Abstract
The purpose of the present study was to assess the effect of heat stress-induced changes in systemic circulation on intra- and extracranial blood flows and its distribution. Twelve healthy subjects with a mean age of 22±2 (s.d.) years dressed in a tube-lined suit and rested in a supine position. Cardiac output (Q), internal carotid artery (ICA), external carotid artery (ECA), and vertebral artery (VA) blood flows were measured by ultrasonography before and during whole body heating. Esophageal temperature increased from 37.0±0.2°C to 38.4±0.2°C during whole body heating. Despite an increase in Q (59±31%, P<0.001), ICA and VA decreased to 83±15% (P=0.001) and 87±8% (P=0.002), respectively, whereas ECA blood flow gradually increased from 188±72 to 422±189 mL/minute (+135%, P<0.001). These findings indicate that heat stress modified the effect of Q on blood flows at each artery; the increased Q due to heat stress was redistributed to extracranial vascular beds.
Keywords: arterial pressure, cardiac output, Doppler ultrasound, humans, hyperthermia
Introduction
Cerebral blood flow (CBF) regulation is different from the regulation of other regional circulations such as renal, splanchnic, muscle, and cutaneous blood flows.1 For example, the brain has distinct blood flow regulatory mechanisms, i.e., cerebral autoregulation and cerebral carbon dioxide (CO2) reactivity.1, 2 In addition, the arterioles of most organs are innervated by sympathetic adrenergic nerve fibers, whereas cutaneous vasculatures are innervated by both sympathetic adrenergic and sympathetic cholinergic nerves.3, 4 However, it is unlikely that the sympathetic nervous system regulates cerebral vasculature directly.5, 6 These differences in blood flow regulation between the brain and other organs may be associated with the fact that the brain is a specific organ of the central nervous system. As the cerebrovascular bed is small, at any time CBF should be regulated precisely to preserve an adequate blood supply to the brain for maintaining cerebral neural activity or metabolism.1, 7 However, these differences may make an understanding of the mechanism of CBF regulation more complicated to achieve.
We have previously manipulated central blood volume via blood volume infusion or lower body negative pressure and found that the changes in cardiac output (Q) influence CBF, and its regulation is independent of cerebral autoregulation.8 Simply, an increase or decrease in Q augments or reduces CBF at rest and during exercise, respectively. Moreover, arterial baroreflex mediated-Q changes (cardiac baroreflex function) affects CBF regulation.9 These findings suggest that systemic blood flow or blood pressure regulation is one of the important physiologic factors in CBF regulation. In contrast, heat stress reduced rather than augmented CBF despite an increase in Q,10 indicating that the role of systemic circulation is unlikely to have an important role in CBF regulation during heat stress. The mechanism of the decrease in CBF during heat stress has been explained partly by heat stress-induced hypocapnia via hyperventilation,11 but it has not been understood completely.11, 12
It is well established that heat stress modified the blood distribution of Q to the systemic circulation by an increase in cutaneous blood flow for thermoregulation.4, 13, 14, 15 Similar to systemic circulation, the contribution of Q to CBF may be altered by heat stress. In the previous study, we have demonstrated that blood distribution in the brain (posterior and anterior CBF) was altered by several physiologic conditions, i.e., dynamic exercise7 and orthostatic stress.16 Therefore, we hypothesized that heat stress also modified the intra- and extracranial blood flows and the modified blood flow distribution might affect reduced CBF despite the increased Q. To test our hypothesis, we measured changes in Q, intra- and extracranial blood flows (i.e., internal and external carotid arteries and the vertebral artery) and calculated the distributions of Q to these arteries.
Materials and Methods
Twelve healthy subjects with a mean age of 22±2 years (mean±s.d.), height of 172±8 cm, and weight of 65±13 kg voluntarily participated in this study. Each subject provided written informed consent after all potential risks and procedures were explained. All experimental procedures and protocols conformed to the Declaration of Helsinki and were approved by the Human Subjects Committee of Nara Women's University. The subjects were free of any known cardiovascular or pulmonary disorders and were not using any prescribed or over–the-counter medications. In addition, they were not engaged in endurance training on a regular basis (<5 hours/week). Before the experiment, each subject gave written informed consent and visited the laboratory for familiarization with the techniques and procedures. Subjects were requested to abstain from caffeinated beverages for 12 hours and strenuous physical activity and alcohol for at least 24 hours before the day of the experiment.
Experimental Protocol
Experiments were performed in a temperature-controlled laboratory (26±1°C). Subjects arrived at the laboratory at least 2 hours after a light meal. Then each subject dressed in a water-perfused, tube-lined suit (Med-Eng, Ottawa, ON, Canada). The water-perfused suit covered the entire body except for the head, face, hands, and feet. After instrumentation, the subjects laid supine on a hospital bed for ∼30 minutes under normothermic condition, while breathing room air through a mouthpiece. Thermoneutral condition was maintained by perfusing 33°C water through the suit. After this equilibrium period, the baseline data were collected for 10 minutes during spontaneous respiration, followed by Q measurement via Doppler techniques. Subjects were then exposed to heat stress by perfusing 50°C water through the suit for a duration (∼45 to 90 minutes) sufficient to increase esophageal temperature to ∼1.5°C. Throughout heat stress, the following physiologic parameters were continuously measured and the Doppler measurements were repeated at four stages; +0.3°C (Heat1), +0.7°C (Heat2), +1.1°C (Heat3), and +1.5°C (Heat4) from the baseline.
