Abstract
Stroke patients with hyperglycemia (HG) develop higher volumes of brain edema emerging from disruption of blood–brain barrier (BBB). This study explored whether inductions of protein kinase C-β (PKC-β) and RhoA/Rho-kinase/myosin-regulatory light chain-2 (MLC2) pathway may account for HG-induced barrier damage using an in vitro model of human BBB comprising human brain microvascular endothelial cells (HBMEC) and astrocytes. Hyperglycemia (25 mmol/L D-glucose) markedly increased RhoA/Rho-kinase protein expressions (in-cell westerns), MLC2 phosphorylation (immunoblotting), and PKC-β (PepTag assay) and RhoA (Rhotekin-binding assay) activities in HBMEC while concurrently reducing the expression of tight junction protein occludin. Hyperglycemia-evoked in vitro barrier dysfunction, confirmed by decreases in transendothelial electrical resistance and concomitant increases in paracellular flux of Evan's blue-labeled albumin, was accompanied by malformations of actin cytoskeleton and tight junctions. Suppression of RhoA and Rho-kinase activities by anti-RhoA immunoglobulin G (IgG) electroporation and Y-27632, respectively prevented morphologic changes and restored plasma membrane localization of occludin. Normalization of glucose levels and silencing PKC-β activity neutralized the effects of HG on occludin and RhoA/Rho-kinase/MLC2 expression, localization, and activity and consequently improved in vitro barrier integrity and function. These results suggest that HG-induced exacerbation of the BBB breakdown after an ischemic stroke is mediated in large part by activation of PKC-β.
Keywords: cytoskeletal remodeling, hyperglycemia, protein kinase C, RhoA, Rho-kinase, tight junction
Introduction
Ischemic strokes develop through an interference with blood supply to the brain and continue to be the leading cause of morbidity in the world. Cerebral ischemia reperfusion triggers a series of events such as oxidative stress and inflammation, which collectively compromise the integrity and function of blood–brain barrier (BBB).1 Disruption of the BBB in turn leads to severe neurologic deficits through aggravation of hemorrhagic transformation or vasogenic edema.2 Although subtle increases in cerebral water content may be apparent within hours of stroke onset, the clinical signs of unattended brain edema peak typically during the following 1 to 5 days. Uncontrolled cerebral edema constitutes the leading cause of death within the first week after ischemic strokes.3, 4 As the incidence and severity of BBB damage is markedly higher in stroke patients with diabetes or acute hyperglycemia (HG) than those without, it is thought that HG has a pivotal role in the development and exacerbation of this defect.5, 6 However, the mechanisms involved remain largely unexplored and are of vital importance to develop efficacious novel therapeutic regimens.
The BBB is composed of brain microvascular endothelial cells, capillary basement membranes, and astrocyte end-feet. The restraining role of the BBB is in large part attributed to continuous presence of tight junctions, formed by several transmembrane and associated cytoplasmic proteins, notably occludin and zonula occludens-1 (ZO-1).7 Given the pronounced roles of occludin and ZO-1 in tightening junctional complex and sustaining endothelial phenotype,8 it is safe to suggest that any pathologic stimulus affecting the expression and localization of these proteins will profoundly affect the integrity of the BBB.
Hyperglycemia-mediated activation of protein kinase C (PKC), especially PKC-β, represents a key step in peripheral vasculopathies and indeed accounts for vascular leakage in organs that manifest ischemic injury- or diabetes-mediated damage such as heart and retina.9, 10 Naturally, attenuation of PKC-β activity has been coupled to amelioration of HG-induced retinal and glomerular microvascular complications to which inhibitions of ZO-1 and occludin translocation and the small GTPase RhoA post-translational modification appear to contribute.11, 12, 13 It is noteworthy in this context that the increased presence of PKC-β protein has also been documented in the brain infarcts of deceased ischemic stroke patients.14
Guanosine triphosphate-binding proteins, in particular RhoA, have crucial roles in many cellular processes, including the regulation of endothelial barrier integrity and function.15, 16 Once activated, RhoA binds to its downstream effector Rho-kinase and as a consequence induce myosin-regulatory light chain-2 phosphorylation (p-MLC2) and actin stress fiber formation in a sequential manner. Because MLC2 destabilizes cell junctions to increase paracellular flux, the inhibition of Rho/Rho-kinase pathway may improve microvascular endothelial function and suppress barrier permeability.15, 16
In light of the above, the aims of the current study were threefold. First, it was to explore whether PKC-β and RhoA/Rho-kinase/p-MLC2 pathway are involved in HG-mediated cerebral barrier damage using a cell culture model of human BBB. Second, it was to study the nature of correlation between PKC-β and the components of RhoA/Rho-kinase/p-MLC2 pathway in a comprehensive fashion to discover new targets that may be utilized in clinical settings to prevent BBB damage in hyperglycemic ischemic stroke. Finally, it was to assess whether normalization of glucose levels could improve barrier function through modulation of RhoA/Rho-kinase/p-MLC2 pathway, cytoskeleton, or tight junction assembly.
Materials and Methods
Cell Culture
Human brain microvascular endothelial cells (HBMEC, >5 × 105 cells) and human astrocytes (>5 × 106 cells) isolated from the cerebral cortex and cryopreserved at passage 1 were purchased from TCS CellWorks (Buckingham, UK). Cells between passages four and seven were cultured in their respective specialized media to ∼90% confluence before exposure to normoglycemia (NG, 5.5 mmol/L D-glucose) or HG(25 mmol/L D-glucose) for 72 hours. Experiments with equimolar concentrations of D-mannitol assessed the impact of HG-induced hyperosmolality. To investigate the effects of glucose normalization on different parameters, HBMEC were successively exposed to equal periods (72 hours) of HG and NG (HG/NG).
In Vitro Model of Human Blood–Brain Barrier
Human astrocytes were seeded overnight on the outside of polyester membrane (0.4 μm pore size) transwell inserts (Corning Costar, High Wycombe, UK) directed upside down in the culture chamber. On the following day, HBMEC were seeded onto the inner parts of the membranes and cells were grown to confluence.
