Abstract
Stroke induces inflammation that can aggravate brain damage. This work examines whether interleukin-10 (IL-10) deficiency exacerbates inflammation and worsens the outcome of permanent middle cerebral artery occlusion (pMCAO). Expression of IL-10 and IL-10 receptor (IL-10R) increased after ischemia. From day 4, reactive astrocytes showed strong IL-10R immunoreactivity. Interleukin-10 knockout (IL-10 KO) mice kept in conventional housing showed more mortality after pMCAO than the wild type (WT). This effect was associated with the presence of signs of colitis in the IL-10 KO mice, suggesting that ongoing systemic inflammation was a confounding factor. In a pathogen-free environment, IL-10 deficiency slightly increased infarct volume and neurologic deficits. Induction of proinflammatory molecules in the IL-10 KO brain was similar to that in the WT 6 hours after ischemia, but was higher at day 4, while differences decreased at day 7. Deficiency of IL-10 promoted the presence of more mature phagocytic cells in the ischemic tissue, and enhanced the expression of M2 markers and the T-cell inhibitory molecule CTLA-4. These findings agree with a role of IL-10 in attenuating local inflammatory reactions, but do not support an essential function of IL-10 in lesion resolution. Upregulation of alternative immunosuppressive molecules after brain ischemia can compensate, at least in part, the absence of IL-10.
Keywords: cytokines, IL-10, inflammation, myeloid cells, microglia, stroke
Introduction
Stroke induces inflammation that is considered as detrimental in the acute phase. Insufficient oxygen supply triggers a transcriptional program designed to promote cellular adaptation to hypoxia.1, 2 Ischemic conditions can induce the expression of inflammatory mediators in microglia,3, 4 and upregulate the expression of Toll-like receptors (TLRs)5 and other danger signal receptors.6 Necrotic cell death induces secondary inflammatory reactions by liberating intracellular molecules to the extracellular environment7 where they act as danger signals activating nearby glial cells. This process causes a second release of proinflammatory mediators8 amplifying the initial inflammatory response to ischemia. Inflammatory mediators can be neurotoxic9 and they induce chemoattraction of blood leukocytes, which, in turn, can release proteases and prooxidant agents further exacerbating the lesion.10 However, the inflammatory reaction after acute brain damage is a complex dynamic process naturally set up to clear the necrotic tissue and evolving through various steps from initiation to resolution. Macrophages carry out an essential role in the process of tissue damage resolution by clearing dead cells and cell debris.11 Although numerous molecules have been identified as players in the multifaceted inflammatory response to acute brain damage, more knowledge is needed to understand the critical molecules orchestrating its dynamics.
Interleukin-10 (IL-10) is a crucial antiinflammatory cytokine that suppresses proinflammatory signals and immune responses. Several lines of evidence support that the cerebral expression of IL-10 is beneficial after experimental stroke, as IL-10 transgenic mice overexpressing IL-10 in astrocytes, microglia, and endothelial brain cells showed smaller infarcts.12 Furthermore, IL-10 gene transfer using adenoviral vectors reduced infarct volume in a model of photothrombotic ischemia in rats.13 Accordingly, mice deficient in IL-10 showed larger infarctions after permanent middle cerebral artery occlusion (pMCAO).14 In agreement with these findings, low levels of circulating IL-10 in patients with lacunar stroke were associated with a worse outcome.15 Animals deficient in IL-10 lack the antiinflammatory drive of this cytokine, and therefore provide a good model to test whether and how IL-10 is involved in stroke outcome. Here, we studied the inflammatory response of IL-10 knockout (IL-10 KO) mice to brain ischemia to better understand the role of this molecule in acute brain damage.
Materials and methods
Animals
Animal work was performed in agreement with the local regulations and in compliance with the Spanish Directives (Real Decreto 53/2013) that follow Directives of the European Community. Experimental procedures were approved by the Ethical Committee (CEEA) of the University of Barcelona (UB). Interleukin-10 KO and wild-type (WT) mice on a C57BL/10j background were obtained from the Jackson Laboratory. In an initial pilot study, we found that some of the IL-10 KO mice kept under conventional animal house conditions developed signs of colitis. To avoid this pathology, IL-10 KO and WT mice were subjected to embryo transfer and were kept in a specific pathogen-free (SPF) zone in the animal house of the School of Medicine (UB). Under SPF conditions, IL-10 KO mice did not develop overt signs of colitis at 3 to 4 months, in agreement with previous reports.16
Brain Ischemia
Permanent distal occlusion of the right middle cerebral artery (pMCAO) was performed in adult (3 to 4 months) male WT (n=78) and IL-10 KO (n=68) mice under isoflurane anesthesia in 30% O2 and 70% N2O. After drilling a small hole in the cranium at the level of the distal portion of the middle cerebral artery, the artery was occluded by cauterization. Flow obstruction was visually verified. Animals showing subdural hemorrhages or signs of incorrect surgery were immediately excluded from the study (<5% in each group). After surgery, animals were allowed to recover from the anesthesia and were returned to their cages.
Infarct Volume by Magnetic Resonance Imaging
Infarct volume was assessed by magnetic resonance imaging (MRI) in a 7.0T BioSpec 70/30 horizontal animal scanner (Bruker BioSpin, Ettlingen, Germany), equipped with a 12-cm inner diameter actively shielded gradient system (400 mT/m). The receiver coil was a phased array surface coil for mouse brain. Mice were placed in a supine position in a Plexiglas holder with a nose cone for administering anesthesia (isoflurane in a mixture of 30% O2 and 70% N2O), fixed with a tooth bar, ear bars, and adhesive tape and maintained under controlled temperature during the acquisition period. Tripilot scans were used for accurate positioning of the animal's head in the isocenter of the magnet. T2 relaxometry maps were acquired with a multislice multiecho acquisition sequence with 16 effective echo times increasing from 11 to 176 ms, slice thickness=0.5 mm, number of slices=18, repetition time=4,764 ms, field of view=20 mm3, matrix size 256 pixels and spatial resolution 0.078 mm2/pixel. Data were processed using the Paravision 5.0 software (Bruker). Infarct volume was calculated from the images using the NIH Image-J software (http://rsb.info.nih.gov/ij). The area of infarction was measured in each brain slice, and the total infarct volume was obtained by integration of the infarcted areas. A correction for edema was made in each area by multiplying the infarct area by the ratio of the contralateral to the ipsilateral hemisphere. Mice were scanned longitudinally at 1, 4, and 7 days, or only once before they were killed. Mice were assigned a code that did not reveal the identity of the groups and images were analyzed in a blind manner.
Measurements of Infarct Volume After Tissue Histology
In a group of mice, infarct volume was determined by two methods, i.e., in vivo MRI at day 7 was followed by postmortem histology in the same animals. After MRI, mice were anesthetized and transcardially perfused with saline followed by 4% paraformaldehyde. Brains were fixed overnight with this fixative, washed in phosphate buffer, cryoprotected in 30% sucrose, and frozen. Serial coronal brain sections were obtained every 500 μm in a cryostat. After cresyl violet staining, the area of infarction in each section was measured using the Image-J software. Areas were integrated to calculate infarct volume.
Assessment of Brain Edema
The brain water content was measured 4 days after pMCAO using the wet/dry weight method. The cerebellum was excluded and the forebrain was divided into two hemispheres (ipsilateral and contralateral). The two brain pieces were immediately weighed (wet weight), dehydrated at 65°C for 72 hours and reweighed (dry weight). The difference between wet and dry weight was normalized to the initial size of the tissue by dividing by the corresponding wet weight value. The ratio between the ipsilateral and contralateral water content was calculated and values are expressed as the percentage of increase in tissue water content induced by ischemia.
Behavioural Test
At day 3, the adhesion/removal tape test that evaluates sensorimotor deficits was performed as previously described.17 Briefly, an adhesive tape is placed in one of the forelimbs. The animal naturally tries to remove it and the time duration of the following three steps is measured: time of forelimb shaking, time until contact with the mouth, and time until tape removal. Animals were trained the 2 days before ischemia.