Instrumentations and Measurements
Hemodynamic variables
Esophageal and skin temperatures were measured by thermocouples. Mean skin temperature was calculated via the weighted average of six thermocouples attached to the skin.17 External canal temperature was measured by infrared sensor (Nipro CE Thermo, NIPRO, Osaka, Japan). Heart rate was obtained from an electrocardiogram (Biomulti 1000, NEC, Tokyo, Japan). Continuous beat-by-beat arterial blood pressure was recorded from a finger using the Penaz method (Portapres, Finapres Medical Systems, Amsterdam, The Netherlands). Intermittent arterial blood pressure was also measured by auscultation of the brachial artery via electrosphygmomanometry (STBP-780, Colin, Komaki, Japan). Skin blood flow was measured via laser-Doppler flowmetry using an integrating flow probe (moor VMS-LDF2, Moor Instruments, Exeter, UK) attached to the forehead and forearm. Middle cerebral artery (MCA) mean blood velocity (Vmean) was continuously measured using transcranial Doppler ultrasonography (WAKIe, Atys Medical, France). A 2-MHz Doppler probe was adjusted over the temporal window until an optimal signal was identified. The probe was then fixed and held in place using a headband strap.
Respiratory variables
The subject breathed through a mouthpiece attached to a different pressure flow meter. Respiratory and metabolic data during the experiments were recorded by an automatic breath-by-breath respiratory gas analyzing system (ARCO2000-MET, Arcosystem, Chiba, Japan). We digitized expired flow, CO2, and oxygen concentrations, and derived tidal volume, respiratory rate, minute ventilation, and end-tidal CO2 partial pressure (PETCO2). Flow signals were computed using single breath data and matched to gas concentrations identified as single breaths using PETCO2, after accounting for the time lag (350 milliseconds) in gas concentration measurements. The corresponding oxygen uptake and CO2 output values for each breath were calculated from inspired–expired gas concentration differences, and by expired ventilation, with inspired ventilation being calculated by N2 correction. During each protocol, minute ventilation and PETCO2 were recorded continuously at 200 Hz. In addition, some subjects performed a 5% CO2 re-breathing test to confirm whether Q estimated by cardiac echo was accurate. Cardiac output was determined using Fick's equation modified to accommodate the CO2 content in mixed venous blood, CO2 output, and arterial CO2 content. The CO2 content in mixed venous blood was estimated using the CO2 re-breathing equilibrium technique,18 in which a Hans Rudolf valve (model 8200, Hans Rudolf, Shawnee, KS, USA), a re-breathing attachment and an automatic breath-by-breath respiratory gas analyzing system were used (ARCO2000-MET, Arcosystem). The re-breathing Q measurement was performed at the baseline and at the end of heating.
Doppler Measurements
Blood flow in each artery in the head
Blood flow was measured before and during heat stress at a given level (Heat1 to 4, see above) for 1 minute. The representative blood flow values at each stage are the average of all 1-minute recordings. The right internal carotid artery (ICA) and vertebral artery (VA) blood flow were measured with a color-coded ultrasound system (Vivid-e; GE Healthcare, Tokyo, Japan) equipped with a 10 MHz linear transducer. ICA blood flow measurements were performed ∼1.0 to 1.5 cm distal to the carotid bifurcation on the right ICA while the subject's chin was slightly elevated. Vertebral artery blood flow was measured between the transverse processes of the C3 and subclavian artery. In addition, external carotid artery (ECA) blood flow was measured with the same Doppler ultrasound system mentioned above. These measurements were generally taken ∼1.0 to 1.5 cm above the carotid bifurcation on the right ECA. Common carotid artery blood flow was estimated by the sum of ICA and ECA blood flow. We have confirmed the validity of this analysis by comparison between measured common carotid artery and the sum of ICA and ECA blood flows in several subjects. We did not observe a large difference in this value between these. In addition, total circulatory blood flow toward head (THBF) was presumed by the equation; (common carotid artery blood flow+VA blood flow) × 2.
For all blood flow measurements, we first used the brightness mode to measure the mean vessel diameter of each vessel in a longitudinal section, and the Doppler velocity spectrum was subsequently identified by pulsed wave mode. The systolic and diastolic diameters were measured in detail, and then the mean diameter (cm) was calculated in relation to the blood pressure curve: mean diameter=((systolic diameter × 1/3))+((diastolic diameter × 2/3)). The time-averaged mean flow velocity obtained in pulsed wave mode was defined as the mean blood flow velocity (cm/second). The measurements of blood flow velocity were made from the average of ∼10 to 20 cardiac cycles to eliminate the effects caused by the breathing cycle.
When making blood flow velocity measurements, care was taken to ensure that the probe position was stable, that the insonation angle did not vary (∼60° in most cases), and that the sample volume was positioned in the center of the vessel and adjusted to cover the width of the vessel diameter. Finally, blood flow was calculated by multiplying the cross-sectional area (π × (mean diameter/2)2) with mean blood flow velocity; blood flow=mean blood flow velocity × area × 60 (mL/minute). All blood flow measurements were performed by the same operator.
Cardiac output
The best echocardiography image for each subject was selected at each stage for Q analysis. Stroke volume (SV) was estimated from aortic flow calculated as the product of beat-to-beat measurements of the Doppler velocity time integral and aortic root diameter (Vivid-i; GE Healthcare). Aortic root diameter was determined using two-dimensional echocardiogram imaging obtained in the left parasternal long axis view with the subject in the supine position and SV calculated as: SV=π (D/2)2 × velocity time integral. Cardiac output was calculated from the product of SV and HR. Total vascular conductance was calculated on a beat-to-beat basis from the ratio of Q to MAP (mean arterial pressure) (total vascular conductance=Q/MAP).
