Abstract
The rates of glucose oxidized at glycolysis and pentose–phosphate pathway (PPP) in neurons are controversial. Using [3-3H]-, [1-14C]-, and [6-14C]glucose to estimate fluxes through these pathways in resting, intact rat cortical primary neurons, we found that the rate of glucose oxidized through PPP was, apparently, ∼14% of total glucose metabolized. However, inhibition of PPP rate-limiting step, glucose-6-phosphate (G6P) dehydrogenase, increased approximately twofold the glycolytic rate; and, knockdown of phosphoglucose isomerase increased ∼1.8-fold the PPP rate. Thus, in neurons, a considerable fraction of fructose-6-phosphate returning from the PPP contributes to the G6P pool that re-enters PPP, largely underestimating its flux.
Keywords: astrocytes, energy metabolism, glucose, neurochemistry, neuronal–glial interaction
Introduction
In neurons, pentose–phosphate pathway (PPP) is very active and contributes to the regeneration of glutathione redox status.1 However, the actual value of the rate of glucose-6-phosphate (G6P) entering the PPP in neurons is yet elusive; moreover, it has recently been reported, on the basis of 13C isotopomer enrichments,2 levels of PPP activity considerably lower than those previously estimated.1, 3, 4, 5 In view of this apparent controversy and the critical role of PPP in neuronal protection against oxidative stress, here we aimed to accurately determine the fluxes of glucose oxidation through PPP and glycolysis, both under normal and acutely inhibited key regulatory enzymes. We conclude that the estimated values for PPP activity in neurons are largely underestimated because of the fast equilibrium between PPP-derived fructose-6-phosphate (F6P) and G6P.
Materials and Methods
Neurons and Astrocytes in Primary Culture
All protocols with animals were approved by the Bioethics Committee of the University of Salamanca following the Spanish (RD1201/2005) and European (609/CEE) directives on research animal protection and experimentation. Cortical neurons in primary culture were prepared from fetal Wistar rats (E16), seeded at 2.5 × 105 cells per cm2 in the bottom surface of 25-ml flasks, previously coated with poly-D-lysine (15 μg/mL) in DMEM (Sigma, Madrid, Spain) supplemented with 10% (v/v) fetal calf serum (Roche Diagnostics, Heidelberg, Germany). Cells were incubated at 37°C in a humidified 5% CO2-containing atmosphere. At 48 hours after plating, the medium was replaced with DMEM supplemented with 5% horse serum (Sigma), 20 mM D-glucose and, on day 4, cytosine arabinoside (10 μM) to prevent non-neuronal proliferation.1 Under these conditions, ∼85% to 95% of the cells were microtubule-associated protein-2-positive (neurons); an ∼3% of the cells were glial fibrillary acidic protein-positive (astrocytes) (not shown). Astrocytes in the primary culture were obtained from rat pups from 0 to 24 hours of age after the same procedure as for neurons. Cell suspension was seeded at 2.5 × 105 cells/cm2 in DMEM supplemented with 10%, v/v, FCS. Culture medium was renewed twice per week and astrocytes were used after 2 weeks.1
Glycolysis and Pentose–Phosphate Pathway Determinations
To determine the glycolytic and the pentose–phosphate pathway fluxes, attached intact cells were washed with PBS and incubated in the presence of either 5 μCi/ml of D-[3-3H]glucose, 0.5 μCi/mL of D-[1-14C]glucose or 1 μCi/mL of [6-14C]glucose in 1.5 mL of a Krebs–Henseleit buffer (11 mmol/L Na2HPO4, 122 mmol/L NaCl, 3.1 mmol/L KCl, 0.4 mmol/L KH2PO4, 1.2 mmol/L MgSO4, 1.3 mmol/L CaCl2; pH 7.4) supplemented with 5 mmol/L D-glucose at 37°C with shaking in a sealed flask. Before sealing the flask with a rubber cap, a 1.5-mL Eppendorf tube containing 1 mL of water (for 3H2O trapping) or 0.8 mL of benzethonium hydroxide (for 14CO2 trapping) was fixed inside the flask by holding it from the flask tab using a rib, and the flask atmosphere gassed with a O2:CO2 (95:5) mixture for 20 seconds. Flasks were incubated in the air-thermostatized chamber of an orbital shaker (Forma Benchtop Orbital Shaker, Model 420, Thermo Fischer, Marietta, OH, USA). In preliminary experiments (not shown), we observed that 3H2O and 14CO2 collection in the holding tube was linear with time for up to 90 minutes; therefore, all remaining incubations were performed for this period. The reactions were stopped by adding 0.2 mL of 20%, v/v, HClO4 through the rubber cap, and flasks were further incubated for 96 hours to allow equilibration of 3H2O between water and the incubation medium, or for 1.5 hours to trap 14CO2 into the benzethonium hydroxide. In the preliminary experiments using 3H2O or NaH14CO3, we observed that the 3H2O equilibrated between both compartments was 28% (hence reflecting an actual 70% of recovery efficiency); the efficiency of 14CO2 trapped was 75%. Both efficiency values (28% for 3H2O and 75% for 14CO2), were taken into account for the calculations. Results were expressed as nmol/L of D-[3-3H]glucose incorporated into 3H2O per minute and per milligram protein (rate of glycolysis), or difference between [1-14C]glucose and [6-14C]glucose incorporated into 14CO2 per minute and per milligram protein (rate of PPP).