Microglia Cultures
Microglial cultures were obtained from the cerebral cortices of 1-day-old neonatal mice (detailed information is provided in Supplementary Materials), as previously described.18 Cells were subjected to ischemic conditions for 3 hours in an anoxia incubator (GalaxyR/RS Biotech, New Brunswick, Eppendorf, Enfield, CT, USA) containing an atmosphere of 95% N2, and 5% CO2 at 37°C, and they were kept for 3 hours more under normoxic conditions. Other cells were exposed to 10 ng/mL lypopolysaccharide (LPS) (Escherichia coli 055:B5) (Sigma-Aldrich Química, S.L., Madrid, Spain) for 6 hours. In all, 10 ng/mL recombinant murine IL-10 (#210-10; PreproTech, Rocky Hill, NJ, USA) was added to the cell cultures 30 minutes before the above challenges.
qRT–PCR
Total RNA was extracted using a Purelink RNA Kit (Invitrogen; Life Technologies S.A., Alcobendas, Madrid, Spain). RNA quantity and purity were determined using the ND-1000 micro-spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). One microgram of total RNA was reverse transcribed using a mixture of random primers (High Capacity cDNA Reverse Transcription kit; Applied Biosystems; Life Technologies S.A.). TaqManR primer sequences were used to evaluate the expression of IL-10 (Mm00439614_m1) and CTLA-4 (Mm00486849_m1) mRNA (Life Technologies S.A.). Quantification was performed by normalizing Ct (Cycle threshold) values with Ct of the TaqManR primer sequence for glyceraldehyde-3-phosphate dehydrogenase (Mm99999915_g1), and the data were analyzed with the 2-ΔΔCT method. The rest of PCR primers (see list in Supplementary Table 1) were designed with the Primer3 software to bridge the exon–intron boundaries within the gene of interest to exclude amplification of contaminating genomic DNA. Primers were purchased from IDT (Laboratorios Conda S.A., Torrejon de Ardoz, Spain). Real-time quantitative RT–PCR analysis was performed by SYBR green I dye detection (#11761500; Invitrogen) using the iCycler iQTM Multicolor Real-Time Detection System (Bio-Rad, Hercules, CA, USA). Optimized thermal cycling conditions were 1 minute at 50°C, 8 minutes and 30 seconds at 95°C and 40 cycles of 15 seconds at 95°C and 30 seconds at 60°C. Data were collected after each cycle and were graphically displayed (iCycler iQTM Real-time Detection System Software, version 3.1; Bio-Rad). Melt curves were performed on completion of the cycles to ensure specificity of the product amplification. Housekeeping gene for normalization was succinate dehydrogenase complex subunit A (SDHA). Quantification was performed using the standard dilution calibration curve and values were normalized to the reference gene. For comparison purposes, values of all samples are expressed as fold versus the mean (n=3 to 6) control value.
ELISA Immunoassays
The concentration of cytokines and chemokines in plasma was analyzed with Q-Plex Mouse Array IR assays (#130951MS; Quansys Bioscience, Logan, UT, USA) and measures were obtained in an Odyssey System (LI-COR, Lincoln, NE, USA). Conventional ELISAs were performed to measure tumor necrosis factor-α (TNF-α) (#88-7324-88; eBioscience, San Diego, CA, USA) and IL-6 (#861.020.005; Diaclone, San Diego, CA, USA) in the medium of cultured microglia.
Isolation of Cells from Tissues
Ischemic (4 days after pMCAO) (WT n=10 and IL-10 KO n=10) mice and controls (n=6 WT and n=6 IL-10 KO) were anesthetized and transcardially perfused with 40 mL saline. The ischemic parietal cortex (ipsilateral) and the corresponding cortex of the nonischemic hemisphere (contralateral) were dissected out and analyzed separately. The dissected contralateral or ipsilateral regions of two mice were pooled together and incubated for 30 minutes at 37°C in 5 mL RPMI-1640 medium (Life Technologies S.A.) containing 100 U/mL collagenase IV and 50 U/mL DNAse I, and pressed through a cell strainer (40 μm; BD Bioscience, San Agustin de Guadalix, Madrid, Spain). Cells were recovered after centrifugation at 400 g for 10 minutes and separated from myelin and debris in a 70% and 30% isotonic Percoll gradient (GE Healthcare Spain, Alcobendas, Madrid, Spain) prepared in Hank's Balanced Salt Solution without calcium or magnesium. Samples were centrifuged at 1,000 g for 30 minutes without acceleration or brake. Cells were collected from the interface, washed once with Hank's Balanced Salt Solution, and processed for flow cytometry.
Spleens were obtained 4 and 7 days after pMCAO. They were dissected in 1 mL RPMI-1640 medium and pressed through a 40-μm cell strainer (BD Bioscience). Cells were incubated for 5 minutes in red blood cell lysis buffer (150 mmol/L NH4Cl, 10 mmol/L KHCO3, 0.1 mmol/L EDTA) and washed twice in phosphate-buffered saline.
Flow Cytometry
Isolated brain cells were washed with fluorescence-activated cell sorting (FACS) buffer (phosphate-buffered saline, 2 mmol/L EDTA, 2% fetal bovine serum), incubated at 4°C for 10 minutes with FcBlock (1/200; Clone 2.4G2; BD Pharmingen; BD Bioscience), and incubated with primary antibodies in FACS buffer for 30 minutes at 4°C. The antibodies used were rat anti-mouse CD11b (clone M1/70, Alexa Fluor 647; BD Pharmingen), CD45 (clone 30-F11, FITC; BD Pharmingen), F4/80 Pan macrophages (clone BM8, FITC; Hycult Biotech, Uden, The Netherlands), and Ly6G (clone 1A8, PE-Cy7; BD Pharmingen). Isotype controls were rat IgG2bκ (clone A95-1, Alexa Fluor 647 or FITC; BD Pharmingen), rat IgG2a (FITC; Hycult Biotech), and rat IgG2aκ (clone R35-95, PE-Cy7; BD Pharmingen). Data acquisition was performed in a BD FacsCantoII cytometer (BD Bioscience) using the FacsDiva software (BD Bioscience). Cells were morphologically identified by linear forward scatter (FSC-A) and side scatter (SSC-A) parameters (Supplementary Figure 1). Data analysis was performed with the FlowJo software (version 7.6.5; TreeStar Inc., Ashland, OR, USA). Again, cells were plotted on forward versus side scatter, and single cells were gated on FSC-A versus FSC-H linearity. Flow-Count Fluorospheres (Beckman-Coulter España, Madrid, Spain) were used for absolute quantification.
Splenocytes were incubated in FACS buffer with FcBlock for 10 minutes at 4°C and then with the following antibodies for 30 minutes at 4°C: Armenian hamster anti-mouse CD3 (clone 145-2C11, Brilliant Violet-421; BioLegend, San Diego, CA, USA), rat anti-mouse CD4 (clone GK1.5, FITC; AbDserotec, Kidlington, UK), Armenian hamster anti-mouse CD152 (clone UC10-4B9, PE; BioLegend), and rat anti-mouse CD25 (clone PC61, Alexa Fluor-647; AbDserotec). After surface staining, cells were prepared for intracellular staining by fixation for 20 minutes and permeabilization for 20 minutes. Fixation and permeabilization buffers were obtained from eBioscience. Cells were then incubated for 30 minutes with rat anti-mouse Foxp3 (clone FJK-16s, PE-Cy7; eBioscience) antibodies. Isotype controls were Armenian hamster IgG (clone HTK888, Brilliant Violet 421 or PE; BioLegend), rat IgG2bκ (clone A95-1, FITC or Alexa Fluor-647; BD Pharmingen), and rat IgG2aκ (clone R35 to 95, PE-Cy7; BD Pharmingen). Data were acquired and analyzed as indicated above.
Immunofluorescence
Cryostat brain sections (14-μm-thick) were fixed with acetone, blocked with rabbit serum, and incubated overnight at 4°C with a rabbit polyclonal primary antibody against IL-10 receptor (IL-10R) (#C-20; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) diluted 1:100. Then, sections were incubated for 2 hours at room temperature with a secondary anti-rabbit antibody (Alexa Fluor-488; Molecular Probes; Life Technologies S.A.). Double immunostaining was performed with a mouse monoclonal antibody against GFAP conjugated with Alexa Fluor-546 (#8152; Cell Signalling Technology, Danvers, MA, USA) diluted 1:50, or with biotin-conjugated tomato isolectin (#L0651; Sigma-Aldrich Química) followed by incubation with Alexa Fluor-546 streptavidin (#S11225; Molecular Probes). Sections were counterstained with Hoechst to visualize the cell nuclei and they were observed under a confocal microscope (Leica SP5, Leica Microsystems, Barcelona, Spain).
Statistical Analyses
Statistics were performed using the GraphPad software (GraphPad Software Inc., La Jolla, CA, USA). One-way ANOVA was used for comparisons between multiple groups followed by the post hoc Bonferroni test. Two-way ANOVA was used for comparisons by genotype and by either brain hemisphere (ipsilateral/contralateral) or by time. Linear regression analysis was used for correlation studies. Sample size for infarct volume measures was calculated taking into account the known standard deviation of the researcher that carried out the ischemia model.
