically relevant stressor on iron transport proteins in a whole animal model. In response to an acute increase in iron [9], the current results indicate that old rats respond appropriately by down-regulating TFR1 protein. Also, we have demonstrated that aging is associated with a delayed and blunted induction of Fpn, which might provide a mechanism for the transient iron accumulation that occurs in old rats after heat stress.
Many of the changes in iron regulatory proteins with aging mimicked the effects of chronic iron loading, despite the fact that hepatic iron concentrations in the old rats were relatively modestly increased compared to the chronically iron-loaded rats used for comparison here. Specifically, TFR1, and DMT1 were down-regulated with aging and chronic iron loading. These results are consistent with a recent study showing that the expression of DMT1 is inversely correlated with iron concentrations in the liver achieved by manipulation of dietary iron [38]. In contrast to the qualitatively similar effects of iron overload and aging on TFR1 and DMT1, we observed a differential effect of aging and iron loading on Fpn levels. Protein levels of Fpn are controlled post-translationally by the iron regulatory hormone, hepcidin. Ferroportin is the receptor for hepcidin, and it is degraded intracellularly after hepcidin binding [20]. We have previously reported that hepcidin mRNA expression is increased 2.5-fold in the cohort of chronically iron-loaded rats used for comparison in this study [30]; however, hepcidin expression was not altered with aging (Bloomer, Kregel, Brown, manuscript submitted). Thus, in the chronically iron-loaded animals, the reduction in levels of Fpn protein is likely attributable to the upregulation in hepcidin. Interestingly, this decrease in Fpn occurred despite a five-fold increase in Fpn mRNA following iron administration (Brown and Mathahs, unpublished), reinforcing the importance of examining the expression of the iron transporters at the protein level. We and others have previously reported that aging results in an increase in the number of Kupffer cells in the liver [41, 42]. Because Kupffer cells strongly express Fpn [16, 21, 22, 43], the increase in Fpn in old rats under nonheated conditions might be due, in part, to the increase in the number of cells in the liver that express Fpn. Since both hepatocytes and Kupffer cells display iron accumulation in old rats [9], an alteration in the cellular localization and/or the function of Fpn with aging might account for the seeming paradox of elevated Fpn expression and concomitant iron deposition. Future studies using immunohistochemistry to assess the sites of Fpn expression may be informative on this point. Also, some of this iron may be deposited in an insoluble form; hence limiting the ability of this iron to be mobilized, despite higher levels of Fpn protein. Since hepatocytes constitute the greatest mass of any cell type of the liver [44], immunoblotting on whole-tissue homogenates from liver tends to detect changes in proteins in this cell type; however, increases in both the hepatocyte and Kupffer cell compartments may contribute to the increased levels of Fpn protein in aged animals.
Aging was associated with an increase in hepatic nonheme iron, and heat stress further increased liver iron content. These increases in iron were associated with physiologically appropriate decreases in hepatic TFR1. Treatment of old rats with the iron chelator, DFO, lowered HIC, and strongly induced TFR1 protein in the nonheated condition. Moreover, in addition to preventing an increase in nonheme iron after heat stress, treatment with DFO also prevented a decrease in TFR1 protein. In contrast, levels of TFR1 were unchanged in young animals, in which there were no increases in hepatic iron content following heating. Taken together, these results strongly suggest that the increase in intracellular iron has a causal role in lowering TFR1 in old rats after heat stress. The reduction in TFR1 may play a protective role by preventing the cell from obtaining excess iron during recovery from heat stress. It will be important to determine the sources of this excess iron after heat stress in old animals. Since there is a net increase in iron in the liver after hyperthermia, and heat stress is associated with intravascular hemolysis [45], we speculate that this iron comes from enhanced influx of hemoglobin from lysed red blood cells.
In contrast to TFR1, there were no changes in DMT1 after heat stress in either age group. Considering that DMT1 protein levels were lower in aged and chronically iron-loaded rats, this suggests that larger changes in iron concentrations are necessary to affect the expression of DMT1. The recent study by Nam and associates supports that long-term changes in iron content are necessary for changes in DMT1 [38]; however, our results do not exclude the possibility of changes in the intracellular iron shuttling activity of DMT1 after heat stress. More functional studies will need to be performed to address whether DMT1 has a role in iron handling during hyperthermia.
