Abstract
Heme, in the presence of hydrogen peroxide, can act as a peroxidase. Intravascular hemolysis results in a massive release of heme into the plasma in several pathophysiological conditions such as hemolytic anemia, malaria, and sickle cell disease. Heme is known to induce heme oxygenase-1(HO-1) expression, and the extent of induction depends on the ratio of albumin to heme in plasma. HO-1 degrades heme and ultimately generates the antioxidant bilirubin. Heme also causes oxidative stress in cells, but whether it causes protein-radical formation has not yet been studied. In the literature, two purposes for the degradation of heme by HO-1 are discussed. One is the production of the antioxidant bilirubin and the other is the prevention of heme-dependent adverse effects. Here we have investigated heme-induced protein-radical formation, which might have pathophysiological consequences, and have used immunospin trapping to establish the formation of heme-induced protein radicals in two systems: human serum albumin (HSA)/H2O2 and human plasma/H2O2.We found that excess heme catalyzed the formation of HSA radicals in the presence of hydrogen peroxide. When heme and hydrogen peroxide were added to human plasma, heme was found to oxidize proteins, primarily and predominantly HSA; however, when HSA-depleted plasma was used, heme triggered the oxidation of several other proteins, including transferrin. Thus, HSA in plasma protected other proteins from heme/H2O2-induced oxidation. The antioxidants ascorbate and uric acid significantly attenuated protein-radical formation induced by heme/ H2O2; however, bilirubin did not confer significant protection. Based on these findings, we conclude that heme is degraded by HO-1 because it is a catalyst of protein-radical formation and not merely to produce the relatively inefficient antioxidant bilirubin.
Keywords: Heme, Nitrone adducts, Peroxidase, Protein radical, Protein oxidation, Oxidative stress
Introduction
Heme is an iron-containing porphyrin with important biological functions [1]. It is synthesized in all cells, and cellular heme levels are tightly controlled by enzymatic synthesis and degradation in a synchronized manner [2,3]. Being hydrophobic, heme readily diffuses through plasma membranes and enhances oxidative stress in cells in the presence of hydrogen peroxide [1]. Release of heme is involved in several pathophysiological conditions such as hemolytic anemia, malaria, sickle cell disease, and rhabdomyolysis.
In these diseases, hemolysis leads to very high concentrations of heme in plasma as erythrocytes contain heme at a concentration of 20 mM and are vulnerable to lysis due to their enucleate status [1]. After lysis, extracellular hemoglobin is easily oxidized to form methemoglobin, which in turn releases heme into the plasma [4]. This released heme predominantly binds to human serum albumin (HSA) and hemopexin [3]. HSA is the most abundant protein in plasma, characterized by its tremendous ligand binding capacity, which provides a reservoir for many compounds present in quantities well beyond their solubility in plasma [5,6]. HSA has a single high affinity binding site for heme in addition to 10 lower affinity ones [4,6].
Excess heme induces HO-1 expression in cells [4]. HO-1 acts as a rate-limiting enzyme in the heme degradation pathway, which leads to the formation of equimolar amounts of biliverdin, iron, and carbon monoxide [7]. Biliverdin is subsequently reduced to bilirubin, which is an antioxidant [8,9].
Under normal conditions, tissue or plasma H2O2 levels are quite low; however, it has been estimated that under conditions of inflammation, the production of H2O2 by activated macrophages and neutrophils can go up sufficiently high to allow some of it to react with heme or hemoproteins [10]. It has been known for decades that hemoproteins react with H2O2,which leads to protein modifications [11], but there are very few reports on heme/H2O2-induced protein oxidation [12]. To the best of our knowledge, nobody has reported heme/H2O2-induced formation of protein radicals.
Heme is degraded in vivo to produce the antioxidant bilirubin [8,9]; however, the prevention of heme-mediated toxicity also appears as a possible purpose for its degradation. We hypothesized that protein-radical formation and subsequent protein oxidation are mechanisms of this toxicity. Therefore, we studied heme-induced oxidation of HSA in the presence of H2O2 using the highly sensitive immuno-spin-trapping technique, which provides direct evidence of protein-radical formation [13].
In the present study we report that heme triggers HSA-radical formation in the presence of H2O2, and in human plasma it is a potent protein oxidant whose action is not efficiently prevented by physiological concentrations of bilirubin. We also report a sacrificial role of HSA to protect other plasma proteins from heme/ H2O2-induced oxidation. We conclude that one purpose for the heme degradation by HO-1 is to eliminate this potent catalyst of protein oxidation.
Materials and methods
Materials
Ascorbic acid, bilirubin, catalase from Aspergillus niger, diethylenetriaminepentaacetic acid (DTPA), dithiothreitol (DTT), gelatin from cold water fish skin, hemin, human serum albumin (HSA), hydrogen peroxide (obtained as a 30% solution), reduced glutathione, Tween-20, and uric acid were from Sigma Chemical Co. (St. Louis, MO). The hydrogen peroxide concentration was determined from its absorbance at 240 nm (ε=39.4 M−1 cm−1). DMPO was obtained from Dojindo Laboratories (Rockville, MD) and used without further purification. Chelex 100® resin was purchased from Bio-Rad Laboratories (Hercules, CA). Human plasma was obtained from the American Red Cross (Charlotte, NC). The PD-10 column was from GE Healthcare Biosciences (Pittsburgh, PA). Mouse monoclonal and rabbit polyclonal anti-DMPO antibodies were developed in our laboratory and used in the immuno-spin-trapping studies.
Methods
Human serum albumin preparation
Globulin and fatty acid free, human serum albumin (20 mg/ml) were dissolved in 100 mM sodium phosphate buffer (pH 7.4) treated with Chelex 100® containing 25 μM DTPA. Preparations of HSA were passed through a prepacked Sephadex G-25 column (PD-10) preequilibrated with Chelex-treated 100 mM phosphate buffer containing 25 μM DTPA to eliminate traces of metals and preservatives.