Blood–Brain Barrier Experiments
Blood–brain barrier integrity was assessed by measurements of transendothelial electrical resistance (TEER) and the flux of Evan's blue-labeled albumin (EBA), a high molecular weight permeability marker (67 kDa), across co-cultures as previously described.16 Briefly, after measuring TEER across the membranes by STX electrodes and EVOM resistance meter (World Precision Instruments, Hertfordshire, UK), the inserts were transferred to fresh 12-well plates containing 2 mL Hank's buffered salt solution in the basolateral compartments. In the apical chambers, culture media was replaced with 500 μL of Hank's buffered salt solution containing EBA (165 μg/mL). Both luminal and abluminal samples were taken after 1 hour of incubation to determine the optical readings of EBA using a plate reader (absorbance: 610 nm). Flux across cell-free inserts were determined and transport was calculated as ‘cleared volume' using the following formula. Cleared volume (μL)=concentration(abluminal reading) × volume(abluminal) × concentration(luminal reading)−1.
Immunoblotting
To evaluate the relative differences in protein levels, immunoblottings were performed as previously described.16
In-Cell Western Analyses
Equal numbers of HBMEC (∼5 × 103) seeded in 96-well plates were subjected to experimental conditions before fixing and permeabilizing them in 3.7% formaldehyde/phosphate-buffered saline and 0.1% Triton X-100/phosphate-buffered saline, respectively. The cells were then successively incubated with primary (5 μg/mL) and infrared dye-tagged species-specific secondary antibodies (Li-Cor Biosciences, Cambridge, UK). The plates were scanned using Odyssey infrared imaging system (Li-Cor BioSciences). Readings representing the aggregate signal of the entire well for target proteins were normalized against those obtained for α-tubulin in the same wells. By simultaneously studying two different targets using spectrally distinct dyes and detection via separate fluorescence detectors and lasers (at 700 and 800 nm), in-cell westerns dramatically enhanced quantification accuracy.
Immunocytochemistry
Human brain microvascular endothelial cells cultured on coverslips were fixed (4% paraformaldehyde/phosphate-buffered saline) and permeabilized (0.1% Triton X-100/phosphate-buffered saline) before visualizing actin cytoskeleton via Rhodamine-labeled phalloidin dye (5 U/mL). To detect RhoA, Rho-kinase, occludin (Santa Cruz Biotech, Dallas, TX, USA) or ZO-1 (Abcam, Cambridge, UK), the cells were successively exposed to the respective primary (overnight at 4°C) and appropriate secondary antibodies. Nuclei were detected by 4,6-diamidino-2-phenylindole staining before viewing cells by fluorescence microscopy.
RhoA Activity Assay
RhoA activity was measured using a commercial kit (Millipore, Billerica, MA, USA). Briefly, HBMEC lysates (∼150 μg) were incubated with Rhotekin Rho-binding peptide immobilized on agarose beads (10 μg). Activated guanosine triphosphate-RhoA bound to Rhotekin was detected by immunoblotting using an anti-RhoA antibody.
Protein Kinase C Activity Assay
Total PKC activity was determined in HBMEC lysates using the PepTag assay kit, which utilizes a brightly colored PKC-specific peptide substrate, PepTag C1 (Promega, Madison, WI, USA). Phosphorylation of peptide substrate by enhanced PKC activity changes the net charge of the peptide from +1 to −1, thereby allowing the separation of phosphorylated and non-phosphorylated peptides via agarose (0.8%) gel electrophoresis. The phosphorylated peptide bands, migrated towards anode, were then excised from the gel under ultraviolet light, melted at 95°C, and solubilized in a mixture containing PepTaq assay gel solubilization solution and glacial acetic acid before reading the absorbances at 570 nm. Total PKC activity (units/mL) was then calculated as per manufacturer's instructions. The specific activity of PKC-β was also measured using the same kit after immunoprecipitation of PKC-β antigen by a specific antibody and Dynabeads protein G (Invitrogen, Paisley, UK).
Small Interfering RNA Knockdown
Human brain microvascular endothelial cells grown to semi-confluence were transfected for 24 hours with DharmaFECT small interfering RNA (siRNA) transfection reagent 4 containing 50 to 100 nmol/L of ON-TARGET plus SMART pool human siRNA against PKC-β and RhoA (Thermo Scientific Dharmacon, Lafayette, CO, USA). Human brain microvascular endothelial cells transfected with non-targeting pool of siRNA served as controls. After 3 days of exposure to experimental conditions, HBMEC were homogenized to assess the levels of protein downregulation by immunoblotting.
Transfection Experiments
Human brain microvascular endothelial cells (5 × 106) were resuspended in 100 μL supplement- and antibiotic-free media and stored on ice in a pre-chilled sterile cuvette. Anti-RhoA IgG (3 μg/cuvette) was added to the cell suspensions before electroporation at 1.8 kV (Electroporator Easyject Prima; Equibio, Ashford, UK). Cells electroporated with equal volumes of vehicle (distilled H2O) containing 3 μg of anti-RhoA IgG served as controls. The viabilities of the electroporated cells were quickly analyzed before plating. As the electroporation process reduced cell viability by ∼39±4%, the number of cells after this procedure was increased by 75% to allow all experimental groups to reach confluence simultaneously. Once the cells are settled, they were subjected to the experimental conditions.
Cell Viability Assay
A small aliquot of cells exposed to different treatment regimens were incubated with 0.1% trypan blue for 5 minutes and viewed under light microscope to determine the level of possible cytotoxicity. The percentage of viable cells was calculated by counting 100 cells.
Statistical Analysis
Data are presented as mean±s.e.m. Statistical analyses were performed using IBM SPSS statistics 20.0 software package. Comparisons of the mean values were carried out by Student's two-tailed t-test or one-way analysis of variance, where appropriate, followed by Dunnett's post hoc testing. P<0.05 was considered significant.