In both age groups, heat stress stimulated the expression of Fpn in the liver. While young rats induced Fpn immediately, the induction of Fpn in old rats was delayed. This delayed and blunted ability to increase the expression of an iron exporter suggests a mechanism for the transient iron retention observed in the livers of old animals, since Fpn protein levels are correlated with cellular iron efflux [19-21, 46]. Furthermore, low Fpn protein expression is associated with iron retention in the liver [22, 23, 24]. Our results are also consistent with a recent study showing that an inability to induce Fpn mRNA is associated with iron accumulation in skeletal muscle of old rats [47]. At 2 and 24 h after heating, the increase in Fpn may permit an increase in iron efflux, thus allowing nonheme iron to return to control values in the old rats. In the young rats, there was a rapid and robust increase in Fpn levels early after heating, and no increase in iron after heat stress. These results may suggest that iron accumulation in young rats was mitigated by an increase in Fpn protein; however, studies that manipulate Fpn are needed to confirm whether it has a functional role in both young and old rats. Additionally, studies utilizing isotopic tracers in this model are necessary to better define iron flux within the liver and other organs after heat stress.
In addition to the delayed and attenuated increase in Fpn, it is noteworthy that hepatocytes isolated from old rats demonstrate an impairment in the induction of heme oxygenase-1 (HO-1) immediately after heat stress [41]. HO-1 is also involved in recycling of iron and its egress from cells [48], and HO-1 knockout mice have attenuated Fpn expression, and increased nonheme iron content in the liver [24]. Therefore, impairments in the induction of both Fpn and HO-1 may contribute to diminished iron export after heat stress in old rats, leading to hepatic iron retention.
The mechanism for the hyperthermia-induced increase in Fpn remains unclear. Hepcidin is thought to be the major regulator of Fpn via hepcidin-mediated internalization and degradation of Fpn [20]; however, in our model, hepcidin mRNA is either unchanged (young) or increased (old) after heat stress (Bloomer, Kregel, Brown, manuscript submitted). Hence, our results suggest hepcidin-independent regulation of ferroportin, which is consistent with the study by Starzynski et al. showing upregulation of both hepcidin mRNA and ferroportin protein in the livers of HO-1 knockout mice [24]. Other exceptions to the general concept that Fpn protein is regulated by hepcidin have been described. For instance, iron and erythrophagocytosis stimulate Fpn protein and mRNA expression in macrophages [26], and hepatic Fpn protein is lowered by an iron-deficient diet [43]. These results suggest that iron stabilizes Fpn through its iron-responsive element (IRE), and Abboud and Haile have demonstrated IRE-dependent regulation of the Fpn promoter in COS7 cells [43]. However, in our investigation, we have observed a robust increase in Fpn protein after heat stress without a change in iron in young rats. Although there were increases in iron in old rats, which might stimulate Fpn via its IRE, we did not observe changes in Fpn protein with iron chelation in this cohort (Fig S1), despite observing an appropriate response of TFR1 to DFO in the same animals (Fig 3). Moreover, there was not an increase in Fpn with short-term iron loading in young rats (Fig S1). Both of these findings are consistent with results from other laboratories showing no effect of iron manipulation on expression of Fpn mRNA in the liver, in RAW264.7 macrophages, and in HepG2 cells [19, 49, 50]. These results suggest redundant control of Fpn by mechanisms independent of its IRE. Since the changes in hepatic iron content in our model were relatively modest, it is possible that larger changes in iron are required to affect protein levels of Fpn. While iron did not stimulate Fpn in these studies, treatment of cells with hemoglobin and hemin resulted in a robust increase in Fpn transcription, which was mediated by the Bach-1 and Nrf2 transcription factors [50]. Taken together, the results of Marro et. al. [50] and our present results suggest that an increase in intracellular heme, possibly from intravascular hemolysis, may have a role in increasing the abundance of Fpn after heat stress.