Data Analysis
Data except for Doppler measurements were sampled at 1 kHz via a data acquisition system (MP150, BIOPAC Systems, Goleta, CA, USA). Data from the last 120 seconds of each period (+0°C, baseline; +0.3°C, Heat1; +0.7°C, Heat2;+1.1°C, Heat3;+1.5°C, Heat4) were averaged for steady-state statistical analyses. Cutaneous vascular conductance (CVC) was indexed from the ratio of skin blood flow to MAP. In addition, each vascular conductance was estimated from the ratio of MCAVmean, ICA, ECA, or VA blood flow to MAP.
Statistical Analysis
Analyses were conducted using SigmaStat (Jandel Scientific Software, SPSS, Chicago, IL, USA). Data were subsequently analyzed using one-way repeated measures analysis of variance test and post hoc Student–Newman–Keul tests. The relationship between PETCO2 and each arterial blood flow was obtained by a simple linear regression of all five points (baseline, Heat1, Heat2, Heat3, and Heat4) in each subject. The relationship between ICA and ECA blood flows was obtained by a simple linear regression of all five points (baseline, Heat1, Heat2, Heat3, and Heat4) in each subject. Data are expressed as mean±s.d. and significance was set at P<0.05.
Results
Typical thermoregulatory and cardiovascular responses before and during passive heat stress were represented in Table 1. Whole body heating increased core and skin temperatures (both P<0.01), and skin blood flow at forehead and forearm increased by direct (forearm) and indirect (forehead and forearm) heating. Cardiac output estimated by cardiac echo gradually increased during heat stress (P<0.001) with increasing heart rate (P<0.001) and no change in SV (P=0.577). Mean arterial pressure was well maintained from the baseline until Heat3, but decreased slightly (−6±6 mm Hg) at Heat4 condition (P=0.024). Total vascular conductance and CVC at forehead or forearm gradually increased during heat stress (P<0.001). In addition, the heat stress caused hyperventilation and subsequent hypocapnia (P<0.001) at the latter half of the heat stress (Heat3 to 4).
Table 1. Cardiorespiratory and cerebrovascular hemodynamic responses during normothermia (baseline) and heat stress (Heat1–4).
| Baseline | Heat1 | Heat2 | Heat3 | Heat4 | ||
|---|---|---|---|---|---|---|
| Tes (°C) | 37.05±0.24 | 37.35±0.27* | 37.73±0.30*# | 38.24±0.30*#$ | 38.41±0.23*#$& | P<0.001 |
| ΔTes (°C) | +0.30±0.10 | +0.69±0.16 | +1.20±0.15 | +1.42±0.08 | ||
| Tty (°C) | 37.01±0.26 | 37.34±0.32* | 37.66±0.32*# | 38.11±0.39*#$ | 38.32±0.36*#$& | P<0.001 |
| Tsk (°C) | 33.52±1.27 | 37.29±1.73* | 38.20±0.88*# | 38.58±0.85*#$ | 38.60±0.70*#& | P<0.001 |
| HR (bpm) | 64±10 | 78±9* | 86±10*# | 93±11*#$ | 96±11*#$ | P<0.001 |
| MAP (mm Hg) | 90±8 | 89±7 | 86±5 | 87±8 | 84±6*# | P=0.024 |
| Q (L/min) | 5.6±1.6 | 7.0±1.9* | 7.9±2.0*# | 8.2±1.9*# | 8.9±2.4*# | P<0.001 |
| SV (ml) | 89±20 | 90±20 | 92±19 | 88±18 | 94±23 | P=0.577 |
| TVC (L/mm Hg) | 0.062±0.016 | 0.079±0.018* | 0.092±0.023*# | 0.094±0.022*# | 0.105±0.030*#$ | P<0.001 |
| PETCO2 (mm Hg) | 40.4±2.8 | 38.3±3.2 | 37.5±4.6 | 34.7±6.2*#$ | 33.7±6.3*#$ | P<0.001 |
| VE (L/min) | 9.1±3.3 | 12.0±4.2 | 12.2±3.6 | 12.7±5.8 | 16.2±6.8* | P=0.021 |
| SkBFforehead (AU) | 74±33 | 150±47* | 202±48*# | 214±57*# | 211±51*# | P<0.001 |
| SkBFforearm (AU) | 17±12 | 112±79* | 161±48*# | 167±42*# | 165±51*# | P<0.001 |
| CVCforehead (AU/mm Hg) | 0.83±0.38 | 1.68±0.50* | 2.35±0.56*# | 2.35±0.54*# | 2.49±0.54*# | P<0.001 |
| CVCforearm (AU/mm Hg) | 0.19±0.12 | 1.24±0.85* | 1.88±0.60*# | 1.98±0056*# | 1.97±0.62*# | P<0.001 |
| MCAVmean (cm/s) | 53±17 | 45±12* | 45±12* | 39±14* | 41±16* | P<0.001 |
| ICA BF (ml/min) | 290±53 | 275±62 | 275±68 | 254±48* | 238±72*#$ | P=0.001 |
| ECA BF (ml/min) | 188±72 | 271±128* | 351±163# | 398±199*# | 442±189*#$ | P<0.001 |
| VA BF (ml/min) | 89±38 | 82±41* | 81±34* | 78±33* | 74±35* | P=0.002 |
| MCAVmean CON (cm/s/mm Hg) | 0.60±0.19 | 0.51±0.22 | 0.47±0.20* | 0.45±0.15* | 0.48±0.20* | P=0.009 |
| ICA CON (ml/min/mm Hg) | 3.29±0.86 | 3.13±0.79 | 3.20±0.83 | 2.95±0.69 | 2.87±1.00 | P=0.037 |
| ECA CON (ml/min/mm Hg) | 2.11±0.81 | 3.03±1.44* | 4.08±1.87*# | 4.60±2.35*# | 5.21±2.16*#$ | P<0.001 |
| VA CON (ml/min/mm Hg) | 1.00±0.44 | 0.93±0.47 | 0.95±0.42 | 0.90±0.41 | 0.88±0.40 | P=0.091 |
BF, blood flow; bpm, beats per minute; CON, conductance; CVC, cutaneous vascular conductance; ECA, external carotid artery; HR, heart rate; ICA, internal carotid artery; MAP, mean arterial pressure; MCAVmean, middle cerebral artery mean blood velocity; min, minute; PETCO2, partial pressure of end-tidal carbon dioxide; Q, cardiac output; s, second; SkBF, skin blood flow; SV, stroke volume; Tes, esophageal temperature; Tsk, mean skin temperature; Tty, external canal temperature; TVC, total vascular conductance; VA, vertebral artery; VE, minute ventilation.
