Abstract
Interleukin 27 (IL-27) is an immunomodulatory cytokine with important roles in both the innate and adaptive immune systems. In the last five years, the addition of exogenous IL-27 to primary cell cultures has been demonstrated to decrease HIV-1 replication in a number of cell types including peripheral blood mononuclear cells (PBMCs), CD4+ T cells, macrophages and dendritic cells. These in-vitro findings suggest that IL-27 may have therapeutic value in the setting of HIV-1 infection. In this review, we describe the current knowledge of the biology of IL-27, its effects primarily on HIV-1 replication but also in other viral infections and explore its potential role as a therapeutic cytokine for the treatment of patients with HIV-1 infection.
Keywords: Interleukin-27, HIV, cytokines, SPTBN1, therapy
1. Introduction
Interleukin 27 (IL-27), a member of the IL-12 family of cytokines, is an immunomodulatory cytokine with important roles in both the innate and adaptive immune systems. Initially typecast as a pro-inflammatory cytokine due to its effect on supporting the differentiation of naïve CD4+ T cells into T helper 1 (Th1) cells, it is now appreciated that it also has important roles to play in inducing the expression of the anti-inflammatory cytokine, IL-10 and the common γ chain cytokine IL-21. In the last five years, IL-27 has been demonstrated to decrease HIV-1 replication in peripheral blood mononuclear cells (PBMCs), CD4+ T cells, macrophages and dendritic cells. These in-vitro findings suggest that IL-27 may have therapeutic value in the setting of HIV-1 infection. In this review, we describe the current knowledge of the biology of IL-27, its effects primarily on HIV-1 replication and explore its potential as a therapeutic cytokine for the treatment of patients with HIV-1 infection.
2. Structure of IL-27 and its receptor
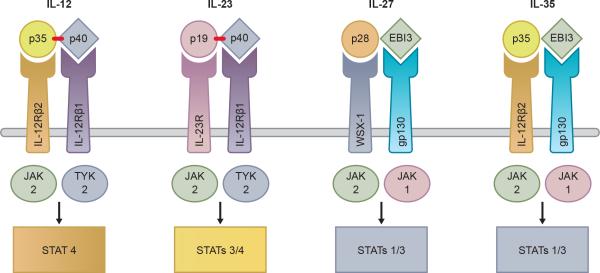
IL-27 belongs to the IL-12 family of cytokines and thus shares some properties with the other members of the group (IL-12, IL-23 and IL-35) [1]. The cytokines from the IL-12 family are made of various combinations of shared α-chains and β-chains [2]. IL-27 is composed of the IL-27p28 subunit (also known as IL-30) and an Epstein-Barr virus induced gene 3 (EBI3) subunit [3] (see Figure 1). These subunits are coordinately expressed in antigen presenting cells (mostly in dendritic cells and macrophages) [3, 4], following exposure to inflammatory mediators (such as CD40L or IL-1β) or by triggering of Toll like receptors (TLRs) with microbial products such as lipopolysaccharide (LPS) [5]. Unlike IL-12 and IL-23, the heterodimers of IL-27 and IL-35 are not linked by disulfide bonds [6]. The disulfide bond linkage aids in secretion efficiency and may account for the lesser stability of IL-27 and IL-35 in comparison to IL-12 and IL-23 [7, 8].
Figure 1. The IL-12 family of cytokines.
The IL-12 family consists of four members: IL-12, IL-23, IL-27 and IL-35. They consist of various heterodimers of shared α-chains and β-chains as shown. The receptors for the IL-12 family of cytokines are also heterodimers of molecules shared amongst the family members as illustrated. Cytokine signaling occurs through the JAK/STAT pathway.
Interestingly, the p28 subunit alone (IL-30), has been reported to bind to the receptors of IL-6 and IL-27 without inducing the same signaling cascades [9]. A recent paper has suggested that IL-30 may mediate trans signaling via the soluble IL-6 receptor by binding to soluble glycoprotein 130 (also known as gp130, IL6ST, IL6-beta or CD130) [10, 11].
IL-27 binds to the IL-27 receptor which is comprised of a heterodimer of IL-27Rα (WSX-1) and gp130 (Figure 1). The gp130 subunit also participates as a subunit for other type 1 cytokine receptors (often referred to as the common gp130 subunit) belonging to the IL-6 receptor family, including IL-6, IL-11, cardiotrophin-like cytokine (CLC), ciliary neurotrophic factor (CNTF), cadiotrophin-1 (CT-1), leukemia inhibitory factor (LIF) and oncostatin M (OSM) [6]. IL-27 directly binds to the WSX -1 component of the IL-27 receptor. Down-stream signaling occurs via gp130. In terms of receptor expression, although gp130 is ubiquitously expressed on many different cell types, IL-27Rα expression is limited to T cells, B cells, monocytes, neutrophils, natural killer (NK) cells, mast cells, and lower levels of expression are seen in macrophages and hepatocytes.
