Abstract
The use of selective serotonin reuptake inhibitors has shown functional improvement after stroke. Despite this, the role of serotoninergic neurotransmission after cerebral ischemia evolution and its involvement in functional recovery processes are still largely unknown. For this purpose, we performed in parallel in vivo magnetic resonance imaging and positron emission tomography (PET) with [11C]DASB and [18F]altanserin at 1, 3, 7, 14, 21, and 28 days after middle cerebral artery occlusion (MCAO) in rats. In the ischemic territory, PET with [11C]DASB and [18F]altanserin showed a dramatic decline in serotonin transporter (SERT) and 5-HT2A binding potential in the cortex and striatum after cerebral ischemia. Interestingly, a slight increase in [11C]DASB binding was observed from days 7 to 21 followed by the uppermost binding at day 28 in the ipsilateral midbrain. In contrast, no changes were observed in the contralateral hemisphere by using both radiotracers. Likewise, both functional and behavior testing showed major impaired outcome at day 1 after ischemia onset followed by a recovery of the sensorimotor function and dexterity from day 21 to day 28 after cerebral ischemia. Taken together, these results might evidence that SERT changes in the midbrain could have a key role in the functional recovery process after cerebral ischemia.
Keywords: cerebral ischemia, PET, MCAO, [11C]DASB, [18F]altanserin, serotonin
Introduction
Brain dysfunction promoted by cerebral ischemia affects several daily life activities such as sensorimotor integration, movement, walking, language, vision, balance, mood and sensory perception.1 Although most of the patients have shown spontaneous behavioral improvements in the first few weeks or months after a stroke,2 the recovery is generally incomplete.3 Therefore, the correct understanding of the mechanisms involved in the process of recovery after stroke is crucial to the design of novel therapies for acute stroke.4 The functional recovery includes some mechanisms, such as neo-formation of brain vessel, increase in axonal sprouting, development of new dendrites, and functional reorganization through recruitment of new brain regions, and neuromodulation to the opposite hemisphere.5 Recently, brain function restoration after brain damage has also been related to changes in the dopaminergic neurotransmission system after cerebral ischemia in rats.6 Likewise, clinical studies have shown that the use of pharmacological therapies that increase brain concentrations of brain amines such as dopamine and serotonin promotes a positive impact on stroke outcomes.7 Moreover, the modulation of serotoninergic neurotransmission by using selective serotonin reuptake inhibitors such as fluoxetine and citalopram improved motor and cognitive recovery in both the clinic8, 9 and the laboratory.10
Positron emission tomography (PET) with [11C]DASB and [18F]altanserin has widely been used to investigate the availability of serotonin transporter (SERT) and serotonin receptor 5-HT2A in both humans and animals.11, 12, 13, 14 This imaging method has proven to be helpful in elucidating the role of serotoninergic mechanisms in aging,15 and neurologic and psychiatric pathologies such as Alzheimer's disease,16 depression17, 18 obsessive-compulsive disorder,19 bipolar disorder,20 and schizophrenia.21 Nevertheless, the role of serotoninergic neurotransmission in stroke evolution using PET imaging has scarcely been explored to date.
The purpose of the present study was to investigate the late postischemic SERT and 5-HT2A receptor changes in the rat brain after transient cerebral ischemia using PET with both [11C]DASB and [18F]altanserin. In particular, we were interested in clarifying the relationship of the serotoninergic system with the recovery of long-term brain function underlying experimental stroke. The results reported here might have a significant practical importance as they could provide novel information about the role of the serotoninergic system in the evolution of cerebral ischemia and may ultimately contribute to a better design of new therapeutic strategies for the improvement of the functional recovery after stroke.
Materials and methods
Animals and Surgery
Adult male Sprague-Dawley rats (300 g body weight; Janvier, France) (n=8) were used. Animal studies were approved by the animal ethics committee of CIC biomaGUNE and local authorities and were conducted in accordance with the Directives of the European Union on animal ethics and welfare. Transient focal ischemia was produced by a 2-hour intraluminal occlusion of the middle cerebral artery (MCA) followed by reperfusion as described elsewhere.22 Briefly, rats were anesthetized with 4% isoflurane in 100% O2, and a 2.6-cm length of 4-0 monofilament nylon suture was introduced into the right external carotid artery up to the level where the MCA branches out and animals were sutured and placed in their cages with free access to water and food. After 2 hours, the animals were reanesthetized and the filament was removed to allow reperfusion. Rats were repeatedly examined before (day 0) and at 1, 3, 7, 14, 21, and 28 days after ischemia. The animals studied at day 0 have been considered as the baseline control group.
Magnetic Resonance Imaging
T2-weighting (T2W) magnetic resonance imaging (MRI) scans were performed in ischemic animals at 24 hours after reperfusion to select the rats (n=8) presenting cortico-striatal lesions to be included in the PET studies. Before the scans, anesthesia was induced with 4% isoflurane and maintained by 2% to 2.5% of isoflurane in 100% O2 during the scan. Animals were placed into a rat holder compatible with MRI acquisition systems and maintained normothermia using a water-based heating blanket at 37°C. Measurements were performed by using an 11.7T Bruker Biospec system (Bruker, Ettlingen, Germany) with a 72-mm volumetric quadrature coil for excitation and a 20-mm surface coil for reception. Acquisition parameters for the T2-weighted spin-echo images were repetition time/echo time=3,300/30 ms, field of view=1.8 × 1.8 cm2, matrix=200 × 200, number of excitations=3, and slice thickness=0.8 mm. Contiguous slices covering all the infarcted volume were acquired and fat suppression was used.
Magnetic Resonance Imaging Image Analysis
The MRI (T2W) images were used to calculate the lesion volume. Regions of interest were manually defined using the Open Source software 3D Slicer image analysis software (Version 3.6.3; www.slicer.org) for each rat on the region of increased signal in the ipsilateral hemisphere. The total lesion volume was calculated by summing the area of the infarcted region of all slices affected by the lesion.
Radiochemistry
For the production of [11C]DASB, [11C]CH4 was directly generated in an IBA Cyclone 18/9 cyclotron and transferred to a TRACERlab FXC Pro synthesis module (GE Healthcare, Waukesha, WI, USA) where [11C]methyl iodide was generated. At the end of the process, [11C]CH3I was distilled under continuous helium flow (20 mL/min) and introduced in a 2-mL stainless steel reaction loop, precharged with a solution of N-Methyl-2-(2-amino-4-cyanophenylthio)-benzylamine (MASB, 1 mg; ABX, Radeberg, Germany) in dimethylsulfoxide (80 μL). The reaction mixture was purified by means of high performance liquid chromatography. The collected fraction was reformulated by dilution with water (20 mL), retention on a C-18 cartridge (Sep-Pak Light, Waters, Milford, MA, USA) and elution with ethanol (1 mL) and saline (9 mL). Filtration through a 0.22-μm sterile filter yielded the final [11C]DASB solution. Typical radiochemical yields and specific activities were 33±5% (end of bombarment) and 135±18 GBq/μmol (end of synthesis), respectively. Radiochemical purity was >98% in all cases.
