Abstract
Explicit and implicit learning and memory networks exist where each network can facilitate or inhibit memory. Clinical evidence suggests that implicit networks are relatively preserved after traumatic brain injury (TBI). Non-spatial pre-training (NSPT) in the Morris water maze (MWM) provides the necessary behavioral components to complete the task, while limiting the formation of spatial maps. Our study utilized NSPT in the MWM to assess implicit and explicit learning and memory system deficits in the controlled cortical impact (CCI) model of TBI. 76 adult male Sprague-Dawley rats were divided: CCI vs. sham surgery, NSPT vs. No-NSPT, and cued vs. non-cued groups. NSPT occurred for 4d prior to surgery (dynamic hidden platform location, extra-maze cues covered, static pool entry point). Acquisition (d14–18), Probe/Visible Platform (d19), and Reversal (d20–21) trials were conducted with or without extra-maze cues. Novel time allocation and search strategy selection metrics were utilized. Results indicated implicit and explicit learning/memory networks are distinguishable in the MWM. In the cued condition, NSPT reduced thigmotaxis, improved place learning, and largely eliminated the apparent injury-induced deficits typically observed between untrained CCI and sham rats. However, among NSPT groups, incorporation of cues into search strategy selection for CCI rats was relatively impaired compared to shams. Non-cued condition performance showed sham/NSPT and CCI/NSPT rats perform similarly, suggesting implicit memory networks are largely intact 2 weeks after CCI. Place learning differences between CCI/NSPT and sham/NSPT rats more accurately reflect spatial deficits in our CCI model compared to untrained controls. These data suggest NSPT as a clinically relevant construct for evaluating potential neurorestorative and neuroprotective therapies. These findings also support development of non-spatial cognitive training paradigms for evaluating rehabilitation relevant combination therapies.
Keywords: traumatic brain injury, controlled cortical impact, implicit memory, water maze, spatial memory, thigmotaxis
1. INTRODUCTION
The Morris water maze (MWM) is a commonly used task for assessing spatial navigation and place learning (D’Hooge & De Deyn, 2001; Vorhees & Williams, 2006). Behavioral factors such as suppression of thigmotaxis and recognition of the hidden platform as an escape are essential to MWM performance, and can be impaired after injury (Cain et al., 2006). These deficits can affect searching behavior, which limits the acquisition of navigation and other skills required to effectively solve the task. The spatial mapping component of the MWM is largely hippocampus-dependent (Morris, 1989; Wolff et al., 2008) and reliant on explicit awareness and incorporation of extra-maze cues in locating the platform, while other implicitly learned, non-specific task components are hippocampus-independent (Cain et al., 2006).
Non-spatial pre-training (NSPT) provides exposure to implicitly learned task components, important for navigation and non-spatial search strategy development. NSPT can suppress thigmotaxis and facilitate acquisition of behaviors associated with successful task completion (Hoh & Cain, 1997). Furthermore, Packard and McGaugh (1996) showed that place- and response-learning can occur concurrently or independently of one another in rats. We hypothesized that NSPT may eliminate or reduce the requirement of learning non-spatial strategies for successful post-injury navigational behavior in the MWM, consistent with previous studies demonstrating that pre-training can alleviate deficits resulting from sex differences (Perrot-Sinal et al., 1996; Beiko et al., 2004), age (Carrasco et al., 2006), lesions (Lukoyanov et al., 2005; Cain et al., 2006), or drug administration (Saucier et al., 1996; Morris, 1989; Dyer & Cain, 2007).
The MWM is used in multiple models of experimental traumatic brain injury (TBI) to demonstrate cognitive deficits (e.g. Hamm et al., 1996; Darrah et al., 2011); and increased platform latencies are typically considered the result of hippocampus-mediated learning and memory deficits. After TBI, rats have difficulty mastering the spatial components of the task and can have a reduced capability to learn the general strategies needed to locate the platform (Saucier et al., 1996). Notably, persistent thigmotaxis is indicative of prominent, long-lasting cognitive impairments post-TBI (Hamm et al., 1992; Bramlett et al., 1997). Unlike lesion studies, the controlled cortical impact (CCI) injury model of TBI results in damage to structures important for both implicit and explicit components of effective spatial navigation in the MWM, including the hippocampus, thalamus, striatum, and amygdala (Packard & Knowlton, 2002; Mair et al., 2003; Wolff et al., 2008; Packard, 2009). Implicit and explicit learning and memory networks have not been studied in experimental TBI. However, some clinical literature suggests implicit networks are less affected than explicit learning and memory networks by TBI (Vakil, 2005; Schmitter-Edgecombe, 2006). Implicit learning strategies are used during cognitive rehabilitation to acquire skills and information relevant to everyday functioning for populations, including those with dementia, amnesia, and TBI (Rothi et al., 2009; Schmitter-Edgecombe, 2006; Steenbergen, et al., 2010; Vakil, 2005). Further, explicit feedback regarding strategies for task completion can be disruptive during activities utilizing implicit learning and/or memory strategies (Jacoby, 1991; Schmitter-Edgecombe, 2006).
Given the lack of knowledge regarding how learning and memory networks are affected by experimental TBI, we used MWM performance to examine these networks in the CCI model. We hypothesized that implicit memory would be relatively preserved and NSPT would facilitate cognitive performance in the MWM where both explicit and implicit memory and learning are required. Also, we hypothesized that the presentation of explicit feedback (i.e. extra-maze cues) to the CCI group receiving NSPT would worsen navigational behavior during specific task conditions, where cognitive flexibility and pliancy are needed to adapt to new task rules (e.g., VP, and reversal trials). We postulated that the VP represents an egocentric cue by which the rat can formulate spatial search strategies to more effectively solve the task. Finally, we hypothesized that elevated platform latencies and reductions in spatial strategy selection for CCI/NSPT rats, compared to Sham/NSPT rats, are more representative of spatial navigation deficits in our CCI model compared to traditional testing, which has implications for interpreting MWM results involving pre-clinical assessment of new treatments and interventions. The work may serve as a starting point to develop experimental cognitive training paradigms that parallel clinical practice and can be evaluated in combination with other rehabilitation relevant treatments to lay the ground work for effective rehabilitation focused clinical trials in TBI.
2. MATERIALS AND METHODS
2.1. Subjects and Experimental Groups
2.1.1. Animals
Animal procedures were carried out with the approval of the University of Pittsburgh Institutional Care and Use Committee. Seventy-six young adult male Sprague-dawley rats with an average weight (343.44+/−3.20g) at injury were used. Rats were housed in pairs in suspended wire mesh cages with ad libitum access to food and water, constant ambient temperature (21±1°C), and 12h light cycle (7:00 a.m. to 7:00 p.m.) Behavioral tests were performed by experimenters blinded to treatment groups.
2.1.2. Experimental Groups
Rats were divided into 8 groups according to injury status (CCI vs. sham), exposure to NSPT (NSPT vs. No-NSPT) in the MWM, and use of extra-maze spatial cues (Cued vs. Non-Cued) during acquisition testing (d14–d18 post injury) with a stationary hidden platform. Probe (d19), followed by visible platform (d19), and reversal trials (d20–21). All MWM testing conditions were performed with each group. Sham groups had 8 rats/group, and injured groups had 11 rats/group.
2.1.3. Controlled Cortical Impact
The CCI injury device is described in detail by Dixon et al. (1991) and was utilized previously by our group (Wagner et al., 2004, 2007a, b; Darrah et al., 2011). Rats underwent unilateral parasagittal CCI or sham surgery. A craniectomy (~6mm) was made between bregma and lambda in the right hemisphere between the central suture and the coronal ridge. The cortical injury was delivered at ~18° angle, such that the impactor was perpendicular with the dural plane. The impact was delivered to a depth of 2.8mm at 4.0m/sec. Shams underwent all procedures except the impact. Post-operative flexion and righting reflexes were monitored as previously described (Dixon et al., 1991; Wagner et al., 2002, 2007a,b).
2.2. Motor Testing
Beam balance and beam walking tasks were completed to assess gross and fine motor function, respectively, as previously described (Dixon et al., 1991; Wagner et al., 2002, 2007a,b). Rats were pre-trained on both beam tasks on the day prior to surgery, and performance was pre-assessed on the day of surgery to determine a baseline score. Rats completed 3 trials daily for both the beam balance and beam walk on d1–6 post surgery. For the beam balance task, the duration of time spent balancing on a narrow beam (1.5 cm width) elevated (90cm) from the ground was assessed (up to 60s per trial). For beam walk trials, latency to traverse a narrow beam (2.5×100-cm) and reach the goal box was recorded. Beam walk trial time began when an adverse stimulus (white noise and bright light) was presented, and stopped upon entry into the goal box. If the rat fell from the beam prior to reaching the goal box, it was assigned a latency of 60s. The average time across trials for each rat was calculated.
2.3. The Morris Water Maze
2.3.1. Apparatus and trial procedures
The water maze consisted of a blue pool measuring 180cm in diameter and 60cm, and an opaque escape platform measuring 10cm in diameter and 26 cm high. The pool was filled with water (26±1° C) to a height of 28cm such that the hidden platform was submerged 2cm below the surface of the water. The pool was located in a room (2.5 × 2.5m) typically with large visual cues (black geometric shapes) fixed to the white walls. For each trial, rats were introduced into the pool hindquarters first, placing the forelimbs on the wall. A 4min inter-trial interval was observed, during which the rats were placed in a heated incubatory box. All trials, with the exception of the probe trial, had a maximum duration of 120s, and a 30s platform habituation. If the rat did not find the platform within the allotted 120s period, it was guided there by hand. During non-cued trials (NSPT, acquisition, probe, VP, and reversal) covers were used to obscure the prominent features of the testing room. White poster board was attached to white walls over the black geometric shapes, a white sheet was draped over the spigot and hose used to fill the pool, and a white curtain covered the door. Efforts were taken to ensure that measures effectively obscured any prominent room features, taking care to eliminate shadows within the testing room. The experimenter collected data from a room adjacent to the testing room, and ambient noise was kept to a minimum.
2.3.2. Non-spatial Pre-training
NSPT provided to CCI and sham rats followed the same general format as described by Cain et al. (2006); consisting of 3 trials/d over the 4 days prior to surgery. During NSPT, all distal spatial cues were covered from view to provide a homogenous environment, and the hidden platform was moved to a different quadrant for each trial. A static entry point was used for NSPT trials. No-NSPT rats were handled daily during the same period prior to surgery as NSPT rats to collect daily weights.
2.3.3. Acquisition Trials
Acquisition trials assessing successful navigation to the hidden platform occurred on d14–18 post surgery, and were completed either with or without extra-maze spatial cues. Four daily acquisition trials were completed, where rats searched for a platform submerged 2cm below the water surface (SW quadrant). Animals were placed into the pool at one of the cardinal positions (N,S,E,W) in a pseudo-randomized sequence. Platform latencies for each trial were recorded and averaged for each acquisition trial day.
2.3.4. Probe, Visible Platform, and Reversal trials
On d19 post-surgery, probe and visible platform (VP) trials were completed. During the probe trial the escape platform was removed, and rats were introduced to the pool at the north entry point. Rats were allowed to navigate the pool for 30 seconds. After the probe trial was completed, the platform was reintroduced into the pool for VP trials. While VP can be considered a control for sensorimotor and motivational deficits, we postulate that the VP represents an egocentric cue by which rats can formulate spatial search strategies to more effectively solve the task. The platform was located in the SW quadrant and raised 2cm out of the water and made visible by wrapping white tape around its edges. Four trials (one entry at each of the cardinal directions) were completed. On d20 and 21, hidden platform reversal (R1 and R2) trials were completed. No new acquisition trials or training was provided, as the goal of reversal trials in our study design was to measure the ability of the animal to adapt to novel testing conditions (i.e. pliancy) and learn new spatial information about the platform location. These trials followed the same procedures as acquisition trials d14–18 post-surgery, except the submerged platform was located in the NW (reversal) quadrant.