Like many other cytokines, signaling occurs through a Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway [12]. In brief, activated JAK proteins phosphorylate STAT transcription factors, which in turn dimerise, translocate to the nucleus and increase transcription of various genes. The binding of IL-27 to its receptor leads to phosphorylation of STATs -1, -3, -4 and -5. The activation status of the CD4+ T cell appears to play a role in which STAT proteins are activated following engagement of the IL27. Naïve T cells preferentially activate STAT1 upon exposure to IL-27. Early activated CD4+ T cells are more likely to activate STATs 1 and 3 in response to IL-27 whereas fully activated CD4+ T cells preferentially phosphorylate STAT3 [13]. In macrophages STATs -1 and -3 are phosphorylated and activated following exposure to IL-27 [14] whereas in naïve B cells, STATs -1 and -3 are strongly phosphorylated [15]. IL-27 also signals via STATs -1 and -3 in monocytes but also requires activation of NF-κB [16]. Recently data suggest that IL-27 can also signal in a JAK/STAT independent manner via the TAK1/MAPK signaling pathway in macrophages [17]. In dendritic cells (DCs), STATs 1,3 and 5 are rapidly phosphorylated following treatment of cells with IL-27 [18].
3. Role in immune function
Like other pleiotropic cytokines, IL-27 has both anti-inflammatory and pro-inflammatory properties. The initial description of IL-27 focused on its role in promoting Th1 responses and hence it was initially described as a pro-inflammatory cytokine [3, 19]. However, subsequent work has shown that IL-27 has a multifaceted role within the immune system with both anti-inflammatory and immunoregulatory properties.
IL-27 has been demonstrated to play important roles in the development and function of a number of different T cell subsets, including Tr1 cells, Tregs, Tfh cells and Th17 cells [20]. Tr1 cells, a type of regulatory T cell, are important producers of the anti-inflammatory cytokine, IL-10 [21]. IL-27 was originally characterized as a cytokine which synergizes with IL-12 to trigger IFN-gamma production in naïve T cells [3]. A further study described that in the presence of IL-27, Tr1 cells up-regulate the expression of the transcription factor c-Maf which can directly activate the Il10 promoter and also increase IL-21 production [22]. IL-27 signaling in Tr1 cells also leads to an increase in expression of the aryl hydrocarbon receptor (AhR) which combines with c-Maf in stabilizing interactions with the IL-10 and IL-21 promoters [23]. The combined production of IL-10 and IL-21 promotes the production and survival of these cells. IL-27, derived from innate immune cells, can also work with IL-2 from CD4+ T cells to promote IL-10 production in cytotoxic T cells in a Blimp-1 dependent manner [24].
Tregs are CD4+CD25+ regulatory T cells that are characterized by the expression of the transcription factor FoxP3 [21]. They arise either from the thymus (natural Tregs) or are generated in the periphery (inducible Tregs) [22]. Exposing inducible Tregs to IL-27 leads to a reduction in FoxP3 expression [23]. Thus IL-27 may be an important regulator of both Treg function and in homeostatic mechanisms controlling inflammation and self-tolerance. T follicular helper cells (Tfh) are located within the lymphoid follicle and are critical regulators of antibody class switching and high affinity antibody production in B cells [25]. IL-27 signaling in Tfh cells increases transcription of IL-21 [26].
Th17 cells are characterized by the production of IL-17 and are important in promoting pathogen clearance but have also been linked to some autoimmune conditions, including multiple sclerosis [27]. IL-27 induction of γ-IFN and IL-10 from Tr1 cells may suppress the development of Th17 cells. Mice lacking the IL-27Rα chain generated more IL-17 producing T helper cells and were shown to be hyper-susceptible to experimental autoimmune encephalomyelitis (an animal model of multiple sclerosis) [28]. IL-27, by affecting a number of important T cell subsets, is increasingly becoming an important player in understanding the pathogenesis of a number of conditions.
4. THE ROLE OF IL-27 IN HIV-1 INFECTION
4.1 IL-27 promotes IFN induced genes that may be protective in HIV-1 infection
The first indication that IL-27 had a protective effect against HIV-1 infection was observed in a model system using non-infectious human papilloma virus like particles (VLPs) as a cervical cancer vaccine [29]. The papilloma virus VLPs had previously been described to bind to dendritic cells via Toll like receptor 4 (TLR4) [30] and induce expression of a number of anti-viral cytokines including Type I interferons (IFN) [30-32]. To determine whether or not VLPs would be safe to administer to HIV-1 positive patients [33], phytohemagglutinin (PHA) stimulated PBMCs were infected with either a CXCR4 or CCR5 HIV-1 tropic virus and then cultured in the presence of VLPs. It was observed that, regardless of tropism, HIV-1 was inhibited in a dose-dependent manner by the VLPs. Culture supernatants from VLP-treated PBMCs or monocyte derived macrophages (MDMs) confirmed the anti-HIV property. This result indicated that soluble factors in the culture supernatants were mediating the anti-HIV effects. Gene expression profiling of PBMCs and monocyte derived macrophages (MDMs) cultured in the presence of VLPs revealed that IL27 rather than Type I IFN was markedly up-regulated in both PBMCs and MDMs. Subsequent experiments using recombinant IL-27 confirmed that HIV-1 replication could be significantly inhibited by IL-27 in a dose-dependent manner in PBMCs, CD4+ T cells and MDMs. A DNA microarray of IL-27 treated MDMs revealed that a number of anti-viral genes were up-regulated, including the following IFN-inducible genes: IFN regulatory factor 1 (IRF1), IRF8, myxovirus resistance 1 (MX1), and 2′-5′ – oligoadenylate synthetase (OAS), without the induction of 20 other IFN family genes [30]. Of note, IL-27 did not induce these anti-viral genes in T cells. To further investigate the mechanism of IL-27 mediated HIV-1 inhibition, HIV-1 infected CD4+ T cells and MDMs were cultured in the presence of IL-27 or IFN-α. IFN-α was able to inhibit HIV-1 replication to a greater degree than IL-27 [34]. A cocktail of neutralizing antibodies to IFN did not interfere with the HIV-1 inhibitory effects of IL-27, whereas they did reduce the HIV-1 inhibitory effects of IFN suggesting that the inhibitory effect of IL-27 was not dependent on production of IFN. Of note IL-27 exposure was not associated with induction of IFN-α. Recent studies have shown that IL-27 is also a potent inhibitor of cis HIV-1 infection in immature dendritic cells (iDCs) and that the inhibition was through an IFN-independent pathway [18]. While it is reported that dendritic cells exert a role in trans HIV infection via DC-SIGN [35], IL-27 had no impact on the expression of DC-SIGN [31], thus it is reasonable to speculate that IL-27 may not have any significant impact on trans-infection of T cells from dendritic cells. However, IL-27 may inhibit HIV-1 replication within T cells following the transfer of HIV-1 from infected dendritic cells.