[18F]altanserin was produced using the TRACERlab FXFN synthesis module (GE Healthcare). The [18F]F− was trapped in a preconditioned QMA cartridge and transferred to the reactor by sequential elution with a solution of K2CO3 (3.5 mg) in water (0.5 mL) and a solution of Kryptofix K2.2.2 (15 mg) in acetonitrile (1 mL). After evaporation to dryness, a solution containing 5 mg of nitro-altanserine (ABX) in 0.5 mL of dimethylsulfoxide was added. The reaction was performed at 150°C for 10 minutes. The reactor was then cooled at room temperature, 3 mL of mobile phase consisting of 0.08 M sodium acetate solution adjusted to pH 5 (70%, v/v), ethanol (10%), and tetrahydrofuran (20%) were added and the mixture was purified by high performance liquid chromatography. The collected fraction was formulated by dilution with water (20 mL), retention on a C-18 cartridge (Sep-Pak Light, Waters) and elution with ethanol (1 mL) and physiologic saline solution (5 mL) containing ascorbic acid (10 mg). Filtration through 0.22 μm sterile filters yielded the final [18F]altanserin solution. Average radiochemical yield was 10.8±3.2% (end of synthesis). Radiochemical purity was higher than 97% in all cases.
Positron Emission Tomography Scans and Data Acquisition
The PET scans were repeatedly performed before (day 0) and at 1, 3, 7, 14, 21, and 28 days after reperfusion using a General Electric eXplore Vista CT camera (GE Healthcare). Scans were performed in rats anesthetized with 4% isoflurane and maintained by 2% to 2.5% of isoflurane in 100% O2. The tail vein was catheterized with a 24-gauge catheter for intravenous administration of the radiotracer. Animals were placed into a rat holder compatible with PET acquisition system and maintained normothermic using a water-based heating blanket. Animals were subjected to two PET scans at all seven time points to assess SERT binding ([11C]DASB) and 5-HT2A receptor binding ([18F]altanserin) at every time point before and after ischemia onset. First, around 20 MBq of [11C]DASB was injected concomitantly with the start of the PET acquisition. Brain dynamic images were acquired (34 frames: 6 × 5, 6 × 15, 6 × 60, 8 × 120, 8 × 300 seconds) in the 400 to 700 keV energetic window, with a total acquisition time of 64 minutes, providing a 175 × 175 matrix with a pixel size of 0.887 mm and 61 slices. Second, after at least 180 minutes (∼9 half-lives of 11C), animals were reanesthetized and placed on the PET camera and around 20 MBq of [18F]altanserin was injected concomitantly with the start of the PET acquisition. The acquisition protocol was the same as for [11C]DASB. After each PET scan, computed tomography acquisitions were also performed (140 μA intensity, 40 kV voltage), providing anatomic information of each animal as well as the attenuation map for the later image reconstruction. Dynamic acquisitions were reconstructed (decay and computed tomography-based attenuation corrected) with filtered back projection using a Ramp filter with a cutoff frequency of 0.5/mm.
Positron Emission Tomography Image Analysis
The PET images were analyzed using the PMOD image analysis software (PMOD Technologies Ltd, Zürich, Switzerland). To verify the anatomic location of the signal, PET images were coregistered to the anatomic data of an MRI rat brain template. Two type of volumes of interest (VOIs) were established as follows: (1) A first set of VOIs were defined to study the whole-brain [11C]DASB and [18F]altanserin nondisplaceable binding potential (BPND) evolution. Whole-brain VOIs were manually drawn in both the entire ipsilateral and contralateral hemispheres containing the territory irrigated by the MCA on slices of a template MRI (T2W) rat brain template from the PMOD software. (2) A second set of VOIs were automatically generated in midbrain, cortex, striatum, and cerebellum by using the regions proposed by the PMOD rat brain template to study the evolution of BPND of the tracers to the specific regions in both ipsilateral and contralateral cerebral hemispheres. The simplified reference-tissue model23 from the PMOD software was used to assess BPND. This model relies on a two-tissue reversible compartment for a target region (ipsilateral or contralateral VOI) and a single-tissue compartment for a reference region (cerebellum).
Neurologic and Behavioral Testing
Two main neurologic tests were used to assess neurologic and behavioral deficits after cerebral ischemia in rats. The assessment of neurologic outcome induced by cerebral ischemia was based on the previously reported 9-neuroscore test.24 Four consecutive tests were performed on every ischemic animal before (day 0) and at 1, 3, 7, 14, 21, and 28 days after MCA occlusion (MCAO) as follows: (1) spontaneous activity (moving and exploring=0, moving without exploring=1, no moving=2); (2) left drifting during displacement (none=0, drifting only when elevated by the tail and pushed or pulled=1, spontaneous drifting=2, circling without displacement, or spinning=3), (3) parachute reflex (symmetric=0, asymmetric=1, contralateral forelimb retracted=2), and (4) resistance to left forepaw stretching (stretching not allowed=0, stretching allowed after some attempts=1, no resistance=2). Total score could range from 0- (normal) to a 9-(highest handicap) point scale.
The adhesion/removal tape test was performed as described elsewhere25 to evaluate the deficits and behavioral recovery on every ischemic animal before (day 0) and at 1, 3, 7, 14, 21, and 28 days after MCAO. In brief, it consists of applying adhesive tape on each forepaw of the animal and measuring the time-to-contact (the duration time till contact with the mouth) and the time-to-remove them, until a maximum of 2 minutes. Animals were trained in this task 2 days before ischemia. This behavior implies correct paw and mouth sensitivity (time-to-contact) and correct dexterity (time-to-remove).
Statistical Analyses
For BPND values, the statistical analysis was performed as follows: BPND values for each animal, brain region (whole brain, striatum, midbrain, and cortex), and brain hemisphere (ipsilateral and contralateral) were calculated at each time point. Values of BPND within each region, time point, and hemisphere were averaged and compared with the averaged baseline control values (before MCAO) using one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison tests for post hoc analysis. Neurologic outcome comparisons were performed as follows: animals were subjected to the 9-neuroscore test before MCAO and at 1, 3, 7, 14, 21, and 28 days after cerebral ischemia. The results within each time point were averaged and compared with baseline average values using Mann–Whitney U-tests. Behavioral outcome comparisons by forepaw (ipsilateral and contralateral) were performed as follows: animals were subjected to the adhesion/removal tape test before MCAO and at different time points after cerebral ischemia. The results within each time point and forepaw (ipsilateral and contralateral) were averaged and compared with baseline values using Mann–Whitney U-tests. To assess the differences between forepaws (ipsilateral versus contralateral), behavioral outcome averaged values at each time point and forepaw were compared using two-way ANOVA. Statistical analyses were performed with the GraphPad Prism software (La Jolla, CA, USA).
Results
Serotonin transporter and the postsynaptic receptors 5-HT2A binding were explored by PET imaging after a 2-hour MCAO in rats. All the images were quantified in standard units, i.e., BPND for both [11C]DASB and [18F]altanserin PET. The images with normalized color scales illustrate the evolution of the PET signals at days 0 (control), 1, and 28 after cerebral ischemia (Figures 1 and 2).