Results
Permanent Middle Cerebral Artery Occlusion Induces the Expression of Interleukin-10 and Interleukin-10 Receptor in Brain Tissue of Wild-Type Mice
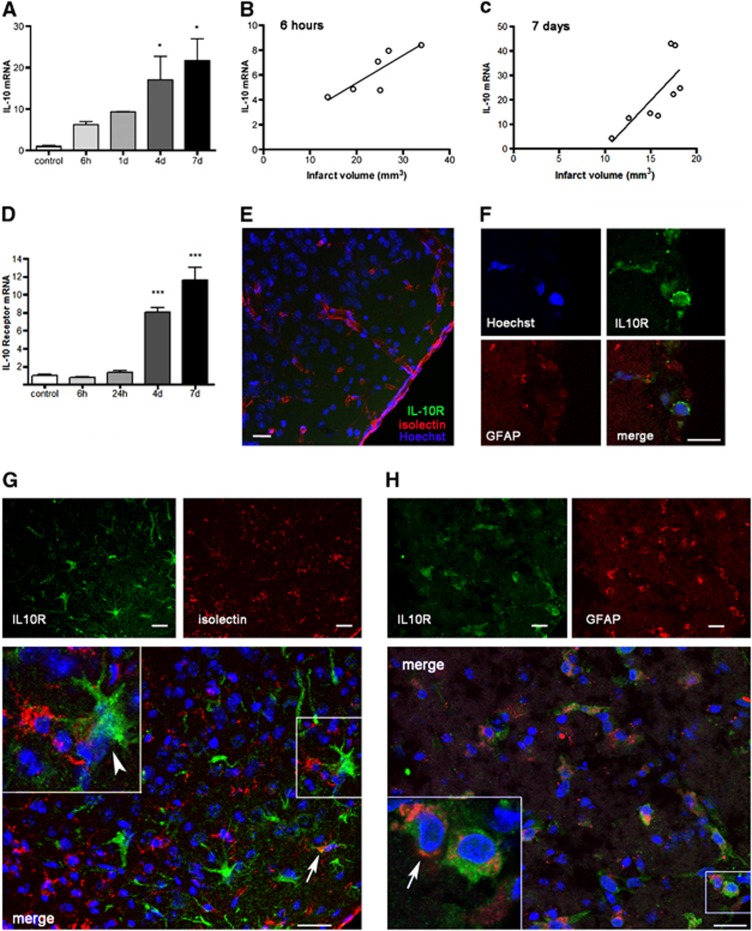
The expression of IL-10 mRNA progressively increased in the ipsilateral cortex of WT mice after pMCAO (Figure 1A). The magnitude of IL-10 mRNA expression was positively correlated (P<0.05) with infarct volume, as assessed by MRI in the same animals, 6 hours (Figure 1B) and 7 days (Figure 1C) after pMCAO, suggesting that it was dependent on the severity of ischemia. A previous study showed that the numbers of cells expressing IL-10 mRNA in the ischemic tissue were correlated with the size of the lesion from 6 hours to 6 days after pMCAO in rats.19
Figure 1.
Ischemia upregulates the expression of interleukin-10 (IL-10) and IL-10 receptor (IL-10R) in wild-type (WT) mice. (A) The expression of IL-10 mRNA progressively increases in brain tissue after permanent middle cerebral artery occlusion (pMCAO) from 6 hours (h) to 7 days (d). (B, C) The extent of IL-10 mRNA expression correlates (linear regression analysis, P<0.05) with infarct volume that was measured by magnetic resonance imaging (MRI) (T2) in the same animals 6 hours (B) or 7 days (C) after pMCAO. (D) Increased expression of IL-10R is apparent from 4 days after ischemia, but not within the first day. mRNA values are expressed as fold increase versus control nonischemic brain tissue. (E to H) Immunofluorescence staining of IL-10R (green) in the cortex of controls (E) and after pMCAO (F to H). (E) IL-10R immunoreaction (green) is not detected in the control cortex. Isolectin (red) staining shows resident microglia and blood vessels. (F) Isolated IL-10R-positive cells are detected in the subarachnoid space on the top of the ischemic cortex after pMCAO. (G) IL-10R immunoreactive cells (green) are seen in superficial cortical layers from 4 days after ischemia. These cells are rarely positive (arrow) for isolectin (red) and most of the IL-10R+ cells have morphology (arrowhead) compatible with that of reactive astrocytes. (H) Double staining with GFAP (red) shows that most of the IL-10R+ cells are astrocytes (arrow). The cell nuclei were stained with Hoechst (blue). Images in (F to H) correspond to days 1, 4, and 7 after pMCAO, respectively. Bar scale: 20 μm. n=4 to 6 mice per group. *P<0.05 and ***P<0.001.
Permanent middle cerebral artery occlusion also upregulated the expression of IL-10R mRNA, but strong increases were not detected until day 4 (Figure 1D). Immunofluorescence against IL-10R showed no immunoreactivity in the control cortex (Figure 1E). One, four, and seven days after ischemia, IL-10R immunoreactive cells were occasionally seen in the subarachnoid space on the surface of the infarcted cortex (Figure 1F). However, it was not until day 4 that the expression of IL-10R was strongly upregulated in the ischemic tissue. From this time point, IL-10R immunoreactivity was very prominent in reactive astrocytes of cortical layers I and II within the infarcted zone and at the subpial glia limitans (Figures 1G and 1H). Isolectin-positive myeloid cells immunoreactive for IL-10R were only occasionally detected (arrow in Figure 1G). These results show that reactive astrocytes are strongly receptive to IL-10 and might be involved in modulating inflammatory and immune responses after stroke.
Interleukin-10 Deficiency Slightly Increases Lesion Volume and Neurologic Deficits After Permanent Middle Cerebral Artery Occlusion
Focal ischemia by distal pMCAO in C57 mice causes a lesion restricted to a portion of the cortical middle cerebral artery territory (mainly the parietal cortex) (Figure 2A). Permanent middle cerebral artery occlusion did not cause mortality within the first 7 days, neither in WT mice nor in IL-10-deficient mice that were housed in SPF. However, in a preliminary experiment carried out in animals maintained under conventional housing conditions (not SPF) (WT n=9, IL-10 KO n=8) we observed no mortality in WT mice but 50% mortality in the IL-10 KO mice within 7 days after pMCAO (χ2=5.88, P<0.05). Interleukin-10 KO mice are known to spontaneously develop colitis when they are kept under conventional animal housing conditions.16 Poststroke mortality in the IL-10 KO mice was associated with the presence of overt signs of colitis as assessed after visual observation of variable degrees of rectal prolapse, which shows a rather severe colitic condition. Furthermore, IL-10 KO mice housed in the conventional animal house had smaller (P<0.001) body weight (mean±s.d., n) (16.7±3.1 g, n=9) than the WT mice (27.5±2.5 g, n=10) housed in the same conditions. This effect was not observed in IL-10 KO mice (27.8±3 g, n=51) and WT mice (29.1±3.8 g; n=45) housed in SPF. The finding that the presence of pathogens and ongoing peripheral inflammation in the IL-10 KO mice increased stroke mortality is in agreement with the notion that systemic inflammation aggravates stroke outcome.20 To explore the consequences of IL-10 deficiency in the stroked brain without confounding systemic inflammatory pathology, the following experiments were performed after the mouse colonies (both IL-10 KO and WT mice) were transferred to an SPF environment. The concentration of proinflammatory cytokines in the plasma of IL-10 KO and WT mice (naïve and 6 hours after pMCAO) that were kept in SPF conditions was examined to verify the absence of signs of subclinical inflammatory pathology (Supplementary Figure 2).
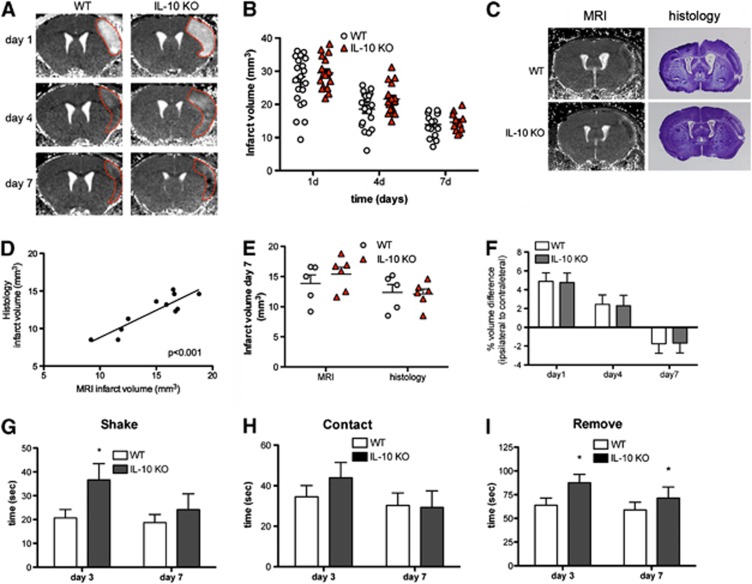
Figure 2.
