Abstract
Alpha klotho (known as klotho) is a multifunctional protein that may be linked to age-associated decline in tissue homeostasis. The original klotho hypomorphic (klothohm) mouse, produced on a mixed C57BL/6 and C3H background, is short lived and exhibits extensive aging-like deterioration of several body systems. Differently, klothohm mice on a pure C57BL/6 background do not appear sickly nor die young, which has permitted us to gain insight into the effect of klotho deficiency in adult life. First, analyzing klotho transcript levels in the kidney, the main site of klotho production, we demonstrated a 71-fold decline in klothohm females compared to wildtype females versus only a 4-fold decline in mutant males. We then examined the effect of klotho deficiency on muscle-related attributes in adult mice, focusing on 7–11 month old females. Body weight and forelimb grip strength were significantly reduced in klothohm mice compared to wildtype and klotho overexpressing mice. The female mice were also subjected to voluntary wheel running for a period of 6 days. Running endurance was markedly reduced in klothohm mice, which exhibited a sporadic running pattern that may be characteristic of repeated bouts of exhaustions. When actually running, klothohm females ran at the same speed as wildtype and klotho overexpressing mice, but spent about 65 % less time running compared to the other two groups. Our novel results suggest an important link between klotho deficiency and muscle performance. This study provides a foundation for further research on klotho involvement as a potential inhibitor of age-associated muscle deterioration.
Keywords: Aging, Alpha klotho, EFmKL46 transgene, Hypomorph, Klotho, Muscle strength, Running endurance, Sarcopenia, Skeletal muscle
Introduction
Aging is a complex degenerative process characterized by the diminished capacity for tissue maintenance and gradual decline in organ systems. In the context of skeletal muscle, there is an age-associated decline in both muscle mass and strength, a condition termed sarcopenia (Rosenberg 1997; Ryall et al. 2008; Thompson 2009; Janssen 2010). More recently, the definition of sarcopenia has been broadened, not only to indicate a decline in muscle strength, but an overall decline in muscle function (Fielding et al. 2011; Rosenberg 2011). In humans, sarcopenia can begin as early as the 4th decade of life (Walston 2012). At the histological level, as seen in humans and in animal models, muscle aging is associated with a gradual elimination of myofibers, atrophy of remaining myofibers, and the progressive replacement of skeletal muscle with adipose and connective tissue (Faulkner et al. 2007; Zamboni et al. 2008; Serrano and Muñoz-Cánoves 2010). The regenerative capability of skeletal muscle also decreases with age (Rader and Faulkner 2006; Barberi et al. 2013). This may be due in part to the decline in number and function of myogenic stem cells (i.e., satellite cells) in old muscle tissue as seen in the context of limb muscles (Renault et al. 2002; Brack et al. 2005; Conboy et al. 2005; Shefer et al. 2006, 2010, 2013; Yablonka-Reuveni 2011). Overall, sarcopenia represents one of the most dramatic declines in tissue function seen in human health (Rosenberg 1997), leading to increased frailty and loss of independence with age (Evans et al. 2010; Walston 2012). Understanding the factors contributing to this condition is essential for developing therapies to slow age associated muscle deterioration.
Both systemic and muscle tissue intrinsic factors are thought to contribute to the age-associated decline in muscle quality (Carlson and Faulkner 1989; Brack et al. 2007; Walston 2012; Barberi et al. 2013; Bucci et al. 2013). Indeed, age-associated changes in the cellular environment can be detrimental to satellite cell performance (Conboy et al. 2005; Shefer et al. 2006; Chakkalakal et al. 2012; Barberi et al. 2013). Once isolated from the aging muscle and maintained ex vivo in a rich mitogenic environment or transplanted into a young host environment, satellite cells from old rodents exhibit good regenerative potential (Carlson and Faulkner 1989; Conboy et al. 2005; Shefer et al. 2006; Collins et al. 2007; Carlson et al. 2009). Parabiotic pairing between young and old mice has identified circulating factors in young animals that are capable of improving muscle regeneration in aged tissue (Conboy and Rando 2005). Such parabolic studies have shown that both Notch and growth differentiation factor 11 (GDF11) decline with age and have the capacity to reduce age-associated phenotypic changes in skeletal and cardiac muscle, respectively (Conboy et al. 2005; Loffredo et al. 2013). Conversely, transforming growth factor β1 (TGF-β1) and Wnt increase with age and promote fibrotic changes in skeletal muscle (Brack et al. 2007; Carlson et al. 2008).
One circulating multifunctional factor that has broad effects on an array of tissues and interacts with a number of growth factor pathways is alpha klotho (klotho) (Liu et al. 2007; Cha et al. 2008). Klotho is one of two members of the klotho gene family (i.e., alpha and beta) (Kurosu and Kuro-o 2009). The main site of klotho expression is the kidney, but it is also expressed, albeit at a much lower level, in other tissues (Kuro-o et al. 1997; Takeshita et al. 2004; Fon Tacer et al. 2010; Zhou et al. 2013). Klotho functions as both a transmembrane protein and a secreted humoral factor (Chen et al. 2007; Bloch et al. 2009; Tomiyama et al. 2010). The membrane-bound form of klotho is an obligate co-receptor for some of the endocrine members of the fibroblast growth factor (FGF) super family (Wu et al. 2007; Tomiyama et al. 2010). Klotho is particularly important for the function of FGF23 signaling, which regulates vitamin D metabolism and subsequent phosphate excretion in the kidney (Razzaque 2009). The secreted form, which is shedded from the transmembrane protein, is believed to be responsible for the systemic influence of the klotho protein, since it is capable of regulating various growth factor signaling pathways throughout the body (Imura et al. 2004, Kuro-o 2009).