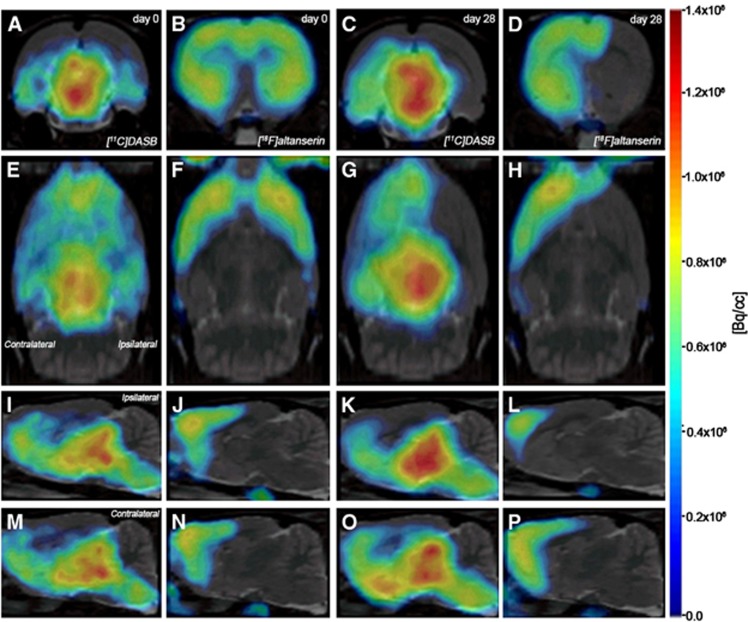
Figure 1.
Normalized positron emission tomography (PET) images of [11C]DASB and [18F]altanserin PET at day 0 and day 28 after middle cerebral artery occlusion (MCAO). PET images of axial (A to D), coronal (E to H), sagittal at the level of the ipsilateral (I to L) and contralateral (M to P) hemispheres are coregistered with a magnetic resonance imaging (MRI) (T2-weighting (T2W)) rat template to localize anatomically the PET signal. Images correspond to the same representative animal for each time condition and radiotracer.
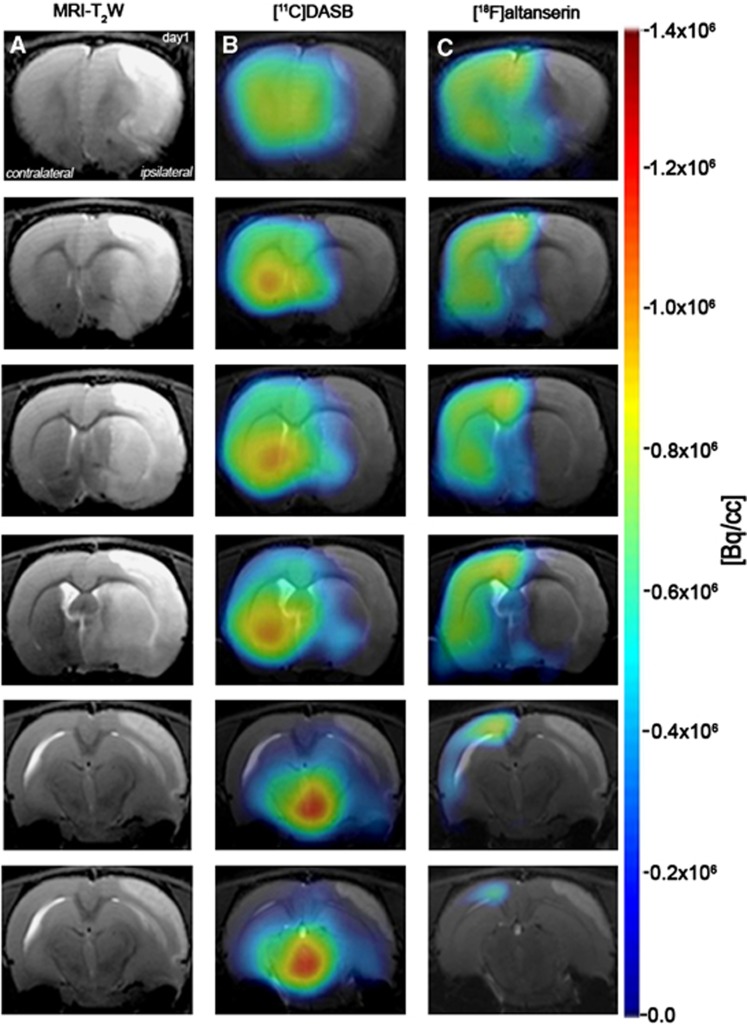
Figure 2.
Magnetic resonance imaging (MRI) (T2-weighting (T2W)) and positron emission tomography (PET) images of [11C]DASB and [18F]altanserin at day 1 after cerebral ischemia. Serial MRI (T2W) (A), serotonin transporter (SERT) (B) and 5-HT2A PET binding (C) images of axial planes at the level of the lesion. PET images are coregistered with the MRI (T2W) of the same animal to localize the PET signal. Images correspond to the anterior–posterior representative brain slices covering the extension of the lesion.
Brain Damage Assessment after Cerebral Ischemia
The extent of brain damage after cerebral ischemia was assessed using T2W MRI at 1 day after ischemia onset. Hyperintensities of T2W images showed similar infarct extents as well as locations affected. All ischemic rats subjected to nuclear studies showed cortical and striatal MRI alterations (mean±s.d.=277.54±83.89 mm3). Coregistration of PET to the MRI images of the same animal showed the decline in both [11C]DASB and [18F]altanserin binding in relation to the injured area at 24 hours after MCAO. Despite this decline, [11C]DASB images showed a minor binding decrease in the striatum than those showed by the [18F]altanserin (Figure 2). These findings were also observed throughout the entire study (Figures 3 and 4).
