Abstract
To assess cerebral energetics in transgenic mouse models of neurologic disease, a robust, efficient, and practical method for quantification of cerebral oxygen consumption is needed. 17O magnetic resonance spectroscopy (MRS) has been validated to measure cerebral metabolic rate of oxygen (CMRO2) in the rat brain; however, mice present unique challenges because of their small size. We show that CMRO2 measurements with 17O MRS in the mouse brain are highly reproducible using 16.4 Tesla and a newly designed oxygen delivery system. The method can be utilized to measure mitochondrial function in mice quickly and repeatedly, without oral intubation, and has numerous potential applications to study cerebral energetics.
Keywords: cerebral metabolic rate of oxygen, magnetic resonance spectroscopic imaging, mouse, oxygen-17
Introduction
Despite its relatively small size (∼2% of body weight), the brain consumes ∼20% of all oxygen used in the body. Impairments in the ability of mitochondria to fuel brain function are implicated in many neurodegenerative diseases.1 However, in vivo confirmation of mitochondrial dysfunction in these diseases is largely lacking.
Techniques to assess cerebral energetics in vivo include 13C magnetic resonance spectroscopy (MRS),2 31P MRS,3 and positron emission tomography.4 13C magnetic resonance spectroscopy bears substantial challenges for studies in the mouse, including the need to place arterial and venous catheters, which precludes longitudinal measurements, and the small blood volume of mice, which precludes blood draws in the scanner (needed for proper analysis of the data). 31P magnetization transfer MRS yields ATP metabolism rates, however, requires long data acquisition times in the mouse brain because of low sensitivity. 18F-fluorodeoxyglucose positron emission tomography imaging can assess hypometabolism in neurologic diseases,4 however, involves repeated radiation exposure in longitudinal studies. 15O positron emission tomography has been used to image cerebral oxygen utilization rate in the human brain;5 however, it is difficult to map cerebral metabolic rate of oxygen (CMRO2) in mouse brain by this method because of limited spatial resolution. In addition, the 15O radioactive tracer has very short half-life (∼2 minutes), thus, requires on-site tracer generation. Therefore, a robust and practical method that can directly quantify cerebral oxygen consumption and that can be applied repeatedly in transgenic mouse models of neurologic disease is highly needed to address the involvement of cerebral energetics in these diseases.
17O MRS imaging (MRSI) can be utilized to map CMRO2,6 and has been rigorously validated in rat brains7 and applied to small animal models including healthy cats8 and ischemic mice.9 17O is a stable, MR-active isotope of oxygen. Despite its low isotopic enrichment (0.037%), the natural abundance 17O-water (H217O) can be detected quickly with high sensitivity because the concentration of H217O at natural abundance is ∼20mmol/L. Cerebral metabolic rate of oxygen is measured from an increase in the H217O signal above natural abundance that results from mitochondrial metabolism of 17O2 gas inhaled over 2 to 3 minutes.6 Thereby the methodology produces three-dimensional maps of CMRO2 quickly and reliably. However, mice present unique challenges for 17O MRSI because of their small size. First, oral intubation, which has been essential for controlled 17O gas delivery in rats and cats, is substantially more challenging in mice. Mouse intubations are feasible,10 however, require extensive training and practice, making the measurements dependent on an individual skilled in this procedure and preventing practical and routine applicability of the method. Second, minimization of the dead space between the 17O reservoir and the mouse is required for rapid gas switching and to limit the use of the expensive 17O2 gas (currently ∼$3,000 for 1 L of 70% enriched gas). One study demonstrated the feasibility of 17O MRSI at 11.7T in stroke mice,9 where each mouse serves as their own control and CMRO2 can be compared bilaterally. Critically, the detection sensitivity for the H217O signal increases in a supra-linear fashion with increasing magnetic field.6, 11 Here, our goal was to develop robust, efficient, and practical methodology to measure regional CMRO2 in the mouse brain at 16.4T and to assess the reproducibility of the measurements.