Interleukin-10 (IL-10) deficiency slightly increases infarct volume and neurologic deficits. (A) Brain infarction was longitudinally monitored by T2 magnetic resonance imaging (MRI) in the same mice at days 1, 4, and 7 after ischemia and a representative animal per group is shown. (B) Infarct volume is slightly larger in IL-10 knockout (KO) mice (n=17) than in the wild-type (WT) (n=20) (two-way ANOVA by genotype and time; P<0.05 for genotype effect; P<0.001 for time effect). (C) In an additional group of mice, MRI was followed by histology in the same mice. T2 maps of representative brain slices at day 7 after permanent middle cerebral artery occlusion (pMCAO) are shown with their corresponding histologic staining (cresyl violet). (D) Infarct volume measured by MRI and by histologic means in the same mice shows a good correlation (linear regression analysis, r2=0.8, P<0.001). (E) The infarct volume measured by histologic means is significantly smaller (P<0.001) than the corresponding MRI measures. However, with either method, genotype group differences at day 7 are not statistically significant. (F) At day 1, the ratio of the volume of the ipsilateral to the contralateral hemisphere is above 1 due to edema. The effect is attenuated at day 4, whereas at day 7 the ipsilateral hemispheric volume is reduced. However, hemispheric volume differences between WT and IL-10 KO mice are similar along time. (G to I) Measure of the time to shake (G), contact (H), and remove (I) steps of the adhesion/removal tape test shows worse neurologic deficits at day 3, as shown by a significant delay in the time to shake (G) and remove (I) for the contralateral (left) forepaw, but the worsening effects of IL-10 deficiency are attenuated at day 7. Animals in (F to I) are the same as in (B), *P<0.05.
The progression of brain damage was assessed in IL-10 KO and WT mice by performing a longitudinal MRI study at days 1, 4, and 7 after pMCAO using T2 relaxometry maps (Figure 2A). Deficiency of IL-10 increased the size of the MRI lesion, but group differences were small and they were at the limit of statistical significance (two-way ANOVA by genotype and time, P=0.049 for genotype effect, and P<0.001 for time effect) (Figure 2B). The maximal infarct volume increase versus WT was 16% at day 4. Notably, IL-10 deficiency did not impair the process of involution of the MRI lesion, as infarct volume decreased at day 7 in both WT and IL-10 KO mice (Figure 2B). A successive reduction in infarct volume within the first 2 weeks after pMCAO has previously been reported in this experimental stroke mouse model.21, 22 However, the histopathologic and molecular correlates of this lesion involution are not known. For histologic validation of the MRI findings, we performed ischemia in an additional group of WT mice and IL-10 KO mice to evaluate infarct volume in vivo by MRI at day 7 followed by histologic evaluation in the same mice (Figure 2C). The correlation between MRI infarct volume and the corresponding histology volume was very good (linear regression r2=0.8, P<0.001) (Figure 2D). Two-way ANOVA by genotype and volume method (MRI versus histology) showed no significant effect of genotype but a significant effect of the volume method (P<0.001) due to smaller infarct volumes detected by histology (Figure 2E). This effect was attributable to postmortem and postprocessing brain volume changes and to larger errors when estimating the distance between sections after cryostat sectioning than after in vivo MRI, where slice thickness and distance between sections are precisely and automatically set.
To assess whether the degree of edema within the first few days after ischemia and the extent of shrinkage of the damaged tissue at later time points were different in the WT and IL-10 KO groups, we measured the ratio of the ipsilateral versus the contralateral MRI hemispheric brain volumes. At day 1, the volume of the ipsilateral hemisphere was larger than that of the contralateral hemisphere due to edema (Figure 2F). This increase was attenuated at day 4, but at day 7 the volume of the ipsilateral hemisphere was smaller than that of the contralateral hemisphere due to tissue loss (Figures 2A and 2F). However, these changes in brain volume were similar in the WT and IL-10 KO groups (Figure 2F), suggesting that the absence of IL-10 interfered neither with ischemia-induced edema nor with lesion involution. In support of these findings, measures of brain water content at day 4 using the wet/dry weight method showed that ischemia increased the water content in the ipsilateral versus the contralateral hemisphere by (mean±s.d.) 0.98±0.27% and 0.92±0.37% in WT (n=5) and IL-10 KO (n=2) groups, respectively.
Permanent middle cerebral artery occlusion induced only mild neurologic deficits that could be detected by the adhesion/removal tape test, which evaluates sensorimotor alterations, but not by the neurologic score tests23 often used in models of transient intraluminal MCAO (not shown). The neurologic deficit observed with the adhesion/removal tape test was greater in IL-10 KO mice than in WT mice. The test showed a worse performance of the contralateral forepaw (left) in IL-10 KO mice 3 days after pMCAO (Figures 2G to 2I). However, at day 7, the worsening effect of IL-10 deficiency was attenuated (Figures 2G to 2I), supporting that the effects of IL-10 were mainly manifested during the first days after stroke.
Interleukin-10 Deficiency Did Not Impair the Cerebral Inflammatory Response Early After Permanent Middle Cerebral Artery Occlusion
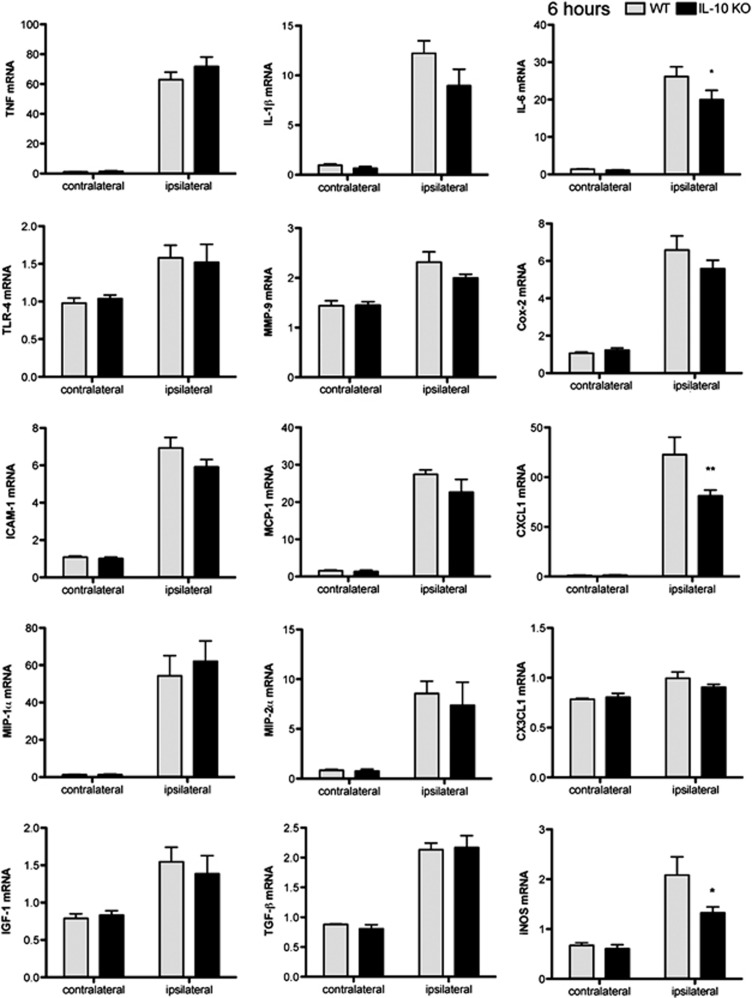
We examined the inflammatory response in brain tissue early (6 hours) after pMCAO by measuring the mRNA expression of several cytokines, chemokines, adhesion molecules, and other inflammatory molecules with qRT–PCR. In WT mice, ischemia induced the expression of cytokines, such as TNF-α, IL-1β, and IL-6, as well as other inflammatory mediators, such as TLR-4, matrix metalloproteinase-9 (MMP-9), cyclooxygenase-2 (Cox-2), and inducible nitric oxide synthase (iNOS) (Figure 3). Ischemia also upregulated the mRNA expression of chemokines: monocyte chemoattractant protein-1 (MCP-1 or CCL2), neutrophil-activating protein KC (CXCL1), and macrophage inflammatory proteins MIP-1α (CCL3) and MIP-2α (CXCL2); the intercellular adhesion molecule-1 (ICAM-1); and growth factors, such as transforming growth factor-β (TGF-β), and insulin growth factor-1 (IGF-1) (Figure 3). Deficiency of IL-10 showed a non-significant tendency to increase the expression of some of the above molecules in the ischemic tissue, while it induced a slightly lower expression of IL-6 and CXCL1 mRNA compared with that in WT mice (Figure 3). These results showed that IL-10 deficiency did not significantly exacerbate the initial inflammatory reaction of resident cells within the first few hours after pMCAO. Therefore, the inflammatory reaction24 to ischemia does not appear to be strongly regulated by IL-10 within the first hours after stroke onset.
Figure 3.