Chemical reactions
Typically, reactions of 7.5 μM HSA, 30 μM H2O2, and 30 μM hemin were carried out in the presence or absence of 100 mM DMPO in 100 mM Chelex-treated sodium phosphate buffer (pH 7.4) containing 25 μM DTPA, in a total volume of 300 μM. The reaction mixture was incubated for 2 h at 37 °C. Samples were diluted and used for electrophoresis and immuno-spin-trapping experiments using an anti-DMPO antibody.
Dialyzed human plasma preparation
Human plasma (3 ml) was dialyzed against three changes of 500 ml of 10 mM Chelex-treated phosphate buffer, pH 7.4 at 4 °C, using a 10-kDa cutoff Slide-A-Lyzer Dialysis Cassette as per the manufacturer's instructions (Pierce, Rockford, IL).
Preparation of HSA-depleted human plasma
HSA-depleted human plasma was prepared using a Swell Gel Blue albumin removal kit (Pierce, Rockford, IL) according to the manufacturer's instructions, using 50 μ1 of regular or dialyzed plasma per disk.
Peroxidase activity
The peroxidase activity of heme was measured using Amplex Red (Invitrogen, Carlsbad, CA). In brief, reactions containing various concentrations of heme and HSA with 50 μM Amplex Red were prepared. Reactions were initiated by the addition of H2O2 and absorbance was followed at 571 nm for 8 min. Chelex-treated 100 mM phosphate buffer containing 25 μM DTPA, pH 7.4, was used to make up the specified volume. To rule out the possible artifactual oxidation of Amplex Red by the spectrophotometer instrumental light [14] the resorufin formation was also tested using dark-incubated experiments. Final absorbance measurements of those samples showed the same resorufin yield as detected in the continuous monitoring experiments.
SDS-PAGE, Coomassie blue stain, and immuno-spin trapping
Reaction mixtures were resolved under reducing conditions in 4-12% Bis Tris NuPage acrylamide gels (Invitrogen, Carlsbad, CA) and either subjected to Coomassie blue staining or transferred to nitrocellulose membranes. Subsequently, membranes were blocked with 4% cold water fish gelatin solution in 100 mM (bi) carbonate buffer, pH 9.6, for 90 min at room temperature. Membranes were washed once with washing buffer (0.2 % fish gelatin and 0.05% Tween-20 in Tris-buffered saline, pH 7.4) and then incubated with mouse monoclonal anti-DMPO antibody (1:100, diluted in washing buffer). After a 1-h incubation, membranes were washed 4 times and incubated with fluorescent secondary anti-mouse antibody (1:15,000, diluted in washing buffer) labeled with infrared dyes for 1 h at room temperature in the dark. After incubation, traces of unbound antibody were eliminated by washing. An Odyssey infrared imaging system (Li-Cor Biosciences, Lincoln, NE) was used to acquire the images [15]. In some experiments where the concentration-dependent effect of hydrogen peroxide was investigated (Fig. 2A and B), reaction mixtures were resolved under nonreducing conditions (without DTT) to rule out the possible artifactual generation of hydrogen peroxide by the dithiothreitol oxidation during the heating step for the electrophoresis sample preparation.
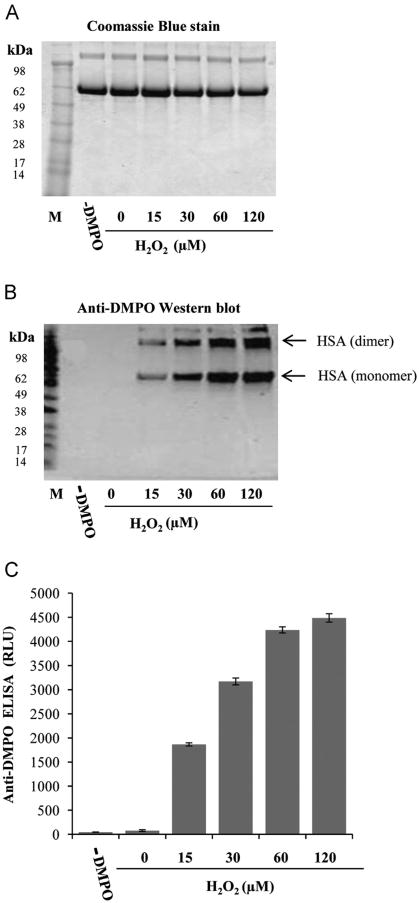
Fig. 2.
Effect of H2O2 on the formation of HSA radical by heme. (A) Coomassie blue-stained gel to show equal loading, (B) anti-DMPO blotting, and (C) anti-DMPO ELISA. Reactions contained 7.5 μM HSA, 30 μM heme, 100 mM DMPO, and different concentrations of H2O2. The reaction mixture was incubated for 2 h at 37 °C in Chelex-treated, 100 mM phosphate buffer (pH 7.4) containing 25 μM DTPA. Gels were run under nonreducing conditions (2.5 μg of protein per lane) followed by anti-DMPO Western blotting. Data show representative gel/blot or mean values ± SE from at least three experiments in duplicate (n=3).
Enzyme-linked immunosorbent assay (ELISA)
Rabbit polyclonal anti-DMPO antibody was used to detect DMPO-protein-derived nitrone adducts using a standard ELISA as described previously [16]. In brief, samples were immobilized by incubating reaction mixtures (2.5 μg/well) in 100 mM (bi)carbonate buffer, pH 9.6, for 90 min at 35 °C. The plate was washed once and blocked (4% fish gelatin in PBS) for 90 min at 35 °C. Following another wash, rabbit polyclonal anti-DMPO antibody (1:5000) was added and incubated for 60 min. After incubation, plate wells were washed four times followed by incubation with anti-rabbit, IgGalkaline phosphatase-conjugated secondary antibody (1:5000) for 60 min at 35 °C. After another four washes, CDP star (25 μM) was added and the chemiluminescent product was detected using a Tecan SpectraFluor-Plus multifunction plate reader with Xfluor software (Tecan US, Research Triangle Park, NC, USA).