2.3.5. Analysis
In addition to recording latencies to find the platform in each of the testing conditions, MWM trials were recorded using a SMART video tracking system (San Diego Instruments Inc.), and multiple data endpoints were extracted for analysis. In addition to the classical quadrant zones (NW, NE, SW, SE, figure 1a), each comprising 25% of total pool area, other zone maps (see section 2.3.6.) were introduced. Time allocation (TA) in relevant zones was measured during acquisition, probe, VP, and reversal trials.
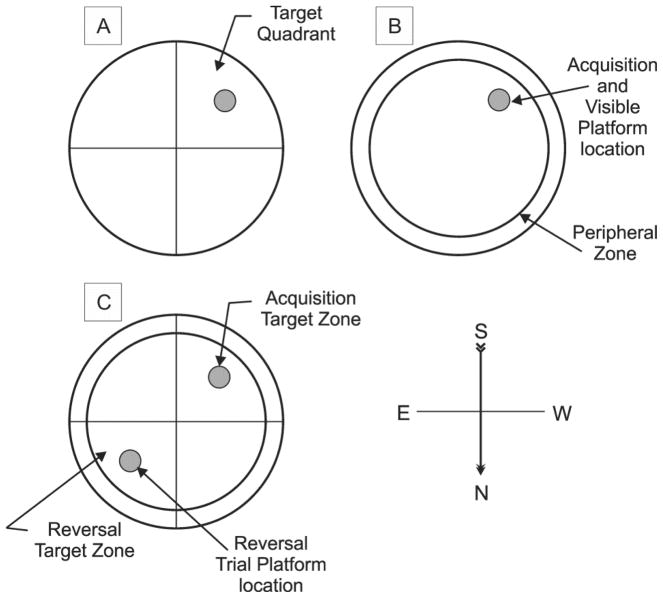
Figure 1.
Schematic for the Morris water maze a) acquisition trials platform location and peripheral zone representing thigmotaxis b) target quadrant for probe trial c) target zone for acquisition/probe trial and platform location and associated target zone for reversal trial.
2.3.6. Zone Definitions
To assess thigmotaxis quantitatively during MWM trials, TA in the outermost edge of the pool (peripheral zone [PZ]; 29% of total pool area) was recorded. No portion of the escape platform was located in the PZ during any trial condition (see figure 1b). To preclude the possibility that thigmotaxic swimming patterns contribute to target quadrant TA, time spent in the PZ was removed from the acquisition and reversal target quadrants to create the acquisition target zone (TZ) and reversal TZ, respectively. Each target zone comprised 17.75% of total pool area, and contained the hidden platform location as appropriate for each testing day. This measure was used in place of target quadrant measures (see figure 1c). TZ, and PZ TA were measured during acquisition, probe, VP, and reversal trials.
2.3.7. Swim Strategy Assignments
A qualitative analysis of swim strategy was completed for D14 and D18 of acquisition, VP, Probe, and both reversal trials (1,434 total trials). The SMART video-tracking software provides a static image of the swim path taken for each trial, which were used to assign each swim path a category. An investigator blinded to rat treatment group categorized the predominant searching strategy exhibited during each trial based on criteria adapted from previously used classification schemes (Graziano et al., 2003; Janus, 2004; Brody & Holtzman, 2006). Swim strategies were divided into 3 primary categories characterized as spatial, nonspatial, or thigmotaxic. These primary categories were further subdivided into 7 strategies that more precisely defined searching behavior (Graziano et al., 2003; Brody & Holtzman, 2006). While assignments are useful for comparison among injury and NSPT groups, the swim strategy classifications are simply observationally consistent with categories identified in other studies, and strategy utilization was not specifically validated with confirmation testing paradigms. Examples of the 7 categories, with representative strategies obtained from this study, are provided in Figure 2. If a rat switched strategies during a trial, the predominant search strategy was assigned (Graziano et al., 2003). For analysis, the percentage of runs for each of the 7 strategy types was calculated, and the 3 primary strategies graphed.
Figure 2.
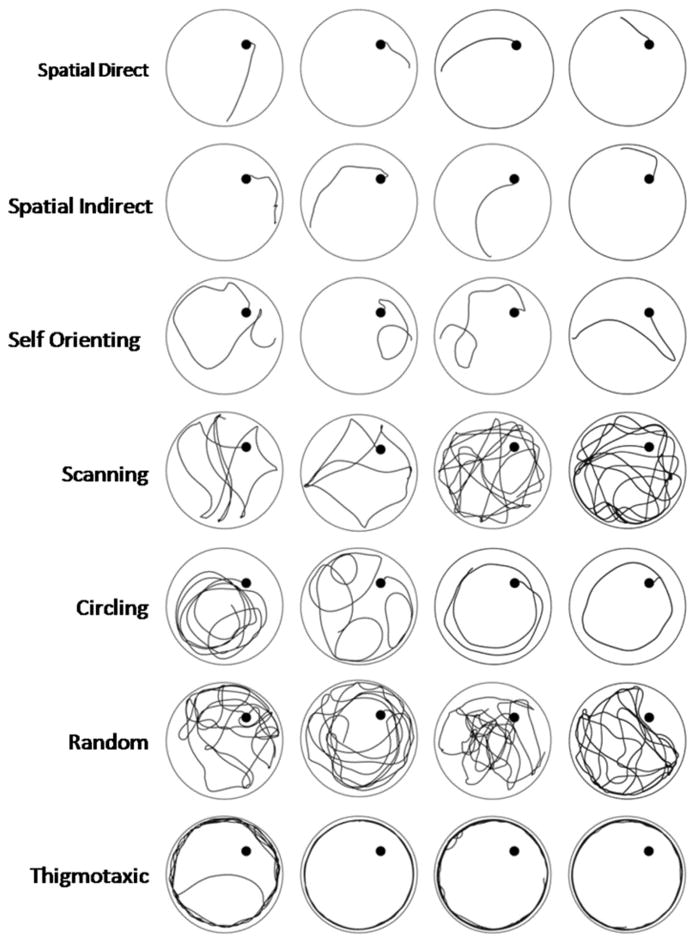
Example swimming strategies from representative rats evaluated in our cohort. The Spatial Swimming category includes spatial direct, spatial indirect, and self-orienting. The Non-spatial category includes scanning, circling, and random strategies. Thigmotaxic swimming was qualified as its own category, as it represents a reflex response. Each run was classified according to the predominant behavior observed during the course of the trial.
Swimming strategies defined as Spatial included: spatial direct (swimming straight to the platform after entry into the pool), spatial indirect (swimming towards the platform while making adjustments to their swimming path) and self-orienting (includes both reorienting after missing the platform and the orientation upon entry into the pool prior to swimming to the platform). The three non-spatial swim strategies included scanning (systematically searching the pool), random (searching the entire pool without a pattern or preference to a specific area) and circling (completing repeated circles at a fixed distance from the pool wall within the zone that the platform is located). Thigmotaxis was defined as swimming in repeated loops at the periphery of the pool while keeping continuous contact with the pool wall. This measure offers a qualitative counterpart to PZ TA (Graziano et al., 2003).
2.4. Histological Characterization
2.4.1. Quantification of Hippocampal Neurons
Twenty-one days after CCI or sham surgery, rats were perfused transcardially under deep anesthesia with pentobarbital (100 mg/kg, i.p.), using 200 ml heparinized 0.1 M phosphate buffered saline (pH 7.4) and 300 mL 10% buffered formalin. Brains were extracted, post-fixed in 10% buffered formalin for at least 1 week, dehydrated with alcohols, and embedded in paraffin. Seven-micrometer thick coronal sections were cut at 0.5 mm intervals through the contusion on a rotary microtome and mounted on glass microscope slides. After drying overnight, the sections were deparaffinized in xylenes, rehydrated, and stained with Cresyl violet. The optical fractionator method in conjunction with a computerized stereology system (Stereologer, Stereology Resource Center, Chester, MD) was used to quantify the number of neurons in the CA1, CA3, and dentate gyrus (DG) sub-regions of hippocampus located under the lesion volume, as detailed previously (Mouton, 2011). To generate an unbiased sample from a total of 12 sections per animal, every fourth section (28uM distance between selected sections) was sampled in a systematic fashion from this total number of sections containing the CA1, CA3, and DG sub regions of hippocampus and underlying the contusion. We counted 15–35 ROIs for CA1, 10–20 ROIs for CA3, and 45–70 ROIs for DG. The reference space was outlined at low power (4X), and neurons with a neuronal phenotype, including a clearly defined cytoplasm and a centrally located nucleolus, were counted at high magnification (60X). Using the dissector principle, thin focal-plane optical scanning was carried out with a virtual 3-D dissection (height=7uM, area=2400uM2) through the section thickness (Mouton, 2011). A guard volume of 3uM was used to avoid incorporating artifacts at the tissue surface. Neurons intersecting the unbiased counting frame without touching the “forbidden lines” were counted. Sampling was continued to a coefficient of error (CE) less than 0.10 (CE<10%). According to the optical fractionator method, the total number of neurons in CA1, CA3, and DG sub-regions were calculated using the following sampling fraction (1) section sampling fraction (1/2), area sampling fraction (0.96), and thickness sampling fraction (0.78) (Mouton, 2011). All data were reported as the percent of total neurons in the ipsilateral (injured) CA1, CA3 and DG regions relative to each contralateral (uninjured) hippocampal region.
2.4.2. Cortical Lesion Volume
Residual tissue volume (mm3) was calculated by outlining the hemispheric tissue for sections taken at 1 mm intervals (MCID, Imaging Research) through the contusion on a rotary microtome. The residual area of the contralateral hemisphere and damaged ipsilateral hemisphere was determined after segmenting each slice along the anatomical midline. The area of each hemisphere was then measured for each section. The residual tissue volume (mm3) in the ipsilateral and contralateral hemispheres was determined by taking the sum of the remaining tissue areas for each hemisphere from each section and multiplying by the distance between sections (1mm). Percent of tissue remaining on the ipsilateral hemisphere was calculated by dividing the tissue volume measured in ipsilateral hemisphere tissue by the tissue volume remaining in the contralateral hemisphere i.e. (ipsilateral/contralateral)×100). This method eliminates the potential bias of shrinkage or swelling caused by histological techniques such as embedding or fixation.
2.5. Statistical Analysis
Statistical analyses were performed using SAS 9.2 and SPSS for windows 19.0. Exploratory data analysis was performed to examine data distributions and check for potential data errors. The normality assumption for each dependent variable was checked using the Kolmogorov-Smirnov test, and the natural log transformation was used when this assumption was violated. Numerical summaries including mean, standard deviations, and medians were computed for all latency and zone data. Data are presented as mean+/−SEM (standard error of the mean) and as percentages of trial time allocation, or TA.