4.2 IL-27 modulates host restriction factors against HIV-1
A number of host restriction factors have been investigated to try and explain the inhibitory actions of IL-27 on HIV-1 infection. Among the best described host restriction factors in HIV-1 infection are the apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like cytidine deaminases (APOBEC proteins), particularly APOBEC3G [36, 37]. A study comparing the effects on HIV-1 infection between IFN-α and IL-27 revealed that IFN-α treated cells were able up-regulate APOBEC3G in both MDMs and CD4+ T cells whereas IL-27 could only up-regulate APOBEC3G in MDMs [34]. To further explore the mechanism behind the decreased HIV-1 replication of macrophages in the presence of IL-27, the kinetics of APOBEC expression were examined in macrophages following administration of IL-27 [14]. APOBEC3A and APOBEC3G were both significantly up-regulated following IL-27 with the maximal effect only seen at 24 hours. Recently IL-27 has also been demonstrated to up-regulate tetherin/bone marrow stromal cell antigen 2 (BST-2) in monocytes and CD4+ T cells, independently of the actions of Type I IFN [38]. BST-2 has recently been found to be an important host restriction factor which prevents HIV-1 virion release and is counteracted by the HIV-1 viral protein, Vpu [39]. Hence, the link between IL-27 and BST-2 up-regulation will need to be further explored to see if this explains any of the HIV-1 inhibitory effects observed with exogenous IL-27 treatment.
4.3 IL-27 inhibits HIV-1 in macrophages by down-regulating SPTBN1
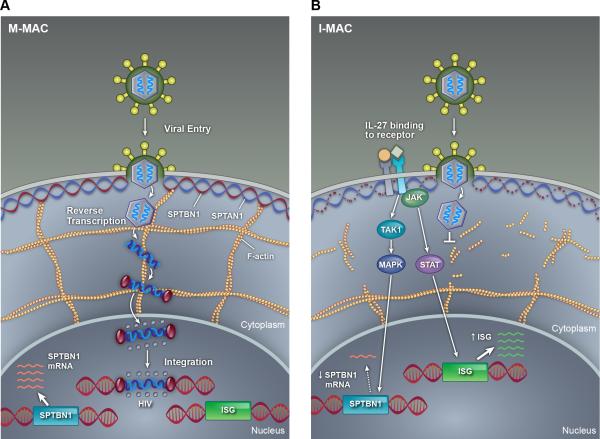
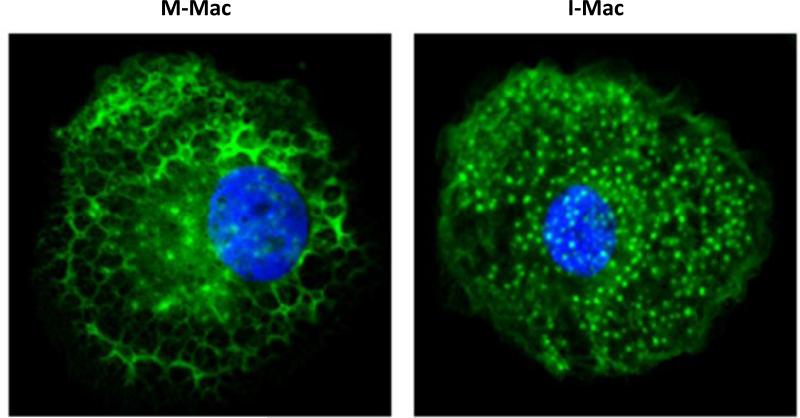
As described above, the mechanism(s) of how IL-27 inhibits HIV-1 infection in either macrophages or T cells are mostly unknown. However, a recent study has revealed a novel IL-27 signaling pathway in macrophages and also characterized an essential host factor which is critical for HIV-1 replication [17]. It is known that macrophages induced from monocytes just with M-CSF (M-Mac) are more susceptible to HIV-1 infection compared to monocytes induced with both IL-27 and M-CSF (I-Mac). Gene profiling of I-Mac versus M-Mac revealed 174 genes which were either up-regulated (118 genes) or down-regulated (56 genes). Comparing these differentially expressed genes with a prior siRNA screen used to identify key genes important in HIV-1 pathogenesis [40], revealed a single match – spectrin beta nonerythrocyte 1 (SPTBN1). SPTBN1 belongs to the family of β spectrin genes (that contains 5 members) and combines with a member of the alpha spectrin family (SPTAN1 or SPTA1) to form spectrin. Spectrin plays a pivotal role in binding and anchoring actin to the plasma membrane. In these experiments, SPTBN1 was down-regulated in I-Mac five-fold compared to M-Mac. Subsequent experiments which over-expressed SPTBN1 in I-Mac, restored susceptibility to HIV-1 (like M-Mac) and knocking down SPTBN1 in M-Mac with short interfering RNAs (siRNAs) made these cells relatively resistant to HIV-1 infection (like I-Mac). SPTBN1 can bind to the HIV-1 gag p55 protein and hence may affect the HIV-1 life-cycle from this perspective. Morphological differences noted on confocal microscopy between M-Mac versus I-Mac, suggest that disruption of the normal lattice actin cytoskeletal structure (particularly near the plasma membrane) by IL-27 down-regulation of SPTBN1, may be a key factor of why HIV-1 replication is affected (Figures 2 and 3). Despite the cytoskeletal changes noted with IL-27 treatment, IL-27 induced macrophages have normal functional activity with regard to phagocytosis and chemotaxis. The IL-27 mediated down-regulation of SPTBN1 was shown to be mediated by the TAK1/MAPK signaling pathway rather than the JAK/STAT signaling pathways previously described for this cytokine. These studies have revealed that SPTBN1 is an important host factor for HIV-1 in macrophages and may herald new therapeutic options targeting and/or arresting HIV-1 replication within the macrophage reservoir.