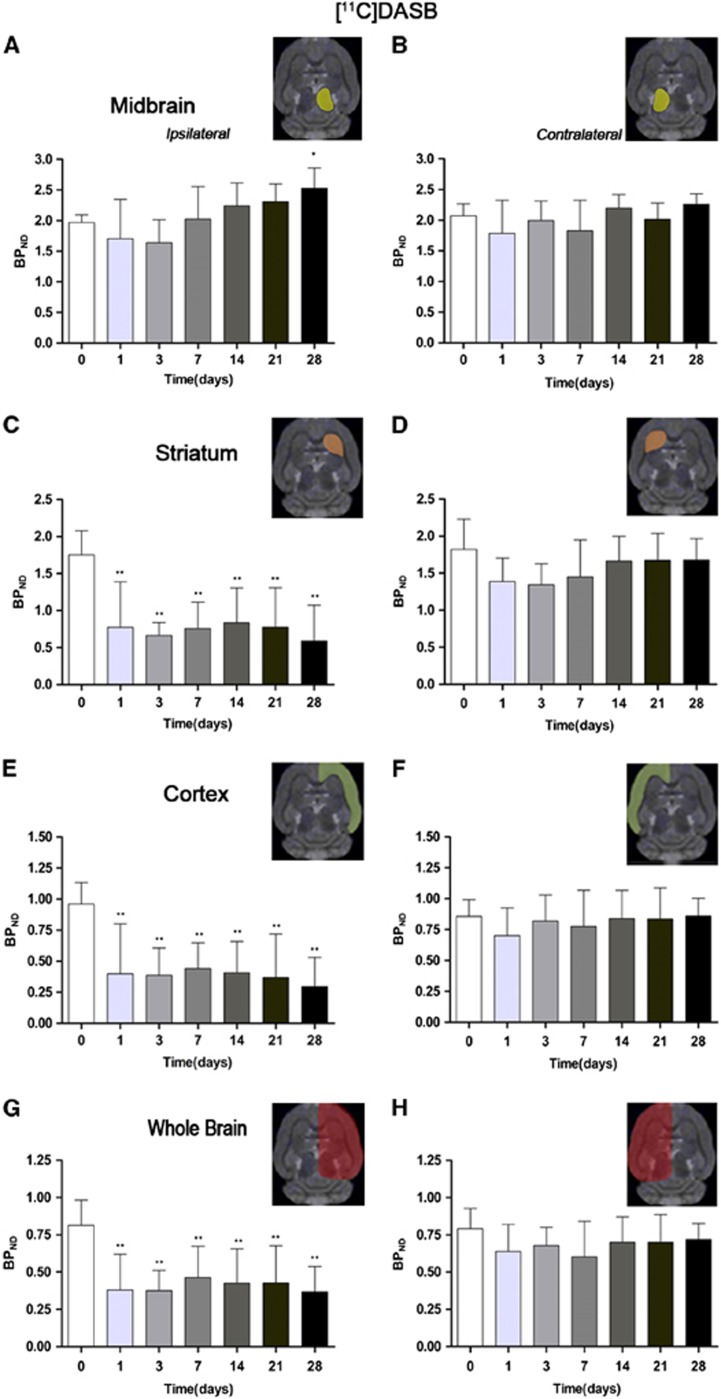
Figure 3.
Time course of the progression of the [11C]DASB positron emission tomography (PET) signal before and after cerebral ischemia. The binding potential nondisplaceable (BPND, mean±s.d.) of [11C]DASB (BP, mean±s.d.) was quantified in four volumes of interest (VOIs) over the ipsilateral (A) and the contralateral (B) midbrain, the ipsilateral (C) and the contralateral (D) striatums, the ipsilateral (E) and the contralateral (F) cortex, and the entire ipsilateral cerebral hemisphere (G) and the contralateral cerebral hemisphere (H). The upper right panels of each figure show the selected brain regions of interest (ROIs) for the quantification defined on a slice of a magnetic resonance imaging (MRI) (T2-weighting (T2W)) template. Rats (n=8) were repeatedly examined by positron emission tomography (PET) before and at 1, 3, 7, 14, 21, and 28 days after ischemia. Statistical analyses were performed by using one-way analysis of variance (ANOVA) followed by a post hoc Dunnett comparison test. Statistically different from control: *P<0.05 and **P<0.01.
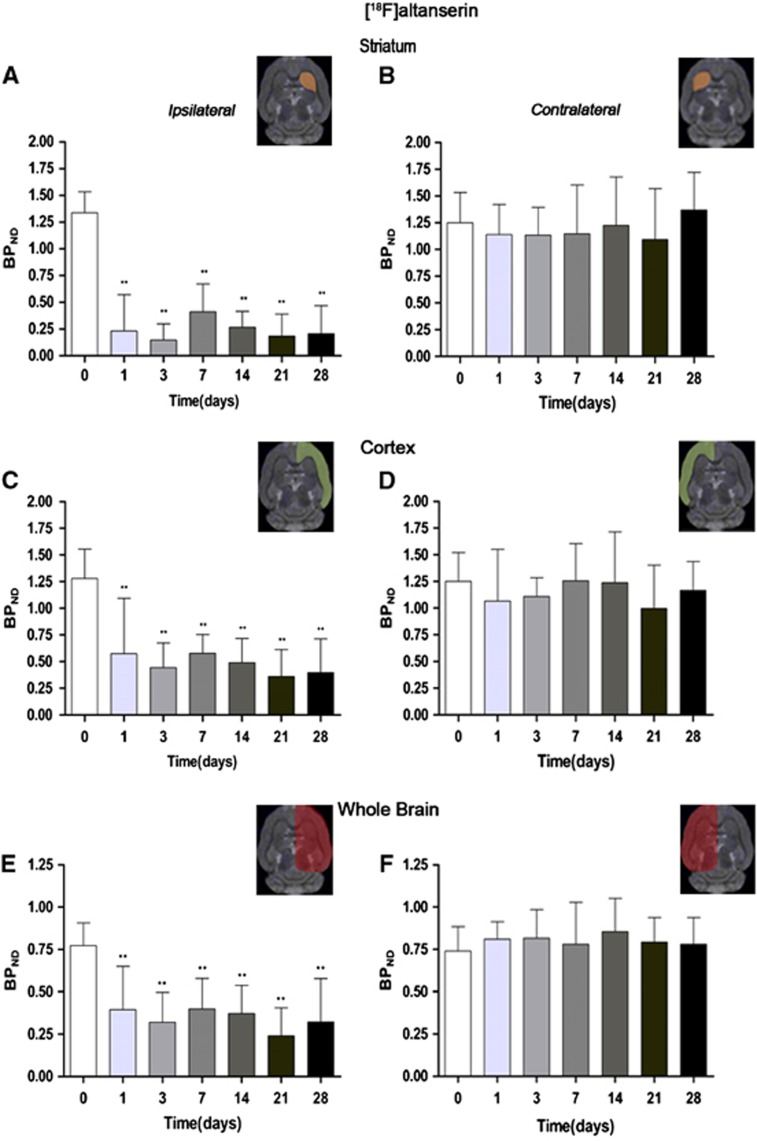
Figure 4.
Time course of the progression of the [18F]altanserin positron emission tomography (PET) signal after cerebral ischemia. The binding potential nondisplaceable (BPND, mean±s.d.) of [18F]altanserin was quantified in three volumes of interest (VOIs) over the entire ipsilateral (A) and the contralateral (B) striatums, the ipsilateral (C) and the contralateral (D) cortex, and the entire ipsilateral cerebral hemisphere (E) and the contralateral cerebral hemisphere (F). The upper right panels of each figure show the selected brain regions of interest (ROIs) for the quantification defined on a slice of a magnetic resonance imaging (MRI) (T2-weighting (T2W)) template. Rats (n=8) were repeatedly examined by PET before and at 1, 3, 7, 14, 21, and 28 days after ischemia. Statistical analyses were performed by using one-way analysis of variance (ANOVA) followed by a post hoc Dunnett comparison test. Statistically different from control: **P<0.01.
