Abstract
In the choroid, there is evidence that blood flow does not only depend on ocular perfusion pressure (OPP), but also on absolute mean arterial pressure (MAP) and intraocular pressure (IOP). The present study included 40 healthy subjects to investigate whether such behavior is also found in the optic nerve head (ONH). The ONH blood flow (ONHBF) was studied using laser Doppler flowmetry during a separate increase in IOP and MAP as well as during a combined elevation. Mean arterial pressure was increased by isometric exercise and IOP by the suction method. During both, the change in ONHBF was less pronounced than the change in OPP indicating autoregulation. Correlation analysis was performed for the combined experiments after pooling all data according to IOP and MAP values. A correlation between ONHBF and MAP was found at IOPs ⩽25 mm Hg (P<0.001), but not at IOPs>25 mm Hg (P=0.79). Optic nerve head blood flow and IOP were significantly correlated (P<0.001), and ONHBF was only slightly dependent on MAP. The data of the present study indicate a complex regulation of ONHBF during combined changes in MAP and IOP. Our results may be compatible with myogenic mechanisms underlying autoregulation, and indicate better ONHBF regulation during an increase in MAP than during an increase in IOP.
Keywords: autoregulation, blood flow, laser Doppler flowmetry, ocular perfusion pressure, optic nerve head
Introduction
Today, glaucoma is the second leading cause of blindness worldwide affecting over 60 million people.1 This neuropathy is characterized by progressive visual field loss due to retinal ganglion cell death. Increased intraocular pressure (IOP) poses the most important known risk factor with a positive correlation between both prevalence and incidence in glaucoma.2, 3, 4 However, some patients deteriorate despite normal IOP indicating that other factors are also at play. As such, vascular factors including altered blood flow have been proposed to contribute to the pathogenesis of glaucoma.5
Autoregulation is a mechanism that keeps blood flow constant during changes in perfusion pressure. This allows for the adequate perfusion of a tissue as well as supply of nutrients and oxygen. The autoregulatory capacity of a vascular bed can be studied by altering the perfusion pressure. Ocular perfusion pressure (OPP) can be calculated as 2/3 × mean arterial blood pressure (MAP)−IOP, meaning that low OPP can be the result of either low blood pressure or elevated IOP. Previous studies have shown that blood flow in the choroid is regulated when OPP is modified.6, 7 More specifically, choroidal blood flow appears to be better regulated during an isometric exercise-induced increase in OPP than during a suction cup-induced decrease in OPP. However, only few data are available in the optic nerve head (ONH).8, 9, 10 Given that reduced ONH perfusion and ischemia have been implicated in the pathogenesis of glaucoma, the knowledge of autoregulatory phenomena in this vasculature is of specific importance.5 Therefore, the present study tested the hypothesis that ONH blood flow (ONBF) is regulated during changes in OPP. Additionally, we investigated whether ONHBF depends only on OPP or also on the absolute values of MAP and IOP.
Materials and methods
The present study was performed adhering to the Declaration of Helsinki and the Good Clinical Practice (GCP) guidelines after approval from the Ethics Committee of the Medical University of Vienna was obtained. All subjects gave written informed consent after the nature of the study was explained to them.
Twenty healthy male and twenty healthy female subjects were included in this prospective, crossover study. The sample size calculation was based on the previous unpublished measurements of ONHBF during isometric exercise in our laboratory using a repeated-measures ANOVA model. Given the variability in our previous experiments, an alpha error of 0.05, a power of 0.80, and a sample size of 40 healthy subjects were allowed to detect changes in ONHBF of 10%. Changes smaller than 10% were considered to be irrelevant.
All subjects passed a prestudy screening, including medical history, physical examination, and a 12-lead electrocardiogram. Additionally, hematologic status (hemoglobin, hematocrit, red blood cell count, mean cellular hemoglobin, and white blood cell count), coagulation status (platelet count, activated partial thromboplastin time, and thrombin time), and clinical chemistry (sodium, potassium, creatinine, alanine transaminase, γ-glutamylamylase, total bilirubin, and total protein) were determined. A complete ophthalmic examination comprising slit-lamp examination, indirect funduscopy, measurement of IOP with Goldmann applanation tonometry, and assessment of best-corrected visual acuity was conducted. All subjects had to be aged between 18 and 35 years and nonsmokers. Main exclusion criteria were ametropia of >1 diopter, use of any medication except hormonal contraceptives in the 3 weeks preceding the study, and normal findings in the screening examination unless the investigator considered an abnormality to be clinically irrelevant.
Two study days were scheduled for all participating volunteers. Subjects had to abstain from alcohol and stimulating beverages containing xanthine derivatives for at least 12 hours before each study day. On the first study day, the effect of an increase in MAP and IOP on ONHONHBF was measured separately in three experiments. Before each measurement, a resting period of at least 20 minutes was implemented to ensure stable hemodynamic conditions. After a 3-minute baseline period, MAP was increased by the means of a 6-minute squatting period. During this period, subjects had to keep their head on the chinrest and remained in a position where the upper and the lower leg almost formed a right angle. In the second experiment, ONHBF was measured during a stepwise artificial increase in IOP (25, 50, 75, and 100 mm Hg of suction for 1 minute each) using the suction-cup method. During these periods, ONHBF was measured continuously with laser Doppler flowmetry (LDF), and blood pressure was assessed every minute. In the third experiment, the suction-cup procedures were repeated and IOP was measured instead of ONHBF every minute at each suction force level.