Results
Effects of Hyperglycemia on RhoA/Rho-Kinase/p-MLC2 Pathway Components
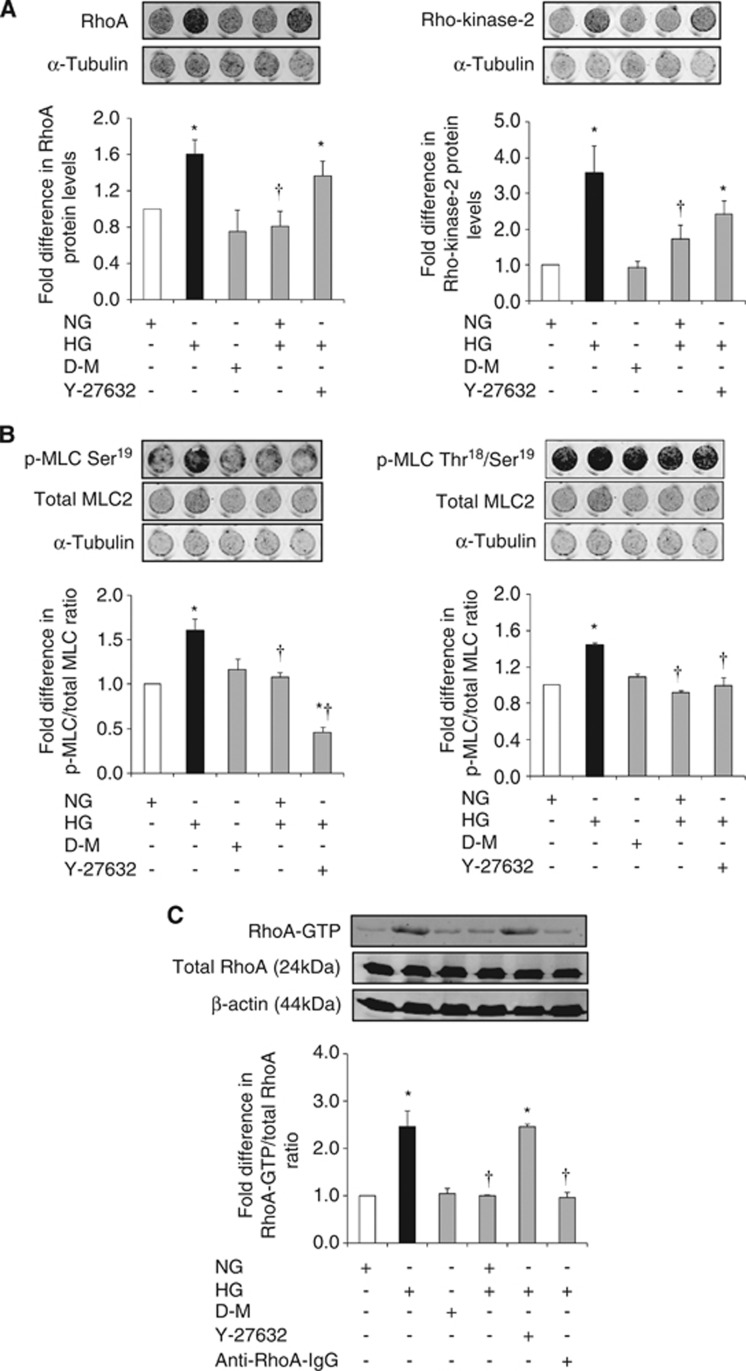
Hyperglycemia significantly increased RhoA and Rho-kinase protein expressions, MLC2 mono/di-phosphorylations (p-MLC2Ser19/p-MLC2Thr18-Ser19), and total RhoA activity. These increases were independent of a rise in osmolality and were completely neutralized by normalization of glucose levels. Although suppression of Rho-kinase activity by its specific inhibitor Y-27632 (2.5 μmol/L) did not affect total RhoA protein expression and activity, it led to marked decreases in p-MLC2Ser19 and p-MLC2Thr18-Ser19. Given that Rho-kinase is a downstream effector of RhoA and an upstream mediator of MLC2, these results were somewhat expected. In contrast, electroporation of anti-RhoA IgG into HBMEC successfully neutralized RhoA activity (Figures 1A and C).
Figure 1.
The effects of glucose normalization, Y-27632 and anti-RhoA immunoglobulin G (IgG) on RhoA/Rho-kinase/p-MLC2 pathway components. Representative in-cell western analyses of RhoA and Rho-kinase (A) and p-MLC2Ser19 and p-MLC2Thr18-Ser19 (B) in human brain microvascular endothelial cells (HBMEC). Representative immunoblotting showing RhoA activity (C) in HBMEC. Data are expressed as mean±s.e.m. from four different experiments. *P<0.05 versus normoglycemia (NG), †P<0.05 versus hyperglycemia (HG). D-M, D-mannitol; GTP, guanosine triphosphate; MLC, myosin-regulatory light chain.
Effects of Hyperglycemia on In Vitro Barrier Integrity and Tight Junction Protein Characteristics
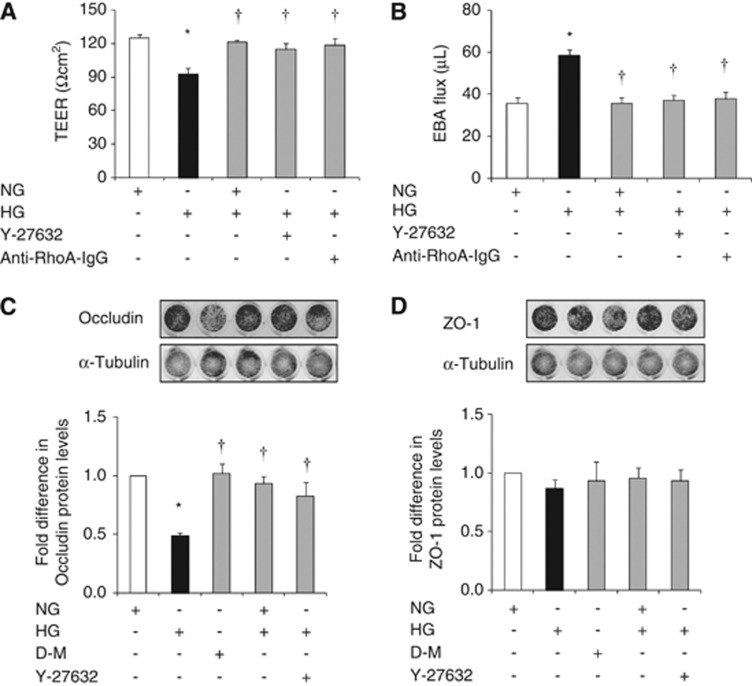
Hyperglycemia compromised in vitro cerebral barrier integrity as evidenced by decreases in TEER and concurrent increases in EBA flux. Normalization of glucose levels and suppressions of RhoA and Rho-kinase activities via electroporation of anti-RhoA IgG and treatments with Y-27632 (2.5 μmol/L), respectively abolished HG-induced barrier dysfunction. Hyperglycemia-mediated decreases observed in occludin levels provided some explanation for the barrier dysfunction. The changes in occludin expression appeared to be independent of an increase in osmolality and were completely prevented by glycemic control and suppression of Rho-kinase activity. Neither HG nor the modulation of glucose levels or Rho-kinase activity had any impact on ZO-1 expression (Figures 2A and D).
