Abstract
Protein kinases Akt1 and Akt3 are considered to be more crucial to brain function than Akt2. We investigated the roles of Akt1 and Akt3 in stroke-induced brain injury and examined their interactions with the Akt/mTOR pathways. Focal ischemia was induced in rats. Lentiviral vectors expressing constitutively active Akt1 and Akt3 (cAkt1 and cAkt3) were injected into the ischemic cortex. Infarct sizes and gene and protein expressions in the Akt/mTOR pathways were evaluated. The results show that Akt1 and Akt3 proteins were degraded as early as 1 hour after stroke, whereas Akt2 proteins remained unchanged until 24 hours after stroke. Lentiviral-mediated overexpression of cAkt1 or cAkt3 reduced neuronal death after in vitro and in vivo ischemia. Interestingly, cAkt3 overexpression resulted in stronger protection than cAkt1 overexpression. Western blot analyses further showed that cAkt3 promoted significantly higher levels of phosphorylated Akt and phosphorylated mTOR than cAkt1. The mTOR inhibitor rapamycin blocked the protective effects of both cAkt1 and cAkt3. In conclusion, Akt isoforms are differentially regulated after stroke and Akt3 offers stronger protection than cAkt1 by maintaining Akt levels and promoting mTOR activity.
Keywords: Akt1, Akt3, cerebral ischemia, mTOR, stroke
Introduction
The PI3K/Akt pathway regulates metabolism, cell growth, and survival.1 PTEN is a phosphatase in the Akt pathway that dephosphorylates Akt. Phosphorylated, active Akt blocks apoptosis by phosphorylating a number of downstream substrates, including the forkhead transcription factor FKHR (FOXO1), GSK3β, PRAS40, mTOR, and p70S6 ribosomal protein (S6K1)1 (Figure 1A). Nonphosphorylated, active FKHR translocates from the cytosol into the nuclei and promotes production of proapoptotic proteins, such as Fas ligand. Nonphosphorylated, active GSK3β also promotes the proapoptotic activity of the Bcl-2-associated X protein, thus inhibiting the mitochondria-associated intrinsic apoptotic pathway.2 Akt can directly phosphorylate mTOR and stimulate its activity.3 It can also indirectly increase mTOR phosphorylation via PRAS40 phosphorylation. Once active, mTOR causes further phosphorylation of downstream proteins such as p70S6 ribosomal protein kinase1 (S6K1), which regulates protein translation and cell growth.4
Figure 1.
Diagram of Akt/mTOR pathways, definition of ischemic penumbra and core, and expression of Akt isoforms after stroke. (A) Diagram shows that the PI3K/Akt pathway closely interacts with the mTOR pathway. (B) Diagram shows the definition of ischemic penumbra and core. Penumbra (P) refers to the ischemic region that can be rescued by various neuroprotectants. Core (C) refers to the region of permanent damage. (C) Representative DNA bands from RT-PCR for Akt1, Akt2, and Akt3 in both the ischemic penumbra and core. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping control gene. Bar graphs represent relative optical densities of DNA bands of Akt isoforms in ischemic brains, normalized to densities in sham rats and expressed as percentages. (D) Representative western blot with protein bands for Akt1, Akt2, and Akt3. A β-actin control ensured equal protein loading. Bar graphs represent relative optical densities of Akt1, Akt2, and Akt3 protein bands, normalized to those in the sham group. N=6/group. *, **, ***, versus sham, P<0.05, 0.01, 0.001, respectively; Φ, ΦΦ P<0.05, 0.01, respectively, between the two indicated groups. n=5 to 6/group.
Akt consists of three isoforms: Akt1, Akt2, and Akt3.5 Although Akt has been extensively studied and is considered to be neuroprotective in stroke,6 the contribution of each Akt isoform remains unclear. Previous studies suggest that Akt1 and Akt3 are more critical to brain function than Akt2. Both Akt1 and Akt3 are expressed abundantly in the brain, whereas Akt2 is predominantly expressed in the heart and brown adipose tissue, with relatively low expression in the brain.7 Genetic knockouts of various Akt isoforms have shown that both Akt1 and Akt3 deficiency lead to smaller brain sizes,8, 9 and the loss of Akt3 is associated with mild neurologic deficits.10 In contrast, the loss of Akt2 leads to insulin-resistant diabetes with hyperglycemia.11 Previous studies used PI3K inhibitors, such as wortmannin and LY294002, which blocked Akt phosphorylation to show that Akt mediates neuroprotective effects.6 However, existing PI3K pharmacologic inhibitors affect multiple signaling pathways and are nonspecific and indirect. Furthermore, genetic manipulation of Akt in stroke models produced controversial results—compared with wild-type controls, stroke in Akt1 knockout12 and Akt transgenic animals13 resulted in smaller infarct volume, possibly due to developmental and global effects of traditional transgenic or gene knockout mouse models.
On the basis of these observations, we investigated the potential roles of Akt isoforms in stroke through genetic approaches that directly manipulate Akt expression in the ischemic penumbra. We focused on Akt1 and Akt3, the critical Akt isoforms in brain function. We investigated their effects in vitro and in vivo, and examined the upstream and downstream molecules of the Akt pathway, including PTEN, FKHR, GSK3β, and mTOR.
Materials and methods
Animal experiments were conducted according to the protocols approved by the Stanford Intuitional Animal Care and Use Committee and the NIH Guidelines for Care and Use of Laboratory Animals, as well as ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines. Animals were housed under a 12:12 hour light–dark cycle with food and water available ad libitum.
Construction of Lentiviral Vectors
We constructed lentiviral vectors containing genes of constitutively active Akt1 and Akt3 (cAkt1 and cAkt3), and dominant-negative Akt (AktDN), but were unable to construct cAkt2 lentiviral vector. Target genes were cloned from the following plasmids: cAkt1-HA (pcDNA3.1) and cAkt3-HA (pcDNA3.1) provided by Dr Richard Roth, Department of Chemical and Systems Biology, Stanford University; and AktDN (pcDNA3) purchased from Addgene (AktDN: 16243, HA-AktDN K197M; Cambridge, MA, USA). These genes were cloned into the lentiviral backbone plasmid, pHR'tripCMV-IRES-eGFP, which contains a CMV promoter and an IRES sequence between its multiple cloning sites and eGFP. The IRES sequence enables independent expression of both the target gene and eGFP simultaneously. Restriction enzymes used for cloning were SalI/SacII (for cAkt1), SacII (for cAk3), and SalI/BamHI (for AktDN). The lentiviral plasmid backbone inserted with only eGFP was used as the control vector. The cAkt1 and cAkt3 were fused with a Gag polypeptide and lacked the pleckstrin homology domain; the expression of a myristoylation signal enabled Akt1 and Akt3 proteins to attach to the cell membrane and become constitutively phosphorylated, thus activated. The AktDN gene is an Akt gene where a point mutation in the 179th amino acid from the N terminal converts a lysine into a methionine. This mutated form of the Akt protein can still be phosphorylated at the S473 site, but loses its ability to affect downstream factors.