On the second study day, subjects had two periods of combined MAP and IOP elevation. This allowed for separate measurement of ONHBF using LDF and IOP during the two identical experimental procedures. After 20 minutes of resting and baseline readings of ONHBF, subjects performed squatting for 6 minutes. During the last 4 minutes, the suction cup was applied with the same suction force as on the first day. The subjects' head did not move away from the chinrest, and ONHBF was assessed continuously. Systemic blood pressure and heart rate were recorded every minute. Applanation tonometry was performed immediately before and after ONHBF measurement. Thereafter, a resting period of at least 30 minutes was scheduled and the procedure was repeated. However, instead of ONHBF, IOP was measured every minute.
Systemic Hemodynamics
Systolic blood pressure, diastolic blood pressure, and MAP were monitored on the upper arm by an automated oscillometric device. Pulse rate (PR) was recorded automatically using a finger pulseoximetric device (HP-CMS patient monitor; Hewlett Packard, Palo Alto, CA, USA). The performance of this system has been reported previously.11
Laser Doppler Flowmetry
Continuous measurement of ONHBF was performed by LDF as described previously.12, 13 With this technique, the vascularized tissue is illuminated by coherent laser light. Light scattered by the moving red blood cells undergoes a frequency shift. In contrast, static tissue scatterers do not change the light frequency, but lead to randomization of light directions impinging on red blood cells. Hence, red blood cells receive light from numerous random directions. As the frequency shift is dependent not only on the velocity of the moving red blood cells, but also on the angle between the incident and the scattered light, scattering of the light in tissue broadens the Doppler shift power spectrum. From this spectrum, hemodynamic parameters can be determined based on a theory of light scattering in tissue. Blood flow parameters obtained included blood flow, velocity, and volume. Velocity is the mean velocity of the red blood cells moving in the sampled tissue proportional to the mean Doppler frequency shift. Volume is the number of moving red blood cells in the sampled tissue proportional to the amount of Doppler shifted light. Blood flow was calculated as the product of velocity and volume. Only data with a direct current value of ±15% to the baseline value were included for analysis. For the measurements of ONHBF, a fundus-camera-based system was used.12 The laser beam was directed toward the temporal neurovascular rim. Care was taken to avoid that any visible vessels were within the scattering volume. During all experiments, LDF measurements were performed by two investigators. One investigator controlled the relative position of the instrument to the subjects' eye and adequate fixation. The second investigator controlled the signal on the computer. Data were corrected for different levels of direct current using the technique proposed by Gugleta and coworkers.14, 15
Intraocular Pressure
A slit-lamp mounted Goldmann applanation tonometer was used to measure IOP. Before each measurement, one drop of 0.4% benoxinate hydrochloride combined with 0.25% sodium fluorescein was used for local anesthesia of the cornea.
Suction-Cup Method
The IOP was increased using the suction-cup method described in more detail by Ulrich and Ulrich.16 In this study, we used an automatic suction pump that is connected by plastic tubing to a rigid plastic suction cup. After topically applied anesthesia, a standardized 11-mm diameter suction cup was placed on the temporal sclera with the anterior edge at least 1 mm from the limbus. Intraocular pressure was incrementally increased by a suction cup with a force of 25, 50, 75, and 100 mm Hg for 1 minute each.
Statistics
Ocular perfusion pressure in the sitting position was calculated as OPP=2/3 × MAP−IOP.17 During the IOP elevation experiments, OPP was calculated using the IOP data that were measured during the second suction-cup period. This was performed because it is impossible to measure IOP and blood flow at the same time. We have, however, previously shown in a pilot experiment (unpublished data, n=8) that IOP data during application of a suction cup are well reproducible after a resting period of 30 minutes. During isometric exercise, we obtained IOP levels at the beginning and the end of each squatting period. From these data, the IOP values during every single minute of squatting were calculated using linear regression analysis. During the squatting and suction-cup period, changes versus baseline were analyzed using repeated-measures ANOVA and planned comparisons for post hoc analyses.
In addition, pressure–flow relationships were calculated for the experiments during isometric exercise and artificial IOP increase. For this purpose, the data were expressed as %change in OPP and %change in flow values over baseline. The OPP values were then sorted according to ascending values and grouped into six groups each. For the squatting experiments, 40 values were therefore considered in each of the groups. As such, the first group consisted of those pressure/flow data with the smallest OPP increase, whereas the sixth group consisted of the pressure/flow data with the largest OPP increase. For the IOP experiments, the number of values included in groups 1 to 4 was 27, whereas it was 26 in groups 5 and 6. Again, the first data point consisted of those pressure/flow data with the smallest OPP decrease, whereas the sixth group consisted of the pressure/flow data with the largest OPP decrease. A statistically significant deviation from baseline flow was defined when the 95% confidence interval did not overlap with the baseline value any more.
For evaluation of the correlation of ONHBF and IOP, data from all time points of all subjects were pooled and then grouped into three categories according to the following MAP values: the first group contained ONHBF and IOP data pairs during MAP⩽85 mm Hg, the second group during 85 mm Hg<MAP⩽115 mm Hg, and the third group during MAP>115 mm Hg. Linear regression analysis was performed for all three groups. For the assessment of the relationship between ONHBF and MAP, the same procedure was used but this time, data were group according to their IOP levels into the following categories: IOP⩽15 mm Hg, 15 mm Hg<IOP⩽25 mm Hg, and IOP> 25 mm Hg. Fisher's r to z transformation was used to calculate the significance of the difference between correlation coefficients.