Figure 2.
The effects of glucose normalization, Y-27632 and anti-RhoA immunoglobulin G (IgG) on blood–brain barrier (BBB) integrity and tight junction protein expressions. Transendothelial electrical resistance (A) and Evan's blue-labeled albumin (EBA) flux (B) across the human brain microvascular endothelial cell (HBMEC) astrocyte co-cultures. Levels of occludin (C) and zonula occludens-1 (D) protein expressions in HBMEC. Data are expressed as mean±s.e.m. from four different experiments. *P<0.05 versus normoglycemia (NG), †P<0.05 versus hyperglycemia (HG). D-M, D-mannitol; TEER, transendothelial electrical resistance.
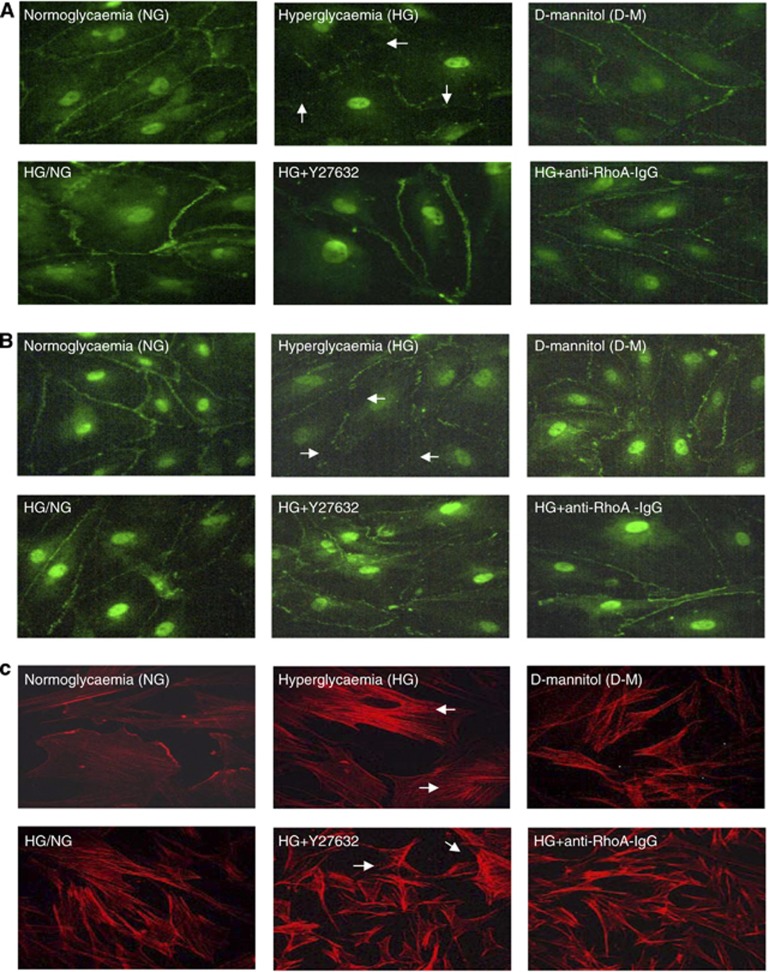
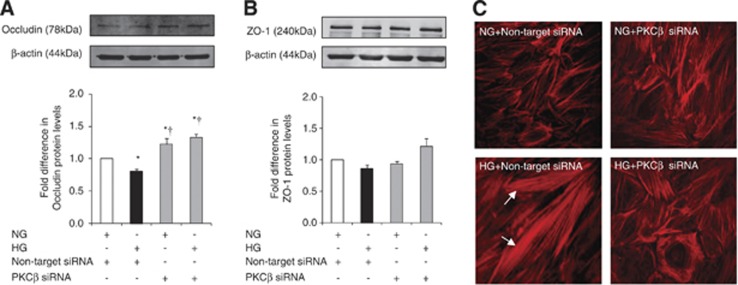
Despite failing to alter ZO-1 levels, HG drastically impaired its endothelial cell membrane localization in a similar pattern to that of occludin. Hyperglycemia also promoted endothelial cells stress fiber formation and morphologic changes characterized with their cuboidal or elongated appearance. Normalization of glucose levels and inhibitions of RhoA and Rho-kinase during hyperglycemic insult reinstated cortical actin staining, normal cellular morphology, and peripheral staining of both occludin and ZO-1 (Figures 3A and C).
Figure 3.
The effects of glucose normalization, Y-27632 and anti-RhoA immunoglobulin G (IgG) on cytoskeleton and tight junction protein localizations. Representative immunocytochemical analyses of occludin (A), zonula occludens-1 (B), and actin cytoskeleton (C) in human brain microvascular endothelial cells; n=3 per group. D-M, D-mannitol; HG, hyperglycemia; NG, normoglycemia.