Cell Treatments
Transfections of primary neurons with small interfering RNA (100 nmol/L) against PGI (siPGI; 5′-CCTTACCAGACGTAGTGTT-3′ Dharmacon Research, Lafayette, Colorado, USA) or against luciferase (siControl; 5′- CTGACGCGGAATACTTCGA-3′ Dharmacon) were performed at day 3 in vitro using the Lipofectamine LTX with Plus Reagent (Invitrogen, Madrid, Spain), following manufacturer's instructions. Experiments and sample collection were done at day 6 in vitro, when we previously confirmed efficient ∼70% knockdown of PGI protein.1 To inhibit PPP activity, we used dehydroepiandrosterone (DHEA; Sigma), a noncompetitive inhibitor of G6PD that, at the concentration used (1 μmol/L), selectively inhibits the PPP without affecting glucose uptake.6, 7
Determination of Glucose-6-Phosphate Concentration
Neurons were lysed in 0.06 mol/L NaOH and the resulting extract was deproteinized and neutralized with the same volume of 1% w/v ZnSO4. After centrifugation of precipitated proteins (12,000 g/10 min), glucose-6-phosphate concentration in the supernatant was determined spectrophotometrically by monitoring the increase in NADPH(H+) absorbance at 340 nm produced by G6PD (0.17 U/mL) and PGI (0.7 U/mL) in 0.2 mol/L trietanolamine, 5 mmol/L MgCl2, 0.2 mM NADP+, pH 7.6, according to Michal.8
Statistical Analysis
Results are expressed as the mean±s.e.m. (standard error of the mean) values for three different culture preparations. Statistical analysis of the results was performed by the Student's t-test. In all cases, P<0.05 was considered significant.
Results and Discussion
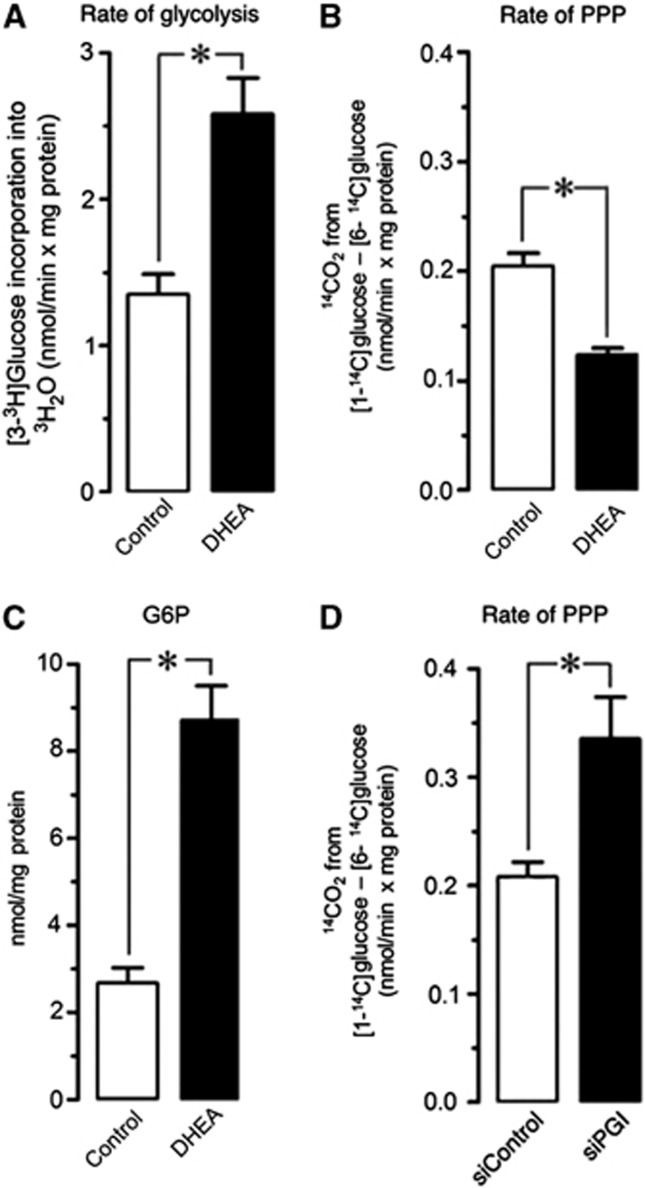
Here, we determined the rate of glucose metabolized through glycolysis as a function of the rate of 3H2O production from [3-3H]glucose, a process that primarily takes place at aldolase and triose-phosphate isomerase, i.e. the glycolytic steps that immediately follow the rate-limiting, 6-phosphofructo-1-kinase-catalyzed reaction.9 We found that, under resting conditions, the rate of glycolysis in neurons was ∼1.2 nmoL/minute × mg protein (Figure 1A), which is considerably lower than that found in attached astrocytes using identical protocol (3.14±0.27 nmoL/min × mg protein; n=3 independent culture preparations, each determined in triplicate), in agreement with previous reports.1 This glycolytic rate value is, however, slightly higher than that found in a previous study,1 a fact that can be ascribed to the fact that those previous determinations were performed in detached, suspended neurons that lost their axon and dendritic processes, which were recently reported to actively perform glycolysis.10 To elucidate if glucose metabolism is dynamic in neurons, we next incubated neurons with DHEA, a well-known inhibitor of G6PD6, 7 –the rate-limiting step of PPP. As shown in Figure 1A, DHEA acutely increased by ∼twofold the rate of glycolysis, thus suggesting that, in principle, ∼50% of glucose fraction oxidized in neurons would take place through the PPP.
