Abstract
The basic processes harvesting chemical energy for life are driven by proton (H+) movements. These are accomplished by the mitochondrial redox complex V, integral membrane supramolecular aggregates, whose structure has recently been described by advanced studies. These did not identify classical aqueous pores. It was proposed that H+ transfer for oxidative phosphorylation (OXPHOS) does not occur between aqueous sources and sinks, where an energy barrier would be insurmountable. This suggests a novel hypothesis for the proton transfer. A lipid-phase-continuity H+ transfer is proposed in which H+ are always bound to phospholipid heads and cardiolipin, according to Mitchell's hypothesis of asymmetric vectorial H+ diffusion. A phase separation is proposed among the proton flow, following an intramembrane pathway, and the ATP synthesis, occurring in the aqueous phase. This view reminiscent of Grotthus mechanism would better account for the distance among the Fo and F1 moieties of FoF1–ATP synthase, for its mechanical coupling, as well as the necessity of a lipid membrane. A unique active role for lipids in the evolution of life can be envisaged. Interestingly, this view would also be consistent with the evidence of an OXPHOS outside mitochondria also found in non-vesicular membranes, housing the redox complexes.
Keywords: ATP, cardiolipin, FoF1–ATP synthase, mitochondria, oxidative phosphorylation, proton transfer
Introduction
Chemical energy for living matter is mostly supplied by the FoF1–ATP synthases (ATP synthases), multimeric proteins, which employ a rotary mechanism driven by proton-motive force. In turn, protons (H+) are translocated by electron transport chain (ETC). The structure and organization of ATP synthases, the ‘splendid nanomolecular machine'1 that mechanically synthesize ATP from ADP and Pi are well described, but how its rotation is driven by proton flow and how this energy is converted into catalysis are less clear.2 F1 moiety is peculiar in that it is the only enzyme as yet known channeling energy to the reactant species by means of mechanical force. The topic of H+ cycling, pivotal in oxidative phosphorylation, is central in energy conversion.3, 4 Mitchell's chemiosmotic theory foresaw the existence of an H+ translocation by the ETC, coupled to the transfer of electrons to oxygen with the formation of water, being the energy involved in the process in the form of H+ flux, converted to ATP by FoF1–ATP synthase nanomotor.3 H+ was also pivotal for the origin of life. The ‘hydrogen hypothesis' for the origin of the first eukaryote posits that an anaerobic hydrogen-dependent nucleus-bearing archaebacterium host phagocytized an α-proteobacterium (that would become a mitochondrion) with an initial benefit represented by molecular hydrogen production by the endosymbiont, thus challenging the traditional view according to which ATP was the reward.5
In his last paper, Mitchell gives us a clue: H+ movements are vectorial, not scalar. ‘In chemiosmotic systems, the pathways of specific ligand conduction are spatially orientated through osmoenzymes and porters in which the actions of chemical group, electron and solute transfer occur as vectorial (or higher tensorial order) diffusion processes down gradients of total potential energy that represent real spatially directed fields of force'.6 Some uncertainty exists about the nano-local pathway of H+ in a respiring membrane, long debated.7, 8, 9 Also, it has recently become clear that in the respiring membranes, the ATP synthases form supramolecular complexes,10 with ETC proteins and lipids such as cardiolipin, central to their functioning.2
Proton Handling Inside Respiring Membranes
H+ do not exist as free species in the aqueous bulk, where they form hydronium ion, with a desolvation cost of more than 500 meV.11 On the other hand, H+ would not be able to freely reside inside the lipid bilayer, as they possess the highest charge/mass ratio than any ion. Moreover, a potential barrier represented by ordered water molecules on the surface of biological membranes would prevent H+ to diffuse.12 How can enough H+ motive force be generated across inner mitochondrial membranes to synthesize ATP? Perhaps, this is a general open question for ATP synthase, as recently pointed out,2 and the crux of the question. The current concept according to which the rotation of the rotor is fueled by H+ translocation in aqueous phase continuity may be inadequate. Starting from the newly acquired structural data on ETC I;13, 14 ETC III;15 and ATP synthase,16 some light is shed on the finely integrated processes of H+ translocation by ETC linked to oxygen reduction, and nanomechanical ATP synthesis.