Materials and Methods
Subjects
All animal experiments were approved by the University of Minnesota Institutional Animal Care and Use Committee and performed according to the National Research Council's Guide for the Care and Use of Laboratory Animals. Five healthy male mice on FVB (N=2, age 20 to 25 weeks; weight 35 to 40 g) and C57B6/CBA (N=3, 7 to 10 weeks; 27 to 30 g) backgrounds were used.
Animal Preparation
Animals were induced with 3% to 4% isoflurane and a 1:1 mixture of O2:N2O. Spontaneously breathing mice were fixed in a custom built mouse holder/nose cone system and maintained anesthetized with 1.2% to 2% isoflurane while monitoring body temperature and respiration rate (SA Instruments, Stony Brook, NY, USA) during MR scanning. Body temperature was maintained at ∼37°C with a circulating warm water system and a heating fan system.
In addition, a MouseOx Plus (Starr Life Sciences, Oakmont, PA, USA) system was used to monitor heart rate, oxygen saturation, breathing rate, and pulse distension when testing the 17O gas delivery system outside the scanner.
Gas Delivery System
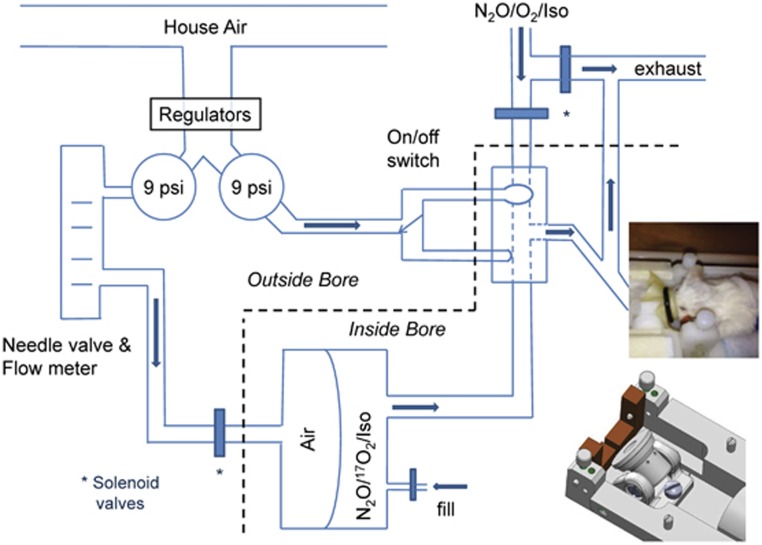
A custom, switchable, magnet-compatible gas delivery system was developed to provide 17O2 to the anesthetized mouse (Figure 1). The mouse's nose was held in place through a flexible membrane into a conical nose cone by an o-ring. The nose cone had an exhaust port vented to the outside and an input port connected to the common port of a manually controlled three-way, pneumatic balloon valve (Hans Rudolph, Shawnee, KS, USA). Pressure (9 psi) for the balloon valve was stepped down from house air. The normally open balloon valve port provided N2O/O2/isoflurane gas from a mixing valve and vaporizer to the nose cone. The N2O/17O2/isoflurane gas mixture was loaded into one side of a custom designed non-diffusible, flexible 250 mL double compartment gas reservoir (Hans Rudolph) and the composition of the gas mixture was ascertained with a capnometer (Capnomac Ultima, ULT-SVI, Datex, Helsinki, Finland). The output port of the reservoir was connected to the alternate input port of the balloon valve. To expel the 17O gas mixture, the back side of the gas reservoir was filled with air. Programmable solenoid valves (ValveBank8, Automate Scientific, Berkeley, CA, USA) gated the flow of low pressure air (9 psi) to fill the back reservoir and vent the normal O2 mixture to the exhaust stream. Timing of the manually switched balloon valve was also triggered by the ValveBank8. The balloon valve and the gas reservoir were positioned as close to the mouse as possible to minimize dead space and speed the delivery of the 17O2 gas mixture. The balloon valve was positioned inside the magnet bore with the pneumatic switch extending into the surrounding control room. The gas reservoir was kept inside the scanner bore or positioned just outside the bore if refilling was needed during the scanning session.