In conclusion, we provide strong evidence to suggest that iron mediates an appropriate decrease in TFR1 protein after heat stress in old rats, demonstrating that responses to acute changes in iron status are intact in the livers of old rats. The decrease in TFR1 was accompanied by an increase in Fpn, which may represent an adaptive mechanism, limiting the accumulation of excess iron in hepatic cells. The physiologic destinations of this iron should be evaluated in future studies, but may include the bone marrow [51], or spleen [52]. In old rats, the increase in Fpn after hyperthermia was delayed compared to young rats; therefore, this blunted ability to induce Fpn might play a role in iron accumulation and subsequent oxidative injury in old rats after heat stress.
Supplementary Material
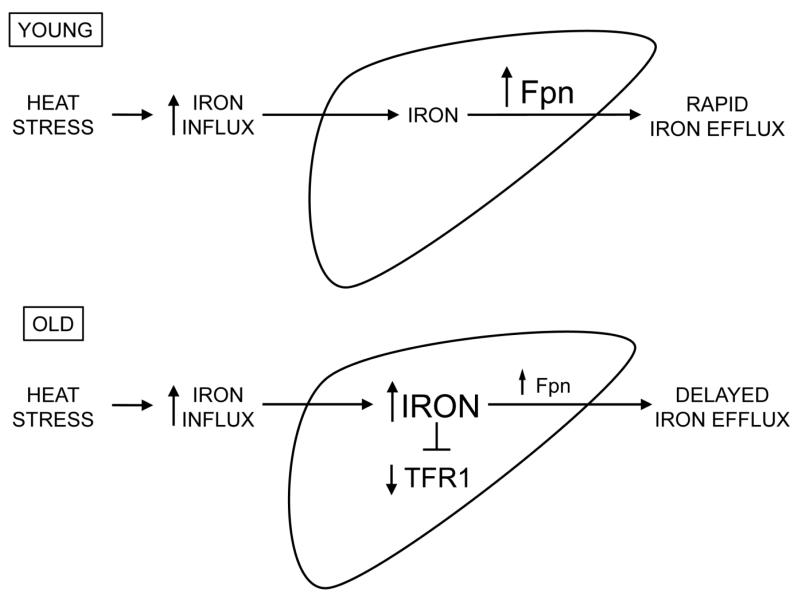
Figure 6.
Proposed model for iron accumulation after heat stress with aging. In both age groups, heat stress increases iron influx through an as yet unidentified mechanism, potentially involving increased influx of hemoglobin from lysed red blood cells. In young animals (upper pathway), an increase in hepatic iron is mitigated by a rapid increase in Fpn. In old animals (lower pathway), the delay in the induction of Fpn leads to transient accumulation of iron, which lowers protein levels of TFR1.
Acknowledgements
The authors thank Rachel Croasdale and Frederick Liaboe for expert technical assistance.
Grants
SAB was supported by start-up funds from Penn State Abington. KEB was supported by a Merit Grant from the Veterans’ Administration. KCK was supported by NIH grant AG12350.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J. Appl. Physiol. 1998;84:1323–1332. doi: 10.1152/jappl.1998.84.4.1323. [DOI] [PubMed] [Google Scholar]
- [2].Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J. Appl. Physiol. 1969;27:673–80. doi: 10.1152/jappl.1969.27.5.673. [DOI] [PubMed] [Google Scholar]
- [3].Stauss HM, Morgan DA, Anderson KE, Massett MP, Kregel KC. Modulation of baroreflex sensitivity and spectral power of blood pressure by heat stress and aging. Am. J. Physiol. - Heart and Circ. Physiol. 1997;272:H776–H784. doi: 10.1152/ajpheart.1997.272.2.H776. [DOI] [PubMed] [Google Scholar]
- [4].Kew M, Bersohn I, Seftel H, Kent G. Liver damage in heatstroke. Am. J. Med. 1970;49:192–202. doi: 10.1016/s0002-9343(70)80075-4. [DOI] [PubMed] [Google Scholar]
- [5].Argaud L, Ferry T, Le Q, et al. Short- and long-term outcomes of heatstroke following the 2003 heat wave in Lyon, France. Arch. Int. Med. 2007;167:2177–2183. doi: 10.1001/archinte.167.20.ioi70147. [DOI] [PubMed] [Google Scholar]
- [6].Semenza JC, Rubin CH, Falter KH, et al. Heat-Related Deaths during the July 1995 Heat Wave in Chicago. New Eng. J. Med. 1996;335:84–90. doi: 10.1056/NEJM199607113350203. [DOI] [PubMed] [Google Scholar]
- [7].Haak JL, Buettner GR, Spitz DR, Kregel KC. Aging augments mitochondrial susceptibility to heat stress. Am. J. Physiol. - Reg. Int. Comp. Physiol. 2009;296:R812–R820. doi: 10.1152/ajpregu.90708.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Oberley TD, Swanlund JM, Zhang HJ, Kregel KC. Aging Results in Increased Autophagy of Mitochondria and Protein Nitration in Rat Hepatocytes Following Heat Stress. J. Histochem. Cytochem. 2008;56:615–627. doi: 10.1369/jhc.2008.950873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bloomer SA, Brown KE, Buettner GR, Kregel KC. Dysregulation of hepatic iron with aging: implications for heat stress-induced oxidative liver injury. Am. J. Physiol. - Reg. Int. Comp. Physiol. 2008;294:R1165–R1174. doi: 10.1152/ajpregu.00719.2007. [DOI] [PubMed] [Google Scholar]