The secreted klotho protein has been shown to directly inhibit the pro-fibrotic TGF-β1 and Wnt pathways in renal tissue (Doi et al. 2011), and could potentially play a similar function in muscle tissue. This makes klotho particularly attractive as a potential regulator of age associated increases in muscle fibrosis. TGF-β1 not only is a master regulator of tissue fibrosis, but also has been shown to inhibit satellite cell activation and myoblast differentiation (Allen and Boxhorn 1987; Yablonka-Reuveni and Rivera 1997; Shefer and Yablonka-Reuveni 2008). Evidence that aging muscle contains higher amount of TGF-β1 and that inhibition of TGF-β1 signaling enhances muscle regeneration, have established a possible role of TGF-β1 in sarcopenia (Carlson et al. 2008; Burks et al. 2011). The secreted form of klotho interacts directly with the TGF-β1 type II receptor (TGF-βR2), reducing the affinity of the endogenous TGF-β1 ligand (Doi et al. 2011). Klotho has also been shown to bind and directly inhibit several Wnt family members in murine small intestine and hair follicle cells (Liu et al. 2007). Like TGF-β1, Wnt family proteins have been implicated in enhancing age related muscle fibrosis (Brack et al. 2007). By targeting TGF-β1 and Wnt pathways, secreted klotho is capable of reducing kidney fibrosis after ureteral obstruction (Doi et al. 2011; Zhou et al. 2013) and has been proposed as a therapy for reducing fibrosis during chronic kidney disease (Sanchez-Niño et al. 2013). Should similar anti-fibrotic effect of klotho extend to muscle tissue, the klotho protein could become an intriguing candidate for reducing the age-associated decline in muscle tissue.
The klotho hypomorphic (klothohm) mutant mouse has reduced klotho expression and develops advanced aging-like symptoms, at a young age, that resemble conditions such as arteriosclerosis, ectopic calcification, osteoporosis, skin atrophy, and emphysema (Kuro-o et al. 1997). These mice are also smaller in size, have decreased physical activity and reduced life span (Kuro-o et al. 1997). The severely compromised healthspan and lifespan features exhibited by the klothohm mouse can however, be rescued by crossing the mutant with a transgenic mouse overexpressing klotho (Kuro-o et al. 1997; Kurosu et al. 2005). With the presence of advanced aging-like symptoms in the klothohm mouse, klotho has gained a reputation of being an anti-aging factor (Kuro-o 2009) but this topic has remained a subject of debate (Miller 2007).
Despite documented effects of klotho deficiency in a diverse range of tissues and evidence of its ability to attenuate tissue damage in the kidney, little is known of the influence of klotho on skeletal muscle (Iida et al. 2011). In this study we have examined the effect of klotho deficiency on muscle strength and running endurance in klothohm mice compared to klotho over-expressing transgenic and wildtype mice. We specifically used klothohm mice from the C57BL/6 background, due to the extensive use of this strain background in aging studies (Goodrick 1975; Turturro et al. 2002; Pettan-Brewer and Treuting 2011). Moreover, as further discussed in the “Results and discussion” section, klothohm C57BL/6 mice do not die at a young age, which has permitted studies with older mice. Our research shows that female klothohm mice on the C57BL/6 background exhibit significantly lower levels of klotho gene expression in the kidney compared to klothohm males. Female klothohm mice also have a significant reduction in body weight, muscle strength and running endurance compared to wildtype females. Our findings offer in-vivo physiological evidence of the effect of klotho deficiency in a muscle context and provide the foundation for further studies on the involvement of this factor in the development of sarcopenia.
Materials and methods
Mouse strains
All mice were from colonies maintained at the University of Washington. Mice were housed in micro-isolator cages in a pathogen-free facility under 12/12-h light/dark cycle and were fed ad libitum Lab Diet 5053. All animal procedures were approved by the University of Washington Institutional Animal Care and Use Committee. Klotho deficient (Klothohm, homozygous males, C57BL/6) and transgenic klotho overexpressing (EFmKL46, homozygous, males and females, C57BL/6) breeders, were generously provided by Dr. Makoto Kuro-o (UT Southwestern Medical Center). Both lines were maintained as homozygous lines for analysis. To establish a local homozygous klothohm line, the original klothohm breeders were first crossed out one generation to wildtype C57BL/6 females and the resulting heterozygous females were backcrossed to the original male breeders.
All mice were genotyped at weaning and again prior to experimentation. Genomic DNA was purified from ear punches with a Qiagen DNeasy, DNA extraction kit and amplified using HotStarTaq (Qiagen) polymerase. Mutant and wildtype alleles were analyzed in separate PCR reactions using a common reverse primer: GGA AGA TTG GAA GTG GAC G and the following allele specific forward primers: mutant, CAA GGA CCA GTT CAT CAT CG; wildtype, TTA AGG ACT CCT GCA TCT GC (Kuro-o et al. 1997). The EFmKL46 mouse was genotyped using the fwd/rev primers: CCT GGT CGA CCA TTT CAG/AGC ACA AAG TCG ACA GAC TTC TGG C (Kuro-o et al. 1997). Genotyping PCR reactions were performed on a C1000 thermal cycler (BioRad) using a protocol of: 95 °C for 15 min, followed by 36 cycles of 95 °C for 30 s, 56 °C for 30 s, and 72 °C for 1 min 30 s with a final extension of 72 °C for 10 min. Presence or absence of an amplicon was determined by running the products on a SYBR Safe (Life Technologies) 1 % agarose gel.