Lentiviral Vector Generation and Titration
We used a 3 plasmid system for lentivirus packaging: the lentiviral transfer vector (pHR'tripCMV-IRES-eGFP) that contains the coding region of various targeted genes as described above; the packaging plasmid (p-delta) that provides all vector proteins driven by the trip CMV promoter, except the envelope protein; and the envelope-encoding plasmid (p-VSVG) that encodes the heterologous vesicular stomatitis virus envelope protein (VSVG).14 Briefly, 293T cells were grown in Dulbecco's modified Eagle medium (DMEM; Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Gibco). A mixture of 45 μg of transfer vectors, 30 μg of packaging plasmids, and 15 μg of envelope-encoding plasmids was transiently transfected into three separate T175 flasks containing 1.5 × 107 HEK-293T cells using the calcium phosphate precipitation method. Supernatants were collected 72 hours after transfection and viral particles were concentrated by ultracentrifugation. Viruses were resuspended in phosphate-buffered saline (PBS) and kept at −80°C until use. The virus particles were titered by the TCID50 method as described previously.15, 16 Virus titers ranged from 1 × 108 to 5 × 108 transduction units/mL and were diluted in PBS to the final concentration of 1 × 108 transduction units/mL before gene transfer was conducted.
In Vitro Oxygen Glucose Deprivation Model, Gene Transfer, and Cell Viability Assay
Primary neuronal cultures were prepared using timed-pregnant Sprague-Dawley rats (E18; Charles River Laboratories International, Wilmington, MA, USA). Briefly, rats were anesthetized with isoflurane and the E18 embryos were removed. The cortical region of the fetal brains was dissected in warm media and pooled together. The cortices were titrated and incubated in papain for 20 minutes at 37°C, then centrifuged at 138 g for 5 minutes at room temperature. Cells were resuspended in MEM (minimal essential medium) (Gibco) containing 10% fetal horse serum (Hyclone, Logan, UT, USA), 2 mmol/L glutamine (Gibco), 25 mmol/L glucose, 1% penicillin/streptomycin (Gibco). Cells were plated onto poly-D-lysine-coated tissue culture plates at 7.5 × 105 cells/mL. Media was completely changed after 24 hours. One-half medium changes were done on day 4. Cultures were incubated at 37°C in a 5% CO2 incubator and experiments were performed after days 9 to 11.
The lentiviral vectors, diluted with PBS to 5 μL for 24-well plates and to 10 μL for 6-well plates, were directly added in the medium of 9-day primary mixed neuron cultures with the multiplicity of infection at 1:5 (cells to virus units). The same amount of PBS was also added to the medium as an experimental control.17 Then, cells were incubated at 37°C in a 5% CO2 incubator for another 2 days before oxygen glucose deprivation (OGD) was conducted. Primary mixed neuron cultures were washed twice with glucose-free balanced salt solution (BSS0, pH 7.4) and the plates were transferred to a modular hypoxic chamber filled with mixed gases of 5% CO2 and 95% N2. Oxygen level was maintained at <0.02% at 37°C. The cells were kept in the hypoxic chamber for 6 hours. Cultures were then restored with glucose at a final concentration of 5.5 mmol/L (BSS5.5, pH 7.4) and recovered at normoxic conditions (37°C, 5% CO2) for ∼18 hours (OGD restoration). The control groups without OGD were washed twice with 5.5 mmol/L glucose in balanced salt solution (BSS 5.5, pH 7.4) with no oxygen deprivation.
Cell viability was quantified by measuring lactate dehydrogenase (LDH) release at 18 hours after OGD restoration using a previously described colorimetric assay.18 Briefly, 100 μL of cell-free supernatant was transferred to 96-well plates. The supernatant was incubated with 150 μL of NADH/phosphate buffer (0.15 mg/mL) for 10 minutes. Then, 30 μL of sodium pyruvate (2.97 mg/mL) was added and the absorbance wavelength was measured at 340 nm using a microplate reader. Background absorbance was subtracted and the percentage of LDH release was calculated based on an LDH standard curve.
Focal Cerebral Ischemia
Focal ischemia was generated as described previously in male Sprague-Dawley rats (250 to 280 g; Charles River Laboratories International, Wilmington, MA, USA).19, 20, 21 Core body temperatures were maintained at 36.5°C to 37.2°C throughout the experiments; heart rate, respiratory rate, and arterial pO2, pCO2, and pH were controlled within normal ranges. Rats were anesthetized with 5% isoflurane and maintained with 2% to 3% isoflurane. A ventral midline incision was made and the two common carotid arteries (CCAs) were isolated. Then, a 2-cm vertical scalp incision was made midway between the left eye and ear. The temporalis muscle was bisected and a 2-mm burr hole was made at the junction of the zygomatic arch and squamous bone. The distal middle cerebral artery was exposed and cauterized above the rhinal fissure at the cross of lateral vein and middle cerebral artery. The bilateral CCAs were occluded for 60 minutes with suture tightening. All animals were randomly assigned into different groups. After surgery, inactive animals and those with hunched posture, unkempt/rough coat, abnormal color of mucous membranes, abnormal respiratory patterns, and severe dehydration were euthanized earlier. In addition, rat brains subjected to stroke but without infarction, and animals with severe bleeding or that died within 48 hours after stroke or virus injection were excluded from the analyses. We estimate that ∼10% of animals were excluded.
In Vivo Lentiviral Vector Injections
All viral vectors were coded, blinding the surgeon who performed both virus injections and stroke models. Lentiviruses were injected into the left cortex 5 days before ischemia. While under isoflurane anesthesia the rats were placed in a stereotaxic frame. A sagittal skin incision of 1 cm was made on the head and the skull was exposed. A burr hole in the left hemisphere was drilled according to the coordinate 0.96 mm posterior, 3.5 mm lateral to the bregma. A 10-μL syringe (Hamilton Company, Reno, NV, USA) with a 26-gauge needle (Cat# 80010) was inserted into the cortex and lowered to the depth of 1.8 mm from the dura. Each viral vector (5 μL) of GFP, cAkt1, cAkt3, or AktDN was injected into the cortex using a micro syringe pump controller at 0.5 μL/minute (World Precision Instruments, Inc., Sarasota, FL, USA) (n=6/group). The same amount of PBS was also injected as an experimental control, in addition to the GFP control vector (n=6/group). After viral vector administration, the needle was left for 10 minutes before being withdrawn. The wound was closed and the animals were allowed to recover.
Cerebral Blood Flow Measurement
Relative regional cerebral blood flow (CBF) was monitored using a laser Doppler probe.19, 22, 23 Briefly, the laser probe with a diameter of 0.5 mm was attached to the surface of the brain through the hole for virus injection to detect CBF values at different time points. Values of CBF were expressed as percentages relative to the baseline (100%). The CBF was measured at 15 minutes before CCA occlusion, immediately after CCA occlusion, immediately after middle cerebral artery occlusion, during ischemia (every 15 minutes after stroke onset), and immediately, 15 and 30 minutes after CCA reperfusion.