Effects of Hyperglycemia on Protein Kinase C-β Activity and Associated Downstream Mediators
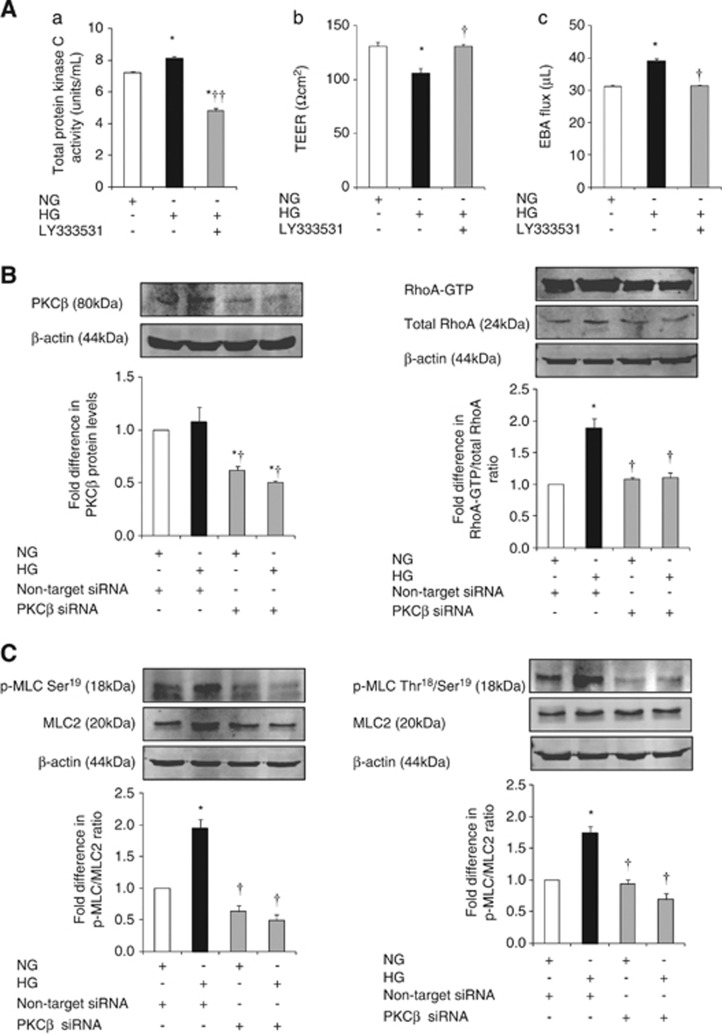
Hyperglycemia significantly increased total PKC activity in HBMEC. Exposure of hyperglycemic cells to LY333531 (5 μmol/L), a selective PKC-β inhibitor, diminished the level of total PKC activity below the levels detected in normoglycemic conditions and proved the efficacy of LY333531 in suppressing both inducible and constitutive PKC activities. Inhibition of PKC-β also improved barrier integrity and function as confirmed by TEER and paracellular flux studies, respectively (Figure 4A).
Figure 4.
The effects of inhibition of protein kinase C-β (PKC-β) activity on blood–brain activity (BBB) integrity, RhoA activity and p-MLC2. (A) Level of total PKC activity (A) in human brain microvascular endothelial cells (HBMEC). Transendothelial electrical resistance (B) and Evan's blue-labeled albumin (EBA) flux (C) across HBMEC astrocyte co-cultures. Representative immunoblotting of PKC-β protein expression and RhoA activity (B) and p-MLC2Ser19 and p-MLC2Thr18-Ser19 (C) in HBMEC. Data are expressed as mean±s.e.m. from ⩾three different experiments. *P<0.05 versus normoglycemia (NG), †P<0.05 versus hyperglycemia (HG), ††P<0.01 versus HG. GTP, guanosine triphosphate; MLC, myosin-regulatory light chain; siRNA, small interfering RNA.
Attenuation of PKC-β activity via specific siRNA neutralized HG-evoked increases in RhoA activity, p-MLC2Ser19 and p-MLC2Thr18-Ser19 and thus proved the direct involvement of this pathway in PKC-β-induced cerebral barrier damage (Figures 4B and C).
Effects of Protein Kinase C-β Inhibition on Tight Junctions and Cytoskeleton
As mentioned above, HG evoked a significant decrease in HBMEC occludin protein expression without affecting that of ZO-1. Small interfering RNA-mediated attenuation of PKC-β activity dramatically increased occludin and ZO-1 expressions under hyperglycemic conditions and produced a selective increase in occludin expression under normoglycemic conditions. Similarly, inhibition of PKC-β activity preserved cortical actin staining and normal cellular structure in cells subjected to HG (Figures 5A and C).
Figure 5.
The effects of protein kinase C-β (PKC-β) activity inhibition on tight junction protein levels and cytoskeleton. Occludin (A) and zonula occludens-1 (B) expressions and actin cytoskeleton staining (C) in human brain microvascular endothelial cells. Data are expressed as mean±s.e.m. from four different experiments. *P<0.05 versus normoglycemia (NG), †P<0.05 versus hyperglycemia (HG). siRNA, small interfering RNA.
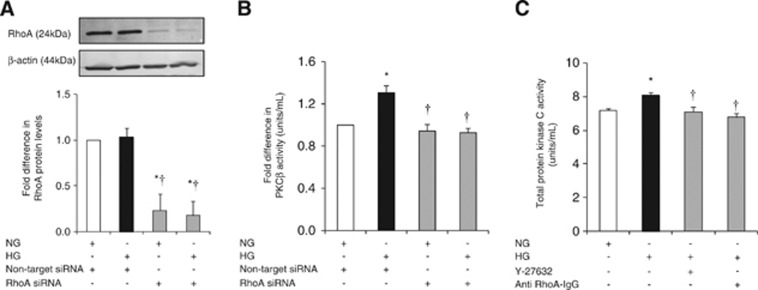
RhoA and Rho-Kinase Regulate Protein Kinase C Activity in a Reciprocal Manner
Suppression of RhoA protein levels in HBMEC abolished HG-mediated increases in PKC-β activity. Furthermore, inhibitions of RhoA and Rho-kinase activities by electroporation of anti-RhoA IgG and Y-27632 (2.5 μmol/L), respectively markedly decreased total PKC activity. Taken together, these findings support counterregulatory effects of RhoA and Rho-kinase on PKC-β activity (Figures 6A and C).