We examined the effect of pre-training, injury status, and presence of extra-maze cues on the latency to find the platform and time spent in the PZ and TZ using mixed effects modeling techniques. The correlation and covariance structures were explored and accounted for in our mixed effect models. Mixed effects regression was used for repeated measures analysis, as there was often deviation from the compound symmetry assumption, precluding the use of an analysis of variance (ANOVA) approach for repeated measures data. Appropriate contrasts were set for specific group comparisons, with Tukey’s adjustment for multiple comparisons as appropriate. The latency to traverse or balance on the beam (motor assessments) and to find the platform on any specific day of MWM testing (including VP trials) was evaluated using the general linear model, with Tukey’s adjustment for multiple comparisons. Improvement during MWM reversal trials was compared between groups by assessing the difference of platform latency or time spent in zone between the two reversal days using the general linear model approach. Main Effects for each of the quantitative parameters noted above is provided in Table 1. Qualitative search strategy classification was analyzed using estimating equations (GEE) for between-group and within-group comparisons.
Table 1.
Main Effects for Quantitative MWM Performance Metrics
| Main Effects | Group Effect | Time Effect | Group*Time Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Trial Type | DF | F-value | P-value | DF | F-value | P-value | DF | F-value | P-value | |
| Acquisition Trials
| ||||||||||
| Latency | Cued Condition | 3,34 | 3.63 | 0.023 | 1,148 | 39.25 | <0.001 | 3,148 | 3.65 | 0.014 |
| No Cues Condition | 3,34 | 10.16 | <0.001 | 1,151 | 22.05 | <0.001 | - | - | - | |
| NSPT Condition | 3,34 | 6.48 | 0.001 | 1,151 | 22.08 | <0.001 | - | - | - | |
| Peripheral Zone | Cued Condition | 3,34 | 21.70 | <0.001 | 1,145 | 19.69 | <0.001 | - | - | - |
| No Cues Condition | 3,34 | 8.87 | <0.001 | 1,148 | 23.80 | <0.001 | - | - | - | |
| NSPT Condition | 3,34 | 1.69 | 0.188 | 1,142 | 18.56 | <0.001 | - | - | - | |
|
| ||||||||||
| Probe Trials
| ||||||||||
| Target Zone | Cued Condition | 3,34 | 7.29 | 0.001 | -- | --- | --- | -- | --- | --- |
| No Cues Condition | 3,32 | 1.87 | 0.155 | -- | --- | --- | -- | --- | --- | |
| NSPT Condition | 3,33 | 4.47 | 0.010 | -- | --- | --- | -- | --- | --- | |
| Peripheral Zone | Cued Condition | 3,33 | 6.00 | 0.002 | -- | --- | --- | -- | --- | --- |
| No Cues Condition | 3,31 | 1.68 | 0.191 | -- | --- | --- | -- | --- | --- | |
| NSPT Condition | 3,32 | 0.97 | 0.418 | -- | --- | --- | -- | --- | --- | |
|
| ||||||||||
| Visible Platform Trials
| ||||||||||
| Latency | Cued Condition | 3,34 | 11.06 | <0.001 | -- | --- | --- | -- | --- | --- |
| No Cues Condition | 3,34 | 6.83 | 0.001 | -- | --- | --- | -- | --- | --- | |
| NSPT Condition | 3,34 | 5.11 | 0.005 | -- | --- | --- | -- | --- | --- | |
| Peripheral Zone | Cued Condition | 3,33 | 6.62 | 0.001 | -- | --- | --- | -- | --- | --- |
| No Cues Condition | 3,32 | 7.54 | 0.001 | -- | --- | --- | -- | --- | --- | |
| NSPT Condition | 3,32 | 0.64 | 0.596 | -- | --- | --- | -- | --- | --- | |
|
| ||||||||||
| Reversal Trials
| ||||||||||
| Latency | Cued Condition | 3,34 | 8.98 | <0.001 | -- | --- | --- | -- | --- | --- |
| No Cues Condition | 3,34 | 6.83 | 0.001 | -- | --- | --- | -- | --- | --- | |
| NSPT Condition | 3,34 | 2.46 | 0.079 | -- | --- | --- | -- | --- | --- | |
| Peripheral Zone | Cued Condition | 3,34 | 7.65 | <0.001 | -- | --- | --- | -- | --- | --- |
| No Cues Condition | 3,34 | 9.14 | <0.001 | -- | --- | --- | -- | --- | --- | |
| NSPT Condition | 3,34 | 4.00 | 0.015 | -- | --- | --- | -- | --- | --- | |
Mean histological comparisons (contusion volume and hippocampal cell survival) were made between sham-surgery and CCI groups using Student’s t-test assuming equal variances Pearson’s correlation coefficients were used to explore relationships between platform latencies, TZ and PZ TA for rats in the cued condition and hippocampal cell survival within CCI groups. Histological assessments of rats with behavioral assessments in the non-cued condition were excluded from this analysis based on the assumption that behavioral performance in this condition represents a hippocampus-independent task.
3. RESULTS
3.1. Effects of CCI and NSPT on Skilled Motor Tasks
We found a significant effect of group, trial day, and group*day interaction on beam balance performance (p<0.001, all comparisons). Post-hoc analysis revealed that CCI/NSPT rats recovered more quickly than CCI/No-NSPT rats over time (p<0.001). Specifically, the CCI/NSPT rats returned to baseline by d5, while the CCI/No-NSPT rats did not return to baseline until d6. There were no differences noted between sham groups (figure 3a). There was a significant effect of injury, testing day, and a group*day interaction on beam walking latencies (p<0.001 all comparisons). Both CCI groups exhibited longer beam walking latencies compared to their respective shams (p<0.001). CCI group performance improved over days, but latencies were higher than sham groups on d6 of testing (p=0.001) (figure 3b).
Figure 3.
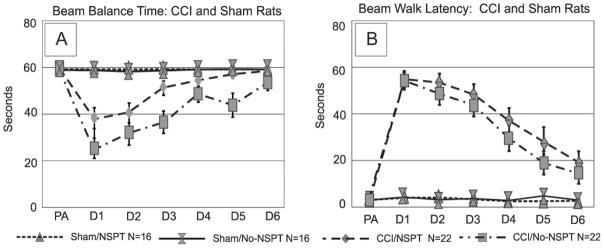
a) Beam Balance Time: Pre-assessment and post-surgical testing days 1-6. CCI/NSPT rats had significantly longer beam balance times compared to CCI/No-NSPT rats on days 3 and 5 days post injury (p<0.003 all comparisons). b) Beam Walk Time: Pre-assessment and post-surgical testing days 1–6. There were no differences between CCI or sham group performances based on NSPT status. (PA=pre-assessment, CCI=controlled cortical impact, NSPT=non-spatial pre-training).
3.2. NSPT, injury status, and extra-maze cues in navigational behavior: Acquisition Trials
3.2.1. Swimming Speed
Consistent with motor testing, NSPT did not have a significant effect on swimming speeds. As such, groups were pooled (CCI vs. sham) for further analysis. Injury status did not affect swim speed on d14 or d18 of MWM testing, suggesting that there were no motivational differences or motor deficits affecting other MWM data endpoints.
3.2.2. Acquisition Trials Platform Latencies and Peripheral Zone TA
3.2.2.1 Cued Condition
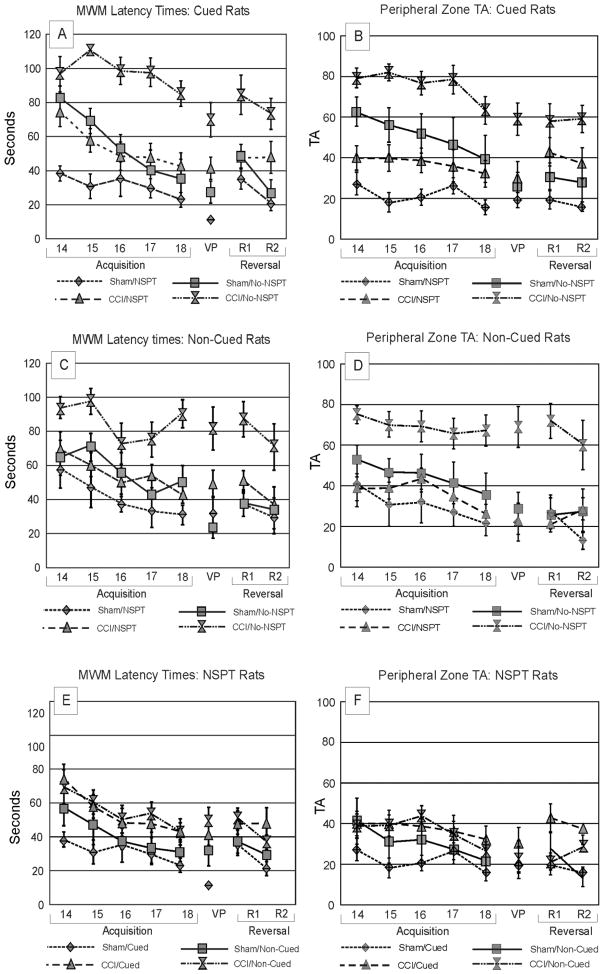
We found a significant main effect of group (p=0.023) and trial day (p<0.001) and a group*day interaction (p=0.014) on latency to find the hidden platform. The CCI/NSPT and sham/No-NSPT groups had significantly larger improvements in latencies across testing days compared to CCI/No-NSPT (p<0.001) and the sham/NSPT (p=0.002) groups, respectively. There was a difference between CCI/NSPT and sham/NSPT rats across days (p=0.026), which likely represents spatial learning deficits in the MWM associated with CCI. No differences were observed between CCI/NSPT and sham/No-NSPT groups across testing days (figure 4a).
Figure 4.
a) MWM Latencies; Cued Rats. Acquisition platform latencies were significantly higher for CCI/No-NSPT versus CCI/NSPT rats (d15–d18; p<0.001 all comparisons). Differences in latencies between CCI/NSPT and sham/NSPT rats were observed on d14–d15; p<0.006 all comparisons. VP latency times were higher for CCI versus sham rats (p<0.009 all comparisons). NSPT improved VP latencies for CCI and sham rats (p<0.027 all comparisons). CCI/NSPT rats R1 latencies were not significantly different from shams. Sham/No-NSPT rats had lower latencies on R2 than on R1 (p<0.002). CCI rats did not exhibit reduced latencies from R1 to R2. There was no NSPT effect between sham groups. b) PZ TA; Cued Rats. CCI/No-NSPT rats exhibited higher PT ZA than CCI/NSPT rats (d14–d18, p<0.011 all comparisons). CCI/NSPT rats exhibited higher PT ZA than sham/NSPT rats d15 (p=0.016), even on days when platform latencies were similar across groups. During VP trials, CCI/No-NSPT groups had higher PT ZA than Sham/No-NSPT rats (p<0.003). CCI/NSPT rats had lower PZ TA than CCI/No-NSPT rats on R2 (p=0.022). No changes in PZ TA were observed between R1 and R2 for any group. c) MWM Latencies: Non-Cued Rats. Platform latencies were different between CCI/No-NSPT and CCI/NSPT rats on d15 (p=0.011) and d18 (p<0.001). NSPT had no effect in the sham condition. During VP trials, there was no difference between CCI/NSPT and Sham/NSPT latencies. Reduced platform latencies across reversal trials were only noted in the CCI/NSPT group (p=0.014). Reversal latency differences for CCI vs. sham groups were not significant within NSPT groups. d) PZTA: Non-Cued Rats. CCI/No-NSPT rats had higher PZ TA than CCI/NSPT rats on d14–18 (p<0.029 all comparisons). During VP trials, CCI/No-NSPT rats had higher PZ TA than other groups (p<0.002 all comparisons). NSPT reduced thigmotaxis for CCI rats (p<0.001). There were no differences between R1 and R2 PZTA for any group. e) MWM Latency Times: NSPT Rats. Extra-maze cues further improved sham performance compared to the non-cued condition on d14 (p=0.043). Sham/cued rats had shorter platform latencies on d14–d17 than CCI/cued rats (p<0.048, all comparisons). For VP trials, allocentric cues did not benefit CCI groups. VP latencies were higher for CCI/cued rats compared to sham/cued rats (p=0.005). However, platform latencies were not different in the non-cued condition. In reversal trials, Latencies were significantly different for CCI/cued and Sham/cued rats on R2 (p=0.009). f) PZ TA: NSPT Rats. CCI rats in both cued and non-cued settings exhibited similar levels of PZ TA, regardless of cues. Sham/cued rats exhibited lower PZ TA than CCI rats on day 15, (p=0.047). During VP trials, PZ TA was similar across CCI and sham groups. During reversal trials there were no differences in PZ TA between R1, R2 for any group (NSPT=non-spatial pre-training, CCI=controlled cortical impact, PT ZA=peripheral time zone allocation).