General Histology and Infarct Size Measurement
Two days after ischemia, rats were perfused transcardially with cold 0.9% saline, followed by 4% paraformaldehyde (PFA) in PBS (pH 7.4). Brains were postfixed in 4% PFA, 20% sucrose for 24 hours, cut into five coronal blocks from rostral (level 1) to caudal (level 5), and frozen at −80°C. The block in each brain containing the needle track was sectioned into 30 μm sections and mounted onto glass slides using a cryostat. The slices with a needle track were selected for infarct measurement. Successful transfection of the lentiviral vectors in the selected slices was confirmed by observing protein expression of eGFP under fluorescent confocal microscopy (Zeiss LSM 510, Thornwood, NY, USA). Sections were stained with cresyl violet. The area of the infarcted cortex was measured by a person who was blinded to the animal's condition, normalized to the contralateral cortex and expressed as a percentage, as described previously.19, 20, 21
RT-PCR
To investigate mRNA expression of Akt isoforms, cortical tissues, ischemic penumbra, and core from ischemic or sham brains were harvested at 1, 5, 9, and 24 hours after stroke onset, and the total RNA was extracted using a QIAGEN RNeasy mini kit (Cat#74104; Invitrogen, Grand Island, NY, USA) (n=5 to 6/group). Ischemic penumbra and core are defined as in our previous studies20, 24, 25 (Figure 1B): ischemic penumbra is the ischemic tissue that can be rescued by certain neuroprotectants, such as hypothermia and ischemic postconditioning, and ischemic core is the ischemic region destined to die despite the application of neuroprotectants. Total RNA was used to synthesize cDNA according to the manufacturer's instructions for the SuperScriptTM First-Strand Synthesis system for RT-PCR (Cat#18080-051; Invitrogen). The PCR was performed using primers specific for Akt1, Akt2, Akt3, and glyceraldehyde-3-phosphate dehydrogenase. The forward and reverse primer sequences were as follows: Akt1, 5′-GCTGATGGACTCAAACGGCA-3′ and 5′-CCCGAAGTCCGTTATCTTGA-3′ Akt2, 5′-CCCTTCTACAACCAGGACCA-3′ and 5′-AGAACTGGGGGAAGTGTGTG-3′ Akt3, 5′-CCTCAAGATGTGGACTTACCT-3′ and 5′-ATGATGGGTTGTAGATGCATC-3′ glyceraldehyde-3-phosphate dehydrogenase, 5′-TGGCCTTCCGTGTTCCTACC-3′ and 5′-TGTAGGCCATGAGGTCCACCAC-3′. The samples were loaded onto 1.5% agarose gels and run at 100 V for 30 minutes. Images were taken by ChemiDoc XRS system (Bio-Rad, Hercules, CA, USA). Semiquantitative measurements were performed using the IMAGEQUANT 5.2 software (GE Healthcare, Buckinghamshire, UK).
Protein Preparation of In Vivo and In Vitro Experiments for Western Blotting
To investigate the effects of in vivo stroke on protein expression of Akt isoforms, ischemic brains corresponding to the ischemic penumbra and core were harvested at 1, 5, 9, and 24 hours after stroke onset (Figure 1). To study the effects of gene transfer on protein changes, brain tissue around the needle track was dissected at 1, 5, and 24 hours after stroke onset (n=6/group). In addition, brain tissue from animals receiving sham surgery without ischemia, with or without transfection of lentiviral vectors, was also prepared for western blotting. Whole cell protein was extracted from the fresh brain tissue, and a western blotting was performed as described with modification.20 Briefly, brain tissue was cut into small pieces and homogenized in a glass homogenizer using seven volumes of the cold cell extraction buffer (Cat# FNN0001; Invitrogen, Eugene, OR, USA), containing 1 mmol/L phenylmethylsulfonyl fluoride and a protease inhibitor cocktail (1:20, Cat# P-2714; Sigma, St. Louis, MO, USA). The homogenate was centrifuged at 10,392 g for 20 minutes at 4°C, and the supernatant was taken for protein detection.
To investigate the effects of gene transfer on protein expression in vitro without OGD, mixed neuron cells were grown in 6-well plates and transfected with lentiviral vectors. The cells were harvested at 48 hours after gene transfer, and homogenized in the cold cell extraction buffer containing 1 mmol/L phenylmethylsulfonyl fluoride and the protease inhibitor cocktail. The homogenate was centrifuged at 10,392 g for 20 minutes at 4°C, and the supernatant was removed for protein detection. Protein concentrations were measured using the Bradford assay.
Western Blotting Procedures and Antibodies
Twenty micrograms of protein was loaded into each lane and subjected to SDS-PAGE using 4% to 15% Ready Gel (catalog #L050505A2; Bio-Rad) at 200 V for 45 minutes. Protein bands were transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA) at 100 V for 2 hours. Membranes were incubated with primary antibodies overnight at 4°C followed by Alexa Fluor 488 donkey antirabbit or antimouse IgG secondary antibody (1:5,000; Invitrogen, Eugene, OR, USA) for 1 hour in the dark room. Table 1 lists each primary antibody, manufacturer, catalog number, and detection method used. Membranes were scanned using Typhoon trio (GE Healthcare). Optical densities of all protein bands were analyzed using the IMAGEQUANT 5.2 software (GE Healthcare). Samples from sham surgery or only GFP gene transfer were used as controls for experimental samples.
Table 1. The information of each primary antibody used in the study.
| Antibodies | Source | Dilutions | Manufacturer | Catalog# | Application |
|---|---|---|---|---|---|
| Akt1 | Rabbit | 1:1,000 | Cell Signaling | 2938 | WB |
| Akt2 | Rabbit | 1:1,000 | Cell Signaling | 3063 | WB |
| Akt3 | Rabbit | 1:500 | Cell Signaling | 3788 | WB |
| pAkt (Ser473) | Rabbit | 1:100/1:1,000 | Cell Signaling | 9271 | IF/WB |
| Akt | Rabbit | 1:1,000 | Cell Signaling | 9272 | WB |
| pFKHR (Ser256) | Rabbit | 1:1,000 | Cell Signaling | 9461 | WB |
| FKHR | Rabbit | 1:50/1:1,000 | Cell Signaling | 2880 | IF/WB |
| pPRAS40 (Thr246) | Rabbit | 1:100/1:1,000 | Cell Signaling | 2997 | IF/WB |
| PRAS40 | Rabbit | 1:1,000 | Cell Signaling | 2691 | WB |
| pmTOR (Ser2448) | Rabbit | 1:1,000 | Cell Signaling | 2971 | WB |
| mTOR | Rabbit | 1:1,000 | Cell Signaling | 2983 | WB |
| GFP | Rabbit | 1:500 | Cell Signaling | 2956 | WB |
| pPTEN (Ser380) | Rabbit | 1:1,000 | Cell Signaling | 9551 | WB |
| PTEN | Rabbit | 1:1,000 | Cell Signaling | 9552 | WB |
| pGSK3β (Ser 9) | Rabbit | 1:1,000 | Cell Signaling | 9336 | WB |
| GSK3β | Rabbit | 1:1,000 | Cell Signaling | 9332 | WB |
| pS6K p70 (Ser371) | Rabbit | 1:500 | Cell Signaling | 9208 | WB |
| HA | Rabbit | 1:100 | Cell Signaling | 3724 | IF |
| MAP-2 | Mouse | 1:200 | Sigma | M-4403 | IF |
| β-Actin | Mouse | 1:300 | Sigma | A-5441 | WB |
IF, immunofluorescence; WB, western blots.