Figure 6.
The impact of RhoA activity suppression on total protein kinase C (PKC) and PKC-β activities. Representative immunoblotting of RhoA expression (A) and PKC-β activity (B) in human brain microvascular endothelial cells (HBMEC) transfected with RhoA small interfering RNA (siRNA). Levels of total PKC activity (C) in HBMEC. Data are expressed as mean±s.e.m. from three different experiments. *P<0.05 versus normoglycemia (NG), †P<0.05 versus hyperglycemia (HG). IgG, immunoglobulin G.
Discussion
Brain edema associated with increased rates of patients' mortality appears to be more prevalent in stroke patients with diabetes or stress HG than those without thereby implying a prominent role for HG in the BBB breakdown.5, 6 Although HG represents a common pathology in ischemic stroke patients, the mechanisms by which it may compromise the cerebral barrier integrity and/or exacerbate vascular damage remain largely unknown. Bearing these in mind, the current study focused on the relevance of two apparently distinct pathways, namely PKC and RhoA/Rho-kinase/MLC2 to HG-mediated barrier damage using an in vitro model of human BBB composed of HBMEC and human astrocytes. It is thought that elucidating the nature of the correlation between these pathways that collectively regulate vasomotor function, cell proliferation, survival, migration, oxidative stress, and immune responses may prove extremely beneficial in identifying novel targets for future therapeutic approaches.17, 18
Exposure of HBMEC to HG led to significant increases in RhoA activity and RhoA and Rho-kinase protein expressions, which were fully mitigated by normalization of glucose levels. Although clinical evidence regarding the necessity and extent of glycemic control during acute ischemic stroke is debatable, it is important to monitor and possibly reduce blood glucose levels in an effective manner considering the close correlation between HG and poor outcomes, typically defined by enhanced mortality or dependence 90 days after a stroke.5, 6 As equimolar concentrations of D-mannitol did not affect cell viability, protein expression, or enzyme activity, it is safe to suggest that the aforementioned increases were a direct consequence of HG itself rather than a rise in osmolality or cell death. A close link between HG and RhoA/Rho-kinase pathway overactivity is also documented in basilar artery sections of type-1 diabetic rats and in endothelial cells originating from different species and parts of vascular tree such as the human saphenous vein and bovine aorta.19, 20, 21 Unlike glycemic control, suppression of the downstream effector of RhoA, i.e., Rho-kinase by its specific inhibitor Y-27632 failed to normalize the increases observed in RhoA protein expression and activity and dismissed the existence of a negative feedback system whereby Rho-kinase bioavailability may dictate the extent of RhoA expression and activity.
Hyperglycemia severely perturbed the cerebral barrier integrity and function in in vitro settings as ascertained by marked decreases in TEER and concomitant increases in EBA flux, respectively. Normalization of glucose levels and inhibitions of RhoA and Rho-kinase eradicated the deleterious effects of HG on the in vitro barrier. Similar to RhoA activity, HG also significantly elevated total PKC and PKC-β activities, which appear to require unabated activities of RhoA and Rho-kinase. Subsequent studies, through assessments of TEER and EBA flux, proved the regulatory function of PKC-β in maintaining the cerebral barrier integrity and function and affirmed the findings of recent studies in which the inhibition of PKC-β substantially improved mouse brain microvascular endothelial cell and human umbilical vein endothelial cell barrier integrities by augmenting cellular anti-oxidant and anti-apoptotic capacities.11, 22 Additional studies designed to assess the specific impacts of PKC-β on RhoA/Rho-kinase/p-MLC2 complex demonstrated dramatic reductions in RhoA and Rho-kinase protein expressions and p-MLC2 in PKC-β-knocked down HBMEC subjected to HG.
The inhibitory reciprocal relationship between total PKC/PKC-β and RhoA activities in hyperglycemic settings necessitated a closer scrutiny of the correlation between RhoA and PKC-β to reveal the true nature of HG-mediated pathologic cascade resulting in the breakdown of cerebral barrier. Analyses of PKC-β activity in HBMEC transfected with specific RhoA siRNA led to selective decreases in enzyme activity and further substantiated the counterregulatory effect of RhoA on PKC-β activity.
Changes in cellular architecture may also contribute to HG-induced barrier failure. Indeed, experiments examining the structural differences in microfilaments, a crucial element of cytoskeleton, showed that HBMEC grown under normal conditions displayed a cortical actin staining whereas those subjected to HG possessed actin stress fibers traversing the cells and took up predominantly elongated and somewhat cuboidal appearance. The appearance of stress fibers coincided with enhanced MLC2 phosphorylation at Ser19 (p-MLC2Ser19) and Thr18-Ser19 (p-MLC2Thr18-Ser19) residues possibly through activations of MLC kinase and Rho-kinase and/or inhibition of MLC phosphatase. Once established, stress fibers create a tensile centripetal force to pull TJ proteins inward to compromise junctional integrity and widen intercellular gaps.16 RhoA/Rho-kinase pathway may also modulate actin filament (de)stabilization by phosphorylating adducin, LIM kinase, and ezrin-radixin-moesin proteins, which promote the binding of cytoskeletal proteins, e.g., spectrin and cofilin to cortical actin.23, 24 In addition to stress fiber formation, the activation of RhoA/Rho-kinase pathway may also disrupt the BBB integrity through attenuations of endothelial nitric oxide synthase expression, phosphorylation (at Ser1177), and activity and consequently deplete the availability of barrier-protective nitric oxide. Indeed, suppression of RhoA activity by statins and greater availability of nitric oxide have been shown to produce the opposite effects.25 Similarly, the inhibitions of RhoA and Rho-kinase before and during hyperglycemic challenge as well as normalization of glucose levels in the current study has also led to normalization of p-MLC2, prevention of stress fiber formation, restoration of actin staining to cell periphery, and abolishment of the barrier-disruptive effects of HG. In concert with its barrier-protective effects, the stress fiber formation was fully mitigated by PKC-β inhibition.