There was a significant main effect of group (p<0.001) and trial day (p<0.001) on PZ TA. NSPT reduced PZ TA for sham (p=0.001) and CCI rats (p<0.001) compared to No-NSPT groups. Additionally, there were differences in PZ TA between CCI/No-NSPT and both Sham groups on R1 as well as between CCI/No-NSPT and all other groups on R2 (p<0.048, all comparisons) (figure 4b). The propensity of the CCI/No-NSPT group to engage in thigmotaxic behavior likely interfered with their ability to learn the platform location.
3.2.2.2. Non-cued Condition
There was a significant main effect of group and trial day (p<0.001 all comparisons) on platform latencies during non-cued acquisition trials. CCI/NSPT rats learned to solve the task more quickly than rats in the CCI/No-NSPT group (p<0.001), (Figure 4c). Importantly, no differences were noted over time between CCI/NSPT and sham groups, indicating that pre-existing non-spatial aspects of navigational behavior developed during NSPT, were not significantly impaired by CCI.
There was also a significant main effect of group (p<0.001) and day (p<0.001) on PZ TA. CCI/No-NSPT rats exhibited greater PZ TA than any other groups across testing days (p<0.007 all comparisons) (figure 4d). There were no differences between CCI/NSPT rats and either sham group. These results suggest that NSPT eliminates the injury-induced deficits in the non-cued version of the MWM such that CCI/NSPT rats perform as well as shams (figure 4d)
3.2.2.3. NSPT Condition
There was a main effect of group and trial day among NSPT groups on platform latencies (p<0.046, all comparisons). Allocentric cues affected performance, such that CCI/Cued rats performed significantly worse than sham/cued rats (p=0.002), while CCI/Non-Cued and Sham/Non-Cued rats exhibited similar platform latencies (figure 4e).
There was a main effect of trial day (p<0.001) on PZ TA between NSPT rats. In the Non-Cued condition, sham/NSPT and CCI/NSPT rats exhibited similar PZ TA across acquisition testing days. However, in the Cued condition, CCI/NSPT rats tended to have higher PZ TA on d15 (p=0.047), d16 (p=0.072), and d18 (p=0.070) compared to sham/NSPT rats (figure 4f). Spatial deficits associated with TBI likely contribute to worse performance in the cued condition, particularly when adjusting for non-spatial deficit contributions to platform latencies through NSPT use.
3.2.3. Acquisition trials: Searching Strategy
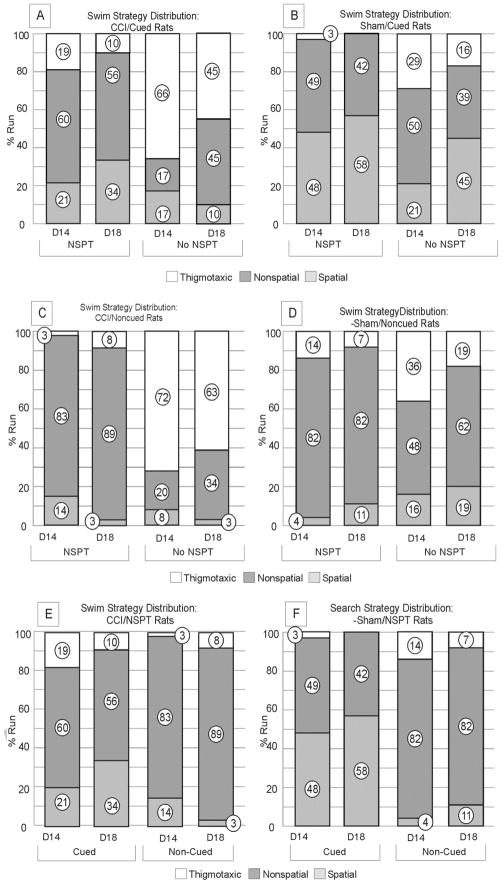
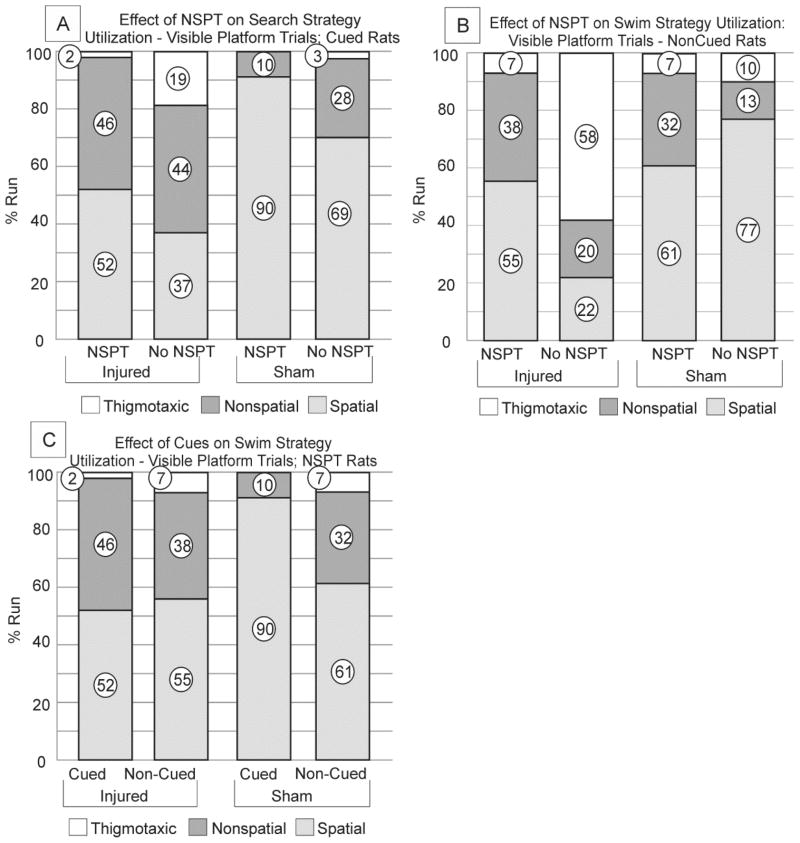
In the cued condition, CCI/NSPT rats exhibited fewer thigmotaxic (p=0.001), and more non-spatial (p=0.008) swim strategies than CCI/No-NSPT rats on d14, though these effects were reduced by d18. CCI/NSPT rats utilized more spatial strategies than CCI/No-NSPT rats on d18 (p=0.020), suggesting that rats exposed to NSPT are better able to utilize spatial cues to solve the place learning task (figure 5a).
Figure 5. Swim strategy distribution; Acquisition trials D14 & D18.
a) CCI/Cued Rats. NSPT was associated with less thigmotaxis on D14 (p=0.001). Also, non-spatial (D14: p=0.008) and spatial (D18: p=0.020) strategies were greater with NSPT b) Sham/Cued Rats. NSPT was associated with less thigmotaxis (p=0.019), and more spatial strategies on D14 (p=0.013). c) CCI/Non-Cued Rats. non-spatial searching strategies predominated with CCI/NSPT rats, compared to CCI/no-NSPT rats. (D14: p<0.001; D18: p<0.001). d) Sham/Non-Cued Rats. NSPT had a significant effect on swim strategy distribution for Sham/non-Cued rats (D14: P<0.001; D18: P<0.001). Thigmotaxis was far more prevalent for CCI rats (c) than sham no-NSPT rats (d) (D14: P=0.002; D18: P=0.012). e) CCI/NSPT Rats. Non-spatial strategies were more prevalent among non-cued groups on both D14 and D18 (D14: p<0.001, D18: p<0.001. f) Sham/NSPT Rats. Spatial strategies predominated among cued groups on D14 and D18 while non-spatial strategies predominated across non-cued groups (p=0.008) (NSPT=non-spatial pre-training, CCI=controlled cortical impact).
The Sham/NSPT group exhibited a significant reduction in thigmotaxic search strategies (p=0.019) and increased spatial strategy utilization (p=0.013) compared to the sham/No-NSPT group on d14, suggesting that Sham/NSPT rats were able to incorporate previously acquired non-spatial behavioral skills with novel cue information to more effectively solve the task (figure 5b).
In the non-cued condition, non-spatial strategies were most prevalent for CCI/NSPT and sham/NSPT groups on d14 and d18 (p<0.001, both comparisons) when compared to their No-NSPT controls (Figure 5c, d). Thigmotaxic search strategies were most prominent for the CCI/No-NSPT group compared to the CCI/NSPT group on both d14 and d18 (p<0.001 all comparisons). Sham/No-NSPT rats exhibited more thigmotaxic search strategies on d14 and d18, compared to the NSPT sham group, but these comparisons did not reach significance. Thus, regardless of injury, NSPT can suppress thigmotaxic search strategies in the non-cued condition, and facilitate the non-spatial strategy use to locate the platform.
In the NSPT condition, extra maze cues did not result in an overall preference for spatial swimming strategies in the CCI/Cued group. In contrast, extra-maze cues elicited more spatial swimming strategies over non-spatial strategies for sham/NSPT rats (d14, p=0.001; d18, p=0.015). Meanwhile, Non-spatial strategies were predominant in the CCI/Non-Cued group on both d14 and d18 (d14: p<0.001, d18: p<0.001). Thigmotaxis was almost non-existent for sham rats in the cued condition (Figures 5e, f). These results suggest that Sham/NSPT rats incorporate extra-maze cues into their searching strategy selection during acquisition trials better than CCI rats. Furthermore, there were residual spatial deficits after TBI, even after minimizing non-spatial deficits with NSPT; this finding is supported by higher platform latencies for CCI/NSPT/Cued compared to sham/No-NSPT/Cued rats.