Interleukin-10 (IL-10) deficiency does not exacerbate the inflammatory reaction 6 hours after ischemia. The mRNA expression for the stated molecules increases in both wild-type (WT) and IL-10 knockout (KO) ischemic brain tissue at 6 hours. Values are expressed as fold versus control WT. n=6 mice per group, *P<0.05 and **P<0.01. TNF-α, tumor necrosis factor-α; TLR, Toll-like receptor; ICAM-1, intercellular adhesion molecule-1; MIP-1α, macrophage inflammatory protein-1α; IGF-1, insulin growth factor-1; MMP, matrix metalloproteinase; MCP-1, monocyte chemoattractant protein-1; TGF-β, transforming growth factor-β; Cox-2, cyclooxygenase-2; iNOS, inducible nitric oxide synthase.
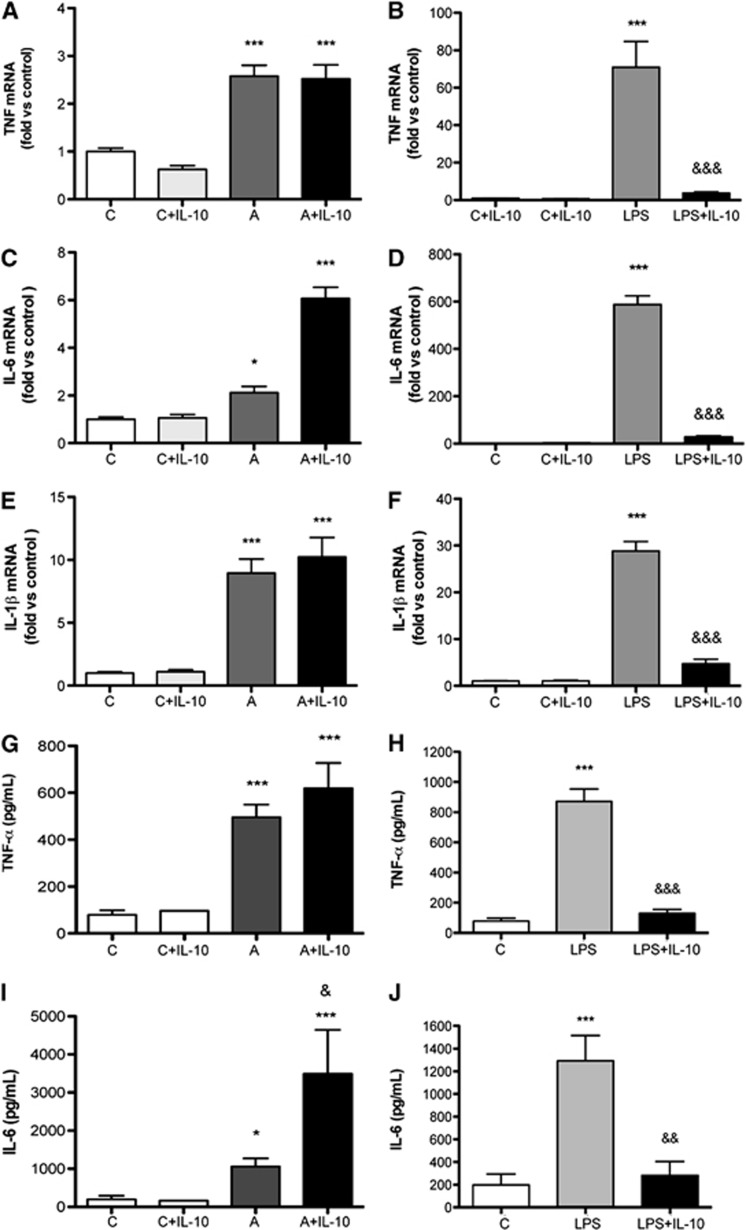
To examine the direct antiinflammatory effect of IL-10, we performed an in vitro study in primary cultures of murine microglia. Cultured microglia (Figure 4) exposed to ischemic conditions showed increased expression of proinflammatory cytokines like TNF-α (Figures 4A and 4G), IL-6 (Figures 4C and 4I), and IL-1β (Figure 4E) 6 hours later. Notably, treatment with recombinant IL-10 did not prevent this inflammatory reaction (Figures 4A, 4C, 4E, 4G, and 4I). In contrast, treatment with IL-10 strongly suppressed the inflammatory response of microglia stimulated with LPS (Figures 4B, 4D, 4F, 4H, and 4J), showing that IL-10 effectively blocked the response to stimuli inducing activation of TLR-4 but not to a lack of oxygen.
Figure 4.
In cultured microglia, interleukin-10 (IL-10) attenuates the inflammatory response to lypopolysaccharide (LPS) but not that induced by exposure to ischemic conditions. Cultured microglia show selective inflammatory responses after stimulation either with 3-hour anoxia (group ‘A') or with 10 ng/mL LPS (group ‘LPS'). (A to F) Cytokine mRNA expression was evaluated by qRT–PCR analysis 6 hours after initiation of the challenge. (G to J) Cytokine concentration in the culture medium was also measured at 6 hours by ELISAs. (A, C, E, G, I) Treatment with recombinant murine IL-10 (10 ng/mL) does not attenuate the response to anoxia (group ‘A+IL-10'). (B, D, F, H, J) However, IL-10 strongly prevents the inflammatory response to LPS (group ‘LPS+IL-10'). Values were obtained in three independent cultures. Symbol * refers to comparison versus control, and symbol & refers to comparison versus either anoxia or LPS. One symbol: P<0.05, two symbols: P<0.01, and three symbols: P<0001. TNF-α, tumor necrosis factor-α.
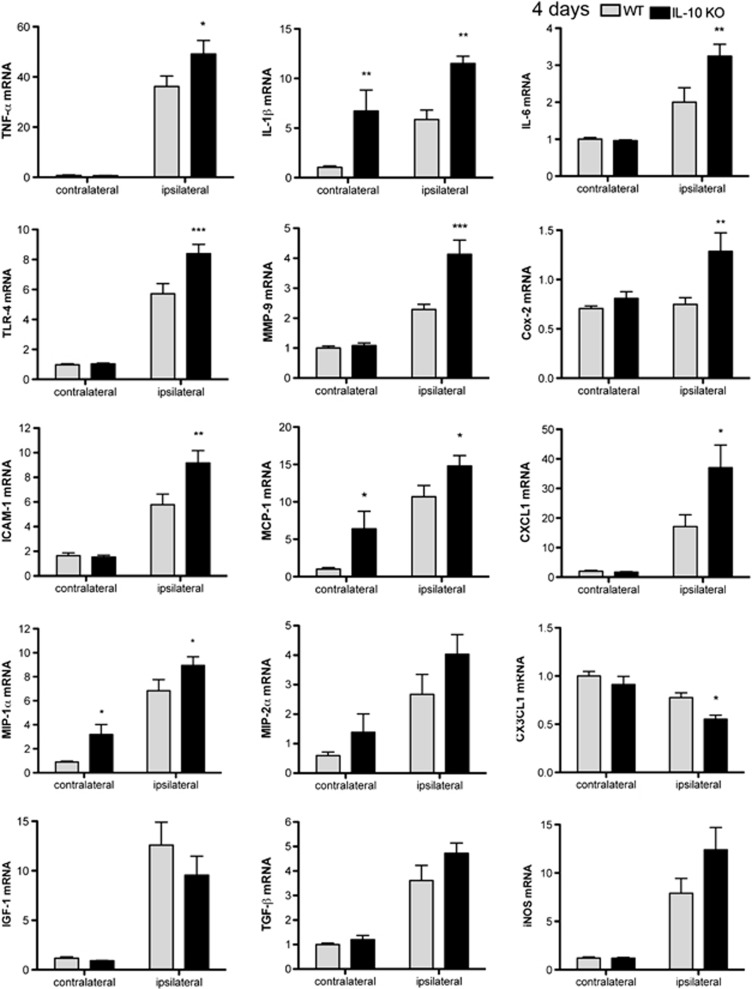
Interleukin-10 Deficiency Induces an Exaggerated Inflammatory Response 4 Days after Permanent Middle Cerebral Artery Occlusion
In contrast to the findings 6 hours after ischemia, at 4 days a significantly higher mRNA expression of TNF-α, IL-1β, IL-6, TLR-4, MMP-9, Cox-2, intercellular adhesion molecule-1, MCP-1, CXCL1, and MIP-1α was found in the ipsilateral cortex of IL-10 KO mice versus the WT mice (Figure 5). Also, the expression of iNOS and MIP-2α mRNA tended to be higher in IL-10 KO mice, but differences were not statistically significant (Figure 5). In contrast to the above increases, the expression of CX3CL1, which is mainly produced by neurons, was reduced in the ipsilateral cortex of IL-10 KO mice versus the WT (Figure 5). In addition, small increases in several molecules (IL-1β and MCP-1 mRNA) were detected at day 4 in the contralateral cortex of IL-10 KO mice versus the WT (Figure 5), suggesting that IL-10 might prevent secondary inflammation in remote brain regions too. The finding of more pronounced inflammatory responses in the IL-10 KO brain at day 4 after ischemia is in agreement with the observation that expression of IL-10 and IL-10R was strongly upregulated at this time point (Figure 1).