- [10].Sohal RS, Wennberg-Kirch E, Jaiswal K, Kwong LK, Forster MJ. Effect of Age and Caloric Restriction on Bleomycin-chelatable and Nonheme Iron in Different Tissues of C57BL/6 Mice. Free Rad. Biol. Med. 1999;27:287–293. doi: 10.1016/s0891-5849(99)00052-0. [DOI] [PubMed] [Google Scholar]
- [11].Cook CI, Yu BP. Iron accumulation in aging: modulation by dietary restriction. Mech. Age. Dev. 1998;102:1–13. doi: 10.1016/s0047-6374(98)00005-0. [DOI] [PubMed] [Google Scholar]
- [12].Jung SH, DeRuisseau LR, Kavazis AN, DeRuisseau KC. Plantaris muscle of aged rats demonstrates iron accumulation and altered expression of iron regulation proteins. Exp. Physiol. 2008;93:407–414. doi: 10.1113/expphysiol.2007.039453. [DOI] [PubMed] [Google Scholar]
- [13].Hofer T, Marzetti E, Xu J, et al. Increased iron content and RNA oxidative damage in skeletal muscle with aging and disuse atrophy. Exp. Gerontol. 2008;43:563–570. doi: 10.1016/j.exger.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Richardson DR, Ponka P. The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim. Biophys. Acta. 1997;1331:1–40. doi: 10.1016/s0304-4157(96)00014-7. [DOI] [PubMed] [Google Scholar]
- [15].Richardson DR, Lane DJR, Becker EM, et al. Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc. Nat. Acad. Sci. 2010;107:10775–10782. doi: 10.1073/pnas.0912925107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Naz N, Malik IA, Sheikh N, et al. Ferroportin-1 is a ‘nuclear’-negative acute-phase protein in rat liver: a comparison with other iron-transport proteins. Lab. Invest. 2012;92:842–856. doi: 10.1038/labinvest.2012.52. [DOI] [PubMed] [Google Scholar]
- [17].Sukumaran A, Venkatraman A, Jacob M. Inflammation-induced effects on iron-related proteins in splenic macrophages and the liver in mice. Blood Cells. Mol. Dis. 2012;49:11–19. doi: 10.1016/j.bcmd.2012.03.002. [DOI] [PubMed] [Google Scholar]
- [18].Cohen LA, Gutierrez L, Weiss A, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574–1584. doi: 10.1182/blood-2009-11-253815. [DOI] [PubMed] [Google Scholar]
- [19].McKie AT, Marciani P, Rolfs A, et al. A Novel Duodenal Iron-Regulated Transporter, IREG1, Implicated in the Basolateral Transfer of Iron to the Circulation. Mol. Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- [20].Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- [21].Donovan A, Brownlie A, Zhou Y, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- [22].Donovan A, Lima CA, Pinkus JL, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell. Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- [23].Viatte L, Nicolas G, Lou D-Q, et al. Chronic hepcidin induction causes hyposideremia and alters the pattern of cellular iron accumulation in hemochromatotic mice. Blood. 2006;107:2952–2958. doi: 10.1182/blood-2005-10-4071. [DOI] [PubMed] [Google Scholar]
- [24].Starzynski RR, Canonne-Hergaux F, Lenartowicz M, et al. Ferroportin expression in haem oxygenase-1 deficient mice. Biochem. J. 2013;449:69–78. doi: 10.1042/BJ20121139. [DOI] [PubMed] [Google Scholar]