Figure 1.
Gas delivery system. A pneumatically remotely controlled balloon switch determines which gas reaches the mouse: the isoflurane and normal O2/N2O mixture or a comparable mixture with 17O2 from a gas-tight two-chamber reservoir placed in front of the mouse inside the bore. Solenoid valves control delivery of air pressure to release the 17O2 over 2 to 3 minutes. The tightly closed mouse nose cone (designed to provide flexibility in the head angle, shown in the inset) prevents leaking of 17O2.
Magnetic Resonance Protocol
All MRI studies were performed using a 16.4 T Varian/Magnex (26 cm bore) system. A home built linear surface 17O coil (14mm diameter, 94.6 MHz) combined with a quadrature 1H coil (16 mm diameter, 700 MHz) designed for minimal cross-talk was used to acquire anatomic 1H images and 17O MRSI data. Axial anatomic 1H images were acquired using a fast spin echo imaging sequence (repetition time TR=4 seconds, echo spacing=12 milliseconds, echo train length=16, echo time TE=48 milliseconds, field-of-view=20 mm × 20 mm, matrix size=256 × 256, slice thickness=2.4 mm, averages=2, total acquisition time=4 minutes). Three-dimensional 17O MRSI data were acquired using a previously described Fourier Series Window MRSI technique9 in which the k-space sampling is weighted according to the Fourier coefficients of a predetermined cylindrical voxel with voxel size of 26 μL (9.3 μL nominal). Acquisition parameters for the 17O MRSI data were: TR/TE=10 milliseconds/0.35 milliseconds, phase encodes=9 × 9 × 5, FOV=20 mm × 20 mm × 12 mm, spectral width=20 kHz, number of slices=5, slice thickness=2.4 mm, total scan number=1,542 and hence, acquisition time=15.4 seconds per 3D-MRSI volume. The free-induction-decays were zero-filled, and a line broadening of 100 Hz was applied before fast Fourier transformation.
For CMRO2 measurements, the 17O2 gas (70% enriched, Cambridge Isotope Laboratories, Tewksbury, MA, USA) was mixed with N2O gas (∼1:1) and isoflurane, and stored in the custom designed gas reservoir. After 17O MRSI data were acquired for 3 minutes during non-labeled O2 inhalation, the respiration gas was switched to the 17O2 mixture while 17O MRSI data were continuously acquired. After ∼2.5 min of 17O2 inhalation, the gas was switched back to the unlabeled O2/N2O gas mixture, and the 17O MRSI acquisition was continued for another 15 minutes. Each mouse underwent a second 17O2 inhalation period (2.5 min) 30 minutes after the completion of the first 17O2 inhalation.
Cerebral Metabolic Rate of Oxygen Calculation
The H217O resonance intensities from each voxel were converted to absolute H217O concentrations using the natural abundance signal (20.35 μmol/g) from the same voxel as an internal reference. Linear regression of the brain H217O concentration time courses during the 17O2 inhalation period was applied and the slopes were used for calculating the CMRO2 values. This simplified model has been previously validated in rat brains with short 17O2 inhalation where fast exchange of the oxygen gas is expected.7, 12 Similarly, this completely non-invasive CMRO2 quantification method was applied to mouse model in the present study.
Results
The newly designed gas delivery system (Figure 1) was tested on the bench and inside the scanner to ensure that there were no gas leaks. No changes were observed in the mouse heart rate, oxygen saturation, breathing rate, and pulse distension during the switch to the 17O gas reservoir. The system ensured efficient gas delivery, with ∼100 to 120 mL 17O2 (70% enriched) required per 2.5-minute inhalation period with enriched gas.