A P value of <0.05 was considered as the level of significance. Statistical analysis was performed with the commercial software (Statistica Version 6.0; Statsoft Inc., Tulsa, OK, USA).
Results
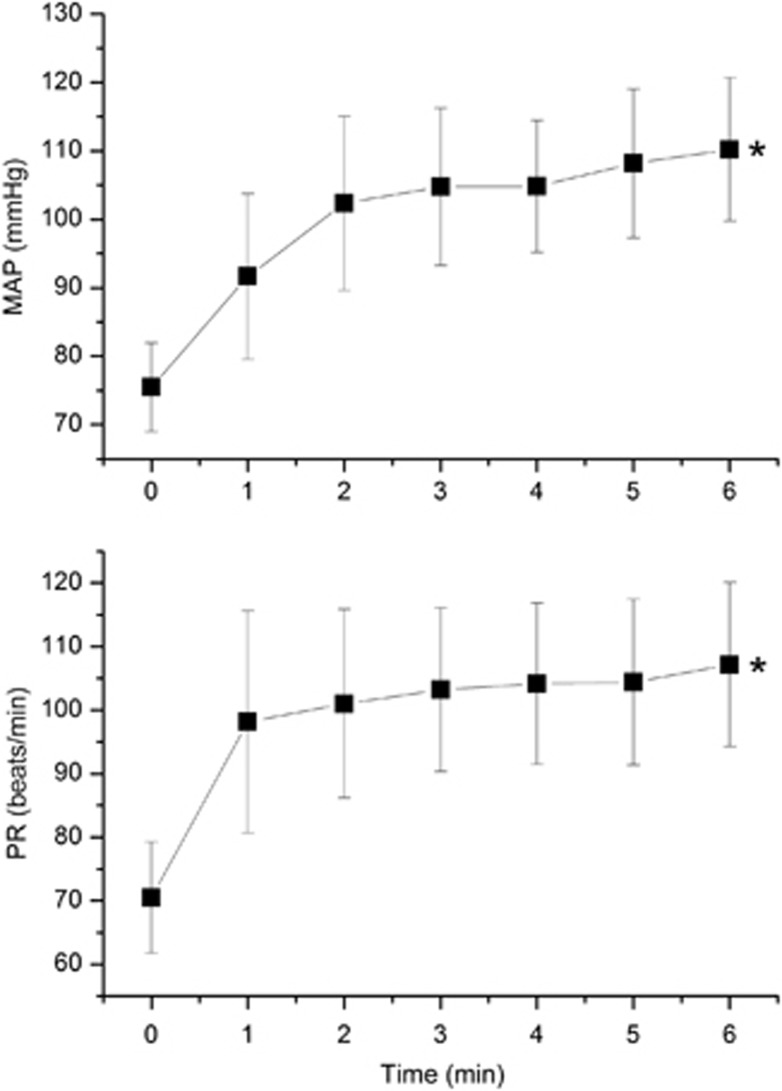
The subjects' age ranged from 18 to 29 years. Baseline data for the included subjects are presented in Table 1. No adverse events were observed except for mild conjunctival hyperemia from the suction-cup period. Isometric exercise significantly elevated both MAP (45.9±11.8%, Figure 1) and PR (52.1±15.3%, P<0.001 each, Figure 1), but had no influence on IOP (P=0.94, data not shown). Therefore, changes in OPP during isometric exercise were essentially influenced by the elevation of MAP. The maximum increase in OPP during squatting was 66.4±22.4%, which was highly significant versus baseline (P<0.001, Figure 2). This increase in OPP was accompanied by an increase in ONHBF (13.1±9.4%, P<0.001, Figure 2), which was, however, smaller than the increase in OPP indicating some degree of autoregulation.
Table 1. Baseline data on the two study days (means±s.d.).
| Day 1 | Day 2 | |
|---|---|---|
| Systolic blood pressure (mm Hg) | 115.8±11.5 | 116.9±11.6 |
| Diastolic blood pressure (mm Hg) | 59.3±8.5 | 61.1±8.0 |
| Mean arterial pressure (mm Hg) | 78.4±8.3 | 80.3±7.6 |
| Heart rate (bpm−1) | 68.3±13.3 | 69.5±12.6 |
| Intraocular pressure (mm Hg) | 14.6±2.4 | 14.7±1.8 |
| Ocular perfusion pressure (mm Hg) | 38.4±5.3 | 40.1±5.0 |
| Optic nerve head blood flow (a.u.) | 20.1±4.9 | 21.0±3.2 |
Figure 1.
Effect of isometric exercise on mean arterial blood pressure (MAP) and pulse rate (PR). Asterisks indicate significant changes versus baseline.
Figure 2.
Effect of isometric exercise on ocular perfusion pressure (OPP) and optic nerve head blood flow (ONHBF). Data are presented as %change from baseline values. Asterisks indicate significant changes versus baseline.
As expected, IOP significantly increased during the suction-cup period (P<0.001) at each suction value (Figure 3). By contrast, MAP (Figure 3) and PR (data not shown) were not altered by the application of the suction cup. Accordingly, we observed a decline in OPP, which reached a maximum level of −67.4±15.4% (P<0.001, Figure 4). This decrease in OPP was paralleled by a smaller decrease in ONHBF (−37.8±8.8%, P<0.001, Figure 4), indicating that autoregulation is also present during these periods.
Figure 3.
Effect of application of the suction cup on intraocular pressure (IOP) and mean arterial blood pressure (MAP). Asterisks indicate significant changes versus baseline.