Figure 2. Proposed mechanisms of IL-27 mediated inhibition of HIV-1 in macrophages.
In M-Mac, there is no block to the HIV-1 life cycle and HIV-1 viral DNA is able to integrate into the host genome. Genes encoding spectrin (SPTBA1 and SPTBN1 for example) are normally transcribed and the cells have a normal cytoskeletal structure with actin being anchored to the plasma membrane by spectrin. The actin cytoskeleton may be also important for the HIV-1 viral cycle. In I-Mac, IL-27 leads to TAK1 activation via gp130 and activates the MAP Kinase pathway which in turn down-regulates SPTBN1.As SPTBN1 is a key component of spectrin, the normal cytoskeletal structure close to the plasma membrane is lost as actin is unable to bind to spectrin. The HIV life cycle is halted prior to completion of reverse transcription. In addition IL-27 mediated activation of the JAK/STAT pathway leads to the induction of interferon stimulated genes (ISG) which may also mediate anti-viral properties.
Figure 3. Confocal image displaying the actin cytoskeleton in M-Mac and I-Mac.
A) The actin cytoskeletal architecture is clearly intact and looks like a lattice, as shown by the green fluorescence in M-Mac. B) The architecture is radically altered in I-Mac where the lattice formation seen in M-Mac gives way to a punctate staining pattern for actin - however, nearly F-actin fibers close to the plasma membrane are still intact. Blue staining is with DAPI and indicates the nucleus; green staining indicates the actin binding dye.
4.4 HIV-1 infection impairs responsiveness to IL-27 in monocytes
The studies described above have looked at the effect of IL-27 on HIV-1 infected PBMCs, CD4+ T cells, MDMs and dendritic cells obtained from healthy donors. Some studies have also looked at whether cells obtained directly from HIV-1 infected donors have an impaired response to IL-27. Exogenous IL-27 was administered to monocytes from HIV-1 infected donors and their responsiveness was compared to monocytes from uninfected controls [41]. In monocytes obtained from HIV-1 infected donors, IL-27 treatment led to lower levels of expression of the IL-27 receptor subunit, gp130, compared to healthy controls. In addition, monocytes from HIV-1 infected donors treated with IL-27 had decreased production of the cytokines IL-6, TNF-α and IL-10, cytokines previously shown to be up-regulated by IL-27 treatment [16, 42]. In HIV-1 viremic individuals, IL-27 mRNA levels were found to be twice as high in CD4+ T cells compared to monocytes from the same individuals whereas the transcript levels were similar between CD4+ T cells and monocytes from HIV negative controls [43]. These results suggest that HIV-1 infection may affect the way in which monocytes and CD4+ T cells respond to IL-27 which may have therapeutic implications if IL-27 enters clinical trials.
4.5 Circulating levels of IL-27 in HIV-1 infection
There are few studies investigating the correlation between systemic levels of IL-27 and correlates of immune dysfunction seen with HIV-1 infection. One study examined the plasma levels of IL-27 in patients with HIV-1 infection alone or co-infected with HIV-1 and Hepatitis C (HCV) [44]. There was a small negative correlation (not significant) between IL-27 plasma levels and HIV-1 viral load noted (those with higher viral loads, had lower levels of IL-27). Patients co-infected with HIV-1/HCV surprisingly had lower levels of IL-27 compared to HIV-1 mono-infected individuals. In a much larger Chinese cohort of HIV-1 patients (120 HIV-1 infected treatment naïve patients versus 108 uninfected controls), IL-27 levels were significantly higher in the HIV-1 cohort compared to the uninfected controls (p<0.001) [45] and a small positive correlation was observed between IL-27 levels and CD4+ T cell count. Unfortunately, correlations of plasma IL-27 levels with HIV-1 viral loads were not investigated and could not be compared with the earlier study. Table 1 summarizes some of the various studies looking at IL-27 in HIV-1 infection.
Table 1.