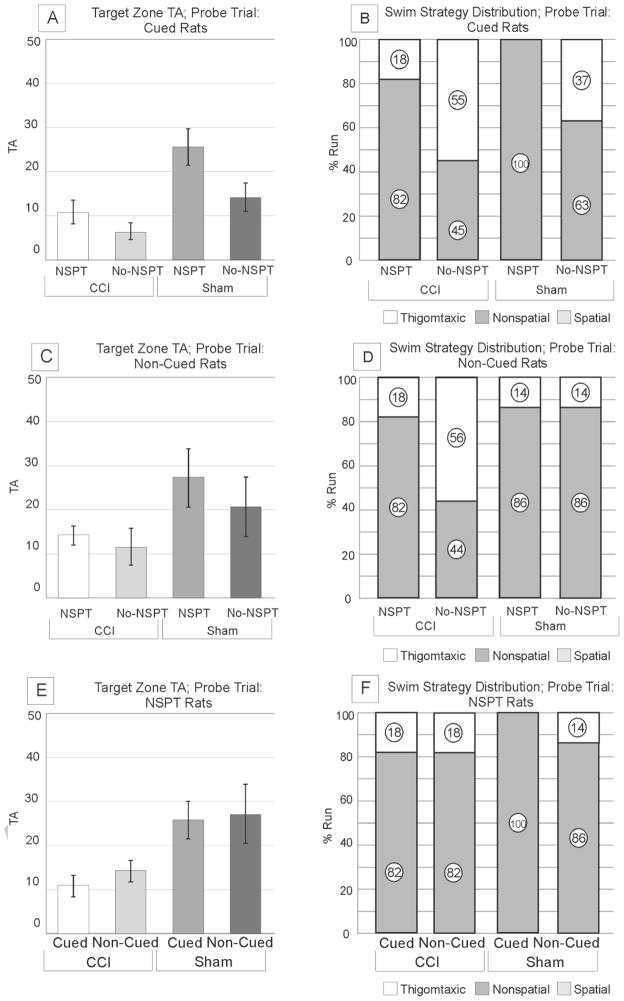
3.4. Effects of NSPT, injury status, and extra-maze cues in navigational behavior: Probe Trials
In the cued condition, there was an effect of group on PZ TA (p=0.002) and TZ TA (p=0.001). CCI/NSPT and Sham/NSPT both exhibited lower PZ TA than their No-NSPT counterparts (p<0.030 all comparisons) (supplementary figure 1a). While there were no differences in TZ TA between CCI/NSPT and CCI/No-NSPT rats, sham/NSPT rats did outperform Sham/No-NSPT rats (p=0.018) (figure 6a). Although typically thought as a measure of spatial awareness, no rats used a predominantly spatial strategy in the probe trial (figure 6b, d, e), suggesting that all groups noted the absence of the escape platform from the expected location, and that results could be confounded by strategy selection bias introduced with platform removal.
Figure 6. Probe Trial.
a) Cued Rats Target zone TA; NSPT enhanced target zone TA for sham rats (p=0.018); improvements in target zone time were not significant for CCI Rats. CCI/NSPT rats performed worse than Sham/NSPT rats (p=0.007). No significant differences were observed between CCI groups and Sham/No-NSPT. b) Cued Rats Swim Strategy Distribution; NSPT suppressed thigmotaxis in the CCI and sham rats, but did not reach significance for either group (p<0.200 all comparisons). No groups used a predominant spatial swimming strategy. c) Non-Cued Rats Target zone TA; NSPT did not significantly improve probe TZ TA performance for CCI or sham rats. d) Non-Cued Rats Swim Strategy Distribution; NSPT did not significantly reduce thigmotaxis in CCI and sham rats. Both NSPT groups tended to use a circling behavior, while no-NSPT rats tended to use scanning behavior. e) NSPT Rats Target zone TA; Regardless of cues, CCI rats exhibited significantly lower target zone TA than Sham rats (p<0.040 all comparisons). Within injury groups, cues had no significant effect on TZ TA. f) NSPT Rats Swim Strategy Distribution; CCI/NSPT rats exhibited similar levels of thigmotaxis, regardless of cues. Thigmotaxis was eliminated for sham rats in the cued condition. Also, no rats had a predominant spatial strategy for this condition. (NSPT=non-spatial pre-training, CCI=controlled cortical impact).
In the non-cued condition, there were no significant group effects TZ or PZ TA. CCI/NSPT rats only tended to have lower TZ TA than sham/NSPT rats, which may be due to similar frequency of thigmotaxis and PZ TA (figure 6c, supplementary figure 1b). In contrast, No-NSPT groups had only modestly reduced TZ TAs, despite differences in thigmotaxic search strategies. Overall, non-spatial strategy utilization was similar between CCI/NSPT and sham/NSPT rats, however, shams tended to use more circling patterns, while the CCI NSPT group used more scanning strategies (data not shown). Also, CCI/NSPT rats had similar TZ TA as CCI/No-NSPT, despite more thigmotaxis in the CCI/no-NSPT group (figures 6c/d). These findings show that different non-spatial search strategy use during probe trials in the non-cued condition is moderated by NSPT status.
When assessing rats with NSPT, there was a significant group effect for TZ, but not PZ TA. CCI/Non-Cued rats exhibited significantly lower TZ TA than sham/Non-Cued rats (p<0.001), despite similar PZ TA (figure 6e, supplementary figure 1c). However, within injury and within sham groups TZ TA was similar, suggesting that cues had little effect if rats have been exposed to NSPT. Sham/NSPT/cued rats utilized a higher frequency of scanning strategies, while Sham/NSPT/Non-Cued rats primarily utilized circling strategies (data not shown) supporting the idea that different distributions of non-spatial patterns were used to probe for the escape platform in NSPT shams as a function of allocentric cues status (see figure 2 for swim strategy examples). No spatial strategies were used in either cued group, suggesting that the probe trial may not be an adequate measure of spatial memory.
3.5. Effects of NSPT, injury status, and extra-maze cues in navigational behavior: Visible Platform Trials
In the cued condition, there was an effect of group where NSPT improved CCI rat performance in both platform latencies (p<0.001) and PZ TA (p<0.001). NSPT reduced latencies (p=0.027), but not PZ TA, between sham groups (figure 4a, b) suggesting that that group differences in latencies were largely due to increased PZ TA for CCI groups compared to sham controls. CCI/NSPT rats used more spatial swim strategies than CCI/No-NSPT rats (p=0.023). In general, though, sham groups more often used spatial swim strategies, while CCI groups used predominantly non-spatial swim strategies (CCI/NSPT vs. sham/NSPT: p<0.001; CCI/No-NSPT vs. sham/No-NSPT: p=0.024) (figure 7a). To determine if CCI rats have difficulty using the VP as a proximal cue to guide them to the platform, we evaluated d18 compared to VP trials (comparisons not shown, refer to figures 5 and 7). Both CCI and Sham rats increased spatial strategy utilization from d18 to VP (p<0.05, all comparisons). However, CCI/NSPT rats exhibited similar non-spatial strategy distributions on both d18 and VP, despite the introduction of the proximal cue. Meanwhile, Sham/NSPT rats decreased their use of non-spatial strategies from 42% to 9% (p<0.001) on these testing days. These data suggest that compared to shams, NSPT does not increase CCI rats’ ability to incorporate a proximal cue into its search strategy selection in the context of extra-maze cues.
Figure 7. VP trials.
a) Cued Rats Swim strategy Distribution; CCI/No-NSPT rats exhibited a higher frequency of thigmotaxis (p=0.034) than CCI/NSPT rats. Sham/NSPT rats utilized more spatial strategies than Sham/No-NSPT rats (p=0.023). CCI rats used spatial strategies less frequently than sham rats, regardless of NSPT status (NSPT CCI vs. sham: p<0.001; No-NSPT CCI vs. sham: p=0.024). b) Non-Cued Rats Swim strategy Distribution; For CCI rats, NSPT effectively reduced thigmotaxis (p=0.002) and increased use of the VP as a spatial cue for identifying platform location (spatial strategies, p=0.045). CCI/No-NSPT rats used the VP as a spatial cue less than sham/No-NSPT rats (p<0.001). c) NSPT Rats Swim strategy Distribution; Allocentric cues did not significantly decrease thigmotaxis or increase spatial strategy use for CCI/NSPT rats. Allocentric cues were associated with greater use of a spatial strategy in Sham/NSPT rats (p=0.009). (NSPT=non-spatial pre-training, CCI=controlled cortical impact, VP= Visible Platform)
In the non-cued condition, there was an effect of group on platform latencies (p<0.001) and PZ TA (p=0.001). CCI/NSPT rats did not differ from Sham rats in latency or PZ TA. CCI/No-NSPT rats had longer latencies (p<0.018 all comparisons) and higher PZ TA (p<0.002 all comparisons) than CCI/NSPT and both sham groups (figure 4c, d). Figure 7b shows that CCI/No-NSPT rats exhibited a higher frequency of thigmotaxis and less spatial strategy utilization than all other groups (p<0.001 all comparisons), showing that this group had little ability to utilize the VP as a proximal cue to swim to the platform. Previous exposure to distal cues was beneficial to CCI/No-NSPT rats (see figure 7 CCI/no-NSPT groups panel b vs. d: p=0.045). In this comparison, cued CCI rats were better able to incorporate the novel proximal cue into their strategy; but the VP may be serving primarily as a goal, rather than a cue, when extra-maze cues were present.
Six rats were unable to find the VP during any of their trials in fewer than 120s, one rat from the CCI/No-NSPT/Cued group, and 5 from the CCI/No-NSPT/Non-Cued group. Generally, these rats performed poorly on all testing days, but 3 were still able to find the hidden platform on acquisition and reversal trials. The imbalance of CCI/No-NSPT/Non-Cued rats that were unable to locate the VP further suggests that rats without pre-injury exposure to training or distal cues have difficulty incorporating the VP as a cue, or utilizing it as a goal, to dictate search strategy selection.
When assessing VP performance among NSPT rats, there was an effect of injury on platform latencies (p<0.001), but not PZ TA, indicating that all rats learned appropriate behavioral strategies through NSPT (figures 4e, f). Spatial strategy use increased in all NSPT groups from d18 to the VP trial (p<0.001 all comparisons). However, CCI/NSPT/Cued rats used fewer spatial strategies than Sham/NSPT/Cued rats (p=0.009), indicating that CCI rats were relatively impaired with incorporating the VP into their swim strategy (figure 7c).
3.6. Effects of NSPT, injury status, and extra-maze cues in navigational behavior: Reversal Trials
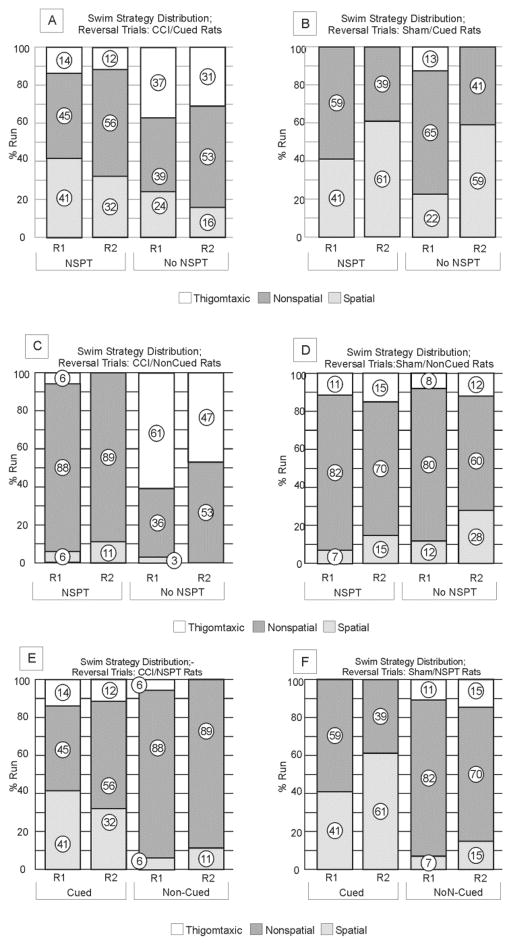
In the cued condition, there was a significant effect of group (p<0.001) on latency to find the reversal platform. CCI/No-NSPT rats exhibited longer platform latencies than either sham group (p<0.001 all comparisons), while CCI/NSPT rats did not differ from either sham group. CCI/NSPT rats had shorter latencies than CCI/No-NSPT rats on R1 (p=0.016), an effect that was reduced by R2 (p=0.065). However, R2 trial latencies were lower than R1 for the Sham/No-NSPT group (p<0.048), indicating improved retention of the new platform location across trials for this group (figure 4a). There was a group effect (p<0.001) on PZ TA. PZ TA was higher for CCI than sham rats (p<0.046, all comparisons), but only trends emerged when comparing CCI/NSPT and CCI/No-NSPT groups. CCI/NSPT rats exhibited similar PZ TA patterns as Sham/No-NSPT rats, indicating that NSPT may suppress thigmotaxis behavior to some degree after CCI even when presented with a new platform location (figure 4b). No significant differences in strategy selection were noted across trials for CCI groups, but NSPT eliminated thigmotaxis in shams during reversal trials, and spatial strategy use increased (p<0.001) across reversal trial days for sham/No-NSPT rats (Figure 8a, b). While NSPT reduced thigmotaxis, its effects on pliancy and flexibility with search strategy selection for learning the new platform location were limited for CCI rats.