Figure 4.
Effect of application of the suction cup on ocular perfusion pressure (OPP) and optic nerve head blood flow (ONHBF). Data are presented as %change from baseline values. Asterisks indicate significant changes versus baseline.
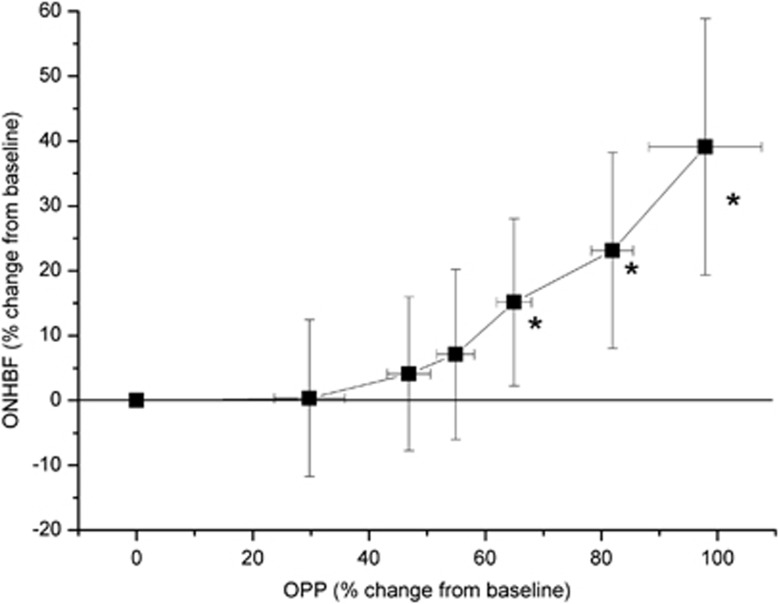
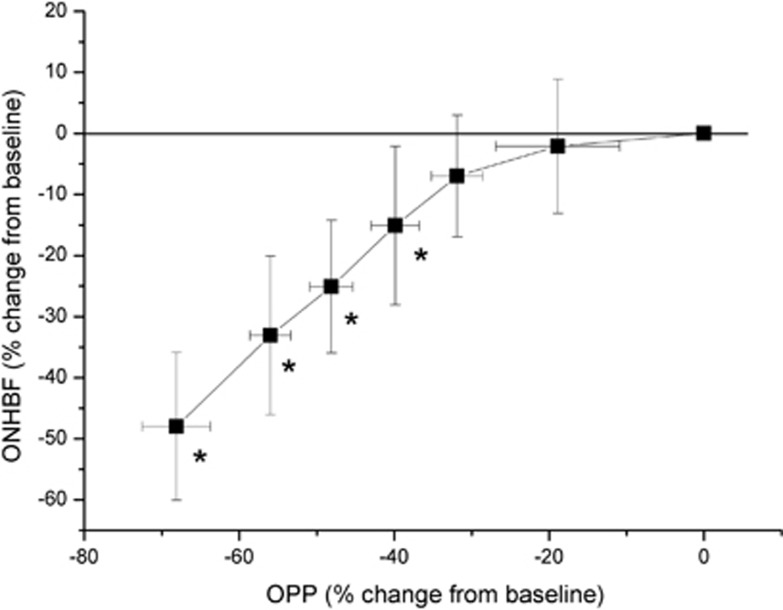
The pressure–flow relationships for isometric exercise and the IOP elevation experiments are shown in Figures 5 and 6, respectively. At OPP values of 55% above, baseline ONHBF values were not significantly different from baseline. Starting at an OPP level of 66% above, baseline ONHBF values almost increased linearly. Whereas ONHBF was not different from baseline at an OPP decrease of 32%, it started to decrease at OPPs below 40% of baseline. At lower OPPs, the pressure–flow relationship became almost linear.
Figure 5.
Pressure–flow relationship for optic nerve head blood flow (ONHBF; n=40) during isometric exercise. Data are sorted according to ascending ocular perfusion pressure (OPP) values, and the means as well as the 95% confidence intervals are shown. Asterisks indicate significant changes from baseline ONHBF.
Figure 6.
Pressure–flow relationship for optic nerve head blood flow (ONHBF; n=40) during an increase in intraocular pressure (IOP). Data are sorted according to descending ocular perfusion pressure (OPP) values, and the means as well as the 95% confidence intervals are shown. Asterisks indicate significant changes from baseline ONHBF.
The data obtained during the combined changes in OPP induced by isometric exercise on the one hand and application of the suction cup on the other hand are presented in Figures 7 and 8, respectively. When data pairs were grouped according to MAP values a highly significant correlation was found between ONHBF and IOP (P<0.001) in all groups (Figure 7). The correlation coefficients were all in the same range (P=0.059 between groups 1 and 2, P=0.052 between groups 2 and 3, and P=0.84 between groups 1 and 3), and the regression lines were almost parallel (MAP⩽85 mm Hg: r=−0.67, P<0.001, k=−1.19; 85 mm Hg<MAP⩽115 mm Hg: r=−0.57, P<0.001, k=−1.22; MAP>115 mm Hg: r=−0.68, P<0.001, k=−1.46). In addition, the ONHBF values were lowest in the group MAP⩽85 mm Hg (90.5±18.3% of baseline), higher in the group 85 mm Hg<MAP⩽115 mm Hg (96.5±20.0% of baseline), and highest in the group MAP>115 mm Hg (106.5±20.6% of baseline, all comparisons P<0.001)
Figure 7.