A new criterion for H+ transfer inside respiring membranes can be envisaged. Evidence on the buffering capacity of the phospholipid head groups has been gathered.9, 17 In his paper presented by Lehninger, Haines said: ‘anionic lipid head groups in biological membranes share protons as acid-anion dimers and anionic lipids thus trap and conduct protons along the head group domain of bilayers that contain such anionic lipids. Protons pumped from the other side of the membrane may enter and move within the head group sheet because the protonation rate of negatively charged proton acceptors is five orders of magnitude faster than that of water'.9 A ‘lateral H+ delivery' i.e. the transfer of H+ along the surface would predominate over transfer between aqueous sources and sinks.11 H+ migrating through acid carriers is in interesting accordance to the structural diffusion mechanism proposed by Theodor von Grotthus (structural diffusion of H+) over 2 centuries ago.18 Lehninger stated that: ‘..the H+ movements occurring during electron transport… do not necessarily mean that such H+ movements also occur between the two bulk phases (the matrix and medium) during the actual normal process of oxidative phosphorylation–translocation of H+ between two bulk phases may not be a necessary event during oxidative phosphorylation; rather, it is possible that charge movements within the membrane are the fundamental processes serving as the vehicle of energy transduction….'.19
When there is the actual need for acidification of the medium, i.e. the transfer of H+ in aqueous phase continuity, proteins that belong to a different class with respect to FoF1–ATP synthase are involved. Notably, biological systems typically use the same process for the same purpose. These are P-type ATP-driven cation transporters such as H+/K+-ATPase. The kidney tubular20 and gastric H, K-ATPase21 exchange ions (H+, chloride ions, and potassium ions) across the cell membrane, extruding H+ through a channel.21
Lipid-Phase-Continuity H+ Transfer
If H+ always reside inside the proteolipid phase of the membrane, a situation already depicted in 1961,22 the process of ATP synthesis occurs with a net phase separation: H+ would flow along an intramembrane pathway, whereas ATP synthesis would be in the aqueous phase. In fact, subunit F1 of ATP synthase is about 10 nm away from the inner mitochondrial membrane (IMM) surface. Such phase separation in turn sets the need for a coupling of the two processes, which is in fact the case. A nanomechanical coupling appears a good solution. Interestingly, it was recently proposed also for ETC I.14
A H+ circuit would be established inside respiring membranes. Negative charges of phosphate groups would lie on both sides of the IMM, whereas positive ones would reside at the nonpolar center of the membrane, where the hydrocarbon tails are compact. Such possibility has been depicted before.23 The possible involvement of the supercomplexes, a supramolecular arrangement of the OXPHOS complexes in the IMM, should also be considered.10 These supercomplexes might funnel H+, also burying cytochrome c. The proteolipid phase appears ideal for hydrophobic compound chemical reactions. Mulkidjanian proposed the existence of ‘shallow ΔpH- and Δψ-sensitive proton traps, mechanistically linked to the functional groups in the membrane interior'.24 An active involvement of plasma membrane environment was recently described in bacteria, mitochondria ancestors, for allocation of diacylglycerol substrate of diacylglycerol kinase.25 This sheds light on the peculiar catalytic mechanisms of membrane enzymes. It is noteworthy that these data come from X-ray studies on lipid mesophase crystal.26
The possibility that H+ are confined to the Helmholtz layer of the IMM, where they diffuse faster was also proposed by Kell,7 and seems confirmed by the recent pivotal advances in the knowledge of the detailed structure of some ETC and ATP synthase,13, 14, 16 which did not prove the existence of a aqueous pores. Instead, the structure of ETC I revealed a coupling mechanism involving antiporter-like subunits acting at distance from the interface with the hydrophilic domain.14 The proteolipid environment was said to thermodynamically stabilize the protonation state of the Fo rotor.16 It may be hypothesized that uncouplers (such as classical 2,4 dinitrophenol) transfer H+ from the phospholipids to the ETC at the center of the membrane.