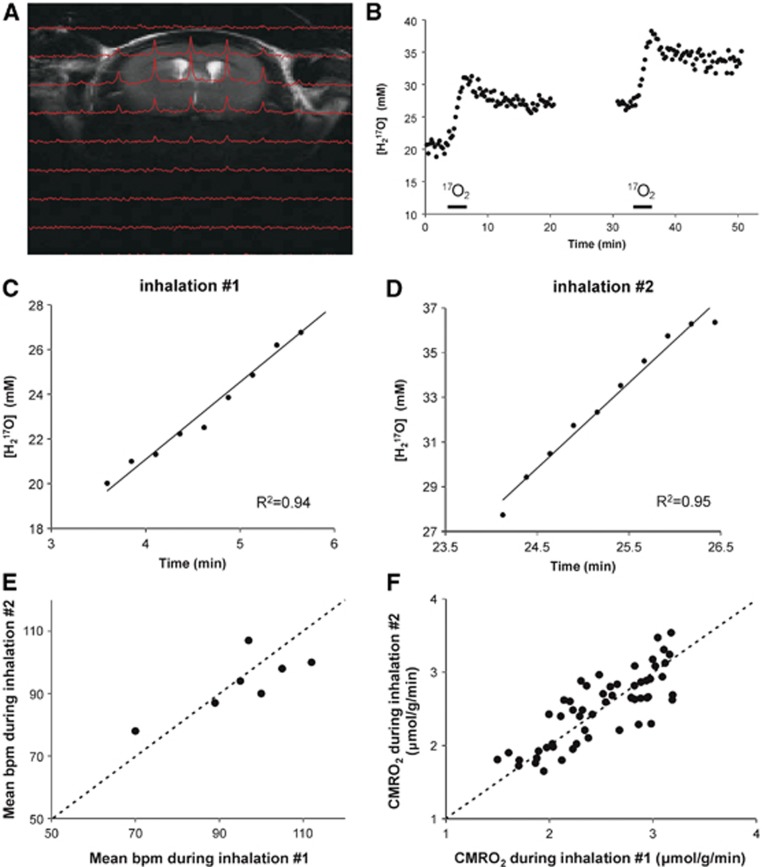
For CMRO2 measurements, the 17O/1H coil was set up such that oxygen utilization could be measured with high sensitivity in a volume encompassing the striatum, a structure of high interest for various neurodegenerative diseases. At a nominal voxel size of 9.3 mm3 (diameter of 2.2 mm and height of 2.4 mm for the cylindrical voxel) and a temporal resolution of 15.4 seconds, the signal-to-noise ratio of the 17O MRSI data were sufficient for reliable CMRO2 measurements in the sensitive area of the coil (Figure 2A).
Figure 2.
Determination of CMRO2 in mouse brain at 16.4T using 17O magnetic resonance spectroscopy imaging (MRSI). (A) 1H T2-weighted coronal anatomic image overlaid with the corresponding slice of the 17O three-dimensional (3D) MRSI image of H217O captured 1.5 minutes into an inhalation in the mouse brain at 16.4T. The signal-to-noise ratio of H217O was increased ∼40% compared with the natural abundance brain H217O signal at this point. The spectral time course has a temporal resolution of 15.4 seconds. Each 3D-MRSI 17O image has a nominal resolution of 9.3 μL. (B) The time course of the H217O signal obtained from a striatal voxel before, during, and after two brief 17O2 inhalations (bars). (C, D) Slopes of the linear fits to each of the 17O2 inhalations yields the cerebral metabolic rate of oxygen (CMRO2). (E) Physiologic conditions (b.p.m.=breaths per minute) were stable during both inhalations. (F) Within-session test–retest reproducibility of CMRO2 measurements as shown by the relationship between two 17O2 inhalations across multiple voxels (N=55). The identity line is also shown in panels E and F.