Optic nerve head blood flow during a combined increase in intraocular pressure (IOP) and mean arterial pressure (MAP). All data were grouped according to MAP values: MAP⩽85 mm Hg (red triangles, r=−0.67, P<0.01, n=290), 85 mm Hg<MAP⩽115 mm Hg (green squares, r=−0.57, P<0.01, n=251), and MAP>115 mm Hg (blue circles, r=−0.68, P<0.01, n=217), r=correlation coefficient.
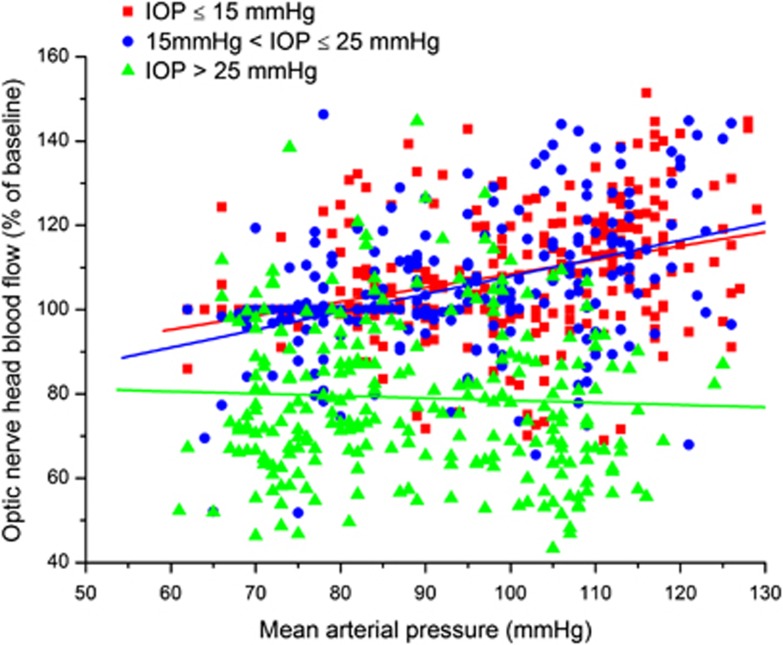
Figure 8.
Optic nerve head blood flow during a combined increase in intraocular pressure (IOP) and mean arterial pressure (MAP). Data were grouped according to IOP values: IOP⩽15 mm Hg (red squares, r=0.39, P<0.01, n=301), 15 mm Hg<IOP⩽25 mm Hg (blue circles, r=0.39, P<0.01, n=224), and IOP>25 mm Hg (green triangles, r=−0.016, P=0.54, n=234), r=correlation coefficient.
When data pairs were grouped according to IOP values the picture was more complex (Figure 8). In the group with IOPs⩽15 mm Hg, a correlation between MAP and ONHBF was observed (r=0.39, P<0.001, k=0.33). A correlation was also found in the group 15 mm Hg<IOP⩽25 mm Hg, and the regression line was almost parallel (r=0.39, P<0.001, k=0.40). By contrast, in the group of highest IOP (>25 mm Hg), ONHBF was independent of MAP (r=−0.016, P=0.79, k=−0.020). The correlation coefficient for this group was significantly different compared with the other two groups (P<0.001 between groups 1 and 3, P<0.001 between groups 2 and 3, and P=1.0 between groups 1 and 2). Mean ONHBF was higher in the groups with IOPs⩽15 mm Hg (107.0±14.3% of baseline) and 15 mm Hg<IOP⩽25 mm Hg (105.2±16.4% of baseline) than in the group with IOP>25 mm Hg (79.7±18.4% of baseline, P<0.001), but not different between the first two groups.
Discussion
The present study confirms the previous data, indicating that ONHBF regulates during both an exercise-induced increase in MAP and a suction cup-induced increase in IOP.8, 9, 10 The data obtained in the present study are generally in good agreement with these previously published data, but it needs to be considered that in the present study more subjects were included and more steps of IOP elevation were used. Data, as obtained in the present cohort, are also in good agreement with our recently published results in another cohort.18 One needs, however, to consider that there is a wide interindividual variability in this response.18 Nevertheless, the pressure–flow relationships presented in this report indicate that ONHBF is autoregulated during both an increase and a decrease in OPP.
In addition, our data extend our knowledge of ONHBF regulation in several ways. During combined increase in MAP and increase in IOP, blood flow in the ONH was generally more dependent on IOP than on blood pressure. Whereas this is in keeping with the previous results we obtained in the choroid, we also found considerable differences between the two vascular beds. In the choroid, blood flow was strongly dependent on IOP when data were grouped according to MAP values during combined isometric exercise and artificial increase in IOP.7 The regression lines between IOP and blood flow were almost similarly independent of the level of MAP. In the present study, the results in the ONH showed in principle a similar behavior with a correlation between blood flow and IOP, but flow values were slightly dependent on the level of MAP. More pronounced differences were, however, found when data were grouped according to IOP. In the choroid, the regression lines between blood flow and MAP were almost horizontal. Blood flow values were, however, generally lower at higher IOPs. In the ONH, we observed a more complex behavior. At the two lower levels of IOP, a small but significant correlation was found between blood flow and MAP, but the level of blood flow was not dependent on IOP. At higher IOPs (>25 mm Hg), blood flow was considerably lower, but became independent of MAP.