Figure 8. Reversal Trials.
a) CCI/Cued Rats Swim strategy Distribution; There were no significant differences in strategy distribution across trials for CCI groups. b) Sham/Cued Rats Swim strategy Distribution; NSPT eliminated thigmotaxis in sham groups during reversal trials. In contrast to CCI groups, spatial strategy use increased (p<0.001) and non-spatial strategies tended to be decreased (p=0.071) between reversal trial days for sham no-NSPT rats. c) CCI/Non-Cued Rats Swim strategy Distribution; NSPT significantly reduced thigmotaxis among CCI rats for each trial day (R1: p<0.001; R2: p<0.001). d) Swim strategy Distribution; Reversal Trials: Sham/Non-Cued Rats. Swim strategies did not differ significantly within groups across days or across groups within days. e) CCI/NSPT Rats Swim strategy Distribution; CCI/Cued rats utilized more spatial search strategies than CCI/non-cued rats on both reversal trial days (Spatial R1: p=0.001, Spatial R2: p=0.011). f) Sham/NSPT Rats Swim strategy Distribution. Cues eliminated thigmotaxis and elicited more spatial strategy use on both testing days (Thigmotaxis R2, p=0.041; Spatial R1: p=0.011, Spatial R2: p=0.003).
In the non-cued condition, there was an effect of group on both platform latency (p=0.001) and PZ TA (p<0.001). Reversal platform latencies were higher for the CCI/No-NSPT group than all other groups on R1 and R2 (p<0.019 all comparisons) (figure 4c). Similarly, PZ TA was higher for the CCI/No-NSPT group than all other groups on R1 and R2 (p<0.019 all comparisons) (figure 4d). NSPT reduced thigmotaxis and increased non-spatial strategy use among CCI rats on both reversal days (R1: p<0.001; R2: p<0.001). NSPT had no effect on sham rat search strategy (figure 8c, d). In the no-cues setting, NSPT eliminated apparent injury effects in reversal TZ TA, and thigmotaxis, even when presented with a novel platform location.
NSPT minimized group effects for R1 and R2 latencies such that they were not significant (p=0.079). However there was a group effect for PZ TA (p=0.015) (figure 4e). The CCI/Cued group had larger PZ TA on R2 than both sham groups (p<0.019 all comparisons), but there were no significant changes in PZ TA from R1 to R2 for CCI vs. sham. CCI/Cued and sham/Non-Cued rats had more thigmotaxic strategies compared to cued shams (figure 8e, f). Also, spatial strategies were higher on R2 for cued sham rats than cued CCI rats. Thus in the NSPT condition, extra-maze cues reduced ability to find the new platform location, suggesting that this explicit feedback is somewhat disruptive in CCI/NSPT groups.
3.7. Histological Findings
Our data (n=9 per CCI group) showed no effects of NSPT on the percent hippocampal cell survival (versus contralateral hemisphere) in the CA1, CA3, and DG regions. Percentage of tissue volume in the hemisphere ipsilateral to the injury site compared to the contralateral cortex ranged from 48% to 77%. Tissue volume of ipsilateral hemisphere ranged from 178.7 mm3 to 234.6 mm3. CCI groups showed no differences in the percent residual tissue volumes as a function of NSPT (CCI/NSPT: 64.8+/−2.2%, CCI/No-NSPT: 63.5+/−3.3%, P=0.78). Also, CCI groups showed no differences in the percent cell sparing in the (CA1, CA3, DG regions) as a function of NSPT (CCI/NSPT CA1: 49.5+/−4.6%, CCI/No-NSPT CA1: 46.3+/−2.7%, P=0.56; CCI/NSPT CA3: 62.3+/−2.7%, CCI/No-NSPT CA3: 58.0+/−3.8%, P=0.38 CCI/NSPT DG: 82.9+/−2.5%, CCI/No-NSPT DG: 79.3+/1.7%, P=0.27). Despite behavioral improvements exhibited by CCI/NSPT and Sham/NSPT rats over their No-NSPT counterparts, there was no correlation between neuronal sparing (CA1, CA3, DG regions) and hidden platform latencies or PZ TA for rats in the cued condition (data not shown), suggesting that behavioral performance improvements were due to task-specific elements of NSPT developing navigational behaviors, as opposed to any histological differences associated with the intervention. Also, no correlations were noted for neuronal counts and VP or reversal latencies or PZ TA.
3.8. Results Summary
In summary, the results of this study suggest that
behaviors learned during NSPT, provided prior to CCI or sham surgery, can be successfully used 2 weeks later to enhance ability to locate a hidden platform in the both the cued and non-cued conditions, eliminating injury effects compared to untrained sham controls.
The differences in behavioral performance between CCI/NSPT/Cued and Sham/NSPT/Cued groups likely incorporate the actual spatial deficits present in the CCI model of experimental TBI.
In the VP trial, extra-maze cues enhanced spatial strategy utilization for all rats compared to D18 of acquisition trials, but the degree of enhancement was less for CCI rats.
Injured rats have difficulty incorporating the novel egocentric cue (VP) into their search strategy, but NSPT provided necessary behavioral elements to better incorporate the VP as a proximal cue.
NSPT did not significantly improve probe trial performance for CCI rats, but did enhance sham performance in the cued condition. However spatial search strategies were not used as a primary strategy by any group during the probe trial.
While NSPT swim strategy use among cued injury and sham groups, extra-maze (allocentric) cues also were associated with more PZ time for CCI rats compared to shams, indicating some competing effects of explicit feedback (cues) with place learning for injured rats.
There were no correlations with HC cell counts and MWM quantitative performance metrics.
4. DISCUSSION
We leveraged NSPT in the MWM to dissociate implicit and explicit learning/memory networks in the CCI model and differentiate apparent spatial and non-spatial learning and memory effects relevant to the cognitive symptoms that occur clinically after TBI. Our results are consistent with our hypotheses and clinical TBI literature that implicitly learned elements used to perform the MWM task are relatively spared after injury (Schmitter-Edgecombe, 2006). Consistent with our hypothesis, NSPT reduced platform latencies associated with injury effects by facilitating use of both spatial and non-spatial strategies more frequently than observed with untrained injured rats. While beneficial for shams, extra-maze cues did not enhance performance for CCI/NSPT rats; in fact during novel test conditions, extra-maze cues hindered CCI/NSPT maze performance, supporting the clinical concept in TBI rehabilitation that explicit feedback can disrupt task performance that incorporates implicitly learned information or strategies (Schmitter-Edgecombe, 2006).
Rodents can flexibly use spatial and non-spatial strategies to shape their navigational behavior, thereby affecting MWM time allocation and latency measures (Cain et al., 2006). NSPT teaches necessary behavioral components of the task while precluding spatial map formation. Furthermore, NSPT teaches rats to suppress thigmotaxis and to utilize the platform as an escape (Hoh & Cain, 1997; Da Cunha et al., 2007). NSPT also likely reduces the emotional stress that animals experience in reaction to a novel task, particularly when completing initial acquisition trials. While each component of NSPT is important, they are not individually sufficient to confer benefit; only the cumulative effect of NSPT improves task performance (Hoh & Cain, 1997). Familiarization with non-spatial MWM elements prior to testing has reversed sex differences in place learning (Perrot-Sinal et al., 1996; Beiko et al., 2004), and alleviated deficits after lesions (Lukoyanov et al., 2005; Cain et al., 2006) and drug administration (Morris, 1989; Dyer & Cain, 2007). While other work suggests that rats have difficulty mastering both behavioral and spatial task components post-injury (Saucier et al., 1996).
We hypothesized that 1) during acquisition trials, CCI rats do not learn the non-spatial strategies of the MWM necessary to effectively generate spatial maps needed for place learning; 2) After CCI, navigational behavior is hindered by both the inability to suppress thigmotaxis as well as search strategy selection deficits. Furthermore, we propose that thigmotaxis in the MWM may be relevant to clinical TBI by representing impaired “response inhibition” or anxiety. Our results also showed that, despite similar platform latencies within NSPT status, apparent searching strategy and time allocation measures can be dynamic and influenced by testing conditions and injury status, suggesting that implicit networks and behaviors can be shaped to improve overall performance. Navigational strategy and time allocation metrics are needed, along with conventional latency analysis, in order to capture this cognitive information effectively in the context of pre-clinical trials.
NSPT has not been evaluated previously in experimental TBI. Although, spatial post-training has been explored using a penetrating model of ballistic brain injury and reported by Shear et al. (2010) who showed that spatial acquisition trials 2-weeks post-TBI improved specific task performance at 4-weeks. However, these same rats demonstrated poor performance when searching for a novel platform location. The Shear et al. (2010) data support the clinical notion of relative inflexibility, or limited pliancy, under new test conditions; similarly our data show increased thigmotaxis and less favorable TA during novel test conditions.
NSPT also may reduce or eliminate an initial “pliancy problem” affecting injured rats. That is, NSPT rats do not have to switch from a less efficient, thigmotaxic, search strategy on D14, as they have already been trained to suppress it; while the CCI/No-NSPT rats have to overcome that behavior. Day et al. (1999) found that rats with frank bilateral hippocampal damage can be taught to swim to a specific location, but do not readily switch strategies when the rules of the task have been changed. This work, and others, suggests that the hippocampus supports “representational flexibility” allowing stored information to be used in new situations (Ramos, 2010). Thus, rats with hippocampal damage may continue to utilize whatever strategy they select first because their deficit is in pliancy rather than spatial awareness. The CCI/No-NSPT rats in our study predominately selected a thigmotaxic strategy upon first exposure to the pool (figure 5a, c); CCI/NSPT rats were trained prior to acquisition trials to utilize more effective non-spatial strategies, and thus, did not need to overcome reflexive thigmotaxic behavior. However, CCI/NSPT rats also performed better on VP and reversal trials, which necessitate a “switch in strategy” away from that which was effective during acquisition trials (figure 7a–d), suggesting that NSPT reduces a “pliancy deficit” in injured rats. Interestingly, CCI/NSPT rats seemed to have a harder time incorporating cues into their searching strategy than sham/NSPT rats, suggesting some decreased pliancy in apparent strategy selection for CCI compared to shams even in the setting of NSPT.