Studies investigating the function of IL-27 in HIV-1 infection
| Cells exposed to IL-27 | Year | Effect of IL-27 | Mechanism | Reference |
|---|---|---|---|---|
| In-vitro: | ||||
| PBMCs, MDMs, CD4+ T cells | 2007 | HIV-1 inhibited | Up-regulation of IFN-related genes | Fakruddin et al [33] |
| MDMs, CD4+ T cells | 2008 | HIV-1 inhibited | IL-27 up-regulates APOBEC3G | Imamichi et al [34] |
| MDMs, CD4+ T cells | 2009 | HIV-1 inhibited | IL-27 induces IFN expression which in turn, up-regulates APOBEC proteins | Greenwell et al[14] |
| Monocytes | 2012 | HIV-1 inhibited | Induction of IL-10 in monocytes | Kwon et al[43] |
| PBMCs, monocytes, CD4+ T cells | 2012 | IL-6, TNF-α, IL-10 levels decreased | IL-27 up-regulates gp130 in monocytes in HIV-1 negative controls to a higher degree compared to HIV-1 positive patients | Guzzo et al [41] |
| MDMs | 2013 | HIV-1 inhibited in IL-27 treated MDMs | Down-regulation of SPTBN1 | Dai et al [17] |
| Dendritic Cells | 2013 | HIV-1 inhibited in IL-27 treated iDCs | Interferon independent | Chen et al [18] |
| In-vivo: | ||||
| Clinical study | 2010 | IL-27 levels negatively correlated with HIV-1 viral loads | N/A | Guzzo et al [44] |
| Clinical study | 2011 | IL-27 levels positively correlated with CD4+ T cell count in HIV-1 infected patients | N/A | He et al [45] |
Legend: APOBEC = apolipoprotein B, mRNA-editing enzyme, catalytic polypeptide-like cytidine deaminases; iDCs = immature dendritic cells; IFN = interferon; MDMs = monocyte derived macrophages; PBMCs = peripheral blood mononuclear cells; SPTBN1 = spectrin beta nonerythrocyte 1,
5. The role of IL-27 in other viral infections
Following studies which demonstrated the anti-viral properties of IL-27 against HIV-1, IL-27 has also subsequently been shown to play various roles in immunity or pathogenesis in other viral infections. Early studies with IL-27 and influenza viruses demonstrated that in vitro infection of macrophage with influenza A virus up-regulate the p28 subunit of the IL-27 heterodimer [46]. More recently, IL-27 expression was also shown to be significantly up-regulated in PBMCs infected with influenza A virus and in the same study, circulating levels of IL-27 were also shown to be significantly elevated in a cohort of patients acutely infected with influenza A as compared to healthy controls [47]. In this latter study, the increased expression of IL-27 in patients with influenza was said to be triggered through protein kinase A and cAMP-response element binding protein signaling. Whilst IL-27 has been mostly demonstrated to mediate its effects on a number of immune cells, it has also been shown that IL-27 treatment can lead to rapid phosphorylation of STATs -1 and -3 in hepatocytes and human hepatoma cells [48] and mostly STAT-1 in hepatic stellate cells [49]. These data suggest that IL-27 may have a role in responding to viral infections of the liver, such as Hepatitis A, B or C. Using an HCV permissive cell line, Huh7.5, IL-27 in a dose dependent manner was shown to potently inhibit HCV infection along with activation of STAT-1 and -3 [50]. Maximal inhibition of HCV with IL-27 occurred with a dose of 1000 ng/ml which is tenfold higher than the dose required for the same maximal inhibitory response in HIV-1 infection. In hepatitis B infection (HBV), serum levels of IL-27 were significantly higher in HBV infected patients compared to uninfected controls and IL-27 was significantly elevated in patients with hepatocellular carcinoma or with acute HBV compared to chronic carriers of HBV [51]. Similar results have been confirmed in other studies [52, 53], although IL-6 was shown to be a better predictor of liver injury compared to IL-27 [52]. It has also been demonstrated that macrophages induced with IL-27 (I-Mac) are relatively resistant to HIV-2, simian immunodeficiency virus (SIV), influenza virus, HSV-2 and KSHV viral infections [17], although the mechanisms of the anti-viral effects of IL-27 in these settings remain unclear. One possible mechanism may be through the induction of microRNAs (miRNAs). IL-27 is able to up-regulate a number of novel miRNAs in macrophages that potentially target the open reading frame of a number of viruses including HSV-1, HSV-2 and KSHV [54]. Further functional experiments are required to see whether over-expressing these novel miRNAs can indeed protect macrophages from infection with these viruses.
6. Therapeutic potential in HIV-1 infection
Due to the general anti-inflammatory properties of IL-27, several groups have proposed that this may be a good therapeutic agent for various disease states, particularly autoimmune diseases such as systemic lupus erythematosus (SLE) [55] and multiple sclerosis [56]. Thus far, no clinical trials have been conducted with this cytokine. IL-27 is a promising therapeutic agent in HIV-1 infection because the anti-HIV effects have been demonstrated in multiple cells targeted by HIV-1 including PBMCs, CD4+ T cells, macrophages and dendritic cells. These in-vitro studies have suggested that the effective concentration required to suppress HIV-1 replication is in the order of 50-100 ng/ml. This is approximately 100-1000 times the levels of IL-27 found in plasma. The key question is whether these concentrations could be achieved in vivo and whether these in-vitro findings can be extended to in-vivo. Given the complicated roles IL-27 has in orchestrating both innate and adaptive immune responses, it is also not known what effect exogenous IL-27 will have on immune function. Trials evaluating the effect of IL-27 on simian immunodeficiency virus (SIV) infected monkeys are currently in advanced stages of planning and are expected to take place in the coming years. Trials utilizing these monkey models with IL-27 will hopefully give more precise information regarding the optimal dose, viral replication kinetics, effect in combination with HAART and possible adverse effects. As IL-27 also targets macrophages, it may prove to be useful to attack HIV-1 in this reservoir. As one can imagine, there is a long path ahead before IL-27 is actually used in the clinical setting for HIV-1 infected patients. Given that human recombinant IL-27 inhibits SIV infection in Rhesus Macaque macrophages (unpublished data) pre-clinical studies using the Rhesus Macaque model of immunodeficiency with SIV infection should be of value.