As such, the data of the present study support our recent findings,18 indicating that ONHBF shows better blood flow regulation during an artificial increase in IOP than choroidal blood flow and less regulatory capacity during an isometric exercise-induced increase in MAP. The finding that at higher IOPs blood flow in the ONH regulates better when MAP is changed than at lower IOPs may be unexpected. Relying, however, on a theoretical model on autoregulation19 this may be predicted. When IOP is artificially increased, subjects start at lower levels of OPP. As such, the autoregulatory reserve toward higher OPPs is increased as compared with physiologic IOP values.
The differences in the regulatory behavior of choroidal and ONH blood flow when OPP is changed may be related to the different anatomic features of these vascular beds. The vascular supply of the ONH is complex, and the anterior and posterior parts are fed by different vessels. The anterior part of the ONH is supplied by the central retinal artery and is not under neural control, because it lacks innervation. The posterior parts of the ONH behind the lamina cribrosa are nourished from the short posterior ciliary arteries either directly or via the circle of Zinn-Haller.20 The LDF signal obtained with the technique used in the present study mainly arises from the anterior part of the ONH vasculature.13 The choroid, however, is richly innervated and neural control appears to have a key role in blood flow regulation.21 This may also explain why the choroid regulates better during an isometric exercise-induced increase in MAP than the ONH related to the activation of the sympathetic system with pronounced choroidal vasoconstriction.
The present study indicates that the level of blood flow is dependent not only on OPP, but also on the absolute levels of IOP and MAP in a complex way. To the best of our knowledge, this is the first study indicating that this is the case in the ONH. There is, however, evidence from a variety of previous animal and human studies that this is the case in the choroid.7, 22, 23, 24, 25, 26, 27
In other vascular beds, little is known about the interaction among perfusion pressure, arterial pressure, and venous pressure. In the brain, most of the research focused on autoregulatory behavior during changes in arterial pressure,28, 29, 30 because fluctuation in venous pressure is considered small. In the eye, this is fundamentally different. When IOP increases the venous pressure increases in parallel and is always slightly higher than IOP in both the retina and the choroid.31, 32 During extremely high IOPs, venous pressure can even exceed arterial pressure leading to complete ischemia of ocular tissues. As such, our results may be of relevance for understanding the pathophysiology of glaucoma.
The most important risk factor for glaucoma is increased IOP, but additional risk factors including low OPP have been identified.33, 34, 35 A complete description of the theory how low OPP may be involved in glaucoma pathogenesis has been summarized in recent reviews.36, 37 With regard to the present data, it is of interest that ONHBF appears to have relatively little regulatory capacity when IOP is increased. This supports the idea that ischemic periods may occur during IOP peaks as has been suggested from diurnal measurements of MAP, IOP, and blood flow in glaucoma patients.38, 39, 40, 41, 42
The results of the present study may be explained based on the myogenic theory of autoregulation, which assumes that changes in transmural pressure result in smooth muscle and constriction in response to changes in perfusion pressure. During an increase in IOP, the decrease in OPP is paralleled by a decrease in perfusion pressure gradient. The situation is different when the OPP is altered via the arterial system during isometric exercise. In this case, the increase in transmural pressure in the arteries supplying the ONH will result in vasoconstriction due to the myogenic response.
A number of limitations need to be mentioned when discussing the results of the present study. Obviously, different subjects start at different MAPs and IOPs at baseline. This makes it difficult to produce pressure–flow relationships. During the experiments in which we increased either MAP or IOP we tried to overcome this limitation by using the data expressed as %change over baseline. During the combined experiments analysis is even more difficult, because ONHBF is obviously not a function of OPP anymore, but depends on OPP, MAP, and IOP in a complex way. As such, we decided to group data according to three different ranges of either MAP or IOP (Figures 7 and 8). Importantly, we then performed linear regression analysis, which does not reflect the pressure/flow curves obtained in Figures 5 and 6. It is, however, obvious that the scattering of data in Figures 7 and 8 is too large to obtain more information on the shape of the curves. Additional data from studies in much larger populations are required to elucidate the exact shape of the association between MAP and ONHBF dependent on IOP. The same holds true for the association between IOP and ONHBF dependent on MAP.
Another limitation relates to the ethical problems of experimentally reducing blood pressure in humans. In the present experiments, we either increased MAP or IOP resulting in either an increase or a decrease in OPP. It would of course be interesting to compare ONHBF at similar OPP reduction if either IOP is increased or MAP is decreased, but such experiments are reserved for experimental animals. This limitation does, however, not affect the key result of the present study, indicating that ONHBF levels may differ at the same level of OPP dependent on absolute MAP and IOP. In addition, our data mimic well the changes in OPP that may occur in daily life.
In conclusion, this study indicates that ONHBF autoregulation during combined changes in MAP and IOP is complex. Blood flow does not only depend on OPP values, but also on the absolute values of MAP and IOP. Generally, ONHBF regulates better during exercise-induced changes in arterial pressure than during an increase in IOP. These results may be compatible with a myogenic response and may have clinical relevance for our understanding of glaucoma pathophysiology.
The authors declare no conflict of interest.
Footnotes
This study was supported by a grant (P21406) from the Austrian Science Fund (Fonds zur Förderung der Wissenschaftlichen Forschung).