Other mechanisms including overt breakings in interendothelial junctional complex may also promote the cerebral barrier failure in hyperglycemic settings. The reduced expression of TJ protein occludin may shed some light on the HG-induced barrier hyperpermeability and support the previous in vivo studies reporting a direct correlation between the levels of TJ proteins and the extent of endothelial barrier permeability.26, 27, 28 The discontinuous appearances of occludin and ZO-1 on cell periphery provide further explanation for the HG-mediated increases in paracellular flux. Consistent with the recent data showing an insulin-mediated elevation in the cerebral contents of occludin and ZO-1, normalization of glucose levels in our study also significantly improved occludin levels and effectively restored occludin and ZO-1 staining to the cell periphery, a set of findings replicated by inhibitions of RhoA or Rho-kinase.26, 27, 28 Subsequent studies focusing on the specific impact of PKC-β on cerebral endothelial cell content of TJ proteins have shown that knockdown of PKC-β significantly increased occludin and ZO-1 protein levels under hyperglycemic conditions and selectively that of occludin also under normoglycemic conditions. Decreases in phosphorylation, ubiquitination, and trafficking of occludin may account for the PKC-β-inhibition-mediated improvements observed in occludin protein levels.29 Likewise, decreases in occludin and claudin-5, another TJ protein, phosphorylation may also account for the barrier-protective effects obtained with inhibition of endothelial Rho-kinase activity.30
Although not investigated in the present study, it is likely that HG may also affect the expression and localization of claudin-5, a key component of the BBB known to prevent interendothelial cell leakage of small molecules (<800 kDa).31 In support of this notion, using an in vitro model of human BBB comprising HBMEC, human astrocytes, and human pericytes, a recent study has shown that HG markedly increases the paracellular flux of sodium fluorescein (376 kDa) through a complex mechanism involving activations of several PKC isoforms, namely PKC-α, β, or βII.32 Bearing HG-induced cytoskeletal reorganization in mind, possible changes exerted on HBMEC-matrix interactions regulated via β1-integrin may also contribute to barrier permeability by directly affecting claudin-5 expression as recently reported.33
In conclusion, the current study demonstrates that activations of PKC-β or RhoA/Rho-kinase/p-MLC2 pathway under hyperglycemic conditions mediate the structural and functional impairment of an in vitro model of human cerebral barrier. Considering the regulatory effects of PKC-β on the expression, phosphorylation, and/or activity of RhoA/Rho-kinase/p-MLC2 pathway components and organization of cytoskeleton and TJ assembly, it is plausible to suggest that PKC-β may be an efficacious novel therapeutic target to stem the cerebral barrier dysfunction in hyperglycemic stroke.
Despite existence of a recent study showing that the inhibition of PKC-βII by CGP53353 reverses enhanced BBB permeability and prevents edema formation in streptozotocin-induced diabetic rats subjected to middle cerebral artery occlusion,34 it remains essential to extend our study to in vivo settings by using a well-established animal model of focal cerebral ischemia with/out diabetes. This is particularly important given the fact that much of the existing animal data with hyperglycemic focal ischemia, although invaluable, are somewhat irrelevant to clinical scenario because of the choice of wrong animal models, excessive blood glucose levels, and inconsistent findings with insulin.35 For instance, although the majority of stroke patients have unrecognized insulin resistance coupled with type-2 diabetes, in most of the experimental studies a streptozotocin-induced model of type-1 diabetes is used.35 Other than being unrepresentative of >90% of clinical cases, streptozotocin has been shown to impair brain microvasculature and increase apoptosis in middle cerebral artery occlusion-induced models of focal cerebral ischemia.36
There are some limitations to the current study. First, a single marker of paracellular transport, i.e., EBA (67 kDa) has been employed throughout this study. The use of fluorescent-labeled Dextran of higher molecular weights may have shed some light on the size of intercellular openings. Furthermore, using a cell culture model of human BBB established with relatively late passage endothelial cells and astrocytes, in this study only one factor, i.e., glucose, out of many involved in ischemic damage could be manipulated, whereas a number of pro-inflammatory cytokines and circulating components during an ischemic stroke, notably tumor necrosis factor-α, interleukin-6, vascular endothelial growth factor, and thrombin, are known to affect BBB permeability.37, 38
The authors declare no conflict of interest.
Footnotes
This study was supported by PhD studentship grants to Dr Bayraktutan.