Immunofluorescence Staining and Confocal Microscopy
For primary neuron cultures, immunofluorescence staining was performed in 24-well plates. Cells were fixed using 4% PFA in PBS for 10 minutes at room temperature and washed three times with PBS. Cells were incubated in blocking solution containing 5% horse serum (Sigma) and 0.1% Triton X-100 in PBS for 2 hours at room temperature followed by incubation with primary antibodies (Table 1) at 4°C overnight. The next day cells were washed with PBS and incubated for 1 hour at room temperature (light shielded) with secondary antibody (Alexa Fluor 647 donkey antirabbit or antimouse IgG, 1:200; Invitrogen, Eugene, OR, USA) and coverslipped with one drop of Vectashield mounting medium with 4′, 6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, USA). Immunofluorescent staining was examined using a Zeiss confocal microscope (Zeiss LSM 510).
For immunofluorescent staining of brain sections, ischemic or sham rats were anesthetized and perfused transcardially with 0.9% saline followed by 4°C PFA in PBS (pH 7.4), then fixed with 4% PFA for 24 hours. Free floating 30 μm sections were cut on a cryostat and stored in antifreeze solution at −20°C. A similar immunofluorescence staining protocol was used as described above.
Statistical Analysis
GraphPad Prism 5.0 software (GraphPad Software Inc., La Jolla, CA, USA) was used for statistical analyses. Two-way ANOVA was used to compare the optical densities of mRNA as well as the protein levels of Akt isoforms of penumbra versus core at the stated time points and to compare LDH levels between no OGD and OGD transfected by each viral vector, followed by the least significant difference post hoc test. For infarction analyses, one-way ANOVA was used followed by the Fisher least significant difference post hoc test. For other western blots, two-way ANOVA was used to analyze the various protein bands from normal, 5-hour, and 24-hour ischemic brain tissues transfected with various viral vectors, followed by the Fisher least significant difference post hoc test. Tests were considered as significant at P values of <0.05. Data are presented as mean±s.e.m.
Results
The Expression of Akt Isoforms Is Differentially Regulated After Stroke
We examined the time course of expression for all Akt isoforms (Akt1, Akt2, and Akt3) after stroke. Akt1 mRNA levels in both the ischemic penumbra and core were significantly reduced at 5 hours and remained low at 9 hours, but at 24 hours, Akt1 mRNA levels remained low in the core but returned to basal levels in the penumbra (Figure 1C). Akt2 mRNA expression in both penumbra and core also decreased from 5 to 24 hours. In contrast, poststroke Akt3 mRNA levels in the core, but not in the penumbra, decreased quickly at 1 hour, and thereafter Akt3 mRNA levels remained low in both the core and penumbra. Both Akt1 and Akt3 proteins had similar patterns of expression in their respective mRNAs (Figure 1D). This contrasts with Akt2 protein levels, which did not reduce until 24 hours after stroke, suggesting that Akt2 may be less relevant to ischemic injury. Unlike Akt1 and Akt3, it is noticed that Akt2 mRNA levels did not correlate with their protein levels, which are not unusual regarding the relationships between mRNA and protein expressions.26 Taken together, our results show that Akt isoforms are differentially regulated and expressed after stroke and suggest that Akt1 and Akt3 are the more relevant isoforms. We focused on Akt1 and Akt3 for the subsequent gene transfer studies and examined their effects on the major signaling molecules in the Akt pathway.
Differential Protective Effects of Constitutively Active Akt1 or Akt3 Gene Transfer Against Both In Vitro and In Vivo Stroke
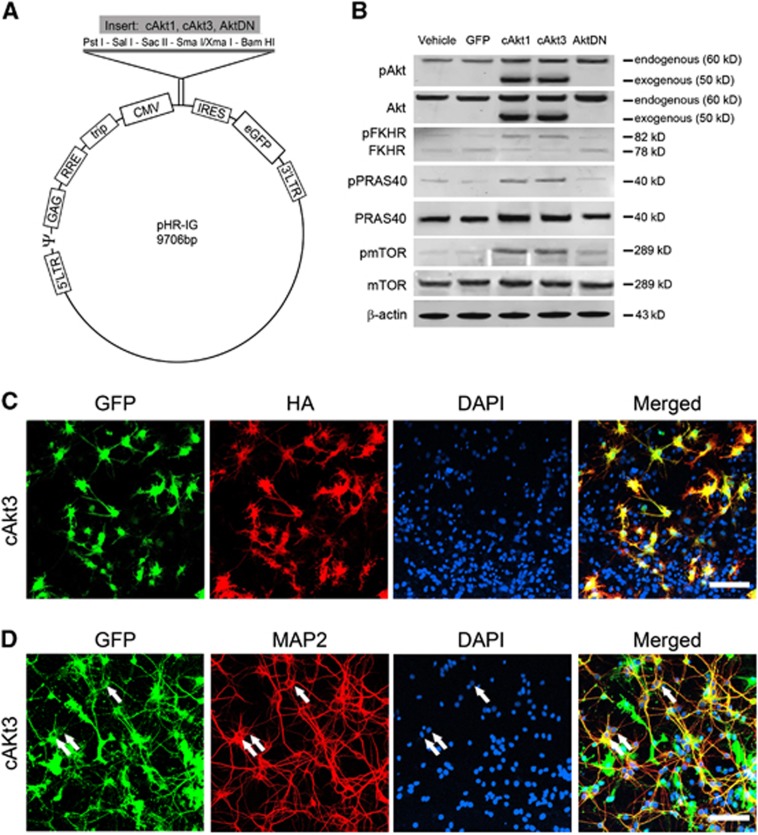
To further study the roles of Akt isoforms in ischemic injury as well as their downstream signaling molecules, such as FKHR, PRAS40, and mTOR, we constructed and confirmed lentiviral vectors expressing cAkt1 or cAkt3 and Akt dominant (AktDN) (Figure 2, Supplementary Figure 1).
Figure 2.