The feasibility of measuring CMRO2 in healthy mice was demonstrated with 2.5-minute inhalations of 17O2. The time course of the H217O concentration obtained from a striatal voxel demonstrates the incorporation of inhaled 17O2 into 17O-water via mitochondrial metabolism (Figure 2B). The oxygen consumption rate was calculated from linear fits to the slopes of rising H217O levels during the inhalations (Figures 2C and 2D ). An average striatal oxygen consumption rate of 2.6±0.4 (SD) μmol/g per minute (N=28) was calculated from 14 bilateral voxels of five healthy mice (two scanned twice). Mice were stable physiologically during all sessions, as illustrated by their spontaneous breathing rates (Figure 2E). Isoflurane levels (1.6±0.2%, mean±s.d.) and body temperature (37.0±0.4°C, mean±s.d.) during the 17O2 inhalations did not vary appreciably between animals. Repeat inhalations demonstrated excellent within-session reproducibility with a mean test–retest coefficient of variance (CV=s.d./mean) of 6.5% for CMRO2 from all voxels in the sensitive area of the coil (Figure 2F) and a CV of 5% from the striatal voxel.
Discussion
Here we demonstrated that efficient and practical 17O MRS measurements of CMRO2 in the mouse brain are feasible with high sensitivity and reproducibility using 16.4T, a surface 1H/17O coil and a newly designed gas delivery system. The method has numerous potential applications to mouse models of human diseases because there is substantial interest in ‘tweaking' energy metabolism to prevent and treat neurologic disorders13 and because such treatments are almost always tested in mouse models first.14, 15
The dynamic change of the H217O signals observed in the mouse brain during and after the 17O2 inhalation was similar to previous observations in the rat and cat brains7, 8 showing the accumulation of the metabolic H217O during the 17O2 inhalation and the H217O signal decay after switching back to non-labeled oxygen gas. Namely, the cerebral H217O concentration reached a new steady state level at the end of the CMRO2 measurement that was higher than the natural abundance or baseline H217O level because of the introduction of the 17O label.
Although one prior study demonstrated the feasibility of CMRO2 measurements by 17O MRSI at high field,9 the issue of efficient gas delivery was not addressed previously. The current study aimed to move the methodology to routine use by optimizing efficient delivery of the expensive 17O2 gas. Namely, normal tidal volumes are ∼0.25 mL in mice. The 200 to 250 mL of N2O/17O2/isoflurane gas mixture flowing during a 2.5-min inhalation provides sufficient airflow to sustain normal respiration without compromising breathing rates or producing rebreathing and results in a per inhalation cost of ∼$350 for 17O gas. In addition, a higher field scanner was utilized in the current study relative to the prior study,9 enabling a higher spatial resolution (9.3 versus 15 μL nominal), which was sufficient to localize, e.g. the striatum. Note, however, that this was not a strict comparison of the 17O sensitivities at the two fields, which is best accomplished by utilizing the same set-up (coil/phantom/animal) at both fields.11
In this pilot study, we utilized two different background strains and mice at different ages to make sure that the O2 delivery system worked with different sized mice and to include any biological variability due to these variables within the measurements. To tease out specific effects of age and strain, a larger and more systematic study should be performed.
The mean striatal CMRO2 obtained here was higher than previously reported CMRO2 in anesthetized rat brain,7 which is expected, as metabolic rates are higher in mice than rats. In addition, the oxygen utilization rate obtained here was consistent with previously reported CMRO2 in anesthetized mouse brain.9 Finally, the excellent reproducibility demonstrated in this study (5% to 7% within-session test–retest CV) indicates that this method can be applied in the future to test responses to physiologic challenges in individual mice.
Acknowledgments
We thank Gilbert Snedden, Todd Carpenter, and Don Forman for helpful discussions during the design of the gas delivery system.
The authors declare no conflict of interest.
Footnotes
This work was supported by the Winston and Maxine Wallin Neuroscience Discovery Fund, the Institute for Translational Neuroscience at the University of Minnesota, the National Institute of Neurological Disorders and Stroke (NINDS) grants R01 NS070815, R01 NS041262, and R01 NS070839 and the Strom Family Fund. The Center for MR Research is supported by National Center for Research Resources (NCRR) biotechnology research resource grant P41 RR008079, National Institute of Biomedical Imaging and Bioengineering (NIBIB) grant P41 EB015894, the Institutional Center Cores for Advanced Neuroimaging award P30 NS076408, and NCRR grant S10 RR025031.