Values are mean±s.d. *P<0.05 different from the baseline; #P<0.05 different from Heat1; $P<0.05 different form Heat2; &P<0.05 different from Heat3.
During heat stress, ICA and VA blood flows as well as MCAVmean decreased (all, P<0.001), while ECA increased. Middle cerebral artery mean blood velocity and VA blood flow decreased immediately at Heat1 from the baseline and these lower values were maintained throughout heat stress. Internal carotid artery blood flow was unchanged from the baseline until Heat2 but it gradually decreased at Heat3 to 4. In contrast, ECA blood flow gradually increased from 188±72 to 422±189 mL/minute (+135%, P<0.001) throughout heat stress. Although PETCO2 gradually decreased during heat stress (Table 1), no significant relationship between decrease in PETCO2 and the changes in ICA and VA blood flows was observed.
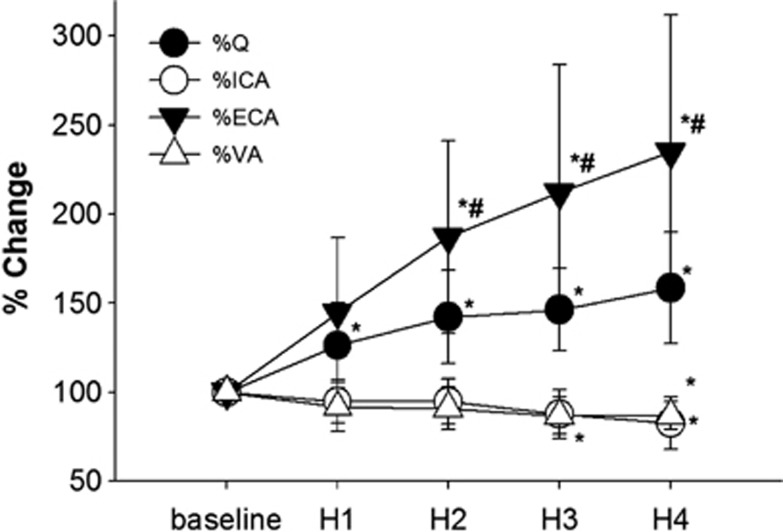
Cardiac output increased during the heat stress, and simultaneously ECA and skin blood flows increased (Table 1). The percentage of change in Q at the end of heating was +59±31% from the baseline, but the percent change in ECA blood flow was much higher than that in Q (+135%, Figure 1). In contrast, both the ICA and VA blood flows decreased to 83±15% and 87±8% of the baseline value at Heat4, respectively. The change in Q was related with changes in ECA (P=0.002) but not ICA blood flow (P=0.227).
Figure 1.
The percentage of changes in the cardiac output (Q), internal carotid artery (ICA), external carotid artery (ECA), and vertebral artery (VA) blood flow from normothermia (baseline) throughout passive heat stress. Values are mean±s.d. *P<0.05 different from the baseline; #P<0.05 different from H1. H, heat.