References
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma Arch Ophthalmol 2002120714–720.discussion 829-730. [DOI] [PubMed] [Google Scholar]
- Leske MC, Wu SY, Honkanen R, Nemesure B, Schachat A, Hyman L, et al. Nine-year incidence of open-angle glaucoma in the Barbados Eye Studies. Ophthalmology. 2007;114:1058–1064. doi: 10.1016/j.ophtha.2006.08.051. [DOI] [PubMed] [Google Scholar]
- Leske MC, Connell AM, Wu SY, Nemesure B, Li X, Schachat A, et al. Incidence of open-angle glaucoma: the Barbados Eye Studies. The Barbados Eye Studies Group. Arch Ophthalmol. 2001;119:89–95. [PubMed] [Google Scholar]
- Flammer J, Orgul S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- Luksch A, Polska E, Imhof A, Schering J, Fuchsjager-Mayrl G, Wolzt M, et al. Role of NO in choroidal blood flow regulation during isometric exercise in healthy humans. Invest Ophthalmol Vis Sci. 2003;44:734–739. doi: 10.1167/iovs.02-0177. [DOI] [PubMed] [Google Scholar]
- Polska E, Simader C, Weigert G, Doelemeyer A, Kolodjaschna J, Scharmann O, et al. Regulation of choroidal blood flow during combined changes in intraocular pressure and arterial blood pressure. Invest Ophthalmol Vis Sci. 2007;48:3768–3774. doi: 10.1167/iovs.07-0307. [DOI] [PubMed] [Google Scholar]
- Movaffaghy A, Chamot SR, Petrig BL, Riva CE. Blood flow in the human optic nerve head during isometric exercise. Exp Eye Res. 1998;67:561–568. doi: 10.1006/exer.1998.0556. [DOI] [PubMed] [Google Scholar]
- Pillunat LE, Anderson DR, Knighton RW, Joos KM, Feuer WJ. Autoregulation of human optic nerve head circulation in response to increased intraocular pressure. Exp Eye Res. 1997;64:737–744. doi: 10.1006/exer.1996.0263. [DOI] [PubMed] [Google Scholar]
- Riva CE, Hero M, Titze P, Petrig B. Autoregulation of human optic nerve head blood flow in response to acute changes in ocular perfusion pressure. Graefes Arch Clin Exp Ophthalmol. 1997;235:618–626. doi: 10.1007/BF00946937. [DOI] [PubMed] [Google Scholar]
- Wolzt M, Schmetterer L, Rheinberger A, Salomon A, Unfried C, Breiteneder H, et al. Comparison of non-invasive methods for the assessment of haemodynamic drug effects in healthy male and female volunteers: sex differences in cardiovascular responsiveness. Br J Clin Pharmacol. 1995;39:347–359. doi: 10.1111/j.1365-2125.1995.tb04462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva CE, Harino S, Petrig BL, Shonat RD. Laser Doppler flowmetry in the optic nerve. Exp Eye Res. 1992;55:499–506. doi: 10.1016/0014-4835(92)90123-a. [DOI] [PubMed] [Google Scholar]
- Riva CE, Geiser M, Petrig BL. Ocular blood flow assessment using continuous laser Doppler flowmetry. Acta Ophthalmol. 2010;88:622–629. doi: 10.1111/j.1755-3768.2009.01621.x. [DOI] [PubMed] [Google Scholar]
- Gugleta K, Orgul S, Flammer I, Gherghel D, Flammer J. Reliability of confocal choroidal laser Doppler flowmetry. Invest Ophthalmol Vis Sci. 2002;43:723–728. [PubMed] [Google Scholar]
- Pemp B, Maar N, Weigert G, Luksch A, Resch H, Garhofer G, et al. Strategies for reducing variance in laser Doppler flowmetry measurements. Graefes Arch Clin Exp Ophthalmol. 2009;247:67–71. doi: 10.1007/s00417-008-0942-0. [DOI] [PubMed] [Google Scholar]
- Ulrich WD, Ulrich C. Oculo-oscillo-dynamography: a diagnostic procedure for recording ocular pulses and measuring retinal and ciliary arterial blood pressures. Ophthalmic Res. 1985;17:308–317. doi: 10.1159/000265391. [DOI] [PubMed] [Google Scholar]
- Robinson F, Riva CE, Grunwald JE, Petrig BL, Sinclair SH. Retinal blood flow autoregulation in response to an acute increase in blood pressure. Invest Ophthalmol Vis Sci. 1986;27:722–726. [PubMed] [Google Scholar]
- Schmidl D, Boltz A, Kaya S, Werkmeister R, Dragostinoff N, Lasta M, et al. Comparison of choroidal and optic nerve head blood flow regulation during changes in ocular perfusion pressure. Invest Ophthalmol Vis Sci. 2012;53:4337–4346. doi: 10.1167/iovs.11-9055. [DOI] [PubMed] [Google Scholar]
- Schmidl D, Garhofer G, Schmetterer L. The complex interaction between ocular perfusion pressure and ocular blood flow—relevance for glaucoma. Exp Eye Res. 2011;93:141–155. doi: 10.1016/j.exer.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Mackenzie PJ, Cioffi GA. Vascular anatomy of the optic nerve head. Can J Ophthalmol. 2008;43:308–312. doi: 10.3129/i08-042. [DOI] [PubMed] [Google Scholar]
- Lutjen-Drecoll E. Choroidal innervation in primate eyes. Exp Eye Res. 2006;82:357–361. doi: 10.1016/j.exer.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Kiel JW. Choroidal myogenic autoregulation and intraocular pressure. Exp Eye Res. 1994;58:529–543. doi: 10.1006/exer.1994.1047. [DOI] [PubMed] [Google Scholar]