[11C]DASB Positron Emission Tomography after Middle Cerebral Artery Occlusion
The time course of SERT was evaluated using [11C]DASB in both the ipsilateral and contralateral midbrain, striatum, cortex, and whole brain at 0 (control), 1, 3, 7, 14, 21, and 28 days after MCAO (Figure 3). Different brain regions showed a different [11C]DASB binding level after long-term focal cerebral ischemia. In the ipsilateral midbrain, the BPND values for [11C]DASB showed a downward trend from day 1 to day 3 in relation to control followed by a slight recovery thereafter at day 7 after reperfusion. Subsequently, [11C]DASB PET signals showed a progressive increase from day 14 to day 21 followed by a statistically significant increase at day 28 after ischemia (P<0.05, Figure 3A). In the contralateral midbrain, the [11C]DASB PET signal showed quasi-normal values after cerebral ischemia (Figure 3B). In the ipsilateral striatum, [11C]DASB PET signal decreased sharply from 65% at day 1 to ca. 75% at day 28 of control values (P<0.01, Figure 3C), whereas the contralateral striatum did not show any significant change. Ipsilateral (occluded MCA) cortex and whole brain showed an abrupt decline in the [11C]DASB PET signal from day 1 to day 28 after cerebral ischemia, similarly to that presented by the striatum (P<0.01, Figures 3E and 3G). Contralateral (nonoccluded MCA) BPND of [11C]DASB also showed pseudo-control values after long-term reperfusion in both cortex and the entire whole region (Figures 3F and 3H). The major contribution of cortex and striatum to the binding potential of [11C]DASB in the entire ipsilateral brain hemisphere is due to the large volume of these specific brain regions in relation to the total brain volume, despite the higher density of 5-HT cell bodies expressing SERT are located in the midbrain raphe nuclei. Interestingly, BPND values evidence an effect of cerebral ischemia over SERT not only on the impaired tissue as cerebral cortex and striatum, but also on remote regions to the lesion as observed in the midbrain.
Time Course of [18F]altanserin Positron Emission Tomography after Middle Cerebral Artery Occlusion
The time course of serotonin receptor 5-HT2A was evaluated using [18F]altanserin in both the ischemic and the contralateral cortex, striatum, and the whole brain at 0 (control), 1, 3, 7, 14, 21, and 28 days after MCAO (Figure 4). Binding potential (BPND) of [18F]altanserin decreased sharply at day 1 after reperfusion in the ipsilateral striatum from day 1 to day 28 after reperfusion (P<0.01, Figure 4C). In contrast, the PET signal showed similar results over time in the contralateral striatum (Figure 4B). In the ipsilateral cortex, [18F]altanserin binding potential decreased from ca. 1.25 to 0.50 from day 0 to day 28 after ischemia (P<0.01, Figure 4C). In contrast, the contralateral cortex did not show any significant postischemic change over time (Figure 4D). Accordingly, ipsilateral and contralateral whole-brain hemisphere evidenced similar BPND profiles to that presented in the ischemic (Figure 4E) and contralateral to the lesion cortex and striatum (Figure 4F).
Time Course of Neurologic Score after Cerebral Ischemia
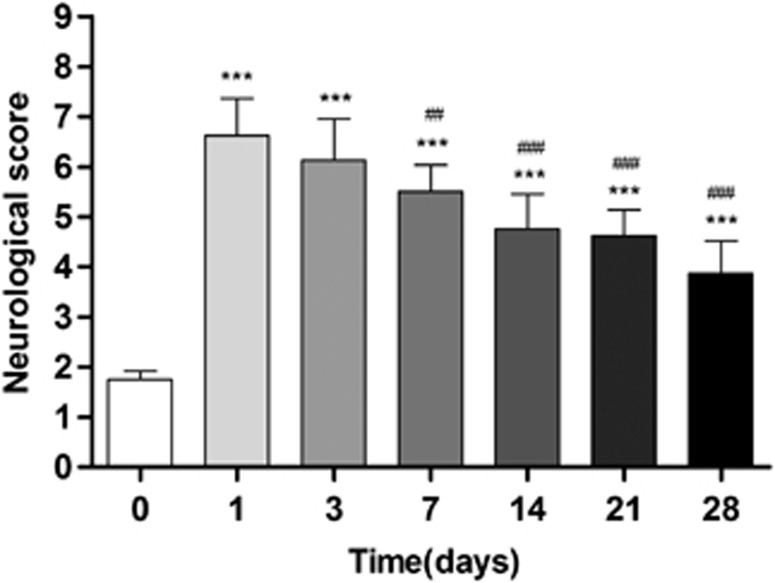
Ischemic animals showed the major neurologic impairment at 1 day after MCAO in relation to control animals (day 0). After day 3, rats showed a trend to a progressive functional recovery over time. The neurologic impairment showed a significant increase versus that in the controls at days 1, 3, 7, 14, 21, and 28 (P<0.001). After day 3, the score decreased at days 7 (P<0.01), 14, 21, and 28 (P<0.001) in relation to day 1 after ischemia (Figure 5).
Figure 5.
Neurologic outcome before and after long-term cerebral ischemia. The neurologic score shows an improvement over time. Statistical analyses were performed by using Mann–Whitney U-tests. Statistically different from control: ***P<0.001. Statistically different from day 1: ##P<0.01 and ###P<0.001.
Time Course of Behavioral Test after Middle Cerebral Artery Occlusion
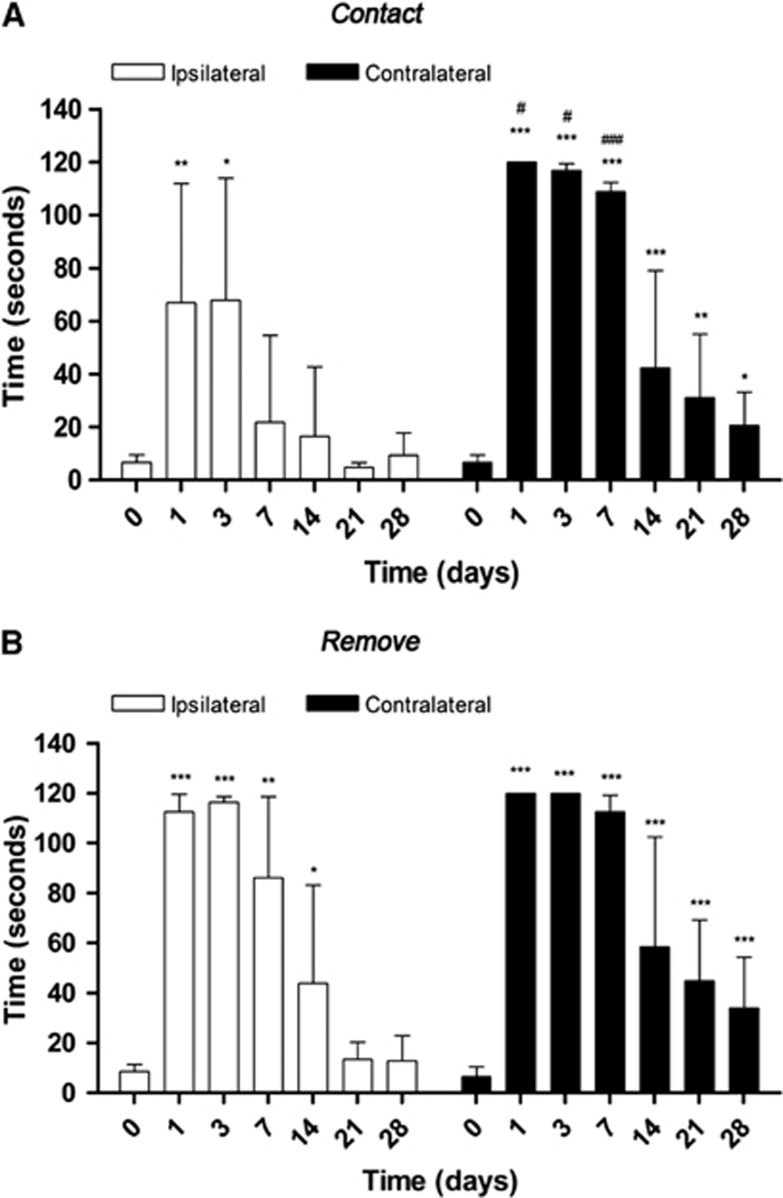
Rats (n=8) were repeatedly subjected to the adhesion/removal tape test before and at 1, 3, 7, 14, 21, and 28 days after cerebral ischemia. Animals showed a worst taping contact (Figure 6A) and remove (Figure 6B) performances of the contralateral in relation to the ipsilateral forepaw. Statistical analyses by using two-way ANOVA by forepaw (ipsilateral and contralateral) and time followed by the Bonferroni test show a significant increase in contact time in the contralateral forepaw at 1, 3, and 7 days with respect to corresponding times in the ipsilateral forepaw (Figure 6A). Statistical analyses by using Mann–Whitney U-tests within the ipsilateral forelimb showed a worst contact performance at 1, 3, and 7 days (P<0.001) followed by an improvement of paw and mouth sensitivity at 14 (P<0.001), 21 (P<0.01), and 28 days (P<0.05) with respect to day 0. In the ipsilateral forepaw, ischemic animals showed an increase in the time-to-contact at 1 (P<0.01) and 3 days (P<0.05) followed by a behavioral recovery from day 7 and onwards. Statistical analyses by using two-way ANOVA did not show statistical differences in the ipsilateral with respect to the contralateral forelimb (Figure 6B). Ischemic animals showed the worst performance of the taping remove test from day 1 to day 3 (P<0.001) followed by a dexterity recovery at 14, 21, and 28 days after ischemia (P<0.001) in the contralateral paw. Subsequently, the ipsilateral forepaw showed the uppermost time-to-remove at 1 and 3 days (P<0.001) followed by a progressive improvement from day 7 (P<0.01) and day 14 (P<0.05) to a pseudo control (day 0) values at days 21 and 28.