References
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Adriany G, Choi IY, Henry PG, Lei H, Öz G. Localized in vivo13C NMR spectroscopy of the brain. NMR Biomed. 2003;16:313–338. doi: 10.1002/nbm.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Zhu XH, Zhang Y, Friedman M, Zhang N, Ugurbil K, et al. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci USA. 2008;105:6409–6414. doi: 10.1073/pnas.0710766105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouquet M, Desgranges B, Landeau B, Duchesnay E, Mezenge F, de la Sayette V, et al. Longitudinal brain metabolic changes from amnestic mild cognitive impairment to Alzheimer's disease. Brain. 2009;132:2058–2067. doi: 10.1093/brain/awp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, Raichle ME, Martin WR, Herscovitch P. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J Nucl Med. 1984;25:177–187. [PubMed] [Google Scholar]
- Zhu XH, Zhang N, Zhang Y, Zhang X, Ugurbil K, Chen W. In vivo17O NMR approaches for brain study at high field. NMR Biomed. 2005;18:83–103. doi: 10.1002/nbm.930. [DOI] [PubMed] [Google Scholar]
- Zhu XH, Zhang Y, Zhang N, Ugurbil K, Chen W. Noninvasive and three-dimensional imaging of CMRO2 in rats at 9.4 T: reproducibility test and normothermia/hypothermia comparison study. J Cereb Blood Flow Metab. 2007;27:1225–1234. doi: 10.1038/sj.jcbfm.9600421. [DOI] [PubMed] [Google Scholar]
- Zhu XH, Zhang N, Zhang Y, Ugurbil K, Chen W. New insights into central roles of cerebral oxygen metabolism in the resting and stimulus-evoked brain. J Cereb Blood Flow Metab. 2009;29:10–18. doi: 10.1038/jcbfm.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XH, Chen JM, Tu TW, Chen W, Song SK. Simultaneous and noninvasive imaging of cerebral oxygen metabolic rate, blood flow and oxygen extraction fraction in stroke mice. Neuroimage. 2013;64:437–447. doi: 10.1016/j.neuroimage.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera B, Miller S, Brown E, Price R. A novel method for endotracheal intubation of mice and rats used in imaging studies. Contemp Top Lab Anim Sci. 2005;44:52–55. [PubMed] [Google Scholar]
- Lu M, Zhang Y, Ugurbil K, Chen W, Zhu XH. In vitro and in vivo studies of 17O NMR sensitivity at 9.4 and 16.4 T. Magn Reson Med. 2013;69:1523–1527. doi: 10.1002/mrm.24386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Zhu XH, Lei H, Ugurbil K, Chen W. Simplified methods for calculating cerebral metabolic rate of oxygen based on 17O magnetic resonance spectroscopic imaging measurement during a short 17O2 inhalation. J Cereb Blood Flow Metab. 2004;24:840–848. doi: 10.1097/01.WCB.0000125885.54676.82. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Kapogiannis D, Greig NH. Tweaking energy metabolism to prevent and treat neurological disorders. Clin Pharmacol Ther. 2010;88:437–439. doi: 10.1038/clpt.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen OA, Dedeoglu A, Ferrante RJ, Jenkins BG, Ferrante KL, Thomas M, et al. Creatine increase survival and delays motor symptoms in a transgenic animal model of Huntington's disease. Neurobiol Dis. 2001;8:479–491. doi: 10.1006/nbdi.2001.0406. [DOI] [PubMed] [Google Scholar]
- Watase K, Gatchel JR, Sun Y, Emamian E, Atkinson R, Richman R, et al. Lithium therapy improves neurological function and hippocampal dendritic arborization in a spinocerebellar ataxia type 1 mouse model. PLoS Med. 2007;4:e182. doi: 10.1371/journal.pmed.0040182. [DOI] [PMC free article] [PubMed] [Google Scholar]