- Kiel JW, Shepherd AP. Autoregulation of choroidal blood flow in the rabbit. Invest Ophthalmol Vis Sci. 1992;33:2399–2410. [PubMed] [Google Scholar]
- Kiel JW, van Heuven WA. Ocular perfusion pressure and choroidal blood flow in the rabbit. Invest Ophthalmol Vis Sci. 1995;36:579–585. [PubMed] [Google Scholar]
- Schmidl D, Weigert G, Dorner GT, Resch H, Kolodjaschna J, Wolzt M, et al. Role of adenosine in the control of choroidal blood flow during changes in ocular perfusion pressure. Invest Ophthalmol Vis Sci. 2011;52:6035–6039. doi: 10.1167/iovs.11-7491. [DOI] [PubMed] [Google Scholar]
- Boltz A, Schmidl D, Weigert G, Lasta M, Pemp B, Resch H, et al. Effect of latanoprost on choroidal blood flow regulation in healthy subjects. Invest Ophthalmol Vis Sci. 2011;52:4410–4415. doi: 10.1167/iovs.11-7263. [DOI] [PubMed] [Google Scholar]
- Fuchsjager-Mayrl G, Georgopoulos M, Hommer A, Weigert G, Pemp B, Vass C, et al. Effect of dorzolamide and timolol on ocular pressure: blood flow relationship in patients with primary open-angle glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci. 2010;51:1289–1296. doi: 10.1167/iovs.09-3827. [DOI] [PubMed] [Google Scholar]
- van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab. 2008;28:1071–1085. doi: 10.1038/jcbfm.2008.13. [DOI] [PubMed] [Google Scholar]
- Claassen JA, Zhang R. Cerebral autoregulation in Alzheimer's disease. J Cereb Blood Flow Metab. 2011;31:1572–1577. doi: 10.1038/jcbfm.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budohoski KP, Czosnyka M, Kirkpatrick PJ, Smielewski P, Steiner LA, Pickard JD. Clinical relevance of cerebral autoregulation following subarachnoid haemorrhage. Nat Rev Neurol. 2013;9:152–163. doi: 10.1038/nrneurol.2013.11. [DOI] [PubMed] [Google Scholar]
- Maepea O. Pressures in the anterior ciliary arteries, choroidal veins and choriocapillaris. Exp Eye Res. 1992;54:731–736. doi: 10.1016/0014-4835(92)90028-q. [DOI] [PubMed] [Google Scholar]
- Glucksberg MR, Dunn R. Direct measurement of retinal microvascular pressures in the live, anesthetized cat. Microvasc Res. 1993;45:158–165. doi: 10.1006/mvre.1993.1015. [DOI] [PubMed] [Google Scholar]
- Leske MC. Ocular perfusion pressure and glaucoma: clinical trial and epidemiologic findings. Curr Opin Ophthalmol. 2009;20:73–78. doi: 10.1097/ICU.0b013e32831eef82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa VP, Arcieri ES, Harris A. Blood pressure and glaucoma. Br J Ophthalmol. 2009;93:1276–1282. doi: 10.1136/bjo.2008.149047. [DOI] [PubMed] [Google Scholar]
- He Z, Vingrys AJ, Armitage JA, Bui BV. The role of blood pressure in glaucoma. Clin Exp Optom. 2011;94:133–149. doi: 10.1111/j.1444-0938.2010.00564.x. [DOI] [PubMed] [Google Scholar]
- Cherecheanu AP, Garhofer G, Schmidl D, Werkmeister R, Schmetterer L. Ocular perfusion pressure and ocular blood flow in glaucoma. Curr Opin Pharmacol. 2013;13:36–42. doi: 10.1016/j.coph.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarieh M, Flammer J. Is there more to glaucoma treatment than lowering IOP. Surv Ophthalmol. 2007;52 (Suppl 2:S174–S179. doi: 10.1016/j.survophthal.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Pemp B, Georgopoulos M, Vass C, Fuchsjager-Mayrl G, Luksch A, Rainer G, et al. Diurnal fluctuation of ocular blood flow parameters in patients with primary open-angle glaucoma and healthy subjects. Br J Ophthalmol. 2009;93:486–491. doi: 10.1136/bjo.2008.148676. [DOI] [PubMed] [Google Scholar]
- Sung KR, Cho JW, Lee S, Yun SC, Choi J, Na JH, et al. Characteristics of visual field progression in medically treated normal-tension glaucoma patients with unstable ocular perfusion pressure. Invest Ophthalmol Vis Sci. 2011;52:737–743. doi: 10.1167/iovs.10-5351. [DOI] [PubMed] [Google Scholar]
- Sung KR, Lee S, Park SB, Choi J, Kim ST, Yun SC, et al. Twenty-four hour ocular perfusion pressure fluctuation and risk of normal-tension glaucoma progression. Invest Ophthalmol Vis Sci. 2009;50:5266–5274. doi: 10.1167/iovs.09-3716. [DOI] [PubMed] [Google Scholar]
- Kochkorov A, Gugleta K, Katamay R, Flammer J, Orgul S. Short-term variability of systemic blood pressure and submacular choroidal blood flow in eyes of patients with primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2010;248:833–837. doi: 10.1007/s00417-009-1277-1. [DOI] [PubMed] [Google Scholar]
- Lee M, Cho EH, Lew HM, Ahn J. Relationship between ocular pulse amplitude and glaucomatous central visual field defect in normal-tension glaucoma. J Glaucoma. 2012;21:596–600. doi: 10.1097/IJG.0b013e31824cfbf7. [DOI] [PubMed] [Google Scholar]