Figure 1.
Glucose fluxes through glycolysis and pentose–phosphate pathway (PPP) in neurons. Incubation of rat cortical intact neurons in primary culture with dehydroepiandrosterone (DHEA) (1 μmol/L) increased by twofold the rate of glycolysis (A), the rate of PPP (B), and the concentrations of glucose-6-phosphate (G6P) (C); knockdown on phosphoglucose isomerase (PGI) increased by 1.8-fold the rate of PPP (D). The rates of glycolysis and PPP were determined as described in Methods. Data represent mean±s.e.m. values for three independent culture preparations. *P<0.05 (Student's t-test).
Next, we aimed to determine the actual fraction of glucose oxidized through the PPP in intact primary neurons. To do so, cells were incubated in the presence of [1-14C]glucose or [6-14C]glucose, and the initial rates of 14CO2 released were quantified. 14CO2 is released from [1-14C]glucose at 6-phosphogluconate decarboxylation by 6-phosphogluconate dehydrogenase, plus at isocitrate and α-ketoglutarate decarboxylations by isocitrate dehydrogenase and α-ketoglutarate dehydrogenase in the tricarboxylic acid cycle; in contrast, 14CO2 is released from [6-14C]glucose exclusively in the tricarboxylic acid cycle. Thus, the difference between the rates of 14CO2 released from [1-14C]glucose and that from [6-14C]glucose is considered acceptable to estimate glucose oxidized through the PPP.11 As shown in Figure 1B, we found that the rate of glucose oxidized through the PPP in neurons was ∼0.2 nmoL/minute × mg protein. This PPP value is, indeed, lower than the one reported in a previous work,1 possibly reflecting the drawbacks of using suspended neurons in that study. However, it does not explain the twofold increase in glycolysis rate observed by DHEA (Figure 1A); thus, according to a PPP value of ∼0.2 nmoL/min × mg protein, glycolysis should only have reached a value of ∼1.4 nmoL/min × mg protein in the presence of DHEA, assuming that this compound blocked PPP. However, we found that DHEA, which efficiently inhibited G6PD activity as judged by the ∼3.5-fold increase in G6P (Figure 1C), triggered a ∼50% inhibition of the rate of PPP (Figure 1B); this could only have accounted for an increase in glycolysis to up to ∼1.3 nmoL/min × mg protein, instead of the observed 2.4 nmoL/min × mg protein. Together, these data indicate that the rate of PPP in neurons herein determined is largely underestimated.