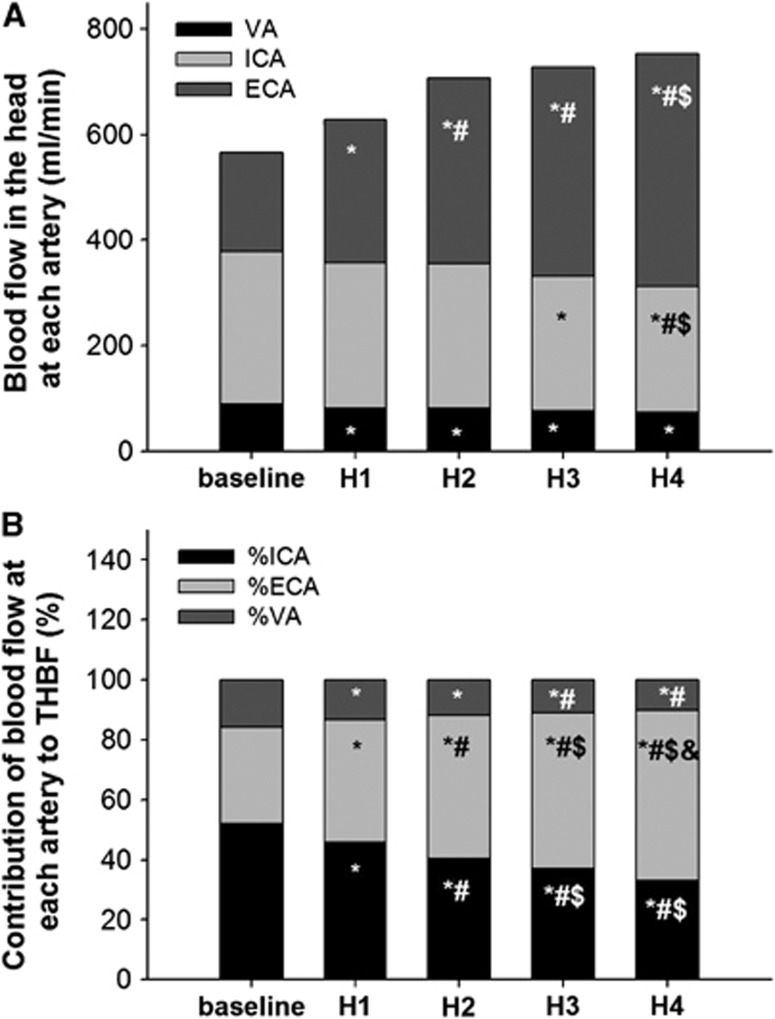
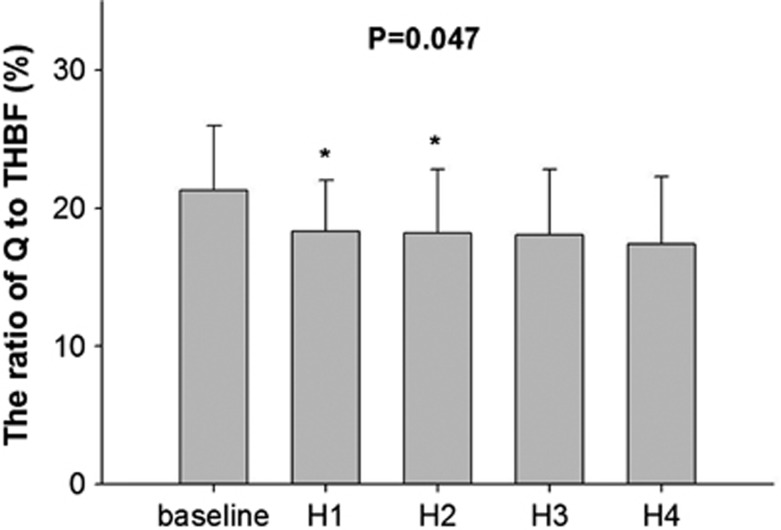
Heat stress-induced increase in THBF was only due to changes in ECA blood flow, because of the decrease in both ICA and VA blood flows (Figure 2A). Therefore, the ratio of THBF to ICA and VA blood flows gradually decreased during heat stress from 52±10% and 16±6% to 33±10% and 10±5%, respectively (Figure 2B). In contrast, the contribution of THBF to ECA blood flow gradually increased from 32±9% to 57±11% throughout the heat stress. Total THBF calculated by ICA, ECA, and VA blood flows also increased from 1.1±0.2 to 1.5±0.5 L/minute, but it was not significant (P=0.141). In the systemic circulation, the ratio of Q to THBF was 21.3±4.7% at the baseline. However, it decreased to 17.4±4.9% at Heat4 (P=0.047, Figure 3). Despite the decrease in percent distribution, increased Q during heat stress augmented THBF, but it was mostly distributed toward ECA (P=0.002) rather than ICA blood flow (P=0.227).
Figure 2.
(A) The changes in the proportion of total blood flow toward head (THBF) distributed to each artery during heat stress; (B) the contribution of each cerebral artery blood flow to THBF. *P<0.05 different from the baseline; #P<0.05 different from H1; $P<0.05 different form H2; &P<0.05 different from H3. ECA, external carotid artery; H, heat; ICA, internal carotid artery; VA, vertebral artery.
Figure 3.
The ratio of cardiac output (Q) to blood flow toward head (THBF) at normothermia (baseline) and during heat stress. Values are mean±s.d. *P<0.05 different from the baseline. H, heat.
Discussion
The main finding of the present study is that heat stress modified the distribution of intra- and extracranial blood flows by decreased vascular resistance on the extra-cranium. This modification appears to contribute the regulation of cerebral (intracranial) blood flow. During heat stress, increased core and skin temperatures gradually increased Q and consequently, THBF was increased (from 1.13 L/minute to 1.5 L/minute at the end of heat stress), whereas the ICA and VA blood flow decreased. However, ECA significantly increased during heat stress. The present observation suggested that despite increased Q due to heat stress increased blood supply toward the head, it mostly distributed to the extracranial area for heat dissipation, and consequently intracranial blood flow did not increase.
Modified Blood Distribution in Hyperthermic Condition
During heat stress, the sympathetic cutaneous active vasodilator system induces cutaneous vasodilation for heat dissipation, which causes reduced vascular resistance.3 In addition, it is well known that heat stress modifies distribution of blood flow toward cutaneous circulation while decreasing renal and splanchnic blood flows by enhanced vasoconstriction.15, 19 Maintained or decreased cerebral blood velocity during heat stress has been reported10, 11, 12, 20, 21, 22, 23 but the mechanism remains unclear. The present study demonstrated that CBF conductance in MCAVmean, ICA, and VA gradually decreased during whole body heating, whereas CVCforehead and ECA conductance increased by 3 times and 2.5 times from the baseline, respectively. External carotid artery blood flow mainly supplies cutaneous circulation on extracranial regions, and cutaneous blood flow has a role for thermoregulation. During heat stress, active cutaneous vasodilator system causes cutaneous vasodilation for heat dissipation, resulting in reduced cutaneous vascular resistance.3 Therefore, for thermoregulation, ECA blood flow gradually increased throughout the heat stress, consequently the decreased vascular resistance in the extracranial regions would modify blood flow distribution. However, changes in ICA blood flow did not correlate with changes in ECA blood flow throughout heat stress (P=0.970). Although the increased ECA might not be a primal factor for reduced CBF during heat stress, the reduced cutaneous vascular resistance in the extra-cranium partially contributes to distribution of blood toward ECA rather than ICA.