The Role of Cardiolipin
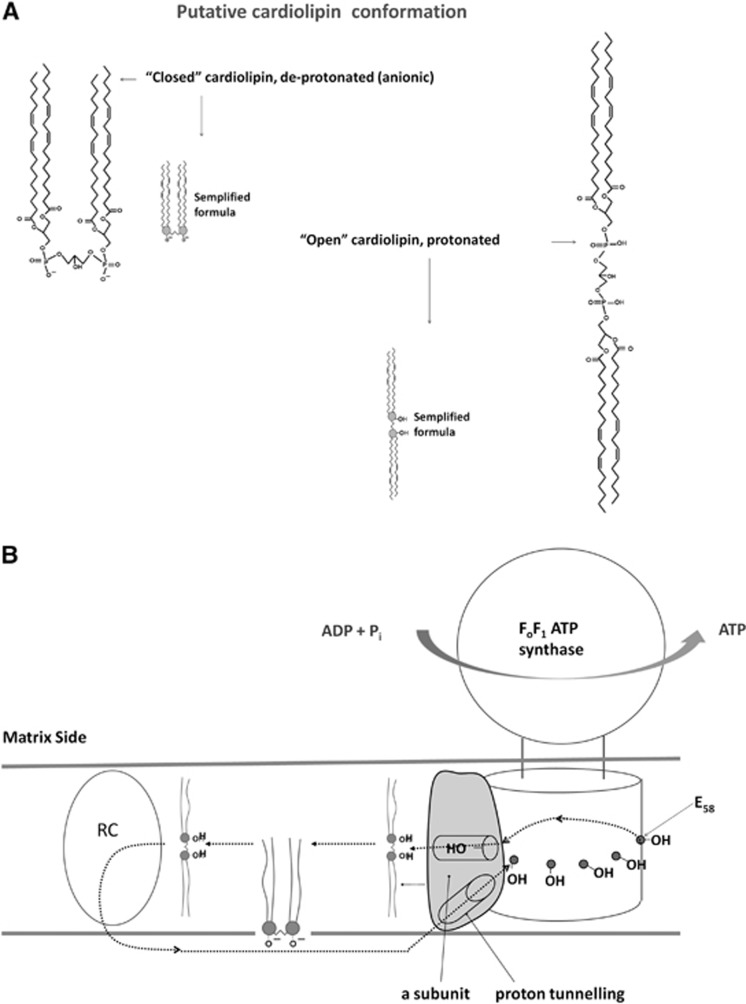
A role in H+ on guidance from the ETC to the phosphates is likely played by cardiolipin (CL). Cardiolipin is inextricably linked to the operation of the OXPHOS proteins,27 due to its unique ability to act as a H+-trap.28 We may speculate that CL, a lipid molecule containing two hydrophobic domains, acts as a H+ shuttle at the centr of the membrane between subunit a of Fo and ETC as tentatively depicted in Figure 1. Panel A illustrates the possibility that the two lipid domains of cardiolipin (CL) molecule can be either in a ‘closed' form, when the phosphates are negatively charged, in one leaflet (probably the periplasmic one29, 30) of the IMM, with the two hydrophobic domains close together, consistent with what reported for complex III analyzed by X-ray diffraction,31 or in an ‘open' conformation when CL accepts H+, in conditions favouring respiration and ATP synthesis. In the latter case, the central part of CL loses polarity and the two lipid diglyceride residue domains would be redistributed inside the two leaflets of the IMM. Therefore, CL would be the only lipid that can be arranged simultaneously in the two layers of the IMM. This is consistent with the data on CL asymmetrical distribution,32 on the existence of multiple binding sites for CL with ETC III,33 and on the fluorescent dye 10-N-nonyl acridine orange.34, 35 In a quantitative assays of CL, authors35 report that 10-N-nonyl acridine orange–cardiolipin fluorescence intensity is maximal in respiratory state 3 during active respiration (i.e.: during active H+ shuttling), decreases in respiratory state 4, reaching a minimum in non-respiring mitochondria. Interestingly, it was reported that 57% of total CL was present in the outer leaflets of inner membranes of isolated mitochondria.30 Figure 1B, based on the knowledge of its structure,36, 37 proposes that subunit a of ATP synthase transfers H+ to Glu 58 at the center of subunit c. Then H+ through an Arg residue (R210 in E. coli38) would pass to respiratory complexes, which, in turn, transfer them to the periplasmic side of the IMM. This putative pathway would be in line with the charged amino acids in subunit a.36, 38 It may also be hypothesized that protonated CL stably realizes a kind of H+ tunneling to the center of the IMM from the center of Fo moiety to the center of the ETC, which translocate H+ to the periplasmic side.
Figure 1.