Gene expression analysis
Klotho expression levels were quantified from kidney and gastrocnemius muscle using SYBR Green reverse transcription quantitative PCR (RT-qPCR) analysis. Total RNA was extracted from the tissue samples using the Qiagen RNeasy Kit, with on column DNase digest, then quantified on a NanoDrop spectrophotometer (Thermo Scientific) and reverse transcribed into cDNA with iScript reverse transcriptase (Bio-Rad). The iTaq Universal SYBR Green Supermix (BioRad) was used for RT-qPCR analysis of 25 ng of total cDNA per sample. The RT-qPCR reactions were performed on an ABI 7300 Real Time PCR machine (Life Technologies) with 500nM of forward and reverse primers under the conditions; 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s, 63 °C for 30 s and 72 °C for 30 s. Eukaryotic translation elongation factor 2 (Eef2) expression was determined for each sample (mean ± SEM, Ct: kidney 17.12 ± 0.09; gastrocnemius muscle 18.51 ± 0.34). Klotho expression was then normalized by subtracting the respective Eef2 expression value for each sample to obtain the ΔCt values used for analysis. All RT qPCR reactions were performed in triplicate with appropriate no template controls. The RT-qPCR primer sets used were (fwd/rev): klotho (398 bp) TAT GCC ACT CGA AAC CGT CCA TGA/CGA CTA CCC AGA GAG TAT GAA G, and Eef2 (123 bp) TGT CAG TCA TCG CCC ATG TG/CAT CCT TGC GAG TGT CAG TGA [PrimerBank ID: 33859482a1, (Spandidos et al. 2010)]. The efficiency for each primer set was validated using a standard curve produced from purified PCR product and RT-qPCR reaction specificity was monitored each reaction by performing a melting curve analysis. Based on the RT-qPCR assay efficiency, gene amplification in gastrocnemius samples at a level higher than 34 cycles (ΔCt of 15) was considered to have no expression.
Body composition
Total lean and fat mass were measured using quantitative magnetic resonance (QMR). Live mice were weighed to the nearest 0.1 g and QMR readings were recorded using an EchoMRI QMR machine (Echo medical systems). The percent lean and fat mass was determined by normalizing to body weight.
Grip strength
Forelimb grip strength was analyzed using a force tension apparatus (San Diego Instruments). Prior to the test, each mouse was weighed to the nearest 0.1 g. Once mice gripped the stationary bar with their forepaws, they were stretched horizontally while held at the base of their tails. Mice were pulled gradually until they let go of the bar. The process was repeated at least 8 times to determine the peak grip force value (gram-force) used for analysis. For an image depicting the procedure go to http://depts.washington.edu/compmed/ivs/grip.html.
Running wheel
Low-profile wireless running wheels (Med Associates, Inc.) were used to compare continuous voluntary running activity. Mice were housed in individual cages and allowed to acclimate to a fixed running wheel for three days after which the wheels were activated to enable rotation. Total revolutions were recorded every min for 6 days. For further details go to http://depts.washington.edu/compmed/ivs/running_wheels.html.
Statistics
Statistical analysis of gene expression data was performed on normalized ΔCt values in order to avoid potential artifacts introduced during ΔΔCt calculation, especially with low expression levels. A t test was used to analyze expression levels and weight data and p values less than 0.05 were considered significant. When comparing physiological outcomes between wildtype, klothohm and EFmKL46 mouse strains, data were first analyzed using a one-way ANOVA, followed by post hoc analysis with individual t tests and Bonferroni correction (p <0.017 considered significant). All average values stated in the text or shown in figures represent mean ± SEM.
Results and discussion
Characterization of the klothohm C57BL/6 mice
Klothohm mice have a deficiency in klotho production due to a transgene insertion, which deleted approximately 8 kb of the promoter region of the klotho gene (Kuro-o et al. 1997, Imura et al. 2004). These mice, which were originally produced on a mixed strain background of C57BL/6J and C3H/J, show an accelerated deterioration of many of their organs and are short-lived, with an average life span of around 60 days. In the current study we used klothohm mice (homozygous for the klotho mutant allele; i.e., klothohm/hm) on a pure C57BL/6 mouse background, which have no noticeable deterioration in health or lifespan. The original male breeders we received (n = 3), lived to an age of around 22 months before being harvested for tissue sampling. To date, klothohm progeny generated in our lab have been followed for up to 14 months of age, and both males and females appear healthy. Notably, klothohm mice on the C57BL/6 background are fertile, while mice on the original strain have been maintained by crossing heterozygous parents (Kuro-o et al. 1997). To our knowledge the lifespan of klothohm mice on the C57BL/6 background has not been reported, and it is possible based on our pilot observation with the original breeders, that their lifespan may not differ significantly from wildtype mice.
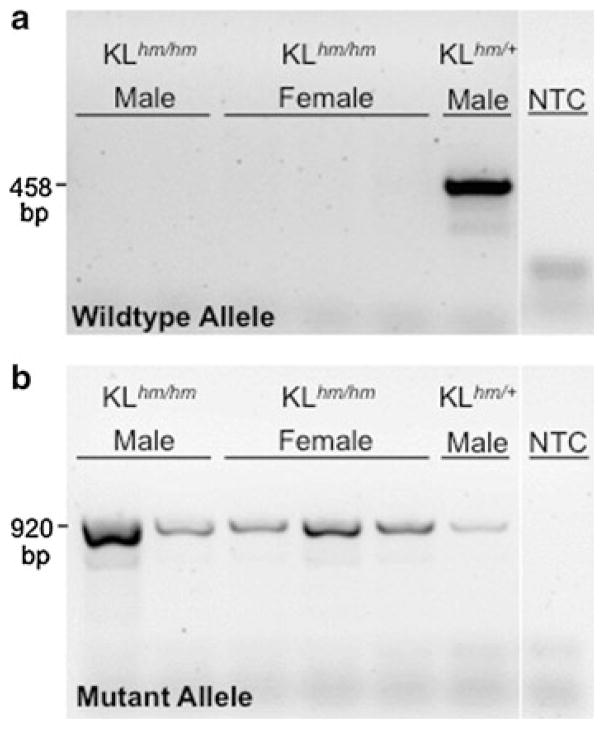
We validated the genotype of our klothohm colony founders and of their progeny using established PCR assays (Kuro-o et al. 1997). All homozygous klothohm mice were found to be negative for the wildtype klotho allele (Fig. 1a) and positive for the klotho mutant allele (Fig. 1b). Heterozygous mice were positive for both wildtype and mutant alleles (Fig. 1a, b). We then characterized our local colony of klothohm C57BL/6 mice for endogenous klotho mRNA expression compared to wildtype mice using RT-qPCR. This analysis was done with RNA isolated from the kidney, the main site of klotho production (Kuro-o et al. 1997). Klotho expression values (ΔCt) were calculated by normalizing klotho expression to endogenous Eef2 expression, determined for each individual RNA sample (mean ± SEM, Ct: kidney 17.12 ± 0.09; gastrocnemius muscle 18.51 ± 0.34).