Previous studies have provided evidence that Q is important for CBF regulation.8, 9, 24, 25, 26 However, heat stress rather reduced than augmented MCAVmean despite an increase in Q.10 Recently, Schlader et al27 demonstrated that reduced MCAVmean during heat stress was unaffected by acute volume expansion (i.e., additional increase of Q) and suggested that the further decreased systemic vascular resistance resulted in no change of cerebrovascular resistance. In this study, change in CVCforearm from the baseline to Heat4 was greater than that in CVCforehead. Therefore, the reduced vascular resistance in the extracranial regions might be smaller than the other regions. Given these observations, coupled with the present finding that heat stress-induced increased Q did not increase ICA blood flow (P=0.227), the influence of changes in Q on CBF might become smaller during heat stress relative to normothermia. Thus altered downstream vascular conductance would be capable of modulating the effect of Q on CBF regulation during heat stress. Especially, in the intra- and extracranial regions, it is likely that large vasodilation of extracranial vessel prevents an increase in intracranial blood flow caused by the increase in Q.
The Effect of Carbon Dioxide Reactivity and Autoregulation on Each Artery During Heat Stress
Similar to some previous studies,28, 29, 30 PETCO2 decreased via hyperventilation during heat stress, especially hyperventilation was observed at Heat3 or later. Cerebral blood flow regulation due to CO2 could be involved in the present results (i.e., reduced ICA, VA, and MCAVmean). Previously, we demonstrated that CO2 reactivity at ICA was much higher than that at ECA.31 Similar to this, during heat stress, ICA blood flow would be more influenced by hyperventilation-induced hypocapnia, whereas ECA blood flow might not be influenced largely by hypocapnia. Thus, the regional difference in CO2 reactivity might affect distribution of these blood flows during heat stress.
Previously, Low et al12 demonstrated unchanged CO2 reactivity of MCAVmean during mild heat stress (internal temperature increased ∼1.0°C). However, their follow-up study showed that MCAVmean did not return to the baseline level during heat stress (internal temperature increased by 1.4°C) even when PETCO2 was clamped to the preheating.11, 12 In the present study, we also calculated the relationship between changes in PETCO2 and changes in ICA and VA blood flows throughout heat stress, but there was no significant relationship. We did not perform any perturbation to change PETCO2 in this study. Although heat stress-induced hypocapnia might affect ICA and VA blood flows during heat stress, the present result could not explain completely why ICA and VA were reduced during heat stress.
Cerebral autoregulation is also an important physiologic mechanism for CBF regulation. Low et al clearly demonstrated that moderate heat stress (internal temperature increased by 1.0°C) does not impair dynamic cerebral autoregulation.32 Also, in the present study, the relationship between MAP and ICA or ECA blood flow (ICA/ECA conductance) was reduced or increased throughout heat stress, indicating that static cerebral autoregulation might be unchanged or attenuated, respectively. Thus, heat stress-induced increase in ECA may be due to the attenuated static cerebral autoregulation. However, a further investigation is required to identify the effect of cerebral CO2 reactivity and cerebral autoregulation on CBF regulation at each artery during heat stress.
The Verified Cerebral Blood Flow Response During Heat Stress
CBF, as indexed by MCAVmean and cerebral vascular conductance, is significantly reduced during passive heat stress.10, 11, 20, 23, 29, 33 Similarly, in the present study, heat stress decreased MCAVmean by −25% from the baseline (P<0.001). In the present study, for the first time, we quantitatively demonstrated that the heat stress decreased both ICA and VA blood flows (Table 1 and Figure 1). However, the percentage of reduction in ICA blood flow from the baseline to Heat4 was smaller (−17%) than those in MCAVmean (−25%). As MCA diameter was unchanged during several physiologic conditions,34, 35 MCA blood velocity has been used as an index of CBF even during heat stress.36 However, in the present study, we observed greater reduction in MCAVmean relative to ICA blood flow during heat stress, indicating that MCAVmean overestimates cerebral hypoperfusion, distribution of blood flow in the Willis circle, or the diameter of MCA might be changed in hyperthermic condition. In contrast, the percent changes in VA blood flow during heat stress (−13%) were similar to that in the posterior cerebral artery Vmean (−10%) in the previous results.10 Thus, posterior cerebral artery diameter may not be changed during heat stress.
Limitations
We were unable to simultaneously measure ipsilateral blood flow in the ECA, ICA, and VA because of the interference caused by the ultrasound beam. As the sum of ICA and ECA blood flows at rest and during heat stress are quantitatively matched with the other side common carotid artery blood flow, our CBF data were therefore reliable. In addition, a linear regression analysis is limited from a mathematical perspective in that it clearly does not disassociate cause from effect. Blood flow was measured before and during heat stress at a given level (Heat1 to 4, see above) for 1 minute in this study. The representative blood flow values at each stage are the average of all 1-minute recordings. The operator confirmed target point in each measurement, and then stored data for 1 minute. However, when subject swallowed saliva or moved with changes in the probe position and the insonation angle of the ultrasound beam, the operator extended the data collection, and then excluded the period subject moved. Thus, it was quite difficult in the present study to collect adequate continuous period for the analysis of dynamic cerebral autoregulation in each artery because of the above technical issue.