Tentative H+ circuit inside respiring inner mitochondrial membrane (IMM). Panel A proposes that cardiolipin (CL) can exist in two conformations: ‘closed', with the two hydrophobic domains close together, when the phosphate residues are deprotonated (anionic) and their polarity prevails so that they lay close to the aqueous milieu; and ‘open' (about 4.8 nm long) with the two hydrophobic domains laying in each of the two leaflets of the IMM, when the phosphate residues are protonated and become less polar. Panel B proposes that the H+ (black dotted line) are transferred to the Glu 58 (E58) at the center of subunit c through a subunit of ATP synthase by proton tunneling. H+ would flow from the periplasmic side, always bound to phospholipid heads. This putative pathway would be in line with the existing charged amino acids in a subunit. Then, after almost one complete turn, H+ would be acquired by an Arg residue and eventually by CL, generating a protonated bis-glycerol phosphate with negligible polarity. This can be arranged in each layer of the membrane. H+ would be shuttled by CL towards the center of each electron transport chain (ETC) (generic respiratory complex, RC in Figure), which in turn transfers them to the periplasmic side of the membrane.
Crystallography studies on cytochrome bc1 complex31 showed that H+ are acquired by CL, co-crystallized with the complex at the center of the IMM. Cardiolipin lies at the center of complex III, involved in H+ transfer.31 The detailed analysis of ATP synthase carried out at the atomic level16 locates the H+ transfer in the central area of the membrane. A direct interaction between ATP synthase and CL has been demonstrated.37 Cardiolipin has also been found on the so-called mitochondria-associated membranes.39 The lack of adequate conceptual chemical–physical tools to examine such processes must be remarked. Indeed, the intrinsic chemical propriety of proteolipid phases of the biological membranes is largely unknown. In fact, the theory of acid/base conversion is referred to water phase. Also, due to the minimal inherent mass of H+, quantum mechanics (change of mass with velocity) may be invoked for H+ movements, reminiscent of the ‘uncertainty principle' proposed by Szent-Györgyi for biological systems.40
Hypothesis of an Intramembrane Potential
The asymmetric charge distribution would generate an intramembrane potential, to fuel the rotation of the ATP synthase. The concept may be introduced of a ‘ H+ pressure' generated by the ETC located inside the proteolipid phase of the IMM, mechanically converted into ATP synthesis. So Fo can be fueled by both a H+ potential applied to the membrane, like in in vitro experiments,41, 42 and a ‘H+ pressure', exclusive of the natural respiring membranes. The inconsistence of a membrane potential-driven backflow of H+ ions has been already discussed.43 This would be in accordance to Mitchell's idea of a conceptual asymmetry of metabolism, which would be better described in terms of vectorial forces rather than ‘conventional scalar energies'.6 Measurements of mitochondrial IMM voltage gradient found a positive charge on the matrix face,44 conflicting with the theoretical polarity needed for H+ translocation toward mitochondrial matrix. Such measurements deserve revaluation because they agree with theoretical calculations.45, 46
Notably, a H+ cycle for OXPHOS confined in the proteolipid phase is in accordance to the coupling of the ETC with oxygen consumption and ATP synthesis. If H+ were translocated to and from the aqueous phase, the ETC would not stop acidifying the extramitochondrial milieu. Instead, it is known that only addition of ADP and Pi (a classical experimental procedure) can unload the ‘H+ pressure'. The concept of a collective H+-tunneling had been also proposed by Bartl et al47 in an FT-IR spectroscopy study of the Fo complex of ATP synthase embedded into CL liposomes. Authors report that: ‘…a proton pathway is present in native Fo, in which the protons are shifted in a hydrogen-bonded chain with large proton polarizability...for collective proton tunneling. Such pathways are very efficient, because they conduct protons within picoseconds'.47 The presence of ‘sequestered domains in which H+ are held in a metastable state out of equilibrium with those in the inner–outer aqueous bulk phases48' has also been reported for the thylakoid membranes.48
Conclusions and Perspectives
Mitchell's chemiosmotic theory3 allowed a huge step forward in the field of bioenergetics. It has been said that:‘.. (Mitchell's) experimental and conceptual work proved extraordinarily successful in providing proof of the general hypothesis and prompting mechanisms of key components..'.4 It is worth pointing out that the model proposed here does not conflict with Mitchell's theory. Rather, it reveals the still unrecognized potential of Mitchell's theory, also in being extendable to the emerging awareness of extramitochondrial OXPHOS.49 New roles emerge for ATP synthases, some of which were recently shown to be ectopically expressed in many cellular membranes where they would conduct extramitochondrial OXPHOS (HUVEC cells,50 rod outer segments,51, 52 rat hepatocytes,53 isolated myelin,54 and cancer cells.38 In particular, the present hypothesis is consistent with the reported possibility to conduct OXPHOS on a plasma membrane, i.e. in the absence of a closed vesicle.38, 53 The proposed proteolipidic H+ translocation would help understand extramitochondrial ATP synthases, acting without any transmembrane H+ gradient.