Fig. 1.
Genotyping of the klothohm mouse. PCR analysis of a wildtype and b mutant alleles in klothohm mice (klothohm/hm) compared to a heterozygous control mouse (klothohm/+). PCR no template control samples (NTC) are included for each primer set
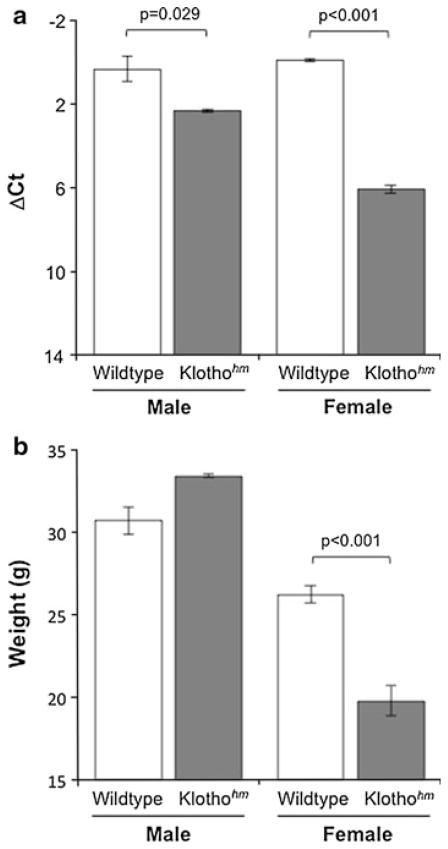
The RT-qPCR analysis of kidney cDNA revealed no differences in klotho expression between wildtype C57BL/6 males and females (p = 0.76, Fig. 2a; mean ± SEM, ΔCt: male, n = 3, 0.34 ± 0.6; female, n = 6, −0.05 ± 0.06). Both male and female klothohm mice, showed significantly reduced klotho expression in the kidney compared to the wildtype mice (Fig. 2a, mean ± SEM ΔCt: male, n = 3, 2.34 ± 0.34; female, n = 3, 6.09 ± 0.2). Surprisingly, there was a significant difference in the extent of decline in klotho expression between male and female klothohm mice. Whereas klothohm males exhibited only 4-fold less klotho expression in the kidney when compared to wildtype male mice, the female klothohm mice had 71-fold less expression than wildtype females (Fig. 2a).
Fig. 2.
Characterization of the klothohm mouse. a Klotho expression level (ΔCt, mean ± SEM) in the kidney of wild type (male n = 3, female n = 6) and klothohm (male n = 3, female n = 3) mice. All samples were independently normalized to the expression of the Eef2 reference gene. b Body weight (mean ± SEM, grams) of wildtype (male n = 9, female n = 6) and klothohm (male n = 4, female n = 7) mice between the ages of 7 and 11 months. Brackets represents significant differences with the indicated p value
In addition to their drastic decline in kidney klotho expression, female klothohm mice were significantly smaller than wildtype females (Fig. 2b, data shown for 7–11 months old animals; mean ± SEM, grams: Klothohm 19.77 ± 0.90; WT 26.2 ± 0.52). There was however, no difference between the weight of mutant and wildtype males (Fig. 2b, 7–11 months old animals; mean ± SEM, grams: Klothohm 33.4 ± 0.09; WT 30.67 ± 0.81). The fact that male klothohm mice did not differ in weight from wildtype males could suggest that the relatively low knockdown of klotho mRNA in male klothohm C57BL/6 mice was insufficient to trigger changes in body weight. Indeed, in the original klothohm mouse, both males and females are significantly smaller than the wildtype mice (Kuro-o et al. 1997).
Characterization of the klotho overexpressing, EFmKL46 C57BL/6 mice
Two transgenic lines that overexpress klotho (driven by the ubiquitous human elongation factor 1 promoter) were previously produced and both showed increased lifespan compared to wildtype mice (Kuro-o et al. 1997; Kurosu et al. 2005). These mice were created on the same mixed mouse background as that described above for the original klothohm mouse. From these two transgenic lines, we focused in the current study on the transgenic strain EFmKL46, which was reported to have transgene expression in skeletal muscle (Kurosu et al. 2005). Klotho serum protein levels have been previously shown to be elevated in EFmKL46 mice concurrent with increased transcript expression (Kurosu et al. 2005). The skeletal muscles of EFmKL46 mice are therefore likely exposed to the direct effects of both secreted and membrane-bound klotho forms.
All EFmKL46 mice used in this study were confirmed to harbor the transgene based on genotyping (see “Materials and methods”). Klotho expression level was then analyzed in both the kidney and gastrocnemius muscle from one male and one female EFmKL46 mouse, to verify klotho overexpression. Per each tissue, no difference in klotho expression was observed between the male and female mice, so genders were combined for analysis. Klotho overexpression in the gastrocnemius muscle of EFmKL46 mice was 51-fold lower than expression levels found in the kidney (mean, ΔCt: gastrocnemius 5.78, kidney 0.10). Nevertheless, the gastrocnemius klotho expression level in EFmKL46 mice was 70-fold higher compared to the low levels of expression present in the gastrocnemius muscle of wildtype mice (mean ± SEM, ΔCt: WT gastrocnemius 11.90 ± 0.65, WT kidney 0.08 ± 0.19, based on mice presented in Fig. 2a). Klotho expression was not detected in the gastrocnemius muscle of klothohm mice (see “Materials and methods”). The low level of klotho expression detected in the gastrocnemius muscle of wildtype mice is consistent with earlier studies that have shown little to no klotho expression in various limb muscles (Kuro-o et al. 1997; Fon Tacer et al. 2010; Stuelsatz et al. 2012, supplemental Fig. S3). It is important to note that klotho gene expression in the muscle tissue does not necessarily arise from myogenic cells. Our unpublished expression data with myogenic and non-myogenic cell populations isolated from adult mouse muscles (Day et al. 2007) attribute low-level klotho expression only to the non-myogenic cell population (K. Day and Z. Yablonka-Reuveni).