Mass spectrometry
The protein bands stained with Coomassie blue were manually excised from the gel, chopped into small pieces, transferred into a 96-well microtiter plate, and subjected to automatic tryptic digestion as described elsewhere [17]. The resulting mixtures were desalted, purified, and analyzed using a 4800 MALDI–TOF/TOF analyzer (AB Sciex, Foster City, CA) operated in the positive ion reflector mode. For all MS analyses, data-dependent spectra were acquired in a fully automated mode, and the MS spectrum was followed by MS/MS analyses of the most abundant ions in the spectrum. Data were analyzed using a Global Protein Server Workstation (AB Sciex) with Mascot software (Matrix Science, London, UK). Searches were performed against the NCBI nonredundant database to search peptide mass fingerprints and MS/MS data as shown in the supplemental data.
Results
Heme-induced formation of HSA radicals in a heme/HSA/H2O2 system
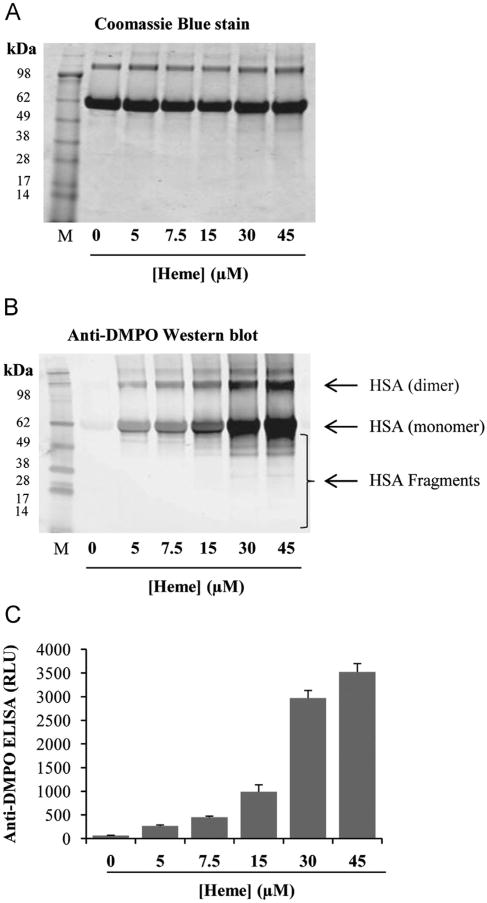
To investigate whether heme induces protein-radical formation, we incubated HSA with 30 μM H2O2, 100 mM DMPO, and various concentrations of heme. The samples were analyzed using the immuno-spin-trapping technique by Western blot and ELISA [13], which detects DMPO-nitrone adducts as a measure of free radical formation. We observed an increase in HSA-radical formation with increasing concentrations of heme (Fig. 1A, B, and C). It is noteworthy that samples with higher concentrations of heme (30 and 45 μM) showed HSA radicals on the dimers and monomers of the protein along with radicals on smaller fragments. The distinct fragmentation of HSA by heme/H2O2 indicates that this oxidizing system acts on multiple sites of HSA and triggers site-specific radical formation [15,18].
Fig. 1.
Concentration-dependent effects of heme on the formation of HSA radical. (A) Coomassie blue-stained gel to show equal loading, (B) anti-DMPO blotting, and (C) anti-DMPO ELISA. Reactions included 7.5 μM HSA, 30 μM H2O2, 100 mM DMPO, and different concentrations of heme. The samples were incubated for 2 h at 37 °C in Chelex-treated, 100 mM phosphate buffer (pH 7.4) containing 25 μM DTPA. Data show representative gel/blot or mean values ±SE from at least three experiments in duplicate (n=3). M shows the molecular weight markers and RLU means relative light units.
The HSA-radical formation was completely dependent on the concentration of H2O2, as can be seen in experiments where samples were prepared with a fixed concentration of heme (30 μM), HSA (7.5 μM), and various concentrations of H2O2 (Fig. 2B and C). In the absence of H2O2, heme did not elicit any HSA-radical formation. When catalase was added to the complete reaction mixture (heme/HSA/H2O2), HSA-radical formation was significantly attenuated, proving that H2O2 plays a significant role in the HSA-radical formation by heme (data not shown).
Effect of HSA or H2O2 on the peroxidase activity of heme
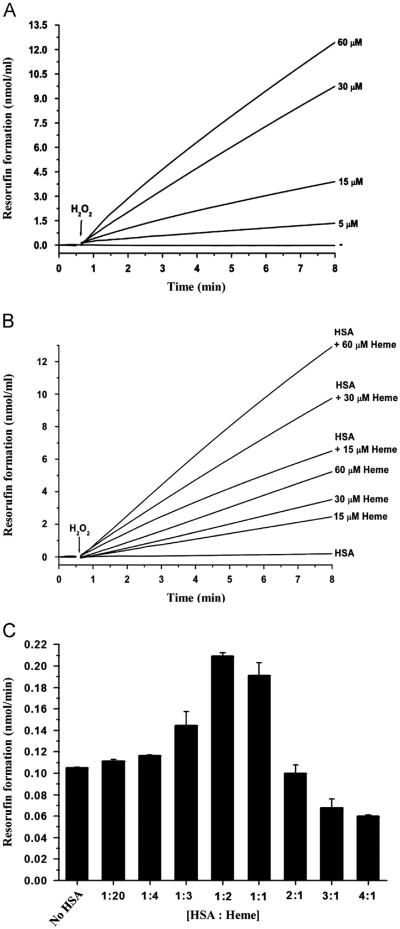
The peroxidase activity of heme is central to heme-induced oxidative damage. To investigate the effect of H2O2 on the peroxidase activity of heme, various concentrations of H2O2 were added to heme/HSA along with 50 μM Amplex Red, a typical peroxidase substrate. Results obtained with resorufin formation demonstrated that H2O2 augmented the peroxidase activity of heme in a concentration-dependent manner (Fig. 3A). The increase in peroxidase activity with increasing concentrations of H2O2 is in concordance with the effect of H2O2 on protein-radical formation (Fig. 2B and C). Taken together, these results link the peroxidase activity of heme to the protein-radical formation.