Figure 6.
Behavioral outcome after cerebral ischemia. The adhesion (A) removal (B) tape test was performed before and at 1, 3, 7, 14, 21, and 28 days after cerebral ischemia. Comparisons by forepaw and time were performed by two-way analyses of variance (ANOVAs). Statistically different from corresponding time to ipsilateral: #P<0.05 and ###P<0.001. Within each forepaw group, comparisons were performed by using Mann–Whitney U-tests. Statistically different from corresponding time to control (day 0): *P<0.05, **P<0.01, and ***P<0.001.
Discussion
This is the first study to evaluate the long-term evolution of both SERT and 5-HT2A receptor by using [11C]DASB and [18F]altanserin after focal cerebral ischemia in rats. The present study showed [11C]DASB binding along the midline of the rat brainstem that projected to virtually the whole of the forebrain (Figure 1). On the contrary, [18F]altanserin binding was observed in the cerebral cortex and striatum (Figure 2). The BPND values for [11C]DASB varied from 2 in control midbrain to ∼1.75 in striatum and to 1 in cerebral cortex (Figure 3). These results are in accordance with the distribution of SERT, which is highly expressed in cell bodies of the dorsal and median raphe nuclei and in relatively low densities in axons and nerve terminals in thalamus, striatum, and cortex.26, 27 Serotonin transporter transports serotonin (5-HT) from synaptic spaces into presynaptic neurons. Therefore, SERT is crucial for the regulation of 5-HT transmission because it controls the 5-HT availability at the site of the postsynaptic receptors.28
Likewise, the serotoninergic neurotransmission is mediated through different postsynaptic receptors such as the 5-HT2A. [18F]altanserin PET imaging studies showed similar BDND of 5-HT2A receptor in both cerebral cortex and striatum. These results are in agreement with the distribution of 5-HT2A reported previously26 (Figure 4).
Serotoninergic Changes after Cerebral Ischemia
Serotoninergic neurotransmission has shown to be affected by cerebral ischemia in both rats29 and humans30 and the modulation of this neurotransmission system has enabled a recovery of movement after stroke.8, 9 Thus, as these findings suggest a key role of serotoninergic neurotransmission in functional recovery after stroke, we sought to evaluate the serotoninergic neurotransmission evolution by using PET after focal cerebral ischemia in rats.
In the present study, T2-weighted MRI images showed hyperintense lesions in both the cortical and striatum during the initial 24 hours of reperfusion after cerebral ischemia (Figure 2). These signal changes have been associated with the histopathologic damage and neuronal loss being considered as a reliable surrogate of tissue infarction.31, 32 Likewise, PET imaging showed a dramatic decrease in SERT and 5-HT2A in the region affected by the ischemic insult that was in agreement with the tissue outcome at 24 hours after reperfusion. Both [11C]DASB and [18F]altanserin showed similar binding reduction in the ipsilateral cortex after cerebral ischemia. In the ipsilateral striatum, PET data showed a major decline in the 5-HT2A availability compared with those of SERT (Figures 3 and 4). These findings could be argued by the heterogeneous binding showed by [11C]DASB over the rat brain in comparison with the well-defined binding of [18F]altanserin in the cortico-striatal area that might promote a major spillover effect of the [11C]DASB PET signal to the striatum from contiguous regions. Interestingly, besides the serotoninergic changes in the brain regions affected by the lesion, increased SERT availability has been observed in remote regions to the injury as the ipsilateral midbrain area (Figure 3). Therefore, a decrease in serotonin levels is expected in the lesioned regions as 5-HT2A receptor binding is considered as a surrogate marker of the levels of 5-HT in the brain.33 Different regulation systems have been proposed to compensate such decrease in 5-HT levels as feedback mechanisms involving both presynaptic and postsynaptic 5-HT1A/5-HT2A autoreceptors located in cell bodies and nerve terminal. Such autoreceptor might be involved in the regulation of the firing of raphe nuclei 5-HT cell neurons.34 As a consequence of this regulatory system, an increase in 5-HT levels may be expected in the ipsilateral serotoninergic pathway promoting a compensatory overexpression of the SERT availability as a key regulator of the synaptic levels of this transmitter.33 Hence, the progressive increase in SERT availability in the ipsilateral raphe nuclei from day 14 to day 21 followed by the uppermost binding at day 28 after cerebral ischemia could be interpreted as a regulatory strategy to compensate the previous decrease in the serotonin levels after the ischemic insult. Likewise, these findings are in agreement with the increase in the serotonin availability observed in the brainstem of migraineurs as a response to altered endogenous 5-HT levels.35
Despite the increase in [11C]DASB binding in the ipsilateral midbrain, a lack of changes in both SERT and 5-HT2A availability has been observed in the contralateral hemisphere after ischemia. These results stand in contrast to the increased D2 dopaminergic receptor availability observed in the contralateral hemisphere after 2-hour MCAO, which could have a key role in the functional recovery after cerebral ischemia.6
Functional Recovery after Stroke
Adult brain after stroke has shown a real capacity to undergo both physiologic and anatomic modifications, which lead to motor and cognitive recovery.1 Recovery processes have been related to proliferation of neural and glial precursors, increased axonal sprouting, and branching of dendrites, and development of new synapses.36 Recent studies have also hypothesized that dopamine D2 receptor might contribute to the recovery of brain function after cerebral ischemia.6 Likewise, the evaluation of long-term functional recovery after cerebral ischemia is a key component in improving the clinical relevance of experimental stroke studies. In the present study, two neurologic tests were performed to assess long-term motor and behavior outcome after cerebral ischemia in rats. The ischemic rats showed the highest neurologic impairment at 24 hours after cerebral ischemia onset. Such sensorimotor outcome was followed by a gradual recovery over time that reached the major recovery 4 weeks later. Subsequently, ischemic animals showed similar skills to those presented by healthy animals (Figure 5). Mouth sensitivity (time-to-contact) and correct dexterity (time-to-remove) were performed by using the adhesion/removal tape test (Figure 6). Ischemic rats showed a trend to worst performance of the left forepaw (contralateral) to that presented by the right (ipsilateral) forepaw due to the ischemic lesion affected part of the right cerebral hemisphere. The time-to-contact the patch on the right forepaw was impaired from day 1 to day 3 followed by a recovery to control values. The left forepaw controlled by the ischemic brain hemisphere showed the worst impairment of both time-to-contact and time-to-remove skills from day 1 to day 3 followed by a sharp decrease from day 14 to day 28 after ischemia onset. Surprisingly, ischemic animals also showed dexterity impairment in the ipsilateral forelimb from day 1 to day 3 followed by a better behavioral recovery to that presented by the contralateral forepaw. These results stand in contrast with studies that did not observe impairment in the ipsilateral forepaw after mild cerebral ischemia in mice.37 Therefore, the degree of impairment experienced by the left forepaw seems to be related to the magnitude of the ischemic damage. Both functional tests performed in the present study showed similar results during the first week after ischemia followed by a gradual recovery of neurologic handicap and a faster improvement of contact/removal patch test from second to third week after reperfusion. Thus, animals experienced a quasi-recovery of functional outcome 1 month after stroke. At this time point, PET showed a significant increase in SERT binding in the ipsilateral rat midbrain, which was preceded by a gradual increase from day 7 after ischemia. Therefore, the present findings may evidence the key role of serotoninergic neurotransmission in the recovery process after cerebral ischemia. Likewise, these results are in agreement with the efficacy of selective serotonin reuptake inhibitors in neurofunctional recovery of nondepressed patients with recent stroke.8, 9, 38, 39
Summary and Conclusions
In summary, we report here for the first time the PET imaging of serotoninergic system using both [11C]DASB and [18F]altanserin and its relationship with the functional recovery after transient focal cerebral ischemia in rats. These results showed a long-term increase in ipsilateral [11C]DASB uptake in the ipsilateral midbrain raphe nuclei and a dramatic decrease in both [11C]DASB and [18F]altanserin in ischemic cortex and striatum. These changes are in accordance with neurologic and behavioral recovery over time. Therefore, these findings might contribute to better understand the role of serotoninergic neurotransmission in stroke evolution, which could foster the development of alternative treatments for the enhancement of the recovery of impaired functions and provide the life quality of persons surviving stroke.
Acknowledgments
The authors would like to thank M González, A Leukona, and M Errasti for technical support in the radiosynthesis; and A Arrieta and T Calvo-Fernández for technical assistance in the PET studies.
The authors declare no conflict of interest.
Footnotes
This study was supported by Departamento de Educación, Universidades e Investigación del Gobierno Vasco (PI2011-3) and the Departamento de Industria, Comercio y Turismo Vasco.
References
- Rossini PM, Calautti C, Pauri F, Baron JC. Post-stroke plastic reorganisation in the adult brain. Lancet Neurol. 2003;2:493–502. doi: 10.1016/s1474-4422(03)00485-x. [DOI] [PubMed] [Google Scholar]
- Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke. 2003;34:1553–1566. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- Cramer SC. Stratifying patients with stroke in trials that target brain repair. Stroke. 2010;41:S114–S116. doi: 10.1161/STROKEAHA.110.595165. [DOI] [PubMed] [Google Scholar]
- Cramer SC. Repairing the human brain after stroke. II. Restorative therapies. Ann Neurol. 2008;63:549–560. doi: 10.1002/ana.21412. [DOI] [PubMed] [Google Scholar]
- Abo M, Chen Z, Lai LJ, Reese T, Bjelke B. Functional recovery after brain lesion—contralateral neuromodulation: an fMRI study. Neuroreport. 2001;12:1543–1547. doi: 10.1097/00001756-200105250-00048. [DOI] [PubMed] [Google Scholar]
- Martín A, Gomez-Vallejo V, San Sebastian E, Padró D, Makuerkiaga I, Llarena I, et al. In vivo imaging of dopaminergic neurotransmission after transient focal ischemia in rats. J Cereb Blood Flow Metab. 2013;33:244–252. doi: 10.1038/jcbfm.2012.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokk J, Salman Roghani R, Delbari A. Effect of methylphenidate and/or levodopa coupled with physiotherapy on functional and motor recovery after stroke—a randomized, double-blind, placebo-controlled trial. Acta Neurol Scand. 2011;123:266–273. doi: 10.1111/j.1600-0404.2010.01395.x. [DOI] [PubMed] [Google Scholar]
- Zittel S, Weiller C, Liepert J. Citalopram improves dexterity in chronic stroke patients. Neurorehabil Neural Repair. 2008;22:311–314. doi: 10.1177/1545968307312173. [DOI] [PubMed] [Google Scholar]
- Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, Lamy C, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 2011;10:123–130. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- Lim CM, Kim SW, Park JY, Kim C, Yoon SH, Lee JK. Fluoxetine affords robust neuroprotection in the postischemic brain via its anti-inflammatory effect. J Neurosci Res. 2009;87:1037–1045. doi: 10.1002/jnr.21899. [DOI] [PubMed] [Google Scholar]
- Houle S, Ginovart N, Hussey D, Meyer JH, Wilson AA. Imaging the serotonin transporter with positron emission tomography: initial human studies with [11C]DAPP and [11C]DASB. Eur J Nucl Med. 2000;27:1719–1722. doi: 10.1007/s002590000365. [DOI] [PubMed] [Google Scholar]
- Huang Y, Hwang DR, Narendran R, Sudo Y, Chatterjee R, Bae SA, et al. Comparative evaluation in nonhuman primates of five PET radiotracers for imaging the serotonin transporters: [11C]McN 5652, [11C]ADAM, [11C]DASB, [11C]DAPA, and [11C]AFM. J Cereb Blood Flow Metab. 2002;22:1377–1398. doi: 10.1097/01.WCB.0000040948.67415.05. [DOI] [PubMed] [Google Scholar]
- Lemaire C, Cantineau R, Guillaume M, Plenevaux A, Christiaens L. Fluorine-18-altanserin: a radioligand for the study of serotonin receptors with PET: radiolabeling and in vivo biologic behavior in rats. J Nucl Med. 1991;32:2266–2272. [PubMed] [Google Scholar]
- Biver F, Goldman S, Luxen A, Monclus M, Forestini M, Mendlewicz J. Multicompartmental study of fluorine-18 altanserin binding to brain 5HT2 receptors in humans using positron emission tomography. Eur J Nucl Med. 1994;21:937–946. doi: 10.1007/BF00238117. [DOI] [PubMed] [Google Scholar]