Figure 5.
Interleukin-10 (IL-10) deficiency exacerbates secondary inflammation at day 4 after permanent middle cerebral artery occlusion (pMCAO). Brain expression of cytokine and chemokine mRNA is significantly increased in IL-10 knockout (KO) than in wild-type (WT) mice at day 4, with the exception of CX3CL1, which decreases in IL-10 KO mice. Values are expressed as fold versus control WT. n=6 mice per group, *P<0.05, **P<0.01, and ***P<0.001. TNF-α, tumor necrosis factor-α; TLR, Toll-like receptor; ICAM-1, intercellular adhesion molecule-1; MIP-1α, macrophage inflammatory protein-1α; IGF-1, insulin growth factor-1; MMP, matrix metalloproteinase; MCP-1, monocyte chemoattractant protein-1; TGF-β, transforming growth factor-β; Cox-2, cyclooxygenase-2; iNOS, inducible nitric oxide synthase.
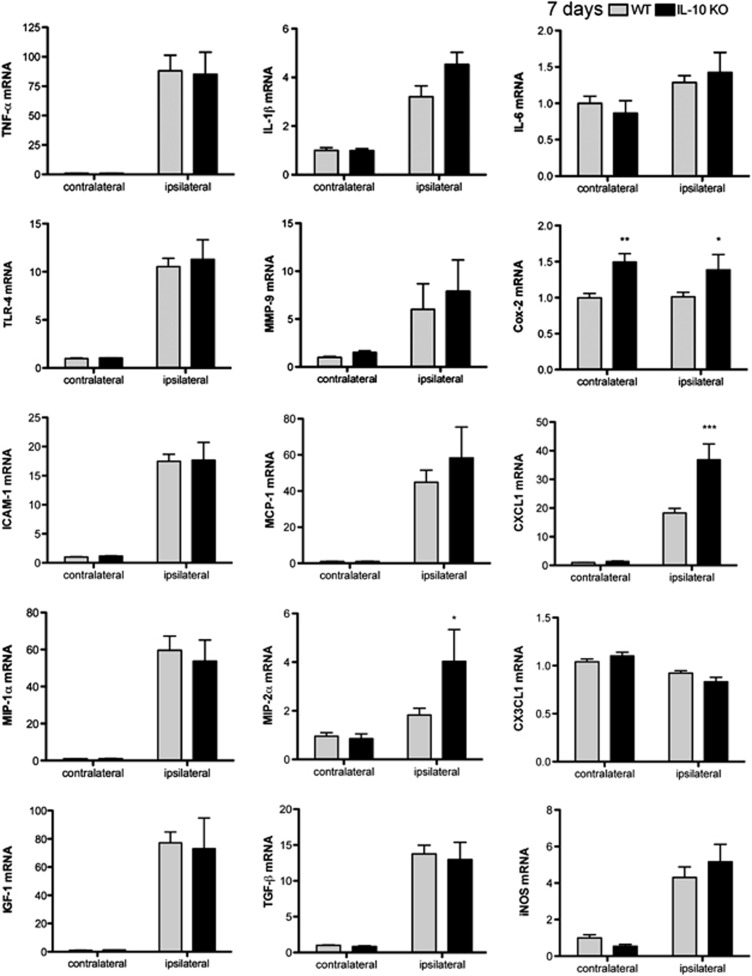
Differences in the Inflammatory Response of Interleukin-10 Knockout and Wild-Type Mice Are Reduced at Day 7
Differences between WT and IL-10 KO groups for the mRNA expression of several proinflammatory molecules, such as TNF-α, IL-1β, IL-6, TLR-4, MCP-1, and MMP-9 (Figure 6), were no longer statistically significant 7 days after ischemia. However, MIP-2α and Cox-2 mRNA was higher in the IL-10 KO mice than in the WT, and an increase in Cox-2 expression also became apparent in the contralateral hemisphere compared with the corresponding WT (Figure 6). At this time point, the mRNA expression of molecules involved in repair mechanisms 11, such as TGF-β and IGF-1, had increased versus previous time points, but this effect was similar in WT and IL-10 KO mice (Figure 6), indicating that resolution of inflammation progressed also in the absence of IL-10.
Figure 6.
Differences between wild-type (WT) and interleukin-10 knockout (IL-10 KO) mice in the brain inflammatory response are attenuated at day 7. Brain expression of most cytokine and chemokine mRNA is no longer significantly different between IL-10 KO and WT mice at day 7. n=6 mice per group. Values are expressed as fold versus control WT. *P<0.05, **P<0.01, ***P<0.001. TNF-α, tumor necrosis factor-α; TLR, Toll-like receptor; ICAM-1, intercellular adhesion molecule-1; MIP-1α, macrophage inflammatory protein-1α; IGF-1, insulin growth factor-1; MMP, matrix metalloproteinase; MCP-1, monocyte chemoattractant protein-1; TGF-β, transforming growth factor-β; Cox-2, cyclooxygenase-2; iNOS, inducible nitric oxide synthase.
Suppressive Mechanisms Are Upregulated in the Interleukin-10 Knockout Mice After Permanent Middle Cerebral Artery Occlusion
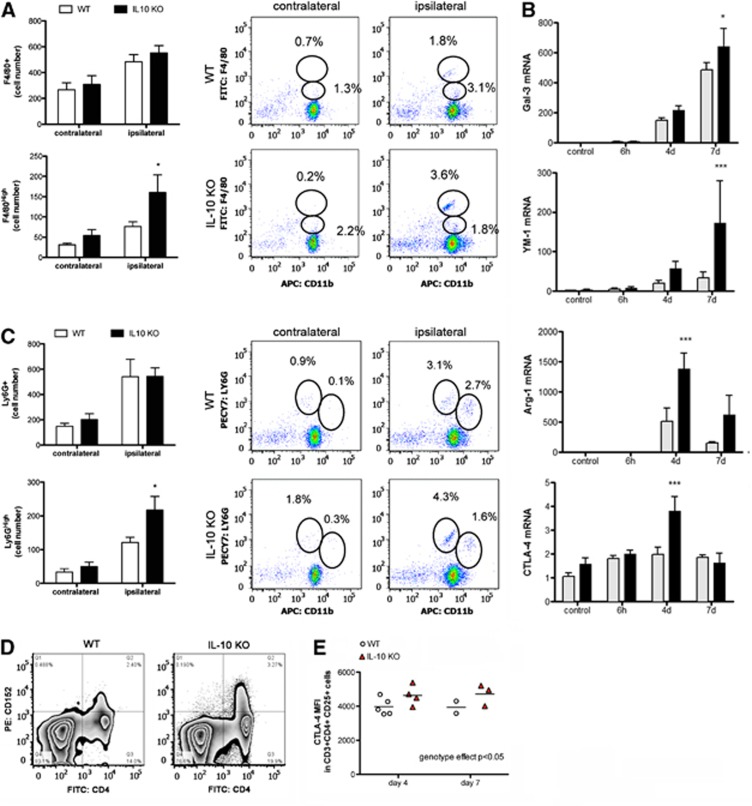
The numbers and features of myeloid cells found in brain tissue of IL-10 KO and WT mice were examined by flow cytometry 4 days after pMCAO. The relative proportion of cells corresponding to microglia (CD11bDimCD45low) decreased in the ischemic tissue of both IL-10 KO and WT mice compared with the contralateral hemisphere or the control brain (Supplementary Figure 3). This effect was due to the presence of leukocytes (CD45High) in the ischemic brain (Supplementary Figure 3). However, the numbers of leukocytes, both CD11b+CD45High (myeloid cells) and CD11b-CD45High (lymphocytes), were similar in IL-10 KO and WT mice (Supplementary Figure 3). Likewise, no differences in the number of F4/80+ cells (a marker of phagocytic microglia/macrophages) were found between the two genotypes (Figure 7A). Nevertheless, IL-10 deficiency induced a shift in the phenotype of these cells due to the presence of significantly higher proportions and numbers of F4/80High cells in the ischemic tissue of IL-10 KO mice than in the WT, while this specific myeloid subpopulation was absent in the contralateral cortex or in controls (Figure 7A). F4/80High cells are considered as mature macrophages undergoing terminal differentiation in the tissue microenvironment.25 As F4/80High cells are phagocytic cells that are expected to actively participate in clearing the damaged tissue, we examined the expression of galectin-3, which in macrophages has a critical role in phagocytosis. Higher galectin-3 mRNA expression was detected in the ischemic tissue of IL-10 KO mice (Figure 7B), supporting that phagocytic activity was enhanced. Furthermore, the ischemia-induced mRNA expression of typical antiinflammatory M2 phenotype markers of myeloid cells, YM1 and arginase-1, was also significantly higher in the IL-10 KO mice (Figure 7B).