In summary, the heat stress increased ECA blood flow with an increase in Q, but decreased MCAVmean, ICA, and VA blood flow. These findings provided evidence that heat stress modified the effect of systemic circulation on intra- and extracranial blood flow or the distribution of Q to each artery. The percent change in ECA blood flow was much higher than that in Q; heat stress-induced increase in Q contributed to changes in ECA rather than ICA blood flow. These findings suggest that the large vasodilation of the extracranial vessel may prevent the increase in intracranial blood flow against the increase in Q during heat stress.
Perspectives
We proposed this project to clarify distribution of blood flow during heat stress. Hyperthermia reduces orthostatic tolerance, which is thought to relate with reduced CBF. The data suggest that a certain amount of blood supplies to the extracranial regions while CBF reduces in hyperthermic condition. Therefore, reduced blood distribution toward extracranial regions may improve decreased CBF. However, the increased ECA blood flow would be important for thermoregulation. Face cooling or fanning, which promote heat dissipation would be required.
Acknowledgments
The authors appreciate the time and effort expended by the volunteer subjects. We also thank MS Gonda, Imai, Ishihara, and Sakamoto for recruitment of subjects and support of this project.
The authors declare no conflict of interest.
Footnotes
This study was supported in part by Grant-in-Aid for Scientific Research B-23300265 (to MS), Grant-in-Aid for Scientific Research B-80553841 (to SO), Grant-in-Aid for young Scientists B-21700704 (to KS), and Grant-in-Aid for young Scientists A-23689014 (to KO).
References
- Ogoh S, Ainslie PN. Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol. 2009;107:1370–1380. doi: 10.1152/japplphysiol.00573.2009. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Ainslie PN. Regulatory mechanisms of cerebral blood flow during exercise: new concepts. Exerc Sport Sci Rev. 2009;37:123–129. doi: 10.1097/JES.0b013e3181aa64d7. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Kellogg DL., Jr. Thermoregulatory and thermal control in the human cutaneous circulation. Front Biosci (Schol Ed) 2010;2:825–853. doi: 10.2741/s105. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Blackmon JR. Lack of sympathetic vasoconstriction in hypoxemic humans at rest. Am J Physiol. 1986;251:H562–H570. doi: 10.1152/ajpheart.1986.251.3.H562. [DOI] [PubMed] [Google Scholar]
- Alm A, Bill A. The effect of stimulation of the cervical sympathetic chain on retinal oxygen tension and on uveal, retinal and cerebral blood flow in cats. Acta Physiol Scand. 1973;88:84–94. doi: 10.1111/j.1748-1716.1973.tb05436.x. [DOI] [PubMed] [Google Scholar]
- Harper AM, Deshmukh VD, Rowan JO, Jennett WB. The influence of sympathetic nervous activity on cerebral blood flow. Arch Neurol. 1972;27:1–6. doi: 10.1001/archneur.1972.00490130003001. [DOI] [PubMed] [Google Scholar]
- Sato K, Ogoh S, Hirasawa A, Oue A, Sadamoto T. The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. J Physiol. 2011;589:2847–2856. doi: 10.1113/jphysiol.2010.204461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoh S, Brothers RM, Barnes Q, Eubank WL, Hawkins MN, Purkayastha S, et al. The effect of changes in cardiac output on middle cerebral artery mean blood velocity at rest and during exercise. J Physiol. 2005;569:697–704. doi: 10.1113/jphysiol.2005.095836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoh S, Tzeng YC, Lucas SJ, Galvin SD, Ainslie PN. Influence of baroreflex-mediated tachycardia on the regulation of dynamic cerebral perfusion during acute hypotension in humans. J Physiol. 2010;588:365–371. doi: 10.1113/jphysiol.2009.180844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MD, Haykowsky MJ, Stickland MK, Altamirano-Diaz LA, Willie CK, Smith KJ, et al. Reductions in cerebral blood flow during passive heat stress in humans: partitioning the mechanisms. J Physiol. 2011;589:4053–4064. doi: 10.1113/jphysiol.2011.212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers RM, Wingo JE, Hubing KA, Crandall CG. The effects of reduced end-tidal carbon dioxide tension on cerebral blood flow during heat stress. J Physiol. 2009;587:3921–3927. doi: 10.1113/jphysiol.2009.172023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low DA, Wingo JE, Keller DM, Davis SL, Zhang R, Crandall CG. Cerebrovascular responsiveness to steady-state changes in end-tidal CO2 during passive heat stress. J Appl Physiol. 2008;104:976–981. doi: 10.1152/japplphysiol.01040.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall CG, Wilson TE, Marving J, Bundgaard-Nielsen M, Seifert T, Klausen TL, et al. Colloid volume loading does not mitigate decreases in central blood volume during simulated haemorrhage while heat stressed. J Physiol. 2012;590:1287–1297. doi: 10.1113/jphysiol.2011.223602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall CG, Wilson TE, Marving J, Vogelsang TW, Kjaer A, Hesse B, et al. Effects of passive heating on central blood volume and ventricular dimensions in humans. J Physiol. 2008;586:293–301. doi: 10.1113/jphysiol.2007.143057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT, Wladkowski SL, Pawelczyk JA, Kenney WL. Age, splanchnic vasoconstriction, and heat stress during tilting. Am J Physiol. 1999;276:R203–R212. doi: 10.1152/ajpregu.1999.276.1.r203. [DOI] [PubMed] [Google Scholar]