Fig. 3.
Peroxidase activity of heme. (A) Effect of H2O2 concentration on the peroxidase activity of heme. Reactions contained 7.5 μM HSA, 30 μM heme, 50 μM Amplex Red, and different concentrations of H2O2 (5, 15, 30, 60 μM). (B) Effect of HSA on the peroxidase activity of heme. Reactions contained 30 μM H2O2, 7.5 μM HSA, 50 μM Amplex Red, and different concentrations of heme (15, 30, 60 μM). (C) Effect of HSA-to-heme ratios on the peroxidase activity of heme. Reactions contained different ratios of HSA to heme as shown in the figure in the presence of 50 μM Amplex Red and 30 μM H2O2. Chelex-treated, 100 mM phosphate buffer (pH 7.4) containing 25 μM DTPA was used to make up volumes.
The addition of HSA to heme/H2O2 augmented the heme peroxidase activity at all concentrations of heme tested (Fig. 3B). To further investigate the effect of HSA on peroxidase activity of heme, we carried out reactions with different ratios of HSA to heme (Fig. 3C), and the peroxidase activity of heme was assessed using resorufin formation. The addition of HSA increased the baseline peroxidase activity of heme. Further increases in the HSA concentration increased the peroxidase activity up to an HSA-to-heme ratio of approximately 1:1. An excess of HSA over heme decreased the peroxidase activity of heme (Fig. 3C). This experiment clearly shows that HSA augments the peroxidase activity of heme, but only when heme is in excess.
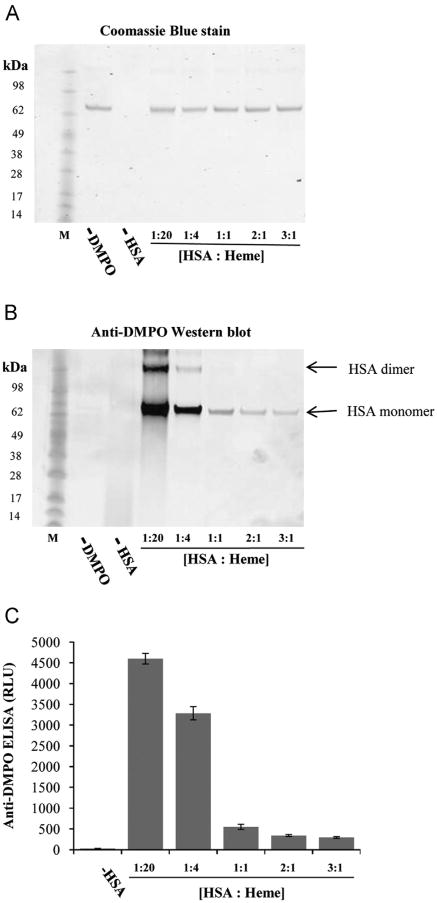
HSA:heme ratio and its effect on HSA-radical formation
Reactions containing different ratios of HSA to heme (Fig. 4) were carried out and analyzed using the immuno-spin trapping technique with Western blot and ELISA (Fig. 4B and C). Samples in which heme was in excess showed remarkably higher HSA-radical formation. The HSA-radical formation was almost completely attenuated when the HSA-to-heme ratio approached 1:1. This experiment clearly shows that protein-radical formation is in line with the modulation of peroxidase activity with a change in HSA: heme ratios.
Fig. 4.
Concentration-dependent effects of HSA on the formation of HSA radical in the presence of heme and H2O2. (A) Coomassie blue-stained gel to show equal loading, (B) anti-DMPO blotting, and (C) anti-DMPO ELISA. Reactions included 30 μM H2O2,100 mM DMPO, 30 μM heme, and different concentrations of HSA (1.5, 7.5, 30, 60, 90 μM). Reaction mixtures were incubated for 2 h at 37 °C in Chelex-treated, 100 mM phosphate buffer, pH 7.4, containing 25 μM DTPA. Data show representative gel/blot or mean values ±SE from at least three experiments in duplicate (n=3).
Heme/H2O2-induced formation of HSA radicals in human plasma
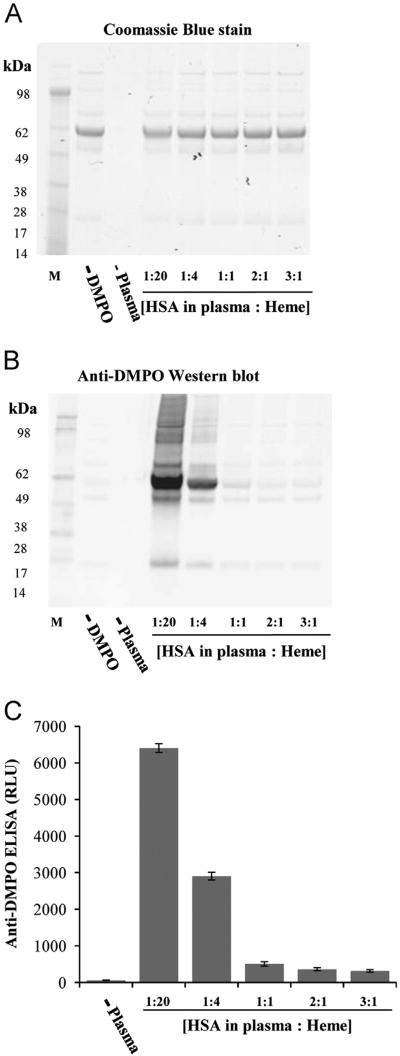
To demonstrate that the peroxidase activity of heme may have biological significance, experiments were performed with human plasma and heme in the presence of H2O2 (Fig. 5). Human plasma was diluted to get the equivalent of 7.5 μM HSA, the same concentration used in the heme/HSA/H2O2 reactions. Reactions containing different ratios of human plasma (expressed as HSA in plasma) to heme (Fig. 5) were made and analyzed using Western blot (Fig. 5B) and ELISA (Fig. 5C). Results were remarkably similar to those obtained with heme/HSA/H2O2, with HSA being the preferential target for protein-radical formation. At a heme to HSA ratio of less than one, HSA-radical formation, even though evident, was very low (Fig. 5B and C). However, noticeably in the reactions with excess heme, several other proteins in addition to HSA were oxidized by heme in the presence of H2O2.