The hypothesis that myelin could act like a mitochondrion to contribute to the energetic metabolism of neurons has been criticized by Harris and Attwell.55 The problem was posed that an arrangement of the OXPHOS proteins is inconsistent with the experimentally measured aerobic ATP synthesis. It was observed that in myelin, the ATP synthase would function in reverse, hydrolyzing rather than synthesizing the ATP.55 It should be noted, however, that the ATP synthase molecular motor is driven by an H+ transfer chain (even if, the mechanism of proton-motive conversion into a mechanical force driving the Fo rotor is largely unknown). Therefore, conversely, a reverse motion of the motor would generate an unlikely backward H+ transfer.
So far, there is no direct evidence for the assumptions we made. Nevertheless, the hypotheses of phase separation and exclusion of aqueous phase continuity for H+ transfer allow to conceive an aerobic ATP synthesis occurring independently of closed compartments where to accumulate H+ is not necessary for ATP synthesis, as also proposed for IMM by Lehninger half a century ago.56 A vectorial transfer of H+ intrinsic to the IMM following the Grotthus mechanism, with a fine integration of H+ translocation and electron transfer for direct coupling of ETC and ATP synthase, thanks to the lipid environment, underlines the unique role of biological membranes in the evolution of life. These would not only have provided a permeability barrier and compartmentation, but had a fundamental role in arranging the proteins involved in gaining chemical energy for life.
Acknowledgments
We thank Giorgio Lenaz (University of Bologna) for his invaluable contribution and Julia J Harris and David Atwell (University College London) for stimulating this basic discussion.
The authors declare no conflict of interest.
Footnotes
The work was supported by grants from Compagnia di San Paolo, for the Neuroscience Program for the research project entitled ‘Energetic metabolism in myelinated axon: a new trophic role of myelin sheath'.
References
- Boyer PD. The ATP synthase—a splendid molecular machine. Annu Rev Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- Walker JE. The ATP synthase: the understood, the uncertain and the unknown. Biochem Soc Trans. 2013;41:1–16. doi: 10.1042/BST20110773. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Rich P. A perspective on Peter Mitchell and the chemiosmotic theory. J Bioenerg Biomembr. 2008;40 (5:407–410. doi: 10.1007/s10863-008-9173-7. [DOI] [PubMed] [Google Scholar]
- Martin W, Muller M. The hydrogen hypothesis for the first eukaryote. Nature. 1998;392:37–41. doi: 10.1038/32096. [DOI] [PubMed] [Google Scholar]
- Mitchell P.Foundations of vectorial metabolism and osmochemistry Biosci Rep 199111297–344.discussion 345-6. [DOI] [PubMed] [Google Scholar]
- Kell DB. On the functional proton current pathway of electron transport phosphorylation, an electrodic view. Biochim Biophys Acta. 1979;549:55–99. doi: 10.1016/0304-4173(79)90018-1. [DOI] [PubMed] [Google Scholar]
- Ferguson SJ. Protons fast and slow. Curr Biol. 1995;5:25–27. doi: 10.1016/s0960-9822(95)00008-x. [DOI] [PubMed] [Google Scholar]
- Haines TH. Anionic lipid headgroups as a proton-conducting pathway along the surface of membranes: a hypothesis. Proc Natl Acad Sci USA. 1983;80:160–164. doi: 10.1073/pnas.80.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaz G, Baracca A, Barbero G, Bergamini C, Dalmonte ME, Del Sole M, et al. Mitochondrial respiratory chain super-complex I-III in physiology and pathology. Biochim Biophys Acta. 2010;1797:633–640. doi: 10.1016/j.bbabio.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Mulkidjanian AY. Proton in the well and through the desolvation barrier. Biochim Biophys Acta. 2006;1757:415–427. doi: 10.1016/j.bbabio.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Cherepanov DA, Junge W, Mulkidjanian A. Proton transfer dynamics at the membrane/water interface: dependence on the fixed and mobile pH buffers, on the size and form of membrane particles, and on the interfacial potential barrier. Biophys J. 2004;86:665–680. doi: 10.1016/S0006-3495(04)74146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efremov RG, Sazanov LA. Structure of the membrane domain of respiratory complex I. Nature. 2011;476:414–420. doi: 10.1038/nature10330. [DOI] [PubMed] [Google Scholar]