7. Conclusions
IL-27 is an important pleiotropic cytokine that modulates both the innate and adaptive immune responses. A number of in-vitro studies have shown that IL-27 can significantly block HIV-1 replication in PBMCs, CD4+ T cells, macrophages and dendritic cells. The mechanism whereby IL-27 inhibits HIV-1 infection in macrophages occurs through the down-regulation of SPTBN1, an essential host factor for HIV- infection. The concentration of IL-27 required to inhibit HIV-1 in-vitro is approximately 100 times the levels found in plasma. Testing of IL-27 in non-human primate models of HIV-1 infection will be needed before any human trials to work out important parameters such as the optimal dose and route of IL-27, kinetics of viral replication following administration and possible adverse effects. If these studies are successful, pre-clinical studies would be the next step to evaluate the effectiveness and potency of IL-27 as a therapeutic agent.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported [in part] by the National Institute of Allergy and Infectious Disease.
Biography
 Sanjay Swaminathan:
Sanjay Swaminathan:
Sanjay Swaminathan received his Bachelor of Medicine, Bachelor of Surgery (Honours) degree from Monash University (Melbourne, Australia) in 1998 and his Ph.D. from the University of New South Wales (Sydney, Australia) in 2011. After completing residency and specialty training in centers in Melbourne, Sydney and Adelaide, he obtained fellowships from both the Royal Australasian College of Physicians (FRACP) and the Royal College of Pathologists of Australasia (FRCPA) in 2008 in the fields of Clinical Immunology/Allergy and Immunopathology, respectively. Sanjay has previously worked as a Clinical Immunologist at Westmead Hospital in Sydney and also as a lecturer at the University of New South Wales in 2011. In the last two years, he has worked as a Post-doctoral Fellow at the Frederick National Laboratory for Cancer Research, where he continues to investigate mechanisms of IL-27 mediated inhibition of HIV-1 infection. His other research interests include understanding the role that microRNAs play in modulating key cytokines important in HIV-1 viral pathogenesis.
 Lue Dai:
Lue Dai:
Dr. Lue Dai received his Ph.D. degree in Immunology and Virology in 2010 from University of Massachusetts Medical School, where his research focused on cytokine manipulation by HIV viral proteins during infection. He then joined the Laboratory of Human Retrovirology at SAIC-Frederick, the Frederick National Laboratory for Cancer Research for post-doctoral training. His work has established an HIV-resistant macrophage subtype promoted by IL-27 and has elucidated the molecular mechanism of the HIV resistance, particularly the interplay between the cytokine, host factors, and HIV infection.
 H.Clifford Lane:
H.Clifford Lane:
H. Clifford Lane received his M.D. degree from the University of Michigan in 1976. He then completed an internship and residency at the University of Michigan Hospital, Ann Arbor, Michigan. In 1979, he came to the National Institutes of Health (NIH) as a clinical associate in the Laboratory of Immunoregulation (LIR) of the National Institute of Allergy and Infectious Diseases (NIAID). He is currently chief of the Clinical and Molecular Retrovirology Section of the LIR and the clinical director, director of the Division of Clinical Research and Deputy Director for Clinical Research and Special Projects of NIAID.
He became one of the first investigators to study immunopathogenic mechanisms of HIV disease, ultimately making a series of observations that helped establish the field of HIV immunopathogenesis. In the clinical arena, he has studied innovative approaches to therapy and has used experimental therapeutic interventions as a means of furthering our understanding of HIV pathogenesis. Among other things, he has investigated the strategies of syngeneic bone marrow transplantation, adoptive transfer of lymphocytes, and role of the cytokines alpha interferon and IL-2 in the treatment of patients with HIV infection.
 Tomozumi Imamichi:
Tomozumi Imamichi:
Dr. Tomozumi Imamichi obtained his Ph.D. degree in Immunology and Biochemistry from the Hokkaido University in Japan in 1991. After completing his post-doctoral training at the National Institute of Dental and Craniofacial Research, in 1995, he joined as a staff scientist at SAIC-Frederick at the Frederick National Laboratory for Cancer Research. He is currently head of the Laboratory of Human Retrovirology and has investigated mechanisms of HIV drug resistance and immunological regulation of virus (HIV-1, HIV-2, SIV, HSV and KSHV) infection and replication.