Construction of lentiviral vectors of constitutively active Akt1 (cAkt1), constitutively active Akt2 (cAkt2), and dominant-negative Akt (AktDN) and their expression in primary neuronal cultures. (A) Schematic backbone of lentiviral vector of pHR'tripCMV-IRES-eGFP. The genes were cloned into the lentiviral backbone plasmid, pHR'tripCMV-IRES-eGFP, which contains a CMV promoter and an IRES sequence between its multiple cloning sites (MCS) and eGFP. The IRES sequence enables independent expression of both the target gene and eGFP simultaneously. Akt1 and Akt3 genes lacking the pleckstrin homology (PH) domain are fused with a Gag polypeptide. A myristoylation signal enables cAkt1 and cAkt3 genes to attach to the cell membrane and be phosphorylated, thus activated. (B) Western blot confirmation of the effects of gene transfer in primary neuronal cultures on protein expression of pAkt, Akt, pPRAS40, PRAS40, pFKHR, FKHR, pmTOR, and mTOR in the Akt/mTOR pathways. Mixed neuron cells were grown in 6-well plates and transfected with lentiviral vectors. The cells were harvested at 48 hours after gene transfer, and homogenized in the cold cell extraction buffer containing 1 mmol/L phenylmethylsulfonyl fluoride and the protease inhibitor cocktail. The homogenate was centrifuged at 13,000 r.p.m. for 20 minutes at 4°C, and the supernatant was removed for protein detection. Protein concentrations were measured using the Bradford assay. The cAkt1 and cAkt3 plasmids lack the PH domain, so proteins translated by the cAkt1 and cAkt3 genes are truncated and have a smaller molecular weight (∼50 kDa). Phosphorylation of FKHR may change its molecular structure and shift its band from 78 to 82 kDa. Thus, pFKHR and FKHR are visible in the same gel as two separate bands. (C) Triple staining of GFP, HA, and 4′, 6-diamidino-2-phenylindole (DAPI) in a mixed primary neuronal culture transfected with cAkt3 vectors. The cAkt1, cAkt3, and AktDN vectors are fused with an HA tag; thus, HA expression is proportional to that of cAkt1. Scale bar, 50 μm. (D) Triple staining of GFP, MAP-2, and 4′, 6-diamidino-2-phenylindole (DAPI) in a mixed primary neuronal culture transfected with cAkt3 confirms the successful transfection of neurons. Triple-stained neurons are labeled by arrows.
We first investigated the effects of cAkt1 or cAkt3 gene transfer on neuronal death induced by OGD in vitro. The LDH release has been used to measure both apoptosis and necrosis as in previous reports.27 Using primary mixed cultures treated with lentivirus expressing cAkt1 or cAkt3, we measured LDH release at 18 hours after OGD. Both cAkt1 and cAkt3 significantly reduced LDH release, whereas AktDN enhanced LDH release and neuronal death (Figure 3; Supplementary Figure 5). However, cAkt3 gene transfer generated a significantly stronger protective effect than cAkt1.
Figure 3.
Effects of gene transfer on cell death in primary neuronal cultures. Effects of gene transfer of constitutively active Akt1 (cAkt1), constitutively active Akt3 (cAkt3), and dominant-negative Akt (AktDN) on neuronal death as measured by lactate dehydrogenase (LDH) release. Cultured mixed neurons were transfected with vectors containing cAkt1, cAkt3, and AktDN; cultures transfected with GFP vectors or treated with vehicle solution served as controls. Cultures were subjected to 6 hours of oxygen glucose deprivation (OGD) 2 days after transfection. LHD release was measured at 16 hours after OGD. All data after OGD were normalized to the values of control, nonOGD samples transfected with the corresponding vectors. n=18 to 24/group. *, **, P<0.05, 0.001, respectively, versus GFP control vector receiving OGD treatment. Φ, ΦΦ, P<0.05, 0.01, respectively, between the two indicated groups.
We then studied the effect of cAkt1, cAkt3, or AktDN gene transfer on infarct size in the in vivo stroke model. Lentiviral vectors were injected into the ischemic cortex and their distribution was presented in Supplementary Figure 2. Gene transfer of these vectors did not alter CBF values during and after stroke (Supplementary Figure 3). Double immunofluorescence staining of MAP-2 (a neuronal marker) and GFP (a transfection marker) indicates lentiviral vector-transfected neurons both in vitro (Figure 3) and in vivo (Supplementary Figure 4). Morphologic analysis also indicates that some astrocytes were transfected in vitro. No blood vessel structures were found to be transfected in vivo. Both cAkt1 and cAkt3 gene transfer reduced infarct size but cAkt3 was significantly more protective, which is consistent with in vitro results (Figures 4A and 4B). Injection of AktDN did not alter infarct size.
Figure 4.
Gene transfer of constitutively active Akt1 and Akt3 (cAkt1 and cAkt3), but not dominant-negative Akt (AktDN), reduced brain infarction after focal ischemia. (A) Representative coronal sections of brains show infarction areas and injection sites. Injection sites are marked by squares in which needle tracks are visible. (B) Average infarct size is determined from brain sections with needle tracts. Infarct area was measured and normalized to the nonischemic contralateral cortex, and expressed as a percentage. **, ***, versus control vector of GFP, P<0.01, 0.001, respectively. ΦΦ, cAkt1 versus cAkt3, P<0.01. (C) Representative immunostaining of GFP in brains transfected with various lentiviral vectors. (D) GFP levels measured by western blot indicate the effects of gene transfer on brain injury. All protein bands presented are derived from the same gel (see Supplementary Figure 5), but were cut and rearranged for convenient comparison. Transfected brain tissues at the needle tracks were dissected for western blotting. The average protein levels of GFP are presented in the bar graphs. N=6/group. ** versus control vector of GFP, P<0.01, respectively. ΦΦ, P<0.05, 0.01, respectively, between the two indicated groups.
GFP levels expressed by the lentivirus are indicators of tissue survival after stroke. We therefore examined the GFP levels by western blot. Our results showed that, compared with control vectors, cAkt1 and cAkt3 gene transfer but not AktDN gene transfer enhanced GFP levels (Figures 4C and 4D). The protective effects of cAkt1 and cAkt3 were further confirmed by reductions in MAP-2 protein expression attenuated by these vector gene transfers (Supplementary Figure 4).