- Baradaran R, Berrisford JM, Minhas GS, Sazanov LA. Crystal structure of the entire respiratory complex I. Nature. 2013;494:443–448. doi: 10.1038/nature11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C, Nett JH, Trumpower BL, Hunte C. Specific roles of protein-phospholipid interactions in the yeast cytochrome bc1 complex structure. EMBO J. 2001;20:6591–6600. doi: 10.1093/emboj/20.23.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogoryelov D, Krah A, Langer JD, Yildiz O, Faraldo-Gomez JD, Meier T. Microscopic rotary mechanism of ion translocation in the F(o) complex of ATP synthases. Nat Chem Biol. 2010;6:891–899. doi: 10.1038/nchembio.457. [DOI] [PubMed] [Google Scholar]
- Grzesiek S, Dencher NA. Dependency of delta pH-relaxation across vesicular membranes on the buffering power of bulk solutions and lipids. Biophys J. 1986;50:265–276. doi: 10.1016/S0006-3495(86)83460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotthuss T. Memoire sur la décomposition de l'eau et des corps qu'elle tient en dissolution à l'aide de l'électricité galvanique. Rome. 1805.
- Lehninger AL, Reynafarje B, Alexandre A, Villalobo A. Respiration-coupled H+ ejection by mitochondria. Ann N Y Acad Sci. 1980;341:585–592. doi: 10.1111/j.1749-6632.1980.tb47200.x. [DOI] [PubMed] [Google Scholar]
- Greenlee MM, Lynch IJ, Gumz ML, Cain BD, Wingo CS. The renal H,K-ATPases. Curr Opin Nephrol Hypertens. 2010;19:478–482. doi: 10.1097/MNH.0b013e32833ce65f. [DOI] [PubMed] [Google Scholar]
- Shin JM, Munson K, Vagin O, Sachs G. The gastric HK-ATPase: structure, function, and inhibition. Pflugers Arch. 2009;457:609–622. doi: 10.1007/s00424-008-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RJ. Possible functions of chains of catalysts. J Theor Biol. 1961;1:1–17. doi: 10.1016/0022-5193(61)90023-6. [DOI] [PubMed] [Google Scholar]
- Ferguson SJ. Chemiosmotic coupling. Protons fast and slow. Curr Biol. 1995;5:25–27. doi: 10.1016/s0960-9822(95)00008-x. [DOI] [PubMed] [Google Scholar]
- Mulkidjanian A. Protons @ interfaces: implications for biological conversion. Biochim Biophys Acta. 2006. p. 1757. [DOI] [PubMed]
- Li D, Lyons JA, Pye VE, Vogeley L, Aragao D, Kenyon CP, et al. Crystal structure of the integral membrane diacylglycerol kinase. Nature. 2013;497:521–524. doi: 10.1038/nature12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey M. Crystallizing membrane proteins for structure determination: use of lipidic mesophases. Annu Rev Biophys. 2009;38:29–51. doi: 10.1146/annurev.biophys.050708.133655. [DOI] [PubMed] [Google Scholar]
- Hoch FL. Cardiolipins and biomembrane function. Biochim Biophys Acta. 1992;1113:71–133. doi: 10.1016/0304-4157(92)90035-9. [DOI] [PubMed] [Google Scholar]
- Haines TH, Dencher NA. Cardiolipin: a proton trap for oxidative phosphorylation. FEBS Lett. 2002;528:35–39. doi: 10.1016/s0014-5793(02)03292-1. [DOI] [PubMed] [Google Scholar]
- Krebs JJ, Hauser H, Carafoli E. Asymmetric distribution of phospholipids in the inner membrane of beef heart mitochondria. J Biol Chem. 1979;254:5308–5316. [PubMed] [Google Scholar]
- Petit JM, Huet O, Gallet PF, Maftah A, Ratinaud MH, Julien R. Direct analysis and significance of cardiolipin transverse distribution in mitochondrial inner membranes. Eur J Biochem. 1994;220:871–879. doi: 10.1111/j.1432-1033.1994.tb18690.x. [DOI] [PubMed] [Google Scholar]