Fig. 5.
Effect of increasing HSA-to-heme ratios on the protein-radical formation in human plasma by heme/H2O2. (A) Coomassie blue-stained gel to show equal loading, (B) anti-DMPO blotting, and (C) anti-DMPO ELISA. Reactions included 30 μM H2O2, 100 mM DMPO, 30 μM heme, and different concentrations of human plasma, expressed here as HSA in plasma (1.5, 7.5, 30, 60, 90 μM). Reaction mixtures were incubated for 2 h at 37 °C in Chelex-treated, 100 mM phosphate buffer, pH 7.4, containing 25 μM DTPA. Data show representative gel/blot or mean values ±SE from at least three experiments in duplicate (n=3).
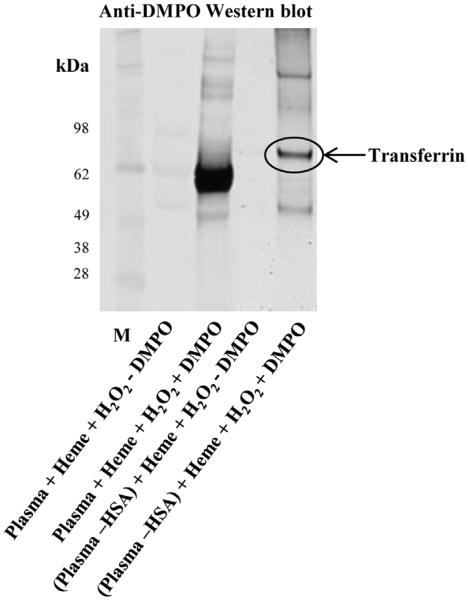
To further elucidate the possible role of HSA in protecting other plasma proteins from heme/H2O2-induced oxidation, HSA-depleted plasma was incubated with heme and H2O2. These samples clearly showed oxidation of other proteins that had not been oxidized in the presence of HSA (Fig. 6). Mass spectrometric analyses (Supplementary Table 1) of the proteins oxidized in the HSA-depleted human plasma identified one of them as transferrin (Fig. 6). To further confirm that transferrin was oxidized by heme/ H2O2, we performed immunoprecipitation of reaction mixture (heme/HSA-depleted plasma/H2O2) using anti-transferrin antibody and analyzed immunoprecipitation products with Western blot using anti-transferrin and anti-DMPO antibodies (Supplementary Fig. 1). These experiments clearly showed oxidation of transferrin in HSA-depleted plasma in the presence of heme/H2O2. When reactions were carried out in regular plasma and HSA was depleted after the reaction, protein-radical formation on these proteins was undetectable (data not shown). These experiments clearly indicate a possible protective role for HSA.
Fig. 6.
Effect of heme/H2O2 on the formation of protein radicals in human plasma. Western blot depicts anti-DMPO immunoreactivity of human plasma (HSA depleted or not) in the presence of heme and H2O2. Reactions included human plasma (HSA depleted or not; 7.5 μM HSA equivalents in plasma and an equal volume equivalent where HSA was depleted), 100 mM DMPO, 30 μM H2O2, and 30 μM heme. Reaction mixtures were incubated for 2 h at 37 °C in Chelex-treated, 100 mM phosphate buffer, pH 7.4, containing 25 μM DTPA. Data show a representative blot from at least three experiments in duplicate (n=3). Encircled band was excised and subjected to mass spectrometric analysis.
Effect of antioxidants on HSA-radical formation in vitro and in dialyzed human plasma
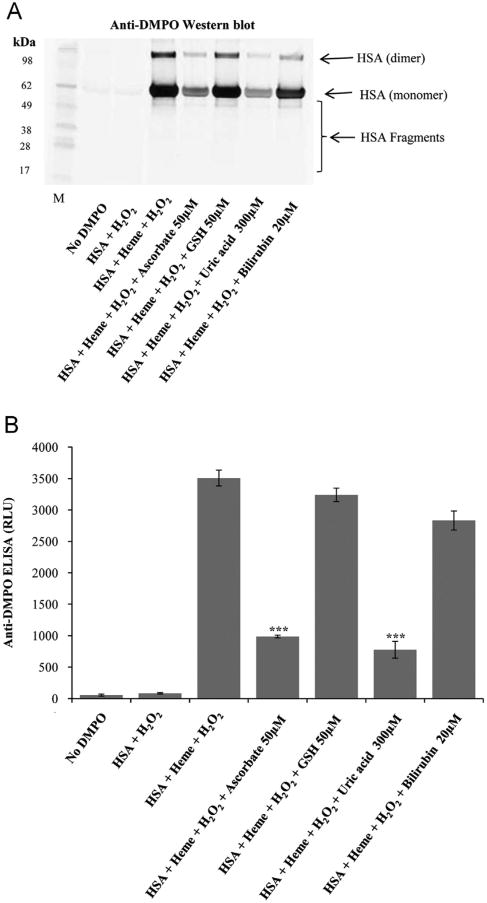
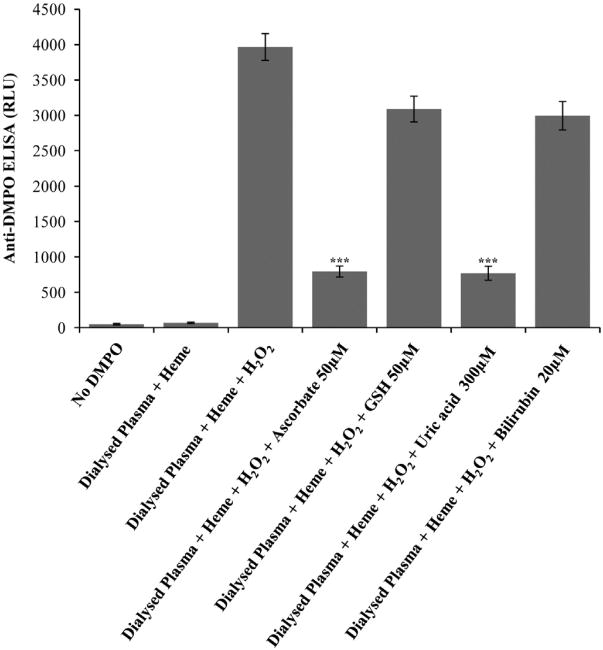
Physiological concentrations of the antioxidants ascorbate, reduced glutathione, uric acid, and bilirubin were used in reactions of heme/HSA/H2O2 to test the possible protection of these smaller antioxidants against HSA-radical formation. Ascorbate and uric acid significantly attenuated the formation of HSA radicals; however, reduced glutathione and bilirubin did not (Fig. 7A and B). When dialyzed plasma was incubated with heme/H2O2 in the presence of these antioxidants, the same trend was observed (Fig. 8). Physiological concentrations of ascorbate and uric acid significantly attenuated the formation of HSA radicals in human plasma; however, bilirubin and reduced glutathione did not confer significant protection at their physiological concentrations. Though, bilirubin and reduced glutathione were evidently effective against protein oxidation, when used at higher concentrations (data not shown).