After TBI, patients’ retention of implicitly learned material is similar to uninjured subjects, particularly for automatized tasks (Shum et al., 1999; Yeates & Enrile, 2005; Schmitter-Edgecombe, 2006). The ability to automatize new skills, or cognitive components of complex skills, occurs more slowly following TBI, possibly due to more attentional resources required during the cognitive phase of learning (McCulloch, 2007). Similarly, rats with impaired place navigation in the MWM following NMDA receptor antagonist treatment or hippocampal lesions (Morris et al., 1982; Morris, 1989) can learn the spatial version of the water maze, but at a slower pace (Moser et al., 1993; Bannerman et al., 1995; Cain & Saucier, 1996). Thus, implicit cognitive training paradigms post-CCI, like non-spatial training could feasibly improve spatial navigation. Future work will specifically assess this concept.
Although CCI/NSPT/Cued rats showed latency and thigmotaxis reductions, strategies consistent with non-spatial navigation were often used, and these rats ostensibly failed to incorporate allocentric cues to formulate more efficient navigational strategies. Conversely, sham/NSPT rats appeared to integrate allocentric cues, which resulted in more efficient strategies, consistent with spatial behaviors and shorter platform latencies. Thus, strategy selection had variable effects on platform latencies observed with NSPT. Although there were injury group effects on target zone TA, there was no significant effect of NSPT on target quadrant TA with probe trial performance. Target zone TA (without the contribution of peripheral zone TA) and swim strategy analysis helped discern injury effects compared to target quadrant analysis alone.
While implicit tasks are more resistant to anxiety or fatigue in a clinical setting, interference with explicit feedback during implicit learning can adversely affect task performance after TBI (Watt et al., 1999; Vakil, 2005; Schmitter-Edgecombe, 2006). Also, the carryover of implicitly learned information to other tasks is often limited (Schmitter-Edgecombe, 2006). In contrast to Sham/NSPT rats, which incorporate extra-maze cues into their swimming strategies to further enhance place learning, thigmotaxis increases for CCI/NSPT/Cued rats over CCI/NSPT/Non-Cued rats (e.g. R1-2). These observations support the idea that implicit-explicit networks can be either cooperative or competitive (White & McDonald, 2002; Poldrack & Packard, 2003). Future work will determine if more “flexible” (pliant) implicit learning constructs can be applied using cognitive training protocols post-injury to suppress thigmotaxis, and to facilitate appropriate incorporation of cues for place learning when exposed to cues and novel testing conditions. CR protocols incorporating implicit (visual) priming strategies may be a clinically relevant approach to enhance cue utilization (Russell et al., 2012, Chen et. al., 1997).
Notably, no rats utilized a predominantly spatial search strategy for the 30s probe trial. Some suggest that the probe trial is not an adequate test of spatial aptitude, as well-trained rats (e.g. Sham/NSPT) may initially navigate to the target zone, but quickly realize that the platform is missing, and search for a new platform location using non-spatial strategies (Choi et al., 2006); Our probe data is consistent with this interpretation, however more work investigating the first few seconds of each probe trial is needed to further evaluate initial strategy selection and directional analysis. Additional work adopting novel probe paradigms outlined by Choi (2006) and others that highlight strategy switching abilities could be useful in assessing spatial retention and associated strategy switching abilities that likely contribute to the appearance of non-spatial strategy utilization within a full 30second probe trial.
Recently, an alternative to O’Keefe and Nadel’s (1978) spatial mapping hypothesis considers a directional based hypothesis. This approach to place learning suggests that rats swim in the direction of familiar cues, rather than create a map onto which landmarks are placed. This is relevant in our MWM paradigm, as rats may utilize this directional information, in conjunction with other information about the maze environment, to effectively locate the platform. While the spatial mapping and directionality hypotheses are not mutually exclusive, Hamilton et al. (2007) suggest that a movement vector (direction) combined with local cues associated with the apparatus (e.g. distance from the pool wall) may influence apparent navigational behavior patterns, particularly in the context of training paradigms (Hamilton et al., 2008) like NSPT. While the directional hypothesis is plausible, this approach aided by putative proximal cues from the arena, would yield a relatively imprecise location, necessitating navigational searching behavior within the target zone, and our swim strategy analysis yielded no such focal swimming behavior.
While the MWM is used extensively in experimental TBI research, most studies do not discern whether platform latency deficits result from deficits in spatial mapping, search strategy, or both. While some interventions improve MWM performance, treatment effects on specific spatial and non-spatial components involved with place learning and retention have not been studied. This point is critical, as pre-clinical testing of neuroprotective strategies using experimental models of TBI has a limited record of translating to effective clinical treatments (Dobkin, 2007). Our results also show that NSPT effectively eliminates the apparent place learning deficits associated with CCI in the standard spatial learning MWM task. Thus, cognitive training in combination with other treatments, may greatly impact recovery, an idea that can be readily tested clinically.
Our injury represented a unilateral parasagittal impact over parietal cortex; given the nature of CCI. Beam balance improvements may be due to possible group differences in transient peri-contusional edema associated with the cortical impact, potentially through neuroprotective effects from brief exercise pre-injury, although pre-injury exercise requirements for neuroprotection are typically greater than the brief exposure that NSPT provided (for review see Kleim et al. 2003). Regional tissue damage analysis after CCI shows diffuse axonal injury within subcortical structures like the thalamus, striatum, and hippocampus (Chauhan et al., 2010), structures involved with both search strategies and spatial navigation in the MWM (Morris, 1989; Mair et al., 2003; Cain et al., 2006; Wolff et al., 2008). Also, cortical damage can influence both proximal and distal cue use in the MWM (DiMattia & Kesner, 1988).
The striatum influences stimulus response learning and VP performance (proximal cue) (Devan & White, 1999; Devan et al., 1999; Brooks et al., 2007; Packard, 2009). Thus, striatal damage after CCI may support the prevalence of thigmotaxis, non-spatial strategies, and inability for CCI rats to incorporate the VP as a proximal cue. Interestingly, NSPT increased apparent spatial strategy use to find the VP after CCI, as evidenced by improved VP target zone TA and increased runs consistent with spatial strategy use. Thalamic damage is associated with MWM performance in a bifrontal CCI model (Goss et al., 2003; Djebaili et al., 2004). However, most experimental TBI studies do not report structure-function correlations linking behavior to regional damage. Our study showed no association between hippocampal damage and platform latencies or peripheral zone TA. Although structures involved in hippocampal independent learning and memory are damaged by CCI, injury does not impair non-spatial skill use. As learned information may be stored in redundant regions, (e.g. contralateral hemisphere), identifying specific structure-function correlations with behavioral effects becomes challenging.
In conclusion, NSPT we report enhanced platform latencies and improved MWM performance after CCI, through suppression of thigmotaxis and the apparent use of spatial and non-spatial strategies to find the platform. Unlike shams, CCI rats had difficulty incorporating both allocentric and egocentric spatial cues into their strategy selection, particularly under novel testing conditions. Place learning deficits exhibited by CCI/NSPT/Cued compared to Sham/NSPT/Cued rats may represent the actual spatial deficits associated with CCI. More work is needed on how implicit learning strategies and behavioral skills training can be applied post-injury to improve neurobehavioral recovery after TBI. Future preclinical trials may consider NSPT, with cued and non-cued testing conditions, as a tool to dissect treatment effects on cognitive performance.
Supplementary Material
a)Cued Rats Peripheral Zone TA; Both CCI/NSPT and Sham/NSPT groups spent significantly less time in the peripheral zone than their No-NSPT counterparts (p<0.030 both comparisons). b) Non-Cued Rats Peripheral Zone TA;Peripheral zone TA was only modestly higher in the CCI/no-NSPT groups compared to CCI/NSPT groups (p=0.108). NSPT did not affect peripheral zone TA for sham rats. c) NSPT Rats Peripheral Zone TA;NSPT tended to result in lower peripheral zone TA for sham compared to CCI rats, in the cue condition (p=0.141). (NSPT=non-spatial pre-training, CCI=controlled cortical impact).
Highlights.
Implicit and explicit learning and memory are dissociable in the Morris Water Maze.
Implicitly learned NSPT skills improve MWM performance up to 3 weeks post-TBI.
MWM performance after NSPT showed increased spatial strategy use in TBI groups.
TBI rats had difficulty using spatial cues to navigate in novel testing conditions.
Novel MWM metrics are needed for a comprehensive behavioral assessment after TBI.