Assessment of muscle strength and running endurance in female mice
In order to examine the effect of klotho deficiency and overexpression in the physiological context of muscle performance, klothohm, EFmKL46, and wildtype C57BL/6 adult females (7–11 month old), were subjected to standard protocols for measuring forelimb grip strength and voluntary wheel running. These physiological assays were preferentially performed on female mice in view of the distinctive effect of klotho deficiency on klothohm females (i.e., a large decline in klotho kidney expression and reduced weight) compared to only a slight effect in klothohm males (i.e., only a small decline in kidney klotho expression). Notably, the reduced body weight of the klothohm females was not due to a change in lean/fat body composition, as shown by QMR analysis (mean ± SEM, %lean, %fat: Klothohm, 76.99 ± 0.46, 11.99 ± 0.66; WT, 74.81 ± 1.57, 14.47 ± 1.41; EF-mKL46, 78.07 ± 1.07, 13.83 ± 1.16).
Klothohm mice had significantly reduced grip strength compared to wildtype and EFmKL46 mice (Fig. 3). The maximum forelimb grip force measured in female klothohm mice was 53 % and 51 % lower than wildtype and EFmKL46 mice, respectively (Fig. 3a). After normalizing raw grip force to body weight, klothohm mice exhibited 37 % less strength than both wildtype and EFmKL46 mice (Fig. 3b). These results were consistently reproduced when assayed 4 independent times with the same animals over a 4-month period.
Fig. 3.
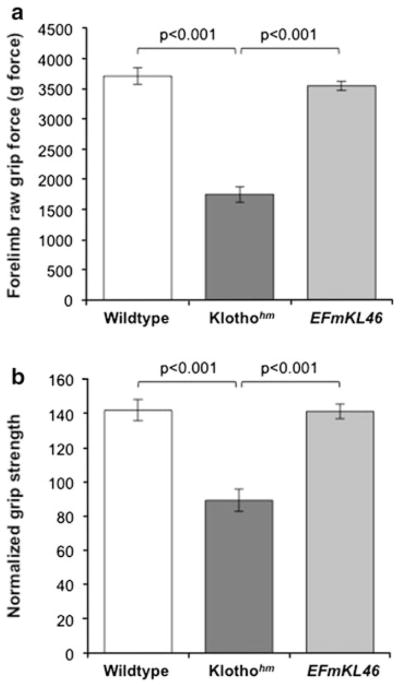
Analysis of forelimb grip strength in adult (7–11 months old) female mice. a Maximum grip strength force (mean ± SEM, grams-force) and b normalized force to body weight ratio (mean ± SEM) of wild type (n = 7), klothohm (n = 7), and klotho transgenic, EFmKL46 mice (n = 8). Significant differences identified by brackets with respective p values
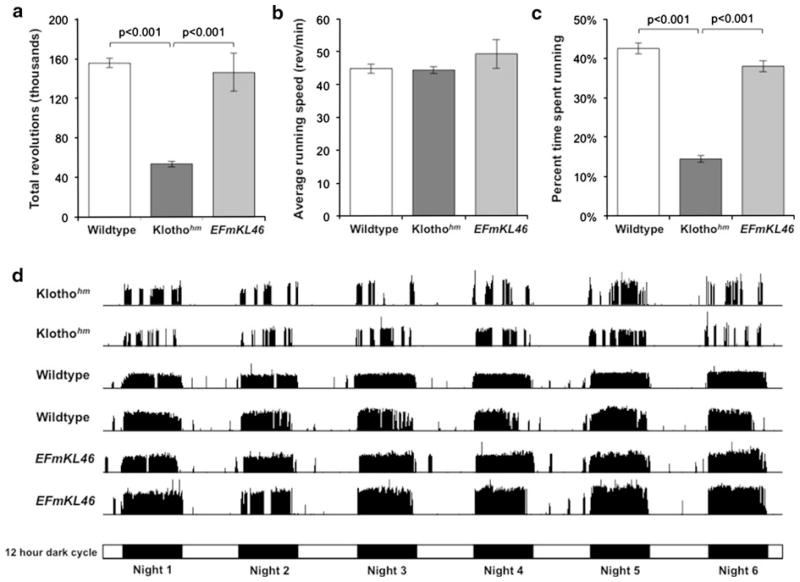
The female mice were additionally examined for their endurance level by submitting the animals to voluntary wheel running for 6 days (Fig. 4). Consistently for all 3 experimental groups, the active running period was during the dark cycle. Whilethere was no difference in the total revolutions ran between wildtype and EFmKL46 mice, the klothohm mice ran 66 and 63 % less than wildtype and EFmKL46 mice, respectively (Fig. 4a). A detailed analysis of klothohm mouse running trends revealed that during their actual running activity there was no difference in the average running speed (i.e., revolutions per minute) compared to wildtype and EFmKL46 mice (Fig. 4b). Klothohm mice however, spent 66 and 62 % less time running than wildtype and EFmKL46 mice, respectively (Fig. 4c). This is readily apparent when analyzing the overall running profile actograms from each mouse strain, which show a sporadic running pattern in klothohm mice (Fig. 4d). Similar results were observed in 2 additional running wheel experiments conducted when the mice were younger in age.
Fig. 4.