Fig. 7.
Effect of physiological concentrations of antioxidants (ascorbate, GSH, uric acid, and bilirubin) on the formation of HSA radical by heme/H2O2. (A) Anti-DMPO Western blot and (B) anti-DMPO ELISA. Reactions contained 7.5 μM HSA, 100 mM DMPO, 30 μM H2O2, and 30 μM heme. Samples were incubated for 2 h at 37 °C in Chelex-treated, 100 mM phosphate buffer, pH 7.4, containing 25 μM DTPA. Data show representative blots or mean values ±SE from at least three experiments in duplicate (n=3), and asterisks indicate statistically significant differences (***P<0.001) with respect to samples marked as HSA+Heme+H2O2.
Fig. 8.
Effect of physiological concentrations of antioxidants (ascorbate, GSH, uric acid, and bilirubin) on the formation of protein radicals in dialyzed human plasma. Anti-DMPO ELISA of samples of dialyzed human plasma exposed to heme/H2O2 in the presence and absence of antioxidants. Reactions contained 7.5 μM HSA equivalents in dialyzed plasma, 100 mM DMPO, 30 μM H2O2, 30 μM heme, and physiological concentration of antioxidants. Samples were incubated for 2 h at 37 °C in Chelex-treated, 100 mM phosphate buffer, pH 7.4, containing 25 μM DTPA. Data show mean values ±SE from at least three experiments in duplicate (n=3), and asterisks indicate statistically significant differences (***P<0.001) with respect to samples marked as Dialysed Plasma+heme+H2O2.
Discussion
Heme plays a crucial role in regulating the physiological functions of a number of specific hemoproteins involved in oxygen transport, mitochondrial respiration, xenobiotic metabolism, steroid biosynthesis, and signal transduction [19,20]. Normally, hemoglobin released in plasma is bound by haptoglobin and efficiently cleared. When excess hemoglobin that is released during pathophysiological conditions overwhelms haptoglobin binding capacity, it is oxidized to methemoglobin, which releases heme in plasma [4]. Heme is potentially toxic, in particular, when high concentrations of free heme occur during pathophysiological conditions, trauma, or hemorrhage [2].
It is known that free heme can generate free radicals, which in turn can cause DNA damage and lipid peroxidation [12,21]. During hemolysis, organisms defend against free heme initially by induction of heme scavengers such as hemopexin, and subsequentlyby expression of cytoprotective genes such as HO-1 and ferritins[1,22]. Despite ample evidence of heme-induced oxidative damage, heme-induced protein-radical formation, especially in the context of plasma proteins, had not been studied. Therefore, we investigated the possible formation of protein radicals in heme/HSA/H2O2 and heme/plasma/H2O2 systems using the highly sensitive immuno-spin-trapping technique [13,23].
HSA-radical formation by heme and H2O2
Heme and H2O2 triggered the formation of HSA radicals. The absence of DMPO adducts when heme was absent indicates the inability of H2O2 per se to induce the formation of HSA radicals (Fig. 1B). Similarly, in the absence of H2O2, heme could not trigger protein-radical formation (Fig. 2B). The inability of heme to trigger protein-radical formation in the absence of H2O2 clearly indicates the role of the peroxidase activity of heme in protein-radical formation. The intrinsic peroxidase activity of heme is dependent on its reaction with H2O2.
HSA binds up to seven molecules of fatty acids with different affinities in each site. In subdomain 1B, a well-characterized fatty acid binding site, heme also binds with a very high affinity [5,17,24]. In aqueous solutions, heme spontaneously forms multimeric species [25]. A decrease in the polarity of the solvent or in the concentration of heme favors the stabilization of the monomeric species. Alterations in the equilibrium of monomeric and multimeric species would, indeed, dramatically change the peroxidase activity of heme since it is known that the monomeric species is the only active form that reacts with H2O2[26,27].
Unbound heme, which occurs when heme is in excess of HSA, generates more protein radicals. The fact that the peroxidase activity of solutions of heme and HSA increases up to a 1:1 heme:HSA ratio shows that concentrations of HSA up to equimolar with heme favor the monomerization of the polymeric species of heme. The heme–HSA complex, however, is less reactive toward H2O2[26], probably due to the binding of the heme iron to tyrosine in the HSA complex instead of histidine as occurs in peroxidases [6]. The lower reactivity of heme bound to HSA is ultimately reflected in the low yields of protein-radical formation in samples with low heme:HSA ratios (Fig. 4B and C).