- Marner L, Knudsen GM, Haugbol S, Holm S, Baare W, Hasselbach SG. Longitudinal assessment of cerebral 5-HT2A receptors in healthy elderly volunteers: an [18F]-altanserin PET study. Eur J Nucl Med Mol Imaging. 2009;36:287–293. doi: 10.1007/s00259-008-0945-4. [DOI] [PubMed] [Google Scholar]
- Marner L, Frokjaer VG, Kalbitzer J, Lehel S, Madsen K, Baare WF, et al. Loss of serotonin 2A receptors exceeds loss of serotonergic projections in early Alzheimer's disease: a combined [11C]DASB and [18F]altanserin-PET study. Neurobiol Aging. 2012;33:479–487. doi: 10.1016/j.neurobiolaging.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Mintun MA, Barch DM, Wilkins C, Snyder AZ, Moerlein SM. Decreased hippocampal 5-HT(2A) receptor binding in older depressed patients using [18F]altanserin positron emission tomography. Neuropsychopharmacology. 2004;29:2235–2241. doi: 10.1038/sj.npp.1300555. [DOI] [PubMed] [Google Scholar]
- Hammoud DA, Endres CJ, Hammond E, Uzuner O, Brown A, Nath A, et al. Imaging serotonergic transmission with [11C]DASB-PET in depressed and non-depressed patients infected with HIV. Neuroimage. 2010;49:2588–2595. doi: 10.1016/j.neuroimage.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto R, Ichise M, Ito H, Ando T, Takahashi H, Ikoma Y, et al. Reduced serotonin transporter binding in the insular cortex in patients with obsessive-compulsive disorder: a [11C]DASB PET study. Neuroimage. 2010;49:121–126. doi: 10.1016/j.neuroimage.2009.07.069. [DOI] [PubMed] [Google Scholar]
- Cannon DM, Ichise M, Fromm SJ, Nugent AC, Rollis D, Gandhi SK, et al. Serotonin transporter binding in bipolar disorder assessed using [11C]DASB and positron emission tomography. Biol Psychiatry. 2006;60:207–217. doi: 10.1016/j.biopsych.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Narendran R, Huang Y, Hwang DR, Lombardo I, Cangiano C, et al. Serotonin transporter availability in patients with schizophrenia: a positron emission tomography imaging study with [11C]DASB. Biol Psychiatry. 2005;57:1510–1516. doi: 10.1016/j.biopsych.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Justicia C, Perez-Asensio FJ, Burguete MC, Salom JB, Planas AM. Administration of transforming growth factor-alpha reduces infarct volume after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 2001;21:1097–1104. doi: 10.1097/00004647-200109000-00007. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Menzies SA, Hoff JT, Betz AL. Middle cerebral artery occlusion in rats: a neurological and pathological evaluation of a reproducible model. Neurosurgery. 1992;31:100–106. doi: 10.1227/00006123-199207000-00014. [DOI] [PubMed] [Google Scholar]
- Bouet V, Boulouard M, Toutain J, Divoux D, Bernaudin M, Schumann-Bard P, et al. The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice. Nat Protoc. 2009;4:1560–1564. doi: 10.1038/nprot.2009.125. [DOI] [PubMed] [Google Scholar]
- Varnas K, Halldin C, Hall H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp. 2004;22:246–260. doi: 10.1002/hbm.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Tao-Cheng JH, Segu L, Patel T, Wang Y. Serotonin transporters are located on the axons beyond the synaptic junctions: anatomical and functional evidence. Brain Res. 1998;805:241–254. doi: 10.1016/s0006-8993(98)00691-x. [DOI] [PubMed] [Google Scholar]
- Blakely RD, De Felice LJ, Hartzell HC. Molecular physiology of norepinephrine and serotonin transporters. J Exp Biol. 1994;196:263–281. doi: 10.1242/jeb.196.1.263. [DOI] [PubMed] [Google Scholar]
- Rogozinska K, Skangiel-Kramska J. Effect of focal cerebral ischaemia on modulatory neurotransmitter receptors in the rat brain: an autoradiographic study. J Chem Neuroanat. 2010;40:232–238. doi: 10.1016/j.jchemneu.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Vera P, Zilbovicius M, Chabriat H, Amarenco P, Kerdraon J, Menard JF, et al. Post-stroke changes in cortical 5-HT2 serotonergic receptors. J Nucl Med. 1996;37:1976–1981. [PubMed] [Google Scholar]
- Ejaz S, Williamson DJ, Ahmed T, Sitnikov S, Hong YT, Sawiak SJ, et al. Characterizing infarction and selective neuronal loss following temporary focal cerebral ischemia in the rat: a multi-modality imaging study. Neurobiol Dis. 2013;51:120–132. doi: 10.1016/j.nbd.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Rojas S, Martin A, Justicia C, Falcón C, Bargalló N, Chamorro A, et al. Modest MRI signal intensity changes precede delayed cortical necrosis after transient focal ischemia in the rat. Stroke. 2006;37:1525–1532. doi: 10.1161/01.STR.0000221713.06148.16. [DOI] [PubMed] [Google Scholar]
- Erritzoe D, Holst K, Frokjaer VG, Licht CL, Kalbitzer J, Nielsen FA, et al. A nonlinear relationship between cerebral serotonin transporter and 5-HT(2A) receptor binding: an in vivo molecular imaging study in humans. J Neurosci. 2010;30:3391–3397. doi: 10.1523/JNEUROSCI.2852-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp T, Boothman L, Raley J, Queree P. Important messages in the ‘post': recent discoveries in 5-HT neurone feedback control. Trends Pharmacol Sci. 2007;28:629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Schuh-Hofer S, Richter M, Geworski L, Villringer A, Israel H, Wenzel R, et al. Increased serotonin transporter availability in the brainstem of migraineurs. J Neurol. 2007;254:789–796. doi: 10.1007/s00415-006-0444-0. [DOI] [PubMed] [Google Scholar]
- Jones TA, Allred RP, Adkins DL, Hsu JE, O'Bryant A, Maldonado MA. Remodeling the brain with behavioral experience after stroke. Stroke. 2009;40:S136–S138. doi: 10.1161/STROKEAHA.108.533653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkaya M, Krober J, Gertz K, Peruzzaro S, Endres M. Characterization of long-term functional outcome in a murine model of mild brain ischemia. J Neurosci Methods. 2013;213:179–187. doi: 10.1016/j.jneumeth.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Mikami K, Jorge RE, Moser DJ, Arndt S, Jang M, Solodkin A, et al. Increased frequency of first-episode poststroke depression after discontinuation of escitalopram. Stroke. 2011;42:3281–3283. doi: 10.1161/STROKEAHA.111.626507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead GE, Hsieh CF, Lee R, Kutlubaev M, Claxton A, Hankey GJ. Selective serotonin reuptake inhibitors for stroke recovery: a systematic review and meta-analysis. Stroke. 2013;44:844–850. doi: 10.1161/STROKEAHA.112.673947. [DOI] [PubMed] [Google Scholar]