6-Phosphofructo-1-kinase catalyzes a rate-limiting reaction–F6P to fructose-1,6-bisphosphate–that represents a glycolysis bottleneck in neurons (Figure 2).1, 12 In contrast, PGI is a near-equilibrium enzyme, very active at converting F6P into G6P in neurons.13 As [1-14C]glucose yields unlabeled F6P, once converted into G6P, it will no longer contribute to 14CO2 detection. To test this possibility, we inhibited PGI activity using a previously validated RNA interference strategy.1 The rate of PPP in PGI-knockdown neurons increased by 1.8-fold (Figure 1D), indicating enrichment of [1-14C]G6P-specific radioactivity likely responsible for the enhancement in apparent PPP activity. Given the high PGI activity in neurons,13 the expected theoretical increase of 1.2 nmoL/min × mg protein in PPP activity by siPGI was not reached (Figure 1D), a fact that is likely due to the still high residual PGI activity after incomplete 70% PGI knockdown. Therefore, the estimated rates of PPP activity reported here may be largely underestimated because of the important contribution of PGI at equilibrating F6P and G6P. Furthermore, the underestimation is independent of the method used to estimate PPP. Thus, it has been reported, by measuring 13C-acetyl-CoA-derived 13C-glutamate and 13C-γ-aminobutyric acid isotopomer abundances from [2-13C]glucose in neurons, that PPP contributes only for a ∼6% of total glucose metabolism (i.e. glycolysis plus PPP).2 Such a low PPP value seems underestimated in view that the contribution of PGI in PPP13 was apparently overlooked. Thus, Brekke et al2 considered [2-13C]glucose to exclusively yield [1-13C]acetyl-CoA through glycolysis and [2-13C]acetyl-CoA through PPP. Unfortunately, it was not taken into account that a fraction of [1,3-13C]F6P formed from PPP, by conversion into [1,3-13C]G6P through PGI, returns [2,3-13C]F6P in the second PPP round. Thus, as from the second PPP round and thereafter –up to the 4 hours of incubation– such [2,3-13C]F6P will label [1-13C]acetyl-CoA hence largely over-estimating glycolysis.2
Figure 2.
Pentose–phosphate pathway (PPP) is an important contributor to glucose consumption in neurons. Schematic representation of the main conclusion of this work, highlighting the critical contribution of phosphoglucose isomerase (PGI)–which converts PPP-derived F6P into glucose-6-phosphate (G6P)–to fueling PPP at the expense of G6P consumption through glycolysis. 6-Phosphofructo-1-kinase (PFK1), which converts fructose-6-phosphate (F6P) into fructose-1,6-bisphosphate (F16BP), is a ‘bottleneck' in neurons due to the low levels of fructose-2,6-bisphosphate (a positive effector of PFK1); this facilitates F6P conversion into G6P. Stoichiometry has been omitted for clarity, and dotted lines represent multiple reactions. GAP, glyceraldehyde-3-phosphate; PYR, pyruvate; R5P, ribulose-5-phosphate.
We therefore conclude that an important proportion of glucose entering neurons is oxidized through the PPP (Figure 2). However, the extent of this metabolic route is yet unrecognized because of misinterpretation in isotopic labeling of metabolites, mostly related to the high activity of PGI in neurons. In contrast to astrocytes, neurons are deficient in de novo glutathione biosynthesis,14 thus by regenerating reduction equivalents as NADPH(H+), the PPP becomes an essential metabolic pathway for the maintenance of the redox status of glutathione and neuronal (and cancer) cell survival.7 This occurs at the expense of a prominent glycolytic rate, a situation that is compatible with the high dependence of neurons on neighboring astrocytes for the supply of oxidative energy substrates, such as lactate,15 to satisfy the high energy demands of neurons. It appears necessary that further work would be required to accurately determine the precise fraction of glucose that neurons oxidize through the PPP and, hence, use for the maintenance of their redox status. Its elucidation will surely allow a better understanding of the mechanisms that coordinate energy substrate disposal with neurotransmission.