Figure 7.
Compensatory immunosuppressive mechanisms in interleukin-10 knockout (IL-10 KO) mice after ischemia. (A) Ischemia increases the number of cells expressing F4/80+ (microglia/macrophages) to a similar extent in both genotypes at day 4. However, more F4/80High phagocytic cells are seen in the brain of IL-10 KO than in wild-type (WT) mice. (B) The ischemic tissue of IL-10 KO mice shows a higher upregulation of the mRNA of galectin-3, Arginase-1, YM-1, and CTLA-4 (n=4 to 6 samples per time group). (C) At day 4, the numbers of Ly6G+ cells (neutrophils) are similar in the ipsilateral cortex of IL-10 KO and WT mice, but higher numbers of Ly6GHigh neutrophils are seen in the ischemic cortex of the IL-10 KO mice. Flow cytometry diagrams shown in the right panels of (A) and (C) correspond to one representative sample out of n=5 independent samples per group. (D) Representative dot plots of CTLA-4 expression (CD152) in splenocytes from WT and IL-10 KO mice 7 days after permanent middle cerebral artery occlusion (pMCAO). Single viable cells are plotted by CD152 versus CD4 expression. (E) Single viable splenocytes are subsequently gated on CD3+ CD4+ and CD25+ cells. CTLA-4 mean fluorescence intensity (MFI) in the latter cells is higher in IL-10 KO mice than in WT mice. Two-way ANOVA by genotype and time. *P<0.05 and ***P<0.001 versus ischemic tissue of WT mice.
Neutrophils (CD11b+Ly6G+) were detected in the ipsilateral cortex to a similar extent in the IL-10 KO mice than in the WT (Figure 7C). However, again the phenotype of these cells shifted in IL-10 KO mice as more Ly6GHigh cells were detected in the latter group. Ly6GHigh and F4/80High cells described above were only found in the ischemic cortex, particularly in IL-10 KO mice, but were not detected in the blood of IL-10 KO or WT mice (not shown), suggesting that this phenotypic shift was induced by the local ischemic brain environment.
Interleukin-10 is important for the immunosuppressive function of regulatory T cells (Treg). For this reason, we examined whether IL-10 deficiency could alter the expression of other T-cell immunosuppressive molecules. Notably, the ischemic tissue of IL-10 KO mice showed a significantly higher expression of the T-cell inhibitory molecule CTLA-4 mRNA at day 4 after ischemia compared with the WT ischemic tissue (Figure 7B). In the spleen, similar percentages of Treg (CD3+CD4+CD25+Foxp3+) were detected in both genotypes 4 and 7 days after ischemia (Supplementary Figure 4). The proportion of CD3+CD4+CD25+ cells that were CTLA-4 (CD152) positive tended to increase in the spleen of IL-10 KO mice, but differences versus the WT were not statistically significant (Supplementary Figure 4). However, the CTLA-4 mean fluorescence intensity in the CD3+CD4+CD25+ cell population (Figure 7D), was significantly higher in the spleen of IL-10 KO mice 4 and 7 days after ischemia (two-way ANOVA by genotype and time showed a significant genotype effect, P<0.05) (Figure 7E), suggesting upregulation of CTLA-4 expression in the Treg population of IL-10-deficient mice. Altogether these findings support that in the absence of IL-10 compensatory alternative immunosuppressive mechanism is upregulated after stroke.
Discussion
The results of this study show that IL-10 deficiency exacerbates brain inflammation after pMCAO, especially at day 4 when IL-10 is expected to exert an action in the ischemic tissue due to the upregulation of this cytokine and its receptor. Strategies increasing antiinflammatory IL-10 in the brain are beneficial in experimental ischemia,12, 13 and mice deficient in IL-10 showed more brain damage than WT mice after pMCAO.14 Here, we found that IL-10 KO mice had slightly larger infarct volumes and worse neurologic deficits than the WT mice during the first few days after ischemia. However, these differences were attenuated at day 7, when in this experimental stroke model the extent of the lesion had markedly regressed.21, 22 This finding supports that IL-10 is not required for the process of lesion involution.
Notably, the increase in infarct volume in IL-10 KO mice found in our study was quite small and it was milder than that previously reported.14 These differences in scale might be attributable to the specific animal housing conditions. Exposure of IL-10 KO mice to environmental pathogens when they were kept under conventional housing conditions triggered the development of apparent signs of colitis, while maintaining the animals in a pathogen-free environment prevented this effect, as previously reported.16 Development of colitis was associated with a worse stroke outcome, in agreement with the described worsening effect of systemic inflammation.20 Therefore, ongoing inflammatory pathology adds a confounding factor exaggerating the direct effects of IL-10 deficiency in stroke.
Expression of IL-10 was upregulated in the ischemic tissue soon after pMCAO, suggesting the involvement of resident cells. The capacity of microglia to secrete IL-10 has been reported after exposure to several agents such as adenosine,26 extracellular ATP,27 or LPS.28 Several lines of evidence support beneficial functions for microglia in stroke,29, 30 but it is unknown whether these effects are dependent on IL-10. Treatment of cultured microglia with recombinant IL-10 did not attenuate the inflammatory reaction triggered by insufficient oxygen supply, but it strongly suppressed cytokine induction after TLR-4 activation. After brain ischemia, the initial inflammatory reaction can be secondarily aggravated by necrotic cell death, as it generates danger signals31 that activate TLR-4, inducing further inflammation8 and contributing to brain damage.32 Here, IL-10 deficiency did not increase the local inflammatory reaction within the first hours after pMCAO, but it did 4 days later. This finding is in accordance with the exacerbated secondary inflammatory process seen in IL-10 KO mice after spinal cord compression injury33 and in a model of brain bacterial infection.34
The neuroinflammatory reaction is a dynamic process and inflammation is also involved in tissue repair mechanisms that are modulated by the complex crosstalk between resident glia and infiltrated immune cells.35 Importantly, appropriate orchestration of the inflammatory responses is critical to allow for lesion resolution. Reactive astrocytes can sense the presence of IL-10 as they showed strong expression of IL-10R at days 4 and 7 after ischemia. Therefore, astrocytes may have a role in controlling inflammatory and immune responses after stroke. Infiltrated macrophages participate through phagocytosis in the process of clearance of the injured tissue.36 In our experimental conditions, the absence of IL-10 facilitated the presence in the ischemic tissue of myeloid cells (F4/80High cells) that are considered mature phagocytic cells. Accordingly, the ischemic tissue of IL-10 KO mice showed higher expression of galectin-3, a protein related to phagocytic activity, and greater upregulation of the antiinflammatory M2 markers arginase-1 and YM-1, compared with the WT mice. Neutrophils also acquired a more mature phenotype in the brain of IL-10 KO mice by showing higher expression of Ly6G. Neutrophils with this phenotypic feature have been described in the context of tumors37 and in bacterial infections,38 where they exert antiinflammatory and immunosuppressive functions. As leukocytes with these features were not detected in the circulation, it is likely that the specific local brain environment was the trigger of the phenotypic changes observed in myeloid cells in the ischemic IL-10 KO brain.
Beneficial effects of Treg in brain ischemia are mediated by IL-10.39 Therefore, the lack of IL-10 could impair the immunosuppressive function of Treg. However, immunosuppressive functions can be promoted by alternative mechanisms, such as that mediated by the T-cell inhibitory receptor CTLA-4,40 which after brain ischemia was more prominent in the IL-10 KO mice than in the WT.
In summary, this study shows that IL-10 attenuates the local inflammatory reaction after brain ischemia as IL-10 deficiency transiently enhanced the expression of proinflammatory cytokines and chemokines and slightly increased the size of the lesion and worsened the neurologic deficits. However, the study does not support a fundamental role for IL-10 in subsequent lesion involution, likely due to compensatory mechanisms able to control immune responses in the absence of IL-10.
Acknowledgments
The authors thank Ms Francisca Ruiz for excellent technical assistance, Dr Tomàs Santalucia for advice on genotyping protocols; and Dr Vanessa Brait for helpful comments. We are indebted to the Image and Cytometry platforms of the Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) for technical help.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by the Spanish Ministry of Economy (SAF2011-30492), and the European Community (FP7, grant agreements: n° 201024 ARISE and n° 278850 InMiND), and the ERANET-NEURON project (PRI-PIMNEU-2011-1342). IPP and EBT had PhD fellowships from the Agència de Gestió d'Ajuts Universitaris i de Recerca (AGAUR) of the Generalitat de Catalunya and the FPU program of the Spanish Ministry of Economy, respectively.