Mouse activity over 6 days of voluntary wheel running. Studies were performed with the adult female groups described in Fig. 3. a Klothohm mice ran significantly less than wildtype and EFmKL46 mice as shown by the total revolutions ran over the 6 days analyzed. b While klothohm mice ran at the same rate as wildtype and EFmKL46 mice (i.e., speed in revolutions/min, per actual time spent running), as shown in c the percentage of time spent running was significantly less compared to the other two groups. d Typical running frequency actograms (showing 2 independent examples per each mouse group), demonstrating a unique sporadic running pattern in klothohm mice characterized by frequent gaps in the nightly running routine. A schematic representation of the light and dark cycle (12/12-h), over the 6 experimental days, is shown below the actograms. a–c Significant differences are noted by brackets and respective p values
Changes in physical activity were indeed evident in the original klothohm mouse from the mixed strain background. These mice demonstrated an altered gait with significantly reduced stride lengths (Kuro-o et al. 1997). They also exhibited 50 % less horizontal and rearing activity than control mice when assayed in an open field experiment (Kuro-o et al. 1997). Interpretations about physical activity in the original klothohm mouse strain however, have been limited by the fact that these mice do not strive and die at a young age from a number of complications. We did not observe any obvious changes in routine cage activity or gait disturbances in klothohm C57BL/6 mice, but these parameters were not measured directly.
A number of conditions could be responsible for the observed decreases in muscle strength and running endurance in klothohm mice. In addition to potentially influencing skeletal muscle, klotho may influence muscle strength by its effect on bone density. Bone mineral density has been consistently shown to be associated with muscle strength in humans (Arden and Spector 1997). The original klothohm mouse strain exhibited a significant reduction in bone mineral density (Kuro-o et al. 1997). This decrease in bone mineral density is believed to be due to the effect of klotho on osteoblast and osteoclast differentiation as well as on calcium and phosphate homeostasis (Kawaguchi et al. 1999; Kuro-o 2006; Nakatani et al. 2009). While further studies would be needed to establish the mechanism involved in the reduced grip strength exhibited by klothohm females, it is interesting to note that a positive correlation between klotho levels and grip strength has been established in a longitudinal study of elderly humans (Semba et al. 2012).
The sporadic running trend exhibited by klothohm mice could also suggest changes in the cardiovascular or respiratory systems, which could induce early onset exhaustion in klotho deficient mice. Cardiovascular and respiratory changes have been documented in the original klothohm mouse strain (Kuro-o et al. 1997). These mice developed arteriolosclerosis, pulmonary emphysema and ectopic calcification of several tissues including bronchial mucosa, alveolar cells, and cardiac muscle (Kuro-o et al. 1997; Suga et al. 2000). Even heterozygous klothohm mice were shown to exhibit endothelial dysfunction (Saito et al. 1998; Takeshita et al. 2004).
There are many intriguing possibilities for why female klothohm mice exhibit such a dramatic decline in muscle-associated functions compared to wildtype and EFmKL46 animals. Whether it involves the musculoskeletal, cardiac or respiratory systems or a combination of factors needs to be further investigated at multiple ages and at the histological level.
Conclusion
Klotho has been shown to have broad reaching effects on a number of body systems. Insight into the role of klotho in skeletal muscle is however, extremely limited. Our results demonstrate that klotho deficiency has a dramatic effect on both muscle strength and running endurance in mice. While it is currently unclear as to the exact underlying cause of the decline in muscle function in klothohm mice, our data provide a critical first step to understanding the impacts of klotho on skeletal muscle. This research also reinforces the importance of mouse strain background and gender in influencing the phenotypic effect of specific genetic modifications. The mouse strain effect is highlighted in this study with the use of klothohm mice from the C57BL/6 mouse background, which do not show any overt pathology and live to an old age. Our research demonstrates that the underutilized klothohm C57BL/6 mouse is a unique model to study the effects of klotho in adult mice without the experimental limitation of an ailing phenotype and a short lifespan. Overall, our findings indicate that klotho deficiency influences muscle strength and running endurance in mice. As the decline in muscle-associated performance is also the hallmark of muscle aging, future research should examine klotho as a potential inhibitor of age-associated muscle deterioration with the goal of understanding the complex mechanisms underlying sarcopenia.
Acknowledgments
We would like to thank the contribution of Heather Hopkins, who helped in the execution of the physiological experiments. We are additionally grateful to Pascal Stuelsatz for his valuable insight on data analysis. We would also like to thank the Nathan Shock Functional Assessment Core and the Department of Comparative Medicine, In-Vivo Services Core facilities for providing essential support for our physiological studies. This research was supported by a grant from the National Institutes of Health to ZYR (AG021566). MP is a recipient of a postdoctoral fellowship from the Genetic Approaches to Aging training grant (T32 AG000057).
Contributor Information
Michael Phelps, Department of Biological Structure, School of Medicine, University of Washington, Health Sciences Building, Room G520, 1959 NE Pacific Street, Box 35740, Seattle, WA 98195, USA.
Christina Pettan-Brewer, Department of Comparative Medicine, School of Medicine, University of Washington, Seattle, WA 98195, USA.
Warren Ladiges, Department of Comparative Medicine, School of Medicine, University of Washington, Seattle, WA 98195, USA.
Zipora Yablonka-Reuveni, Email: reuveni@uw.edu, Department of Biological Structure, School of Medicine, University of Washington, Health Sciences Building, Room G520, 1959 NE Pacific Street, Box 35740, Seattle, WA 98195, USA.