- Sato K, Fisher JP, Seifert T, Overgaard M, Secher NH, Ogoh S. Blood flow in internal carotid and vertebral arteries during orthostatic stress. Exp Physiol. 2012;97:1272–1280. doi: 10.1113/expphysiol.2012.064774. [DOI] [PubMed] [Google Scholar]
- Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol. 1989;66:1586–1592. doi: 10.1152/jappl.1989.66.4.1586. [DOI] [PubMed] [Google Scholar]
- Jones KR. A respiration monitor for use with CT body scanning and other imaging techniques. Br J Radiol. 1982;55:530–533. doi: 10.1259/0007-1285-55-655-530. [DOI] [PubMed] [Google Scholar]
- Escourrou P, Freund PR, Rowell LB, Johnson DG. Splanchnic vasoconstriction in heat-stressed men: role of renin-angiotensin system. J Appl Physiol. 1982;52:1438–1443. doi: 10.1152/jappl.1982.52.6.1438. [DOI] [PubMed] [Google Scholar]
- Brothers RM, Zhang R, Wingo JE, Hubing KA, Crandall CG. Effects of heat stress on dynamic cerebral autoregulation during large fluctuations in arterial blood pressure. J Appl Physiol. 2009;107:1722–1729. doi: 10.1152/japplphysiol.00475.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybo L, Moller K, Volianitis S, Nielsen B, Secher NH. Effects of hyperthermia on cerebral blood flow and metabolism during prolonged exercise in humans. J Appl Physiol. 2002;93:58–64. doi: 10.1152/japplphysiol.00049.2002. [DOI] [PubMed] [Google Scholar]
- Nybo L, Nielsen B. Middle cerebral artery blood velocity is reduced with hyperthermia during prolonged exercise in humans. J Physiol. 2001;534:279–286. doi: 10.1111/j.1469-7793.2001.t01-1-00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Zhang R, Crandall CG. Heat stress reduces cerebral blood velocity and markedly impairs orthostatic tolerance in humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1443–R1448. doi: 10.1152/ajpregu.00712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide K, Boushel R, Sorensen HM, Fernandes A, Cai Y, Pott F, et al. Middle cerebral artery blood velocity during exercise with beta-1 adrenergic and unilateral stellate ganglion blockade in humans. Acta Physiol Scand. 2000;170:33–38. doi: 10.1046/j.1365-201x.2000.00757.x. [DOI] [PubMed] [Google Scholar]
- Hellstrom L, Wahrenberg H, Reynisdottir S, Arner P. Catecholamine-induced adipocyte lipolysis in human hyperthyroidism. J Clin Endocrinol Metab. 1997;82:159–166. doi: 10.1210/jcem.82.1.3664. [DOI] [PubMed] [Google Scholar]
- Ide K, Gullov AL, Pott F, Van Lieshout JJ, Koefoed BG, Petersen P, et al. Middle cerebral artery blood velocity during exercise in patients with atrial fibrillation. Clin Physiol. 1999;19:284–289. doi: 10.1046/j.1365-2281.1999.00178.x. [DOI] [PubMed] [Google Scholar]
- Schlader ZJ, Seifert T, Wilson TE, Bundgaard-Nielsen M, Secher NH, Crandall CG. Acute volume expansion attenuates hyperthermia-induced reductions in cerebral perfusion during simulated hemorrhage. J Appl Physiol. 2013;114:1730–1735. doi: 10.1152/japplphysiol.00079.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers RM, Ganio MS, Hubing KA, Hastings JL, Crandall CG. End-tidal carbon dioxide tension reflects arterial carbon dioxide tension in the heat-stressed human with and without simulated hemorrhage. Am J Physiol Regul Integr Comp Physiol. 2011;300:R978–R983. doi: 10.1152/ajpregu.00784.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JL, Cotter JD, Lucas RA, Thomas K, Wilson L, Ainslie PN. Human cardiorespiratory and cerebrovascular function during severe passive hyperthermia: effects of mild hypohydration. J Appl Physiol. 2008;105:433–445. doi: 10.1152/japplphysiol.00010.2008. [DOI] [PubMed] [Google Scholar]
- Fujii N, Honda Y, Hayashi K, Kondo N, Koga S, Nishiyasu T. Effects of chemoreflexes on hyperthermic hyperventilation and cerebral blood velocity in resting heated humans. Exp Physiol. 2008;93:994–1001. doi: 10.1113/expphysiol.2008.042143. [DOI] [PubMed] [Google Scholar]
- Sato K, Sadamoto T, Hirasawa A, Oue A, Subudhi AW, Miyazawa T, et al. Differential blood flow responses to CO(2) in human internal and external carotid and vertebral arteries. J Physiol. 2012;590:3277–3290. doi: 10.1113/jphysiol.2012.230425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low DA, Wingo JE, Keller DM, Davis SL, Cui J, Zhang R, et al. Dynamic cerebral autoregulation during passive heat stress in humans. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1598–R1605. doi: 10.1152/ajpregu.90900.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Zhang R, Witkowski S, Crandall CG. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. J Appl Physiol. 2002;93:85–91. doi: 10.1152/japplphysiol.01043.2001. [DOI] [PubMed] [Google Scholar]
- Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery. 1993;32:737–742. [PubMed] [Google Scholar]
- Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1473–R1495. doi: 10.1152/ajpregu.91008.2008. [DOI] [PubMed] [Google Scholar]