References
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol. 2004;56:468–477. doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]
- Moulin T, Cattin F, Crépin-Leblond T, Tatu L, Chavot D, Piotin M, et al. Early CT signs in acute middle cerebral artery infarction: predictive value for subsequent infarct locations and outcome. Neurology. 1996;47:366–375. doi: 10.1212/wnl.47.2.366. [DOI] [PubMed] [Google Scholar]
- Ringelstein EB, Biniek R, Weiller C, Ammeling B, Nolte PN, Thron A. Type and extent of hemispheric brain infarctions and clinical outcome in early and delayed middle cerebral artery recanalization. Neurology. 1992;42:289–298. doi: 10.1212/wnl.42.2.289. [DOI] [PubMed] [Google Scholar]
- Baird TA, Parsons MW, Barber PA, Butcher KS, Desmond PM, Tress BM, et al. The influence of diabetes mellitus and hyperglycaemia on stroke incidence and outcome. J Clin Neurosci. 2002;9:618–626. doi: 10.1054/jocn.2002.1081. [DOI] [PubMed] [Google Scholar]
- Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform-βII and diacylglycerol levels in the aorta and heart of diabetic rats - differential reversibility to glycemic control by islet cell transplantation. Pro Natl Acad Sci USA. 1992;89:11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba T, Inoguchi T, Sportsman JR, Heath WF, Bursell S, King GL. Correlation of diacylglycerol level and protein kinase C activity in rat retina to retinal circulation. Am J Physiol. 1993;265:e783–e793. doi: 10.1152/ajpendo.1993.265.5.E783. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Dimitrijevic OB, Keep RF, Andjelkovic AV. Protein kinase Calpha-RhoA cross-talk in CCL2-induced alterations in brain endothelial permeability. J Biol Chem. 2006;281:8379–8388. doi: 10.1074/jbc.M513122200. [DOI] [PubMed] [Google Scholar]
- Chen ML, Pothoulakis C, LaMont JT. Protein kinase C signaling regulates ZO-1 translocation and increased paracellular flux of T84 colonocytes exposed to Clostridium difficile toxin A. J Biol Chem. 2002;277:4247–4254. doi: 10.1074/jbc.M109254200. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Clermont A, Arora V, Davis MD, Sheetz MJ, Bursell SE. Inhibition of PKC beta by oral administration of ruboxistaurin is well tolerated and ameliorates diabetes-induced retinal hemodynamic abnormalities in patients. Invest Ophthalmol Vis Sci. 2006;47:86–92. doi: 10.1167/iovs.05-0757. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Slevin MA, Kumar P, Gaffney J, Kaluza J. Protein kinase C expression and activity in the human brain after ischemic stroke. Acta Neurobiol Exp. 1998;58:13–21. doi: 10.55782/ane-1998-1254. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Allen C, Srivastava K, Bayraktutan U. Small GTPase RhoA and its effector Rho kinase mediate oxygen glucose deprivation-evoked in vitro cerebral barrier dysfunction. Stroke. 2010;41:2056–2063. doi: 10.1161/STROKEAHA.109.574939. [DOI] [PubMed] [Google Scholar]
- Kowluru RA. Diabetes-induced elevations in retinal oxidative stress, protein kinase C and nitric oxide are interrelated. Acta Diabetologica. 2001;38:179–185. doi: 10.1007/s592-001-8076-6. [DOI] [PubMed] [Google Scholar]
- Nonaka A, Kiryu J, Tsujikawa A, Yamashiro K, Miyamoto K, Nishiwaki H, et al. PKC-beta inhibitor (LY333531) attenuates leukocyte entrapment in retinal microcirculation of diabetic rats. Invest Ophthalmol Vis Sci. 2000;41:2702–2706. [PubMed] [Google Scholar]
- Miao L, Calvert J, Tang J, Zhang J. Upregulation of small GTPase RhoA in the basilar artery from diabetic (mellitus) rats. Life Sci. 2002;71:1175–1185. doi: 10.1016/s0024-3205(02)01827-1. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Okamoto R, Kato S, Konishi K, Mizutani H, Yamada N, et al. High glucose induces plasminogen activator inhibitor-1 expression through Rho/Rho-kinase-mediated NF-kappaB activation in bovine aortic endothelial cells. Atherosclerosis. 2008;196:22–28. doi: 10.1016/j.atherosclerosis.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Rikitake Y, Liao J. Rho-kinase mediates hyperglycemia-induced plasminogen activator inhibitor-1 expression in vascular endothelial cells. Circulation. 2005;111:3261–3268. doi: 10.1161/CIRCULATIONAHA.105.534024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Sun D, Yin Z, Yuan Y, Hwang A, Zhang Y, et al. A PKC-beta inhibitor protects against cardiac microvascular ischemia reperfusion injury in diabetic rats. Apoptosis. 2010;15:488–498. doi: 10.1007/s10495-009-0439-2. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem. 2000;275:3577–3582. doi: 10.1074/jbc.275.5.3577. [DOI] [PubMed] [Google Scholar]
- Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, et al. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikitake Y, Liao J. Rho GTPases, statins, and nitric oxide. Circ Res. 2005;97:1232–1235. doi: 10.1161/01.RES.0000196564.18314.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehade J, Haas M, Mooradian A. Diabetes-related changes in rat cerebral occludin and zonula occludens-1 (ZO-1) expression. Neurochem Res. 2002;27:249–252. doi: 10.1023/a:1014892706696. [DOI] [PubMed] [Google Scholar]
- Antonetti D, Barber A, Khin S, Lieth E, Tarbell J, Gardner T. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes. 1998;47:1953–1959. doi: 10.2337/diabetes.47.12.1953. [DOI] [PubMed] [Google Scholar]
- Hawkins B, Lundeen T, Norwood K, Brooks H, Egleton R. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50:202–211. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- Murakami T, Frey T, Lin C, Antonetti DA. Protein kinase C-β phosphorylates occludin regulating tight junction trafficking in vascular endothelial growth factor-induced permeability in vivo. Diabetes. 2012;61:1573–1583. doi: 10.2337/db11-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Ramirez SH, Sato S, Kiyota T, Cerny RL, Kaibuchi K, et al. Phosphorylation of claudin-5 and occludin by Rho kinase in brain endothelial cells. Am J Pathol. 2008;172:521–533. doi: 10.2353/ajpath.2008.070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao B, Bayraktutan U.Hyperglycaemia promotes cerebral barrier dysfunction through activation of protein kinase C-β Diabetes Obes Metab 2013. doi: 10.1111/dom.12120(e-pub ahead of print). [DOI] [PubMed]
- Osada T, Gu YH, Kanazawa M, Tsubota Y, Hawkins BT, Spatz M, et al. Interendothelial claudin-5 expression depends on cerebral endothelial cell-matrix adhesion by β(1)-integrins. J Cereb Blood Flow Metab. 2011;31:1972–1985. doi: 10.1038/jcbfm.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla MJ, Huang Q, Sweet JG. Inhibition of protein kinase Cβ reverses increased blood-brain barrier permeability during hyperglycemic stroke and prevents edema formation in vivo. Stroke. 2011;42:3252–3257. doi: 10.1161/STROKEAHA.111.623991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall NJ, Muir KW. Hyperglycaemia and infarct size in animal models of middle cerebral artery occlusion: systematic review and meta-analysis. J Cereb Blood Flow Metab. 2011;31:807–818. doi: 10.1038/jcbfm.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton M, Rafols J, Alousi S, Dunbar JC. The effects of middle cerebral artery occlusion on central nervous system apoptotic events in normal and diabetic rats. Int J Exp Diabesity Res. 2003;4:13–20. doi: 10.1080/15438600303727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Graham SH. Inflammation after stroke: mechanisms and therapeutic approaches. Transl Stroke Res. 2010;1:74–84. doi: 10.1007/s12975-010-0023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]