Gene Transfer of Constitutively Active Akt3 and Akt1 Differentially Modulated Protein Levels of Endogenous Akt Isoforms and Promoted mTOR Phosphorylation
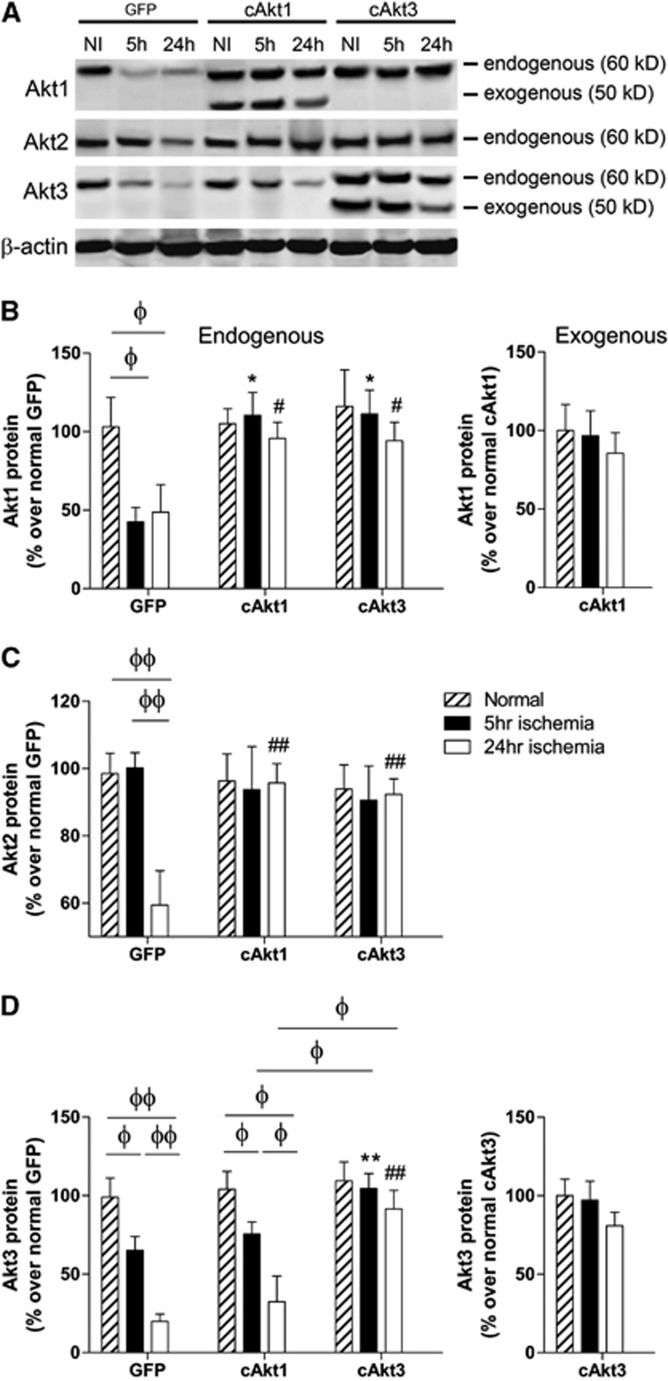
We examined the in vivo effects of cAkt1 and cAkt3 gene transfer on endogenous Akt isoform protein levels after focal ischemia (Figure 5; Supplementary Figure 5). Stroke caused reductions in the endogenous Akt isoforms at 5 and 24 hours after stroke in the GFP-treated group. cAkt1 gene transfer blocked endogenous Akt1 and Akt2 protein degradation, but not Akt3 protein degradation. Interestingly, cAkt3 gene transfer blocked protein degradation in all endogenous Akt isoforms (Akt1, Akt2, and Akt3). Exogenous levels of Akt1 and Akt3 proteins were not significantly reduced by stroke (Figure 5).
Figure 5.
Effects of constitutively active Akt1 and Akt3 (cAkt1 and cAkt3) gene transfer on expression of endogenous and exogenous Akt1, 2, and 3 after stroke. (A) Representative protein bands of Akt1, Akt2, and Akt3 as shown by western blot. β-Actin was used to show even protein loading. (B) Quantified Akt1 protein levels in ischemic brains transfected with GFP, cAkt1, and cAkt3 are presented in bar graphs. Left and right bar graphs show endogenous and exogenous Akt1 protein levels, respectively. (C) Effects of gene transfer on Akt2 protein expression. (D) Effects of gene transfer on both endogenous and exogenous Akt3 expression. N=6/group. *, ** versus GFP, 5 hours, P<0.05, 0.01, respectively; #, ## versus GFP, 24 hours, P<0.05, 0.01, respectively; Φ, ΦΦ show significance difference between the two indicated groups, P<0.05, 0.01, respectively.
To identify proteins relevant to the protective effects of gene transfer, we analyzed and compared the effects of gene transfer of cAkt1, cAkt3, and AktDN on both phosphorylated and total protein levels of PTEN, Akt, FKHR, GSK3β, PRAS40, and mTOR (Figure 6). In the GFP-treated group, protein levels of pPTEN, pFKHR, and pGSK3β were decreased at both 5 and 24 hours after stroke, and pAkt and pmTOR were also decreased at 24 hours. Gene transfer of cAkt1 or cAkt3 similarly blocked reductions in pPTEN, pFKHR, and pPRAS40 protein levels; no significant difference between cAkt1 and cAkt3 gene transfer was detected in their effects on these proteins. pGSK3β protein levels were further reduced in ischemic brains receiving gene transfer of cAkt1 and cAkt3, and no difference between these two groups was detected either. The cAkt3 gene transfer promoted significantly more pAkt and pmTOR protein levels than did cAkt1. In contrast, AktDN gene transfer did not alter protein levels with the exception of pAkt levels, which were increased (Figure 6).
Figure 6.
The effects of constitutively active Akt1 (cAkt1), constitutively active Akt3 (cAkt3), and dominant-negative Akt (AktDN) gene transfer on protein phosphorylation in the Akt/mTOR pathways 5 and 24 hours after stroke. (A) Representative protein bands of critical molecules in the Akt/mTOR pathways, including phosphorylated and total protein expression as measured by western blot. All protein bands presented are derived from the same gel, but were cut and rearranged for convenient comparison (see Supplementary Figure 6). (B) The bar graphs show quantified protein levels of pPTEN, pAkt, pFKHR, pGSK3β, pPRAS40, and pmTOR. N=6/group. & and &&, versus the nonischemic group treated with control GFP vectors, P<0.05, 0.001, respectively; *, ** versus ischemic brain transfected with control GFP vectors 5 hours after stroke, P<0.05, 0.01, respectively; #, ## versus ischemic brain transfected with control GFP vectors 24 hours after stroke, P<0.05, 0.01, respectively; Φ, ΦΦ show significance difference between the two indicated groups, P<0.05, 0.01, respectively.
mTOR Inhibition Blocked the Protective Effects of Constitutively Active Akt1 or Akt3 Gene Transfer Both In Vitro and In Vivo
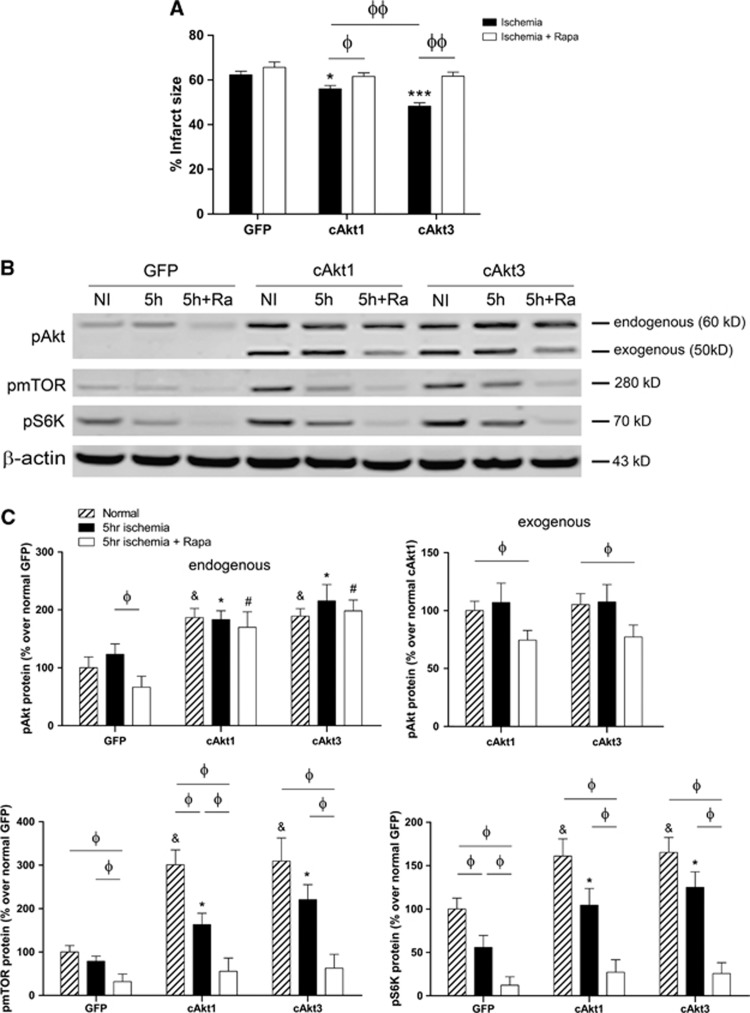
To assess the importance of mTOR in mediating the protective effects of cAkt1 and cAkt3, we tested whether an mTOR inhibitor (rapamycin) could block the protective effects of cAkt1 and cAkt3 in vivo (Figure 7). Administration of rapamycin in vivo significantly blocked the protective effects of cAkt1 or cAkt3 on infarct size (Figure 7A). Rapamycin treatment also affected the protein levels of pAkt, pmTOR, and pS6K at 5 hours after the in vivo stroke model (Figures 7B and 7C). Although rapamycin did not alter endogenous pAkt protein levels in ischemic brains transfected with cAkt1 or cAkt3, rapamycin inhibited exogenous pAkt protein levels in both cAkt1- and cAkt3-transfected brains. Both cAkt1 and cAkt3 gene transfer increased pmTOR and pS6K in control and stroke brains; however, such increases in the ischemic brains were inhibited by rapamycin administration.