References
- Allen RE, Boxhorn LK. Inhibition of skeletal muscle satellite cell differentiation by transforming growth factor-beta. J Cell Physiol. 1987;133:567–572. doi: 10.1002/jcp.1041330319. [DOI] [PubMed] [Google Scholar]
- Arden N, Spector T. Genetic influences on muscle strength, lean body mass, and bone mineral density: a twin study. J Bone Miner Res. 1997;12:2076–2081. doi: 10.1359/jbmr.1997.12.12.2076. [DOI] [PubMed] [Google Scholar]
- Barberi L, Scicchitano BM, De Rossi M, Bigot A, Duguez S, Wielgosik A, Stewart C, McPhee J, Conte M, Narici M, Franceschi C, Mouly V, Butler-Browne G, Musarò A. Age-dependent alteration in muscle regeneration: the critical role of tissue niche. Biogerontology. 2013;14:273–292. doi: 10.1007/s10522-013-9429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, Kaether C. Klotho is a substrate for α-, β- and γ-secretase. FEBS Lett. 2009;583:3221–3224. doi: 10.1016/j.febslet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack AS, Bildsoe H, Hughes SM. Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J Cell Sci. 2005;118:4813–4821. doi: 10.1242/jcs.02602. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Bucci L, Yani SL, Fabbri C, Bijlsma AY, Maier AB, Meskers CG, Narici MV, Jones DA, McPhee JS, Seppet E, Gapeyeva H, Pääsuke M, Sipilä S, Kovanen V, Stenroth L, Musarò A, Hogrel JY, Barnouin Y, Butler-Browne G, Capri M, Franceschi C, Salvioli S. Circulating levels of adipokines and IGF-1 are associated with skeletal muscle strength of young and old healthy subjects. Biogerontology. 2013;14:261–272. doi: 10.1007/s10522-013-9428-5. [DOI] [PubMed] [Google Scholar]
- Burks TN, Andres-Mateos E, Marx R, Mejias R, Van Erp C, Simmers JL, Walston JD, Ward CW, Cohn RD. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci Transl Med. 2011;3:82ra37. doi: 10.1126/scitranslmed.3002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson B, Faulkner J. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol. 1989;256:C1262–C1266. doi: 10.1152/ajpcell.1989.256.6.C1262. [DOI] [PubMed] [Google Scholar]
- Carlson ME, Hsu M, Conboy IM. Imbalance between pSmad3 and notch induces CDK inhibitors in old muscle stem cells. Nature. 2008;454:528–532. doi: 10.1038/nature07034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson ME, Suetta C, Conboy MJ, Aagaard P, Mackey A, Kjaer M, Conboy I. Molecular aging and rejuvenation of human muscle stem cells. EMBO Mol Med. 2009;1:381–391. doi: 10.1002/emmm.200900045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S, Ortega B, Kurosu H, Rosenblatt KP, Kuro-o M, Huang C. Removal of sialic acid involving klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA. 2008;105:9805–9810. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–360. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extra-cellular domain of klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. 2005;4:407–410. doi: 10.4161/cc.4.3.1518. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z. Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev Biol. 2007;304:246–259. doi: 10.1016/j.ydbio.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M. Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286:8655–8665. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WJ, Paolisso G, Abbatecola AM, Corsonello A, Bustacchini S, Strollo F, Lattanzio F. Frailty and muscle metabolism dysregulation in the elderly. Biogerontology. 2010;11:527–536. doi: 10.1007/s10522-010-9297-0. [DOI] [PubMed] [Google Scholar]
- Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol. 2007;34:1091–1096. doi: 10.1111/j.1440-1681.2007.04752.x. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fon Tacer KF, Bookout AL, Ding X, Kurosu H, John GB, Wang L, Goetz R, Mohammadi M, Kuro-o M, Mangelsdorf DJ, Kliewer SA. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol. 2010;24:2050–2064. doi: 10.1210/me.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrick CL. Life-span and the inheritance of longevity of inbred mice. J Gerontol. 1975;30:257–263. doi: 10.1093/geronj/30.3.257. [DOI] [PubMed] [Google Scholar]
- Iida R, Kanko S, Suga T, Morito M, Yamane A. Auto-phagic-lysosomal pathway functions in the masseter and tongue muscles in the klotho mouse, a mouse model for aging. Mol Cell Biochem. 2011;348:89–98. doi: 10.1007/s11010-010-0642-z. [DOI] [PubMed] [Google Scholar]
- Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted klotho protein in sera and CSF: implication for post-translational cleavage in release of klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- Janssen I. Evolution of sarcopenia research. Appl Physiol Nutr Metab. 2010;35:707–712. doi: 10.1139/H10-067. [DOI] [PubMed] [Google Scholar]
- Kawaguchi H, Manabe N, Miyaura C, Chikuda H, Nakamura K, Kuro-o M. Independent impairment of osteoblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteopenia. J Clin Invest. 1999;104:229–237. doi: 10.1172/JCI5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuro-o M. Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens. 2006;15:437–441. doi: 10.1097/01.mnh.0000232885.81142.83. [DOI] [PubMed] [Google Scholar]
- Kuro-o M. Klotho and aging. Biochim Biophys Acta. 2009;1790:1049–1058. doi: 10.1016/j.bbagen.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Kuro-o M. The klotho gene family as a regulator of endocrine fibroblast growth factors. Mol Cell Endocrinol. 2009;299:72–78. doi: 10.1016/j.mce.2008.10.052. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- Loffredo F, Steinhauser M, Jay S, Gannon J, Pancoast J, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach J, Miller C, Singer B, Stewart A, Psychogios N, Gerszten R, Hartigan A, Kim M, Serwold T, Wagers A, Lee R. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. Of aging mice and men. Science. 2007;318:390. doi: 10.1126/science.318.5849.390b. [DOI] [PubMed] [Google Scholar]