He discovered for the first time that an amino acid deletion in HIV reverse transcriptase (RT) is associated with resistance to multiple nucleotide RT inhibitors. Subsequently he identified a novel amino acid insertion in HIV protease also associated with multi-drug resistance. His group first identified IL-27 as an anti-HIV cytokine and discovered a total of six novel IL-27 inducible microRNAs that have potential anti-viral properties against HSV infection. His group has also defined a novel mechanism of innate immune activation via Ku70 that plays as a novel cytosolic DNA sensor protein. Currently his research interests are to innovate novel immune therapies (combining with or without anti-viral reagents) for infectious disease including HIV infection under collaboration with Dr. H. Clifford Lane in NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13:722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones LL, Vignali DA. Molecular interactions within the IL-6/IL-12 cytokine/receptor superfamily. Immunol Res. 2011;51:5–14. doi: 10.1007/s12026-011-8209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 4.Wirtz S, Becker C, Fantini MC, Nieuwenhuis EE, Tubbe I, Galle PR, et al. EBV-induced gene 3 transcription is induced by TLR signaling in primary dendritic cells via NF-kappa B activation. J Immunol. 2005;174:2814–2824. doi: 10.4049/jimmunol.174.5.2814. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Guan X, Ma X. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-gamma-mediated pathways. J Exp Med. 2007;204:141–152. doi: 10.1084/jem.20061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batten M, Ghilardi N. The biology and therapeutic potential of interleukin 27. J Mol Med (Berl) 2007;85:661–672. doi: 10.1007/s00109-007-0164-7. [DOI] [PubMed] [Google Scholar]
- 7.Lupardus PJ, Garcia KC. The structure of interleukin-23 reveals the molecular basis of p40 subunit sharing with interleukin-12. J Mol Biol. 2008;382:931–941. doi: 10.1016/j.jmb.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beyer BM, Ingram R, Ramanathan L, Reichert P, Le HV, Madison V, et al. Crystal structures of the pro-inflammatory cytokine interleukin-23 and its complex with a high-affinity neutralizing antibody. J Mol Biol. 2008;382:942–955. doi: 10.1016/j.jmb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Stumhofer JS, Tait ED, Quinn WJ, Hosken N, Spudy B, Goenka R, et al. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nature Immunology. 2010;11:1119–U1118. doi: 10.1038/ni.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 11.Garbers C, Spudy B, Aparicio-Siegmund S, Waetzig GH, Sommer J, Holscher C, et al. An Interleukin-6 Receptor-dependent Molecular Switch Mediates Signal Transduction of the IL-27 Cytokine Subunit p28 (IL-30) via a gp130 Protein Receptor Homodimer. J Biol Chem. 2013;288:4346–4354. doi: 10.1074/jbc.M112.432955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darnell JE, Jr., Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimura T, Takeda A, Hamano S, Miyazaki Y, Kinjyo I, Ishibashi T, et al. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J Immunol. 2006;177:5377–5385. doi: 10.4049/jimmunol.177.8.5377. [DOI] [PubMed] [Google Scholar]
- 14.Greenwell-Wild T, Vazquez N, Jin W, Rangel Z, Munson PJ, Wahl SM. Interleukin-27 inhibition of HIV-1 involves an intermediate induction of type I interferon. Blood. 2009;114:1864–1874. doi: 10.1182/blood-2009-03-211540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larousserie F, Charlot P, Bardel E, Froger J, Kastelein RA, Devergne O. Differential effects of IL-27 on human B cell subsets. J Immunol. 2006;176:5890–5897. doi: 10.4049/jimmunol.176.10.5890. [DOI] [PubMed] [Google Scholar]
- 16.Guzzo C, Che Mat NF, Gee K. Interleukin-27 induces a STAT1/3- and NF-kappaB- dependent proinflammatory cytokine profile in human monocytes. J Biol Chem. 2010;285:24404–24411. doi: 10.1074/jbc.M110.112599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai L, Lidie KB, Chen Q, Adelsberger JW, Zheng X, Huang D, et al. IL-27 inhibits HIV-1 infection in human macrophages by down-regulating host factor SPTBN1 during monocyte to macrophage differentiation. J Exp Med. 2013;11:517–534. doi: 10.1084/jem.20120572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Q, Swaminathan S, Yang D, Dai L, Sui H, Yang J, et al. Interleukin-27 is a potent inhibitor of cis HIV-1 replication in Monocyte-derived Dendritic Cells via a Type I Interferon-independent pathway. PLoS One. 2013;8:e59194. doi: 10.1371/journal.pone.0059194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, et al. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 20.Wojno ED, Hunter CA. New directions in the basic and translational biology of interleukin-27. Trends Immunol. 2012;33:91–97. doi: 10.1016/j.it.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 22.Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun J, Dodd H, Moser EK, Sharma R, Braciale TJ. CD4+ T cell help and innate-derived IL-27 induce Blimp-1-dependent IL-10 production by antiviral CTLs. Nat Immunol. 2011;12:327–334. doi: 10.1038/ni.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 26.Batten M, Ramamoorthi N, Kljavin NM, Ma CS, Cox JH, Dengler HS, et al. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. J Exp Med. 2010;207:2895–2906. doi: 10.1084/jem.20100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 28.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 29.Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang R, Murillo FM, Cui H, Blosser R, Uematsu S, Takeda K, et al. Papillomavirus-like particles stimulate murine bone marrow-derived dendritic cells to produce alpha interferon and Th1 immune responses via MyD88. J Virol. 2004;78:11152–11160. doi: 10.1128/JVI.78.20.11152-11160.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Pineres AJ, Hildesheim A, Trivett M, Williams M, Wu L, Kewalramani VN, et al. Role of DC-SIGN in the activation of dendritic cells by HPV-16 L1 virus-like particle vaccine. Eur J Immunol. 2006;36:437–445. doi: 10.1002/eji.200535068. [DOI] [PubMed] [Google Scholar]