- [25].Oliveira SJ, Pinto JP, Picarote G, et al. ER Stress-Inducible Factor CHOP Affects the Expression of Hepcidin by Modulating C/EBPalpha Activity. PLOS ONE. 2009;4:e6618. doi: 10.1371/journal.pone.0006618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Knutson MD, Vafa MR, Haile DJ, Wessling-Resnick M. Iron loading and erythrophagocytosis increase ferroportin 1 (FPN1) expression in J774 macrophages. Blood. 2003;102:4191–4197. doi: 10.1182/blood-2003-04-1250. [DOI] [PubMed] [Google Scholar]
- [27].Myśliwska J, Bryl E, Foerster J, Myśliwski A. Increase of interleukin 6 and decrease of interleukin 2 production during the ageing process are influenced by the health status. Mech. Age. Dev. 1998;100:313–328. doi: 10.1016/s0047-6374(97)00154-1. [DOI] [PubMed] [Google Scholar]
- [28].Tacchini L, Poli DF, Bernelli-Zazzera A, Cairo G. Transferrin receptor gene expression and transferrin-bound iron uptake are increased during postischemic rat liver reperfusion. Hepatology. 2002;36:103–111. doi: 10.1053/jhep.2002.33997. [DOI] [PubMed] [Google Scholar]
- [29].Hall DM, Baumgardner KR, Oberley TD, Gisolfi CV. Splanchnic tissues undergo hypoxic stress during whole body hyperthermia. Am. J. Physiol .Gastrointest. Liver Physiol. 1999;276:G1195–1203. doi: 10.1152/ajpgi.1999.276.5.G1195. [DOI] [PubMed] [Google Scholar]
- [30].Brown KE, Broadhurst KA, Mathahs MM, Weydert J. Differential expression of stress-inducible proteins in chronic hepatic iron overload. Toxicol. Appl. Pharm. 2007;223:180–186. doi: 10.1016/j.taap.2007.05.011. [DOI] [PubMed] [Google Scholar]
- [31].Zhang HJ, Xu L, Drake VJ, Oberley LW, Kregel KC. Heat-induced liver injury in old rats is associated with exaggerated oxidative stress and altered transcription factor activation. Faseb J. 2003;17:2293–2295. doi: 10.1096/fj.03-0139fje. [DOI] [PubMed] [Google Scholar]
- [32].Torrance JD, Bothwell TH. Tissue Iron Stores. In: Cook JD, editor. Iron. Methods in Hematology, Churchill Livingstone. New York: 1980. pp. 90–115. [Google Scholar]
- [33].Han O, Kim E-Y. Colocalization of ferroportin-1 with hephaestin on the basolateral membrane of human intestinal absorptive cells. J. of Cell. Biochem. 2007;101:1000–1010. doi: 10.1002/jcb.21392. [DOI] [PubMed] [Google Scholar]
- [34].Dongiovanni P, Fracanzani AL, Cairo G, et al. Iron-Dependent Regulation of MDM2 Influences p53 Activity and Hepatic Carcinogenesis. Am. J. Pathol. 2010;176:1006–1017. doi: 10.2353/ajpath.2010.090249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gunshin H, Mackenzie B, Berger UV, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- [36].Trinder D, Oates PS, Thomas C, Sadleir J, Morgan EH. Localisation of divalent metal transporter 1 (DMT1) to the microvillus membrane of rat duodenal enterocytes in iron deficiency, but to hepatocytes in iron overload. Gut. 2000;46:270–276. doi: 10.1136/gut.46.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Touret N, Furuya W, Forbes J, Gros P, Grinstein S. Dynamic Traffic through the Recycling Compartment Couples the Metal Transporter Nramp2 (DMT1) with the Transferrin Receptor. J. Biol. Chem. 2003;278:25548–25557. doi: 10.1074/jbc.M212374200. [DOI] [PubMed] [Google Scholar]