Supplementary Material
References
- Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med. 2004;36:1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- Geiger K, Leiherer A, Muendlein A, Stark N, Geller-Rhomberg S, Saely CH, et al. Identification of hypoxia-induced genes in human SGBS adipocytes by microarray analysis. PLoS ONE. 2011;6:e26465. doi: 10.1371/journal.pone.0026465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Wang L, Li JW, Gong M, He L, Feng R, et al. Hypoxia induced amoeboid microglial cell activation in postnatal rat brain is mediated by ATP receptor P2 × 4. BMC Neurosci. 2011;12:111. doi: 10.1186/1471-2202-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinman R, Deitch EA, Watkins AC, Abungu B, Colorado I, Kannan KB, et al. HIF-1 mediates pathogenic inflammatory responses to intestinal ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2010;299:G833–G843. doi: 10.1152/ajpgi.00065.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Choi YJ, Joung SM, Lee BH, Jung YS, Lee JY. Hypoxic stress up-regulates the expression of Toll-like receptor 4 in macrophages via hypoxia-inducible factor. Immunology. 2010;129:516–524. doi: 10.1111/j.1365-2567.2009.03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafani M, Schito L, Pellegrini L, Villanova L, Marfe G, Anwar T, et al. Hypoxia-increased RAGE and P2 × 7R expression regulates tumor cell invasion through phosphorylation of Erk1/2 and Akt and nuclear translocation of NF-{kappa}B. Carcinogenesis. 2011;32:1167–1175. doi: 10.1093/carcin/bgr101. [DOI] [PubMed] [Google Scholar]
- Johnson GB, Brunn GJ, Platt JL. Activation of mammalian Toll-like receptors by endogenous agonists. Crit Rev Immunol. 2003;23:15–44. doi: 10.1615/critrevimmunol.v23.i12.20. [DOI] [PubMed] [Google Scholar]
- Gorina R, Font-Nieves M, Marquez-Kisinousky L, Santalucia T, Planas AM. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK and Jak1/Stat1 pathways. Glia. 2011;59:242–255. doi: 10.1002/glia.21094. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 2009;276:13–26. doi: 10.1111/j.1742-4658.2008.06766.x. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- de Bilbao F, Arsenijevic D, Moll T, Garcia-Gabay I, Vallet P, Langhans W, et al. In vivo over-expression of interleukin-10 increases resistance to focal brain ischemia in mice. J Neurochem. 2009;110:12–22. doi: 10.1111/j.1471-4159.2009.06098.x. [DOI] [PubMed] [Google Scholar]
- Ooboshi H, Ibayashi S, Shichita T, Kumai Y, Takada J, Ago T, et al. Postischemic gene transfer of interleukin-10 protects against both focal and global brain ischemia. Circulation. 2005;111:913–919. doi: 10.1161/01.CIR.0000155622.68580.DC. [DOI] [PubMed] [Google Scholar]
- Grilli M, Barbieri I, Basudev H, Brusa R, Casati C, Lozza G, et al. Interleukin-10 modulates neuronal threshold of vulnerability to ischaemic damage. Eur J Neurosci. 2000;12:2265–2272. doi: 10.1046/j.1460-9568.2000.00090.x. [DOI] [PubMed] [Google Scholar]
- Vila N, Castillo J, Dávalos A, Esteve A, Planas AM, Chamorro A. Levels of anti-inflammatory cytokines and neurological worsening in acute ischemic stroke. Stroke. 2003;34:671–675. doi: 10.1161/01.STR.0000057976.53301.69. [DOI] [PubMed] [Google Scholar]
- Kullberg MC, Ward JM, Gorelick PL, Caspar P, Hieny S, Cheever A, et al. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66:5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouet V, Boulouard M, Toutain J, Divoux D, Bernaudin M, Schumann-Bard P, et al. The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice. Nature Prot. 2009;4:1560–1564. doi: 10.1038/nprot.2009.125. [DOI] [PubMed] [Google Scholar]
- Saura J, Tusell JM, Serratosa J. High-yield isolation of murine microglia by mild trypsinization. Glia. 2003;44:183–189. doi: 10.1002/glia.10274. [DOI] [PubMed] [Google Scholar]
- Li HL, Kostulas N, Huang YM, Xiao BG, van der Meide P, Kostulas V, et al. IL-17 and IFN-gamma mRNA expression is increased in the brain and systemically after permanent middle cerebral artery occlusion in the rat. J Neuroimmunol. 2001;116:5–14. doi: 10.1016/s0165-5728(01)00264-8. [DOI] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich-Noack P, Baldauf K, Reiser G, Reymann KG. Pattern of time-dependent reduction of histologically determined infarct volume after focal ischaemia in mice. Neurosci Lett. 2008;432:141–145. doi: 10.1016/j.neulet.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Pialat JB, Cho TH, Beuf O, Joye E, Moucharrafie S, Langlois JB, et al. MRI monitoring of focal cerebral ischemia in peroxisome proliferator-activated receptor (PPAR)-deficient mice. NMR Biomed. 2007;20:335–342. doi: 10.1002/nbm.1157. [DOI] [PubMed] [Google Scholar]
- Cervera A, Planas AM, Justicia C, Urra X, Jensenius JC, Torres F, et al. Genetically-defined deficiency of mannose-binding lectin is associated with protection after experimental stroke in mice and outcome in human stroke. PLoS ONE. 2010;5:e8433. doi: 10.1371/journal.pone.0008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32:1677–1698. doi: 10.1038/jcbfm.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwama A, Wang MH, Yamaguchi N, Ohno N, Okano K, Sudo T, et al. Terminal differentiation of murine resident peritoneal macrophages is characterized by expression of the STK protein tyrosine kinase, a receptor for macrophage-stimulating protein. Blood. 1995;86:3394–3403. [PubMed] [Google Scholar]
- Koscsó B, Csóka B, Selmeczy Z, Himer L, Pacher P, Virág L, et al. Adenosine augments IL-10 production by microglial cells through an A2B adenosine receptor-mediated process. J Immunol. 2012;188:445–453. doi: 10.4049/jimmunol.1101224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo DR, Kim SY, Kim KY, Lee HG, Moon JH, Lee JS, et al. Cross talk between P2 purinergic receptors modulates extracellular ATP-mediated interleukin-10 production in rat microglial cells. Exp Mol Med. 2008;40:19–26. doi: 10.3858/emm.2008.40.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeboer A, Brevé JJ, Wierinckx A, van der Jagt S, Bristow AF, Leysen JE, et al. Expression and regulation of interleukin-10 and interleukin-10 receptor in rat astroglial and microglial cells. Eur J Neurosci. 2002;16:1175–1185. doi: 10.1046/j.1460-9568.2002.02200.x. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustino JV, Wang X, Johnson CE, Klibanov A, Derugin N, Wendland MF, et al. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. J Neurosci. 2011;31:12992–13001. doi: 10.1523/JNEUROSCI.2102-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HE, Raucci A. Alarmin(g) news about danger: workshop on innate danger signals and HMGB1. EMBO Rep. 2006;7:774–778. doi: 10.1038/sj.embor.7400759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- Genovese T, Esposito E, Mazzon E, Di Paola R, Caminiti R, Bramanti P, et al. Absence of endogenous interleukin-10 enhances secondary inflammatory process after spinal cord compression injury in mice. J Neurochem. 2009;108:1360–1372. doi: 10.1111/j.1471-4159.2009.05899.x. [DOI] [PubMed] [Google Scholar]
- Deckert M, Soltek S, Geginat G, Lütjen S, Montesinos-Rongen M, Hof H, et al. Endogenous interleukin-10 is required for prevention of a hyperinflammatory intracerebral immune response in Listeria monocytogenes meningoencephalitis. Infect Immun. 2001;69:4561–4571. doi: 10.1128/IAI.69.7.4561-4571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. Tissue-repairing' blood-derived macrophages are essential for healing of the injured spinal cord: From skin-activated macrophages to infiltrating blood-derived cells. Brain Behav Immun. 2010;24:1054–1057. doi: 10.1016/j.bbi.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Zoglmeier C, Bauer H, Nörenberg D, Wedekind G, Bittner P, Sandholzer N, et al. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin Cancer Res. 2011;17:1765–1775. doi: 10.1158/1078-0432.CCR-10-2672. [DOI] [PubMed] [Google Scholar]
- Zhang X, Majlessi L, Deriaud E, Leclerc C, Lo-Man R. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity. 2009;31:761–771. doi: 10.1016/j.immuni.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Ertelt JM, Buyukbasaran EZ, Jiang TT, Rowe JH, Xin L, Way SS. B7-1/B7-2 blockade overrides the activation of protective CD8 T cells stimulated in the absence of Foxp3+ regulatory T cells. J Leukoc Biol. 2013;94:367–376. doi: 10.1189/jlb.0313118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.