Acknowledgments
Work associated with this study was supported, in part, by the National Institutes of Health (1R21HD071728). The authors would like to acknowledge Christopher Henderson, Jay Fuletra, Krishma Kumar and Scott Ketchman for their assistance in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bannerman DM, Good MA, Butcher SP, Ramsay M, Morris RG. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature. 1995;378(6553):182–186. doi: 10.1038/378182a0. [DOI] [PubMed] [Google Scholar]
- Beiko J, Lander R, Hampson E, Boon F, Cain DP. Contribution of sex differences in the acute stress response to sex differences in water maze performance in the rat. Behavioural Brain Research. 2004;151(1–2):239–253. doi: 10.1016/j.bbr.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Green EJ, Dietrich WD. Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Research. 1997;762(1–2):195–202. doi: 10.1016/s0006-8993(97)00387-9. [DOI] [PubMed] [Google Scholar]
- Brody DL, Holtzman DM. Morris water maze search strategy analysis in PDAPP mice before and after experimental traumatic brain injury. Experimental Neurology. 2006;197(2):330–340. doi: 10.1016/j.expneurol.2005.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SP, Trueman RC, Dunnett SB. Striatal lesions in the mouse disrupt acquisition and retention, but not implicit learning, in the SILT procedural motor learning task. Brain Research. 2007;1185:179–188. doi: 10.1016/j.brainres.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Cain DP, Boon F, Corcoran ME. Thalamic and hippocampal mechanisms in spatial navigation: a dissociation between brain mechanisms for learning how versus learning where to navigate. Behavioural Brain Research. 2006;170(2):241–256. doi: 10.1016/j.bbr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Cain DP, Saucier D. The neuroscience of spatial navigation: Focus on behavior yields advances. Reviews in the Neurosciences. 1996;7(3):215–231. doi: 10.1515/revneuro.1996.7.3.215. [DOI] [PubMed] [Google Scholar]
- Carrasco C, Vicens P, Redolat R. Neuroprotective effects of behavioural training and nicotine on age-related deficits in spatial learning. Behavioural Pharmacology. 2006;17(5–6):441–452. doi: 10.1097/00008877-200609000-00010. [DOI] [PubMed] [Google Scholar]
- Chauhan NB, Gatto R, Chauhan MB. Neuroanatomical correlation of behavioral deficits in the CCI model of TBI. Journal of Neuroscience Methods. 2010;190(1):1–9. doi: 10.1016/j.jneumeth.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Chen SHA, Thomas JD, Gluechauf RL, Bracy OL. The effectiveness of comuter assisted cognitive rehabilitation for persons with traumatic brain injury. Brain Injury. 1997;11(3):197–209. doi: 10.1080/026990597123647. [DOI] [PubMed] [Google Scholar]
- Choi SH, Woodlee MT, Hong JJ, Schallert T. A simple modification of the water maze test to enhance daily detection of spatial memory in rats and mice. Journal of Neuroscience Methods. 2006;156(1–2):182–193. doi: 10.1016/j.jneumeth.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Wietzikoski S, Wietzikoski EC, Silva MH, Chandler J, Jr, Ferro MM, Andreatini R. Pre-training to find a hidden platform in the Morris water maze can compensate for a deficit to find a cued platform in a rat model of Parkinson’s disease. Neurobiology of Learning and Memory. 2007;87(4):451–463. doi: 10.1016/j.nlm.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Darrah SD, Chuang J, Mohler LM, Chen X, Cummings EE, Burnett T, Reyes-Littaua MC. Dilantin therapy in an experimental model of traumatic brain injury: Effects of limited versus daily treatment on neurological and behavioral recovery. Journal of Neurotrauma. 2011;28(1):43–55. doi: 10.1089/neu.2010.1521. [DOI] [PubMed] [Google Scholar]
- Day LB, Weisand M, Sutherland RJ, Schallert T. The hippocampus is not necessary for a place response but may be necessary for pliancy. Behavioral Neuroscience. 1999;113(5):914–924. doi: 10.1037//0735-7044.113.5.914. [DOI] [PubMed] [Google Scholar]
- Devan BD, McDonald RJ, White NM. Effects of medial and lateral caudate- putamen lesions on place- and cue-guided behaviors in the water maze: Relation to thigmotaxis. Behavioural Brain Research. 1999;100(1–2):5–14. doi: 10.1016/s0166-4328(98)00107-7. [DOI] [PubMed] [Google Scholar]
- Devan BD, White NM. Parallel information processing in the dorsal striatum: Relation to hippocampal function. Journal of Neuroscience. 1999;19(7):2789–2798. doi: 10.1523/JNEUROSCI.19-07-02789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Research: Brain Research Reviews. 2001;36(1):60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- DiMattia BD, Kesner RP. Spatial cognitive maps: Differential role of parietal cortex and hippocampal formation. Behavioral Neuroscience. 1988;102(4):471–480. doi: 10.1037//0735-7044.102.4.471. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. Journal of Neuroscience Methods. 1991;39(3):253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123(2):349–359. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Dobkin BH. Curiosity and cure: Translational research strategies for neural repair-mediated rehabilitation. Developmental Neurobiology. 2007;67(9):1133–1147. doi: 10.1002/dneu.20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer K, Cain DP. Water maze impairments after combined depletion of somatostatin and serotonin in the rat. Behavioural Brain Research. 2007;181(1):85–95. doi: 10.1016/j.bbr.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Goss CW, Hoffman SW, Stein DG. Behavioral effects and anatomic correlates after brain injury: A progesterone dose-response study. Pharmacology, Biochemistry and Behavior. 2003;76(2):231–242. doi: 10.1016/j.pbb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Graziano A, Petrosini L, Bartoletti A. Automatic recognition of explorative strategies in the Morris water maze. Journal of Neuroscience Methods. 2003;130(1):33–44. doi: 10.1016/s0165-0270(03)00187-0. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Johnson TE, Rice JP, Candelaria FT, Sutherland RJ, Weisend MP. The relative influence of place and direction in the Morris water task. Journal of Experimental Psychology Animal Behavior Processes. 2008;34(1):31–53. doi: 10.1037/0097-7403.34.1.31. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Weisend MP, Sutherland RJ. How do room and apparatus cues control navigation in the Morris water task? Evidence for distinct contributions to a movement vector. Journal of Experimental Psychology Animal Behavior Processes. 2007;32(2):100–114. doi: 10.1037/0097-7403.33.2.100. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Dixon CE, Gbadebo DM, Singha AK, Jenkins LW, Lyeth BG, Hayes RL. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. Journal of Neurotrauma. 1992;9(1):11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Temple MD, O’Dell DM, Pike BR, Lyeth BG. Exposure to environmental complexity promotes recovery of cognitive function after traumatic brain injury. Journal of Neurotrauma. 1996;13(1):41–47. doi: 10.1089/neu.1996.13.41. [DOI] [PubMed] [Google Scholar]
- Hoh TE, Cain DP. Fractioning the nonspatial pretraining effect in the water maze task. Behavioral Neuroscience. 1997;111(6):1285–1291. doi: 10.1037//0735-7044.111.6.1285. [DOI] [PubMed] [Google Scholar]
- Jacoby JL. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–541. [Google Scholar]
- Janus C. Search strategies used by APP transgenic mice during navigation in the Morris water maze. Learning and Memory. 2004;11(3):337–346. doi: 10.1101/lm.70104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Jones TA, Schallert T. Motor enrichment and the induction of plasticity before or after brain injury. Neurochemical Research. 2003;28(11):1757–1769. doi: 10.1023/a:1026025408742. [DOI] [PubMed] [Google Scholar]
- Lukoyanov NV, Lukoyanova EA, Andrade JP, Paula-Barbosa MM. Impaired water maze navigation of Wistar rats with retrosplenial cortex lesions: Effect of nonspatial pretraining. Behavioural Brain Research. 2005;158(1):175–182. doi: 10.1016/j.bbr.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Mair RG, Burk JA, Porter MC. Impairment of radial maze delayed nonmatching after lesions of anterior thalamus and parahippocampal cortex. Behavioral Neuroscience. 2003;117(3):596–605. doi: 10.1037/0735-7044.117.3.596. [DOI] [PubMed] [Google Scholar]
- McCulloch K. Attention and dual-task conditions: Physical therapy implications for individuals with acquired brain injury. Journal of Neurologic Physical Therapy. 2007;31(3):104–118. doi: 10.1097/NPT.0b013e31814a6493. [DOI] [PubMed] [Google Scholar]
- Morris RG. Synaptic plasticity and learning: Selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. Journal of Neuroscience. 1989;9(9):3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. Journal of Neuroscience. 1993;13(9):3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton PR. Unbiased stereology: A concise guide. Baltimore and London: The Johns Hopkins University Press; 2011. [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Oxford University Press; 1978. [Google Scholar]
- Packard MG. Anxiety, cognition, and habit: A multiple memory systems perspective. Brain Research. 2009;1293:121–128. doi: 10.1016/j.brainres.2009.03.029. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annual Review of Neuroscience. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiology of Learning and Memory. 1996;65(1):65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Kostenuik MA, Ossenkopp KP, Kavaliers M. Sex differences in performance in the Morris water maze and the effects of initial nonstationary hidden platform training. Behavioral Neuroscience. 1996;110(6):1309–1320. doi: 10.1037//0735-7044.110.6.1309. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: Converging evidence from animal and human brain studies. Neuropsychologia. 2003;41(3):245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Ramos JM. Preserved learning about allocentric cues but impaired flexible memory expression in rats with hippocampal lesions. Neurobiology of Learning and Memory. 2010;93(4):506–514. doi: 10.1016/j.nlm.2010.01.008. doi: 10.1016/j.nlm.2010.01.008. Epub 2010 Jan 28. [DOI] [PubMed] [Google Scholar]
- Rothi LJ, Fuller R, Leon SA, Kendall D, Moore A, Wu SS, Crosson B, Heilman KM, Nadeau SE. Errorless practice as a possible adjuvant to donepezil in Alzheimer’s disease. Journal of the International Neuropsychological Society. 2009;15(2):311–322. doi: 10.1017/s1355617709090201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell KC, Arenth PM, Scanlon JM, Kessler L, Ricker JH. Hemispheric and executive influences on low-level language processing after traumatic brain injury. Brain Injury. 2012;26(7–8):948–955. doi: 10.3109/02699052.2012.660513. [DOI] [PubMed] [Google Scholar]
- Saucier D, Hargreaves EL, Boon F, Vanderwolf CH, Cain DP. Detailed behavioral analysis of water maze acquisition under systemic NMDA or muscarinic antagonism: Nonspatial pretraining eliminates spatial learning deficits. Behavioral Neuroscience. 1996;110(1):103–116. [PubMed] [Google Scholar]
- Schmitter-Edgecombe M. Implications of basic science research for brain injury rehabilitation: A focus on intact learning mechanisms. The Journal of Head Trauma Rehabilitation. 2006;21(2):131–141. doi: 10.1097/00001199-200603000-00006. [DOI] [PubMed] [Google Scholar]
- Shear DA, Lu XC, Bombard MC, Pedersen R, Chen Z, Davis A, Tortella FC. Longitudinal characterization of motor and cognitive deficits in a model of penetrating ballistic-like brain injury. Journal of Neurotrauma. 2010;27(10):1911–1923. doi: 10.1089/neu.2010.1399. [DOI] [PubMed] [Google Scholar]
- Shum D, Jamieson E, Bahr M, Wallace G. Implicit and explicit memory in children with traumatic brain injury. Journal of Clinical and Experimental Neuropsychology. 1999;21(2):149–158. doi: 10.1076/jcen.21.2.149.929. [DOI] [PubMed] [Google Scholar]
- Steenbergen B, van der Kamp J, Verneau M, Jongbloed-Pereboom M, Masters RS. Implicit and explicit learning: Applications from basic research to sports for individuals with impaired movement dynamics. Disability and Rehabilitation. 2010;32(18):1509–16. doi: 10.3109/09638288.2010.497035. [DOI] [PubMed] [Google Scholar]
- Vakil E. The effect of moderate to severe traumatic brain injury (TBI) on different aspects of memory: A selective review. Journal of Clinical and Experimental Neuropsychology. 2005;27(8):977–1021. doi: 10.1080/13803390490919245. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nature Protocols. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AK, Kline AE, Ren D, Willard LA, Wenger MK, Zafonte RD, Dixon CE. Gender associations with chronic methylphenidate treatment and behavioral performance following experimental traumatic brain injury. Behavioural Brain Research. 2007a;181(2):200–209. doi: 10.1016/j.bbr.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AK, Kline AE, Sokoloski J, Zafonte RD, Capulong E, Dixon CE. Intervention with environmental enrichment after experimental brain trauma enhances cognitive recovery in male but not female rats. Neuroscience Letters. 2002;334(3):165–168. doi: 10.1016/s0304-3940(02)01103-5. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Postal BA, Darrah SD, Chen X, Khan AS. Deficits in novelty exploration after controlled cortical impact. Journal of Neurotrauma. 2007b;24(8):1308–1320. doi: 10.1089/neu.2007.0274. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Willard LA, Kline AE, Wenger MK, Bolinger BD, Ren D, Zafonte RD. Evaluation of estrous cycle stage and gender on behavioral outcome after experimental traumatic brain injury. Brain Research. 2004;998(1):113–121. doi: 10.1016/j.brainres.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Watt S, Shores EA, Kinoshita S. Effects of reducing attentional resources on implicit and explicit memory after severe traumatic brain injury. Neuropsychology. 1999;13(3):338–349. doi: 10.1037//0894-4105.13.3.338. [DOI] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiology of Learning and Memory. 2002;77(2):125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Wolff M, Gibb SJ, Cassel JC, Dalrymple-Alford JC. Anterior but not intralaminar thalamic nuclei support allocentric spatial memory. Neurobiology of Learning and Memory. 2008;90(1):71–80. doi: 10.1016/j.nlm.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Enrile BG. Implicit and explicit memory in children with congenital and acquired brain disorder. Neuropsychology. 2005;19(5):618–628. doi: 10.1037/0894-4105.19.5.618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
a)Cued Rats Peripheral Zone TA; Both CCI/NSPT and Sham/NSPT groups spent significantly less time in the peripheral zone than their No-NSPT counterparts (p<0.030 both comparisons). b) Non-Cued Rats Peripheral Zone TA;Peripheral zone TA was only modestly higher in the CCI/no-NSPT groups compared to CCI/NSPT groups (p=0.108). NSPT did not affect peripheral zone TA for sham rats. c) NSPT Rats Peripheral Zone TA;NSPT tended to result in lower peripheral zone TA for sham compared to CCI rats, in the cue condition (p=0.141). (NSPT=non-spatial pre-training, CCI=controlled cortical impact).