Figure 7.
The mTOR inhibitor rapamycin blocked the protective effects of gene transfer of cAkt1 and cAkt3. (A) Infarct sizes in animals injected with control GFP, cAkt1, and cAkt3, with and without rapamycin. (B) Western blot was used to detect the effect of rapamycin on protein expression in the Akt/mTOR pathways. Representative protein bands of pAkt, pmTOR, and pS6K in ischemic brain transfected with control GFP vectors, cAkt1, and cAkt3, with and without rapamycin treatment are shown. (C) Bar graphs show the relative average optical densities of protein bands of endogenous pAkt, exogenous pAkt, pmTOR, and pS6K, respectively. N=6 to 8/group. &, *, #, versus corresponding control GFP group, P<0.05; Φ, ΦΦ, compare between the two indicated groups, P<0.05, 0.01, respectively.
Discussion
This study provides the first evidence that Akt isoforms are differentially regulated and have distinct effects after stroke. Although Akt has been investigated in stroke-induced brain injury for more than a decade,28 few studies offer solid and direct evidence of Akt's role in neuronal survival. Our findings provide direct evidence that Akt1 and Akt3 are neuroprotective again stroke, as gene transfer of either cAkt1 or cAkt3 inhibited infarct size in vivo and blocked neuron death induced by OGD in vitro. We found that using an AktDN vector that blocked Akt resulted in increased neuronal death in vitro. Although AktDN vectors did not worsen the infarct size in vivo, this does not exclude a possible detrimental effect for AktDN as the injection site into the ischemic penumbra may have been too close from the infarct core where cells were destined to die after stroke.
The AktDN gene has a point mutation where the 179th amino acid, lysine, from the N terminal is converted into methionine. This mutated form of the Akt protein can still be phosphorylated at the S473 site but loses its ability to phosphorylate its downstream proteins. Thus, AktDN gene transfer in vitro was shown to promote cell death (Figure 3), but AktDN gene transfer could still promote pAkt levels (Figure 6), while the downstream protein levels of Akt, pFKHR, and pPRAS40 were not altered.
Our data show several mechanisms that may underlie the protective effects of cAkt1 and cAkt3 in stroke. Overexpression of cAkt1 or cAkt3 robustly increased endogenous Akt phosphorylation. Overexpression of cAkt1 or cAkt3 increased PRAS40 and FKHR phosphorylation, which strongly suggests that they can phosphorylate downstream molecules. PTEN phosphorylation was promoted by cAkt1 and cAkt3 gene transfer, inhibiting its phosphatase activity. This suggests positive feedback in inhibition of PTEN's upstream phosphatase. Gene transfer of cAkt1 and cAkt3 may protect against brain injury by promoting mTOR activity, as mTOR phosphorylation was increased.
Interestingly, our data revealed that cAkt3 offered stronger protection than cAkt1, as gene transfer of cAkt3 reduced infarct size more than cAkt1 in vivo and attenuated more cell death in vitro. This is consistent with previous studies showing that Akt3 is the predominant isoform in the brain. This differential protection of cAkt1 and cAkt3 is supported by several facts. First, cAkt1 and cAkt3 differ in their ability to block degradation of endogenous Akt isoforms, as gene transfer of cAkt1 inhibited degradation of Akt1 and Akt2 proteins, but not Akt3. In contrast, gene transfer of cAkt3 blocked degradation of all Akt protein isoforms. Second, cAkt3 gene transfer resulted in higher pAkt levels than those after cAkt1 gene transfer at 24 hours after stroke in vivo. Third, although both cAkt1 and cAkt3 increased pPTEN, pFKHR, and pPRAS40 to similar levels, cAkt3 gene transfer resulted in higher pmTOR levels than cAkt1, which is consistent with a previous study showing that Akt3 but not Akt1 is crucial for activation of the mTOR/S6K1 signaling pathway in the brain.10
Active Akt phosphorylates its downstream molecule GSK3β and therefore inhibits apoptosis; but in our study, GSK3β phosphorylation levels were not proportional to the protective outcomes of Akt. Previous studies have shown that, when dephosphorylated, GSK3β becomes active and induces apoptosis.29 In our study, however, protein levels of phosphorylated GSK3β did not appear to be related to ischemic neuronal death or survival nor to the protective effects of Akt gene transfer. We previously showed that stroke caused reduced pGSK3β protein levels20 but stroke followed by moderate hypothermia, which robustly spared infarction, caused greater reductions in pGSK3β.20 Consistent with this finding, gene transfer of cAkt1 and cAkt3 also reduced rather than promoted pGSK3β levels. We therefore conclude that dephosphorylated GSK3β is not a marker of cell death or survival.
We also showed that cAkt1 and cAkt3 transfection increased the levels of endogenous Akt phosphorylation, but the underlying mechanisms are unknown. Of note, needles were used to inject vectors into the brain, potentially causing some traumatic injury and neuronal loss near the site of the needle track. In this case, neurons transfected with Akt vectors might out survive those transfected with control vectors. Thus, western blot results for increased endogenous Akt expression may reflect more neuronal survival in brains transfected with Akt vectors than in animals transfected with control vectors. In addition, p-Akt levels might be increased via a positive feedback mechanism by stimulating mTOR activity. It is known that mTOR forms two complexes with other proteins—mTOR complex 1 and complex 2.30 While mTOR complex 1 promotes protein syntheses and cell survival by phosphorylating S6K protein kinases, mTOR complex 1 promotes Akt phosphorylation.30 We do not exclude the possibility that Akt vectors promoted mTOR complex 1 activity and increased Akt phosphorylation through positive feedback. As related with this, we found that mTOR inhibition with rapamycin inhibited endogenous p-Akt levels in animals receiving control ischemia with or without cAkt1 and cAkt3 gene transfer. This finding is in contrast with previous studies showing that rapamycin promoted p-Akt levels in cancer cell lines.31 Again, the underlying mechanisms for such differences are not known, which are probably due to different responses of the Akt and mTOR activities in different tissues, organs, and pathologic conditions.