The authors declare no conflict of interest.
Footnotes
JPB is funded by Spanish Ministerio de Economia y Competitividad (grant numbers SAF2010-20008 and RETICEF-RD12/0043/0021), European Regional Development Fund, and Junta de Castilla y Leon (SA003U13).
References
- Herrero-Mendez A, Almeida A, Fernandez E, Maestre C, Moncada S, Bolaños JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- Brekke EM, Walls AB, Schousboe A, Waagepetersen HS, Sonnewald U. Quantitative importance of the pentose phosphate pathway determined by incorporation of 13C from [2-13C]- and [3-13C]glucose into TCA cycle intermediates and neurotransmitter amino acids in functionally intact neurons. J Cereb Blood Flow Metab. 2012;32:1788–1799. doi: 10.1038/jcbfm.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yoseph O, Boxer PA, Ross BD. Assessment of the role of the glutathione and pentose phosphate pathways in the protection of primary cerebrocortical cultures from oxidative stress. J Neurochem. 1996;66:2329–2337. doi: 10.1046/j.1471-4159.1996.66062329.x. [DOI] [PubMed] [Google Scholar]
- Ben-Yoseph O, Camp DM, Robinson TE, Ross BD. Dynamic measurements of cerebral pentose phosphate pathway activity in vivo using [1,6-13C2,6,6-2H2]glucose and microdialysis. J Neurochem. 1995;64:1336–1342. doi: 10.1046/j.1471-4159.1995.64031336.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Rodriguez P, Fernandez E, Almeida A, Bolaños JP. Excitotoxic stimulus stabilizes PFKFB3 causing pentose-phosphate pathway to glycolysis switch and neurodegeneration. Cell Death Differ. 2012;19:1582–1589. doi: 10.1038/cdd.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrini S, Natalicchio A, Laviola L, Belsanti G, Montrone C, Cignarelli A, et al. Dehydroepiandrosterone stimulates glucose uptake in human and murine adipocytes by inducing GLUT1 and GLUT4 translocation to the plasma membrane. Diabetes. 2004;53:41–52. doi: 10.2337/diabetes.53.1.41. [DOI] [PubMed] [Google Scholar]
- Vaughn AE, Deshmukh M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat Cell Biol. 2008;10:1477–1483. doi: 10.1038/ncb1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michal G.D-Glucose 6-phosphate and D-fructose 6-phosphateIn: Bergmeyer J, Graßl M (eds).. Methods of Enzymatic Analysis Verlag Chemie: Deerfield Beach, FL; 1985191–198. [Google Scholar]
- Katz J, Rognstad R, Kemp RG. Isotope Discrimination Effects in the Metabolism of Tritiated Glucose. J Biol Chem. 1965;240:PC1484–PC1486. [PubMed] [Google Scholar]
- Zala D, Hinckelmann MV, Yu H, Lyra da Cunha MM, Liot G, Cordelieres FP, et al. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 2013;152:479–491. doi: 10.1016/j.cell.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Hothersall JS, Greenbaum AL, McLean P. The functional significance of the pentose phosphate pathway in synaptosomes: protection against peroxidative damage by catecholamines and oxidants. J Neurochem. 1982;39:1325–1332. doi: 10.1111/j.1471-4159.1982.tb12574.x. [DOI] [PubMed] [Google Scholar]
- Almeida A, Moncada S, Bolaños JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol. 2004;6:45–51. doi: 10.1038/ncb1080. [DOI] [PubMed] [Google Scholar]
- Gaitonde MK, Murray E, Cunningham VJ. Effect of 6-phosphogluconate on phosphoglucose isomerase in rat brain in vitro and in vivo. J Neurochem. 1989;52:1348–1352. doi: 10.1111/j.1471-4159.1989.tb09178.x. [DOI] [PubMed] [Google Scholar]
- Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci. 1999;19:562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2012;32:1152–1166. doi: 10.1038/jcbfm.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]