- [38].Nam H, Wang C-Y, Zhang L, et al. ZIP14 and DMT1 in the liver, pancreas, and heart are differentially regulated by iron deficiency and overload: implications for tissue iron uptake in iron-related disorders. Haematologica. 2013 doi: 10.3324/haematol.2012.072314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xu J, Marzetti E, Seo AY, et al. The emerging role of iron dyshomeostasis in the mitochondrial decay of aging. Mech. Age. Dev. 2010;131:487–493. doi: 10.1016/j.mad.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Xu J, Jia Z, Knutson M, Leeuwenburgh C. Impaired iron status in aging research. Int. J. Mol. Sci. 2012;13:2368–2386. doi: 10.3390/ijms13022368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bloomer SA, Zhang HJ, Brown KE, Kregel KC. Differential Regulation of Hepatic Heme Oxygenase-1 Protein With Aging and Heat Stress. J. Gerontol. A: Biol. Sci. Med. Sci. 2009;64A:419–425. doi: 10.1093/gerona/gln056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hilmer SN, Cogger VC, Couteur DGL. Basal Activity of Kupffer Cells Increases With Old Age. J. Gerontol. A: Biol. Sci. Med. Sci. 2007;62:973–978. doi: 10.1093/gerona/62.9.973. [DOI] [PubMed] [Google Scholar]
- [43].Abboud S, Haile DJ. A Novel Mammalian Iron-regulated Protein Involved in Intracellular Iron Metabolism. J. Biol. Chem. 2000;275:19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- [44].Weibel ER, Stäubli W, Gnägi HR, Hess FA. correlated morphometric and biochemical studies on the liver cell. J. Cell Biol. 1969;42:68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hall DM, Buettner GR, Oberley LW, et al. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H509–521. doi: 10.1152/ajpheart.2001.280.2.H509. [DOI] [PubMed] [Google Scholar]
- [46].Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc. Nat. Acad. Sci. 2005;102:1324–1328. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Xu J, Hwang JCY, Lees HA, et al. Long-term perturbation of muscle iron homeostasis following hindlimb suspension in old rats is associated with high levels of oxidative stress and impaired recovery from atrophy. Exp. Gerontol. 2012;47:100–108. doi: 10.1016/j.exger.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ferris CD, Jaffrey SR, Sawa A, et al. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat. Cell Biol. 1999;1:152–157. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- [49].Lymboussaki A, Pignatti E, Montosi G, et al. The role of the iron responsive element in the control of ferroportin1/IREG1/MTP1 gene expression. J. Hepatol. 2003;39:710–715. doi: 10.1016/s0168-8278(03)00408-2. [DOI] [PubMed] [Google Scholar]
- [50].Marro S, Chiabrando D, Messana E, et al. Heme controls ferroportin1 (FPN1) transcription involving Bach1, Nrf2 and a MARE/ARE sequence motif at position -7007 of the FPN1 promoter. Haematologica. 2010;95:1261–1268. doi: 10.3324/haematol.2009.020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Cook J, Hershko C, Finch C. Storage iron kinetics V: iron exchange in the rat. Br. J. Haematol. 1973;25 doi: 10.1111/j.1365-2141.1973.tb01782.x. [DOI] [PubMed] [Google Scholar]
- [52].Layoun A, Huang H, Calvé A, Santos MM. Toll-Like Receptor Signal Adaptor Protein MyD88 Is Required for Sustained Endotoxin-Induced Acute Hypoferremic Response in Mice. Am. J. Pathol. 2012;180:2340–2350. doi: 10.1016/j.ajpath.2012.01.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.