- Nakatani T, Sarraj B, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, Mohammadi M, Lanske B, Razzaque MS. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23)-mediated regulation of systemic phosphate homeostasis. FASEB J. 2009;23:433–441. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettan-Brewer C, Treuting PM. Practical pathology of aging mice. Pathobiol Aging Age Relat Dis. 2011;1:7202. doi: 10.3402/pba.v1i0.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader EP, Faulkner JA. Effect of aging on the recovery following contraction-induced injury in muscles of female mice. J Appl Physiol. 2006;101:887–892. doi: 10.1152/japplphysiol.00380.2006. [DOI] [PubMed] [Google Scholar]
- Razzaque MS. FGF23-mediated regulation of systemic phosphate homeostasis: is klotho an essential player? Am J Physiol Renal Physiol. 2009;296:F470–F476. doi: 10.1152/ajprenal.90538.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault V, Thorne L, Eriksson P, Butler-Browne G, Mouly V. Regenerative potential of human skeletal muscle during aging. Aging Cell. 2002;1:132–139. doi: 10.1046/j.1474-9728.2002.00017.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127:990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- Rosenberg IH. Sarcopenia: origins and clinical relevance. Clin Geriatr Med. 2011;27:337–339. doi: 10.1016/j.cger.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology. 2008;9:213–228. doi: 10.1007/s10522-008-9131-0. [DOI] [PubMed] [Google Scholar]
- Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, Matsumura Y, Masuda H, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998;248:324–329. doi: 10.1006/bbrc.1998.8943. [DOI] [PubMed] [Google Scholar]
- Sanchez-Niño M, Sanz A, Ortiz A. Klotho to treat kidney fibrosis. J Am Soc Nephrol. 2013;14:273–292. doi: 10.1681/ASN.2013030294. [DOI] [PubMed] [Google Scholar]
- Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, Guralnik JM, Ferrucci L. Relationship of low plasma klotho with poor grip strength in older community-dwelling adults: the InCHIANTI study. Eur J Appl Physiol. 2012;112:1215–1220. doi: 10.1007/s00421-011-2072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano AL, Muñoz-Cánoves P. Regulation and dysregulation of fibrosis in skeletal muscle. Exp Cell Res. 2010;316:3050–3058. doi: 10.1016/j.yexcr.2010.05.035. [DOI] [PubMed] [Google Scholar]
- Shefer G, Yablonka-Reuveni Z. Ins and outs of satellite cell myogenesis: the role of the ruling growth factors. In: Schiaffino S, Partridge T, editors. Skeletal Muscle Repair and Regeneration. Vol. 3. Springer; Netherlands: 2008. pp. 107–144. Advances in Muscle Research. Chapter 6. [Google Scholar]
- Shefer G, de Mark Van, Daniel P, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer G, Rauner G, Yablonka-Reuveni Z, Benayahu D. Reduced satellite cell numbers and myogenic capacity in aging can be alleviated by endurance exercise. PLoS One. 2010;5:e13307. doi: 10.1371/journal.pone.0013307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer G, Rauner G, Stuelsatz P, Benayahu D, Yablonka-Reuveni Z. Moderate-intensity treadmill running promotes expansion of the satellite cell pool in young and old mice. FEBS J. 2013;280:4064–4073. doi: 10.1111/febs.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010;38:D792–D799. doi: 10.1093/nar/gkp1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuelsatz P, Keire P, Almuly R, Yablonka-Reuveni Z. A contemporary atlas of the mouse diaphragm: myogenicity, vascularity, and the Pax3 connection. J Histochem Cytochem. 2012;60:638–657. doi: 10.1369/0022155412452417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga T, Kurabayashi M, Sando Y, Ohyama Y, Maeno T, Maeno Y, Aizawa H, Matsumura Y, Kuwaki T, Kuro-O M, Nabeshima Y, Nagai R. Disruption of the klotho gene causes pulmonary emphysema in mice. defect in maintenance of pulmonary integrity during postnatal life. Am J Respir Cell Mol Biol. 2000;22:26–33. doi: 10.1165/ajrcmb.22.1.3554. [DOI] [PubMed] [Google Scholar]
- Takeshita K, Fujimori T, Kurotaki Y, Honjo H, Tsujikawa H, Yasui K, Lee J, Kamiya K, Kitaichi K, Yamamoto K, Ito M, Kondo T, Iino S, Inden Y, Hirai M, Murohara T, Kodama I, Nabeshima Y. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation. 2004;109:1776–1782. doi: 10.1161/01.CIR.0000124224.48962.32. [DOI] [PubMed] [Google Scholar]
- Thompson LV. Age-related muscle dysfunction. Exp Gerontol. 2009;44:106–111. doi: 10.1016/j.exger.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama K, Maeda R, Urakawa I, Yamazaki Y, Tanaka T, Ito S, Nabeshima Y, Tomita T, Odori S, Hosoda K, Nakao K, Imura A, Nabeshima Y. Relevant use of klotho in FGF19 subfamily signaling system in vivo. Proc Natl Acad Sci USA. 2010;107:1666–1671. doi: 10.1073/pnas.0913986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turturro A, Duffy P, Hass B, Kodell R, Hart R. Survival characteristics and age-adjusted disease incidences in C57BL/6 mice fed a commonly used cereal-based diet modulated by dietary restriction. J Gerontol A Biol Sci Med Sci. 2002;57:B379–B389. doi: 10.1093/gerona/57.11.b379. [DOI] [PubMed] [Google Scholar]
- Walston JD. Sarcopenia in older adults. Curr Opin Rheumatol. 2012;24:623–627. doi: 10.1097/BOR.0b013e328358d59b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Ge H, Gupte J, Weiszmann J, Shimamoto G, Stevens J, Hawkins N, Lemon B, Shen W, Xu J, Veniant MM, Li YS, Lindberg R, Chen JL, Tian H, Li Y. Co-receptor requirements for fibroblast growth factor-19 signaling. J Biol Chem. 2007;282:29069–29072. doi: 10.1074/jbc.C700130200. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z. The skeletal muscle satellite cell: still young and fascinating at 50. J Histochem Cytochem. 2011;59:1041–1059. doi: 10.1369/0022155411426780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rivera AJ. Proliferative dynamics and the role of FGF2 during myogenesis of rat satellite cells on isolated fibers. Basic Appl Myol (BAM) 1997;7:189–202. [PMC free article] [PubMed] [Google Scholar]
- Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovas. 2008;18:388–395. doi: 10.1016/j.numecd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Zhou L, Li Y, Zhou D, Tan RJ, Liu Y. Loss of klotho contributes to kidney injury by derepression of wnt/β-catenin signaling. J Am Soc Nephrol. 2013;24:771–785. doi: 10.1681/ASN.2012080865. [DOI] [PMC free article] [PubMed] [Google Scholar]