One major limit of this study is that cAkt2 is not included, although we have realized that Akt2 is not critical for brain function, possibly due to its relatively low expression in the brain. Despite this, we had planned to study all three Akt isoforms using lentiviral vectors in our pilot studies, but we were, unfortunately, unable to construct cAkt2 lentiviral vectors. As a result, the actual role of Akt2 in ischemic brain injury requires further study. In addition, animals with Akt isoform gene knockout or overexpression should be examined in the future.
Conclusion
We showed that stroke resulted in differential expression of Akt isoforms, with rapid degradation of Akt1 and Akt3 along with delayed reductions in Akt2. Our data reveal crucial roles for Akt1 and Akt3 in promoting neuron survival after stroke through mediation of multiple signaling pathways, such as enhancing the downstream factors PRAS40 and mTOR. Furthermore, Akt3 may be the most promising Akt isoform against poststroke injury, as overexpression of cAkt3 was more protective than cAkt1.
Acknowledgments
The authors thank Elizabeth Hoyte for figure preparation, and Cindy H Samos and Greta Beekhuis for manuscript editing.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by American Heart Association (AHA) grant in aid 10GRNT4200024 and 1R01NS064136-01 (HZ).
Supplementary Material
References
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altomare DA, Wang HQ, Skele KL, De Rienzo A, Klein-Szanto AJ, Godwin AK, et al. AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene. 2004;23:5853–5857. doi: 10.1038/sj.onc.1207721. [DOI] [PubMed] [Google Scholar]
- Dunlop EA, Tee AR. Mammalian target of rapamycin complex 1: signalling inputs, substrates and feedback mechanisms. Cell Signal. 2009;21:827–835. doi: 10.1016/j.cellsig.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Hers I, Vincent EE, Tavare JM. Akt signalling in health and disease. Cell Signal. 2011;23:1515–1527. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol. 2006;34:249–270. doi: 10.1385/MN:34:3:249. [DOI] [PubMed] [Google Scholar]
- Lee RS, House CM, Cristiano BE, Hannan RD, Pearson RB, Hannan KM. Relative expression levels rather than specific activity plays the major role in determining in vivo AKT isoform substrate specificity. Enzyme Res. 2011;2011:720985. doi: 10.4061/2011/720985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, et al. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- Li J, Lang J, Zeng Z, McCullough LD. Akt1 gene deletion and stroke. J Neurol Sci. 2008;269:105–112. doi: 10.1016/j.jns.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba N, Kiryu-Seo S, Maeda M, Muraoka M, Ishii M, Kiyama H. Transgenic mouse overexpressing the Akt reduced the volume of infarct area after middle cerebral artery occlusion. Neurosci Lett. 2004;359:159–162. doi: 10.1016/j.neulet.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Hu Q, Chen C, Khatibi NH, Li L, Yang L, Wang K, et al. Lentivirus-mediated transfer of MMP-9 shRNA provides neuroprotection following focal ischemic brain injury in rats. Brain Res. 2011;1367:347–359. doi: 10.1016/j.brainres.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Apolonia L, Waddington SN, Fernandes C, Ward NJ, Bouma G, Blundell MP, et al. Stable gene transfer to muscle using non-integrating lentiviral vectors. Mol Ther. 2007;15:1947–1954. doi: 10.1038/sj.mt.6300281. [DOI] [PubMed] [Google Scholar]
- Breckpot K, Dullaers M, Bonehill A, van Meirvenne S, Heirman C, de Greef C, et al. Lentivirally transduced dendritic cells as a tool for cancer immunotherapy. J Gene Med. 2003;5:654–667. doi: 10.1002/jgm.400. [DOI] [PubMed] [Google Scholar]
- Cheng MY, Sun G, Jin M, Zhao H, Steinberg GK, Sapolsky RM. Blocking glucocorticoid and enhancing estrogenic genomic signaling protects against cerebral ischemia. J Cereb Blood Flow Metab. 2009;29:130–136. doi: 10.1038/jcbfm.2008.105. [DOI] [PubMed] [Google Scholar]
- Dugan LL, Bruno VM, Amagasu SM, Giffard RG. Glia modulate the response of murine cortical neurons to excitotoxicity: glia exacerbate AMPA neurotoxicity. J Neurosci. 1995;15:4545–4555. doi: 10.1523/JNEUROSCI.15-06-04545.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Sapolsky RM, Steinberg GK. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1114–1121. doi: 10.1038/sj.jcbfm.9600348. [DOI] [PubMed] [Google Scholar]
- Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, et al. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–9806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 2003;85:1026–1036. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- Dinapoli VA, Rosen CL, Nagamine T, Crocco T. Selective MCA occlusion: a precise embolic stroke model. J Neurosci Methods. 2006;154:233–238. doi: 10.1016/j.jneumeth.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Gao X, Ren C, Zhao H. Protective effects of ischemic postconditioning compared with gradual reperfusion or preconditioning. J Neurosci Res. 2008;86:2505–2511. doi: 10.1002/jnr.21703. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhang H, Takahashi T, Hsieh J, Liao J, Steinberg GK, et al. The Akt signaling pathway contributes to postconditioning's protection against stroke; the protection is associated with the MAPK and PKC pathways. J Neurochem. 2008;105:943–955. doi: 10.1111/j.1471-4159.2008.05218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Zhang H, Steinberg G, Zhao H. The Akt pathway is involved in rapid ischemic tolerance in focal ischemia in Rats. Transl Stroke Res. 2010;1:202–209. doi: 10.1007/s12975-010-0017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4:117. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barczyk K, Kreuter M, Pryjma J, Booy EP, Maddika S, Ghavami S, et al. Serum cytochrome c indicates in vivo apoptosis and can serve as a prognostic marker during cancer therapy. Int J Cancer. 2005;116:167–173. doi: 10.1002/ijc.21037. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Tan Y, Comb M, Liu CL, Martone ME, Siesjo BK, et al. Survival- and death-promoting events after transient cerebral ischemia: phosphorylation of Akt, release of cytochrome C and Activation of caspase-like proteases. J Cereb Blood Flow Metab. 1999;19:1126–1135. doi: 10.1097/00004647-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Kaytor MD, Orr HT. The GSK3 beta signaling cascade and neurodegenerative disease. Curr Opin Neurobiol. 2002;12:275–278. doi: 10.1016/s0959-4388(02)00320-3. [DOI] [PubMed] [Google Scholar]
- Yang Z, Ming XF. mTOR signalling: the molecular interface connecting metabolic stress, aging and cardiovascular diseases. Obes Rev. 2012;13 (Suppl 2:58–68. doi: 10.1111/j.1467-789X.2012.01038.x. [DOI] [PubMed] [Google Scholar]
- Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback asctivation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.