Protein-radical formation in plasma
Fresh blood plasma has been used to study the effect of heme/H2O2 as an in vitro model for many pathophysiological conditions such as hemolytic anemia, malaria, and sickle cell disease [27,28]. When ratio experiments were extended to human plasma, protein-radical formation was remarkably similar to that of the heme/HSA/H2O2 system. At a slight excess of heme, only HSA was detectably oxidized and, thus, conferred protection to the other proteins in plasma. However, at higher concentrations of heme, HSA could not confer total protection (Fig. 5B). At high concentrations of heme, the capacity of HSA to retain heme is overwhelmed [28], and free heme efficiently targets other proteins, which are present in lower concentrations (Fig. 5B). The addition of heme to HSA-depleted plasma induced protein-radical formation on other proteins such as transferrin (Fig. 6). Radicals on transferrin and other proteins were formed by low heme concentrations in HSA-depleted human plasma, but radicals on these proteins were detected in regular human plasma containing HSA only when exposed to high concentrations of heme (at heme-to-HSA ratio of 20:1). These results clearly show that HSA mediates the protection of other plasma proteins that are often involved in pathophysiology [29].
Specifically, plasma transferrin is a potent chelator capable of maintaining iron homeostasis by binding iron tightly, but reversibly [30]. Transferrin also plays an indirect defensive role against infection by chelating extracellular iron [31]. In view of their vital functions, heme/H2O2-induced radical formation on transferrin and other proteins may impair iron dishomeostasis and other related functions [32].
Implications of protein oxidation for heme degradation
Activation of HO-1 is a ubiquitous response for cellular defense from oxidative damage [9,33,34]. In the literature, two purposes are discussed for the degradation of heme by HO-1 [2]. One is the production of the antioxidant bilirubin [9], and the other is the prevention of heme-dependent adverse effects. Despite ample evidence of bilirubin being an antioxidant [35], it appeared to be a relatively inefficient antioxidant, in the context of preventing protein oxidation by heme (Fig. 8). Bilirubin contains an extended system of conjugated double bonds and a reactive hydrogen atom and thus shows antioxidant properties against lipid peroxides and their breakdown products [9,35], and does not appear as effective against protein oxidation. Inefficiency of bilirubin to mitigate HSA oxidation is not due to competition with heme because heme binds to HSA on different sites than that of bilirubin and so heme does not compete with bilirubin for its binding sites on HSA [36]. This inefficiency of bilirubin supports the view that heme degradation by HO-1 is beneficial mainly because it eliminates heme as a catalyst of protein-radical formation. The induction of HO-1 would then be beneficial because it safeguards the protein pool from heme-catalyzed oxidation.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Dr. Ann Motten and Mary Mason for their valuable help in the preparation of the manuscript. This work has been supported by the Intramural Research Program of the National Institutes of Health and the National Institute of Environmental Health Sciences. Saurabh Chatterjee is also supported by a K99-R00, NIH, and Pathway to Independence Award (ES01-19875A). The authors declare no conflict of interest.
Abbreviations
- DMPO
5,5-dimethyl-1-pyrroline N-oxide
- HSA
human serum albumin
- HO-1
heme oxygenase-1
- M
molecular weight markers
- RBC
red blood cells
- RLU
relative light units
Footnotes
Appendix A. Supplementary Information: Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.freeradbiomed.2013.04.026
References
- 1.Balla J, Vercellotti GM, Jeney V, Yachie A, Varga Z, Jacob HS, Eaton JW, Balla G. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid Redox Signal. 2007;9:2119–2137. doi: 10.1089/ars.2007.1787. [DOI] [PubMed] [Google Scholar]
- 2.Wijayanti N, Katz N, Immenschuh S. Biology of heme in health and disease. Curr Med Chem. 2004;11:981–986. doi: 10.2174/0929867043455521. [DOI] [PubMed] [Google Scholar]
- 3.Miller YI, Shaklai N. Kinetics of hemin distribution in plasma reveals its role in lipoprotein oxidation. Biochim Biophys Acta. 1999;1454:153–164. doi: 10.1016/s0925-4439(99)00027-7. [DOI] [PubMed] [Google Scholar]
- 4.Hanson MS, Piknova B, Keszler A, Diers AR, Wang X, Gladwin MT, Hillery CA, Hogg N. Methaemalbumin formation in sickle cell disease: effect on oxidative protein modification and HO-1 induction. Br J Haematol. 2011;154:502–511. doi: 10.1111/j.1365-2141.2011.08738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fasano M, Curry S, Terreno E, Galliano M, Fanali G, Narciso P, Notari S, Ascenzi P. The extraordinary ligand binding properties of human serum albumin. IUBMB life. 2005;57:787–796. doi: 10.1080/15216540500404093. [DOI] [PubMed] [Google Scholar]
- 6.Ascenzi P, Fasano M. Allostery in a monomeric protein: the case of human serum albumin. Biophys Chem. 2010;148:16–22. doi: 10.1016/j.bpc.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassu AM, Bonaparte D, Cavalcante MM, Chora A, Ferreira A, Marguti I, Cardoso S, Sepulveda N, Smith A, Soares MP. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med. 2010;2:1–12. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- 8.Stocker R, Glazer AN, Ames BN. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci USA. 1987;84:5918–5922. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 10.Grisham MB, Gaginella TS, von Ritter C, Tamai H, Be RM, Granger DN. Effects of neutrophil-derived oxidants on intestinal permeability, electrolyte transport, and epithelial cell viability. Inflammation. 1990;14:531–542. doi: 10.1007/BF00914274. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez DC, Chen YR, Mason RP. Immunochemical detection of hemoglobin-derived radicals formed by reaction with hydrogen peroxide: involvement of a protein-tyrosyl radical. Free Radic Biol Med. 2003;34:830–839. doi: 10.1016/s0891-5849(02)01437-5. [DOI] [PubMed] [Google Scholar]