- 32.Pinto LA, Viscidi R, Harro CD, Kemp TJ, Garcia-Pineres AJ, Trivett M, et al. Cellular immune responses to HPV-18, -31, and -53 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. Virology. 2006;353:451–462. doi: 10.1016/j.virol.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Fakruddin JM, Lempicki RA, Gorelick RJ, Yang J, Adelsberger JW, Garcia-Pineres AJ, et al. Noninfectious papilloma virus-like particles inhibit HIV-1 replication: implications for immune control of HIV-1 infection by IL-27. Blood. 2007;109:1841–1849. doi: 10.1182/blood-2006-02-001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imamichi T, Yang J, Huang DW, Brann TW, Fullmer BA, Adelsberger JW, et al. IL-27, a novel anti-HIV cytokine, activates multiple interferon-inducible genes in macrophages. AIDS. 2008;22:39–45. doi: 10.1097/QAD.0b013e3282f3356c. [DOI] [PubMed] [Google Scholar]
- 35.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances transinfection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 36.Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- 37.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 38.Guzzo C, Jung M, Graveline A, Banfield BW, Gee K. IL-27 increases BST-2 expression in human monocytes and T cells independently of type I IFN. Sci Rep. 2012;2:974. doi: 10.1038/srep00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, et al. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brass AL, Dykxhoorn DM, Benita Y, Yan N, Engelman A, Xavier RJ, et al. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319:921–926. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- 41.Guzzo C, Hopman WM, Che Mat NF, Wobeser W, Gee K. IL-27-Induced Gene Expression Is Downregulated in HIV-Infected Subjects. PLoS One. 2012;7:e45706. doi: 10.1371/journal.pone.0045706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009;183:2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwon DS, Angin M, Hongo T, Law KM, Johnson J, Porichis F, et al. CD4+ CD25+ regulatory T cells impair HIV-1-specific CD4 T cell responses by upregulating interleukin-10 production in monocytes. J Virol. 2012;86:6586–6594. doi: 10.1128/JVI.06251-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guzzo C, Hopman WM, Che Mat NF, Wobeser W, Gee K. Impact of HIV infection, highly active antiretroviral therapy, and hepatitis C coinfection on serum interleukin-27. AIDS. 2010;24:1371–1374. doi: 10.1097/QAD.0b013e3283391d2b. [DOI] [PubMed] [Google Scholar]
- 45.He L, Zhao J, Gan Y, Chen L, He M. Upregulation of interleukin-27 expression is correlated with higher CD4+ T cell counts in treatment of naive human immunodeficiency virus-infected Chinese. Journal of AIDS and HIV Research. 2011;3:6–10. [Google Scholar]
- 46.Pirhonen J, Siren J, Julkunen I, Matikainen S. IFN-alpha regulates Toll-like receptor-mediated IL-27 gene expression in human macrophages. J Leukoc Biol. 2007;82:1185–1192. doi: 10.1189/jlb.0307157. [DOI] [PubMed] [Google Scholar]
- 47.Liu L, Cao Z, Chen J, Li R, Cao Y, Zhu C, et al. Influenza A virus induces interleukin-27 through cyclooxygenase-2 and protein kinase A signaling. J Biol Chem. 2012;287:11899–11910. doi: 10.1074/jbc.M111.308064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bender H, Wiesinger MY, Nordhoff C, Schoenherr C, Haan C, Ludwig S, et al. Interleukin-27 displays interferon-gamma-like functions in human hepatoma cells and hepatocytes. Hepatology. 2009;50:585–591. doi: 10.1002/hep.22988. [DOI] [PubMed] [Google Scholar]
- 49.Schoenherr C, Weiskirchen R, Haan S. Interleukin-27 acts on hepatic stellate cells and induces signal transducer and activator of transcription 1-dependent responses. Cell Commun Signal. 2010;8:19. doi: 10.1186/1478-811X-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frank AC, Zhang X, Katsounas A, Bharucha JP, Kottilil S, Imamichi T. Interleukin-27, an anti-HIV-1 cytokine, inhibits replication of hepatitis C virus. J Interferon Cytokine Res. 2010;30:427–431. doi: 10.1089/jir.2009.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu C, Zhang R, Liu L, Rasool ST, Mu Y, Sun W, et al. Hepatitis B virus enhances interleukin-27 expression both in vivo and in vitro. Clin Immunol. 2009;131:92–97. doi: 10.1016/j.clim.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 52.Kao JT, Lai HC, Tsai SM, Lin PC, Chuang PH, Yu CJ, et al. Rather than interleukin-27, interleukin-6 expresses positive correlation with liver severity in naive hepatitis B infection patients. Liver Int. 2012;32:928–936. doi: 10.1111/j.1478-3231.2011.02742.x. [DOI] [PubMed] [Google Scholar]
- 53.Wang HL, Zhang HY, Zhai ZL, Zhou X. The correlation between hepatitis B virus infection and IL-27. Biomed Mater Eng. 2012;22:187–193. doi: 10.3233/BME-2012-0706. [DOI] [PubMed] [Google Scholar]
- 54.Swaminathan S, Hu X, Zheng X, Kriga Y, Shetty J, Zhao Y, et al. Interleukin-27 treated human macrophages induce the expression of novel microRNAs which may mediate anti-viral properties. Biochem Biophys Res Commun. 2013 doi: 10.1016/j.bbrc.2013.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pan HF, Tao JH, Ye DQ. Therapeutic potential of IL-27 in systemic lupus erythematosus. Expert Opin Ther Targets. 2010;14:479–484. doi: 10.1517/14728221003769911. [DOI] [PubMed] [Google Scholar]
- 56.Fitzgerald DC, Rostami A. Therapeutic potential of IL-27 in multiple sclerosis? Expert Opin Biol Ther. 2009;9:149–160. doi: 10.1517/14712590802646936. [DOI] [PubMed] [Google Scholar]