- Lange C, Nett JH, Trumpower BL, Hunte C. Specific roles of protein-phospholipid interactions in the yeast cytochrome bc1 complex structure. EMBO J. 2001;20:6591–6600. doi: 10.1093/emboj/20.23.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallet PF, Petit JM, Maftah A, Zachowski A, Julien R. Asymmetrical distribution of cardiolipin in yeast inner mitochondrial membrane triggered by carbon catabolite repression. Biochem J. 1997;324 (Pt 2:627–634. doi: 10.1042/bj3240627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnarez C, Mazat JP, Elezgaray J, Marrink SJ, Periole X. Evidence for cardiolipin binding sites on the membrane-exposed surface of the cytochrome bc1. J Am Chem Soc. 2013;135:3112–3120. doi: 10.1021/ja310577u. [DOI] [PubMed] [Google Scholar]
- Kaewsuya P, Danielson ND, Ekhterae D. Fluorescent determination of cardiolipin using 10-N-nonyl acridine orange. Anal Bioanal Chem. 2007;387:2775–2782. doi: 10.1007/s00216-007-1135-0. [DOI] [PubMed] [Google Scholar]
- Garcia FMI, Ceccarelli D, Muscatello U. Use of the fluorescent dye 10-N-nonyl acridine orange in quantitative and location assays of cardiolipin: a study on different experimental models. Anal Biochem. 2004;328:174–180. doi: 10.1016/j.ab.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Vik SB, Long JC, Wada T, Zhang D. A model for the structure of subunit a of the Escherichia coli ATP synthase and its role in proton translocation. Biochim Biophys Acta. 2000;1458:457–466. doi: 10.1016/s0005-2728(00)00094-3. [DOI] [PubMed] [Google Scholar]
- Acehan D, Malhotra A, Xu Y, Ren M, Stokes DL, Schlame M. Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys J. 2011;100:2184–2192. doi: 10.1016/j.bpj.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi VK, Girvin ME. Structural changes linked to proton translocation by subunit c of the ATP synthase. Nature. 1999;402:263–268. doi: 10.1038/46224. [DOI] [PubMed] [Google Scholar]
- Lebiedzinska M, Szabadkai G, Jones AW, Duszynski J, Wieckowski MR. Interactions between the endoplasmic reticulum, mitochondria, plasma membrane and other subcellular organelles. Int J Biochem Cell Biol. 2009;41:1805–1816. doi: 10.1016/j.biocel.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi A. lntroduction to a Submolecular Biology. Academic Press, Inc.: New York; 1960. [Google Scholar]
- Sone N, Yoshida M, Hirata H, Kagawa Y. Adenosine triphosphate synthesis by electrochemical proton gradient in vesicles reconstituted from purified adenosine triphosphatase and phospholipids of thermophilic bacterium. J Biol Chem. 1977;252:2956–2960. [PubMed] [Google Scholar]
- Turina P, Samoray D, Graber P. H+/ATP ratio of proton transport-coupled ATP synthesis and hydrolysis catalysed by CF0F1-liposomes. EMBO J. 2003;22:418–426. doi: 10.1093/emboj/cdg073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie RJ, Azzone GF, Conover TE. Bulk phase proton fluxes during the generation of membrane potential in rat liver mitochondria. J Biol Chem. 1991;266:803–809. [PubMed] [Google Scholar]
- Tupper JT, Tedeschi H. Mitochondrial membrane potentials measured with microelectrodes: probable ionic basis. Science. 1969;166:1539–1540. doi: 10.1126/science.166.3912.1539. [DOI] [PubMed] [Google Scholar]
- Harris EJ, Pressman BC. The direction of polarity of the mitochondrial trans-membrane potential. Biochim Biophys Acta. 1969;172:66–70. doi: 10.1016/0005-2728(69)90092-9. [DOI] [PubMed] [Google Scholar]
- Zoratti M, De Marchi U, Biasutto L, Szabo I. Electrophysiology clarifies the megariddles of the mitochondrial permeability transition pore. FEBS Lett. 2010;584:1997–2004. doi: 10.1016/j.febslet.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Bartl F, Deckers-Hebestreit G, Altendorf K, Zundel G. The F0 complex of the ATP synthase of Escherichia coli contains a proton pathway with