- 12.Vincent SH. Oxidative effects of heme and porphyrins on proteins and lipids. Semin Hematol. 1989;26:105–113. [PubMed] [Google Scholar]
- 13.Mason RP. Using anti-5,5-dimethyl-1-pyrroline N-oxide (anti-DMPO) to detect protein radicals in time and space with immuno-spin trapping. Free Radic Biol Med. 2004;36:1214–1223. doi: 10.1016/j.freeradbiomed.2004.02.077. [DOI] [PubMed] [Google Scholar]
- 14.Zhao B, Summers FA, Mason RP. Photooxidation of Amplex red to resorufin: implications of exposing the Amplex red assay to light. Free Radic Biol Med. 2012;53:1080–1087. doi: 10.1016/j.freeradbiomed.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez DC, Mejiba SE, Mason RP. Copper-catalyzed protein oxidation and its modulation by carbon dioxide: enhancement of protein radicals in cells. J Biol Chem. 2005;280:27402–27411. doi: 10.1074/jbc.M504241200. [DOI] [PubMed] [Google Scholar]
- 16.Detweiler CD, Deterding LJ, Tomer KB, Chignell CF, Germolec D, Mason RP. Immunological identification of the heart myoglobin radical formed by hydrogen peroxide. Free Radic Biol Med. 2002;33:364–369. doi: 10.1016/s0891-5849(02)00895-x. [DOI] [PubMed] [Google Scholar]
- 17.Deterding LJ, Ramirez DC, Dubin JR, Mason RP, Tomer KB. Identification of free radicals on hemoglobin from its self-peroxidation using mass spectrometry and immuno-spin trapping: observation of a histidinyl radical. J Biol Chem. 2004;279:11600–11607. doi: 10.1074/jbc.M310704200. [DOI] [PubMed] [Google Scholar]
- 18.Aft RL, Mueller GC. Hemin-mediated oxidative degradation of proteins. J Biol Chem. 1984;259:301–305. [PubMed] [Google Scholar]
- 19.Ponka P. Cell biology of heme. Am J Med Sci. 1999;318:241–256. doi: 10.1097/00000441-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Muller-Eberhard U, Fraig M. Bioactivity of heme and its containment. Am J Hematol. 1993;42:59–62. doi: 10.1002/ajh.2830420112. [DOI] [PubMed] [Google Scholar]
- 21.Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000;28:289–309. doi: 10.1016/s0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 22.Choi AM, Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- 23.Keszler A, Mason RP, Hogg N. Immuno-spin trapping of hemoglobin and myoglobin radicals derived from nitrite-mediated oxidation. Free Radic Biol Med. 2006;40:507–515. doi: 10.1016/j.freeradbiomed.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Das A, Hecht MH. Peroxidase activity of de novo heme proteins immobilized on electrodes. J Inorg BioChem. 2007;101:1820–1826. doi: 10.1016/j.jinorgbio.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van der Zee J, Barr DP, Mason RP. ESR spin trapping investigation of radical formation from the reaction between hematin and tert-Butyl hydroperoxide. Free Radic Biol Med. 1996;20:199–206. doi: 10.1016/0891-5849(95)02031-4. [DOI] [PubMed] [Google Scholar]
- 26.Grinberg LN, O'Brien PJ, Hrkal Z. The effects of heme-binding proteins on the peroxidative and catalatic activities of hemin. Free Radic Biol Med. 1999;27:214–219. doi: 10.1016/s0891-5849(99)00082-9. [DOI] [PubMed] [Google Scholar]
- 27.Wagner JR, Motchnik PA, Stocker R, Sies H, Ames BN. The oxidation of blood plasma and low density lipoprotein components by chemically generated singlet oxygen. J Biol Chem. 1993;268:18502–18506. [PubMed] [Google Scholar]
- 28.Ascenzi P, Bolli A, di Masi A, Tundo GR, Fanali G, Coletta M, Fasano M. Isoniazid and rifampicin inhibit allosterically heme binding to albumin and peroxynitrite isomerization by heme-albumin. J Biol Inorg Chem. 2011;16:97–108. doi: 10.1007/s00775-010-0706-2. [DOI] [PubMed] [Google Scholar]
- 29.Lim W, Jeong W, Kim JH, Lee JY, Kim J, Bazer FW, Han JY, Song G. Differential expression of alpha 2 macroglobulin in response to dietylstilbestrol and in ovarian carcinomas in chickens. Reprod Biol Endocrinol. 2011;9:137–146. doi: 10.1186/1477-7827-9-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gkouvatsos K, Papanikolaou G, Pantopoulos K. Regulation of iron transport and the role of transferrin. Biochim Biophys Acta. 2012;1820:188–202. doi: 10.1016/j.bbagen.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Brock JH, Mainou-Fowler T, McGregor SJ. Transferrins and defence against infection. Ann Ist Super Sanita. 1987;23:935–941. [PubMed] [Google Scholar]
- 32.Crane FL, Low H. The oxidative function of diferric transferrin. Biochem Res Int. 2012;2012:1–7. doi: 10.1155/2012/592806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirkby KA, Adin CA. Products of heme oxygenase and their potential therapeutic applications. Am J Physiol Renal Physiol. 2006;290:F563–F571. doi: 10.1152/ajprenal.00220.2005. [DOI] [PubMed] [Google Scholar]
- 34.Vercellotti GM, Balla G, Balla J, Nath K, Eaton JW, Jacob HS. Heme and the vasculature: an oxidative hazard that induces antioxidant defenses in the endothelium. Artif Cells Blood Substit Immobil Biotechnol. 1994;22:207–213. doi: 10.3109/10731199409117415. [DOI] [PubMed] [Google Scholar]
- 35.Stocker R. Antioxidant activities of bile pigments. Antioxid Redox Signal. 2004;6:841–849. doi: 10.1089/ars.2004.6.841. [DOI] [PubMed] [Google Scholar]
- 36.Ostrow JD, Mukerjee P, Tiribelli C. Structure and binding of unconjugated bilirubin: relevance for physiological and pathophysiological function. J Lipid Res. 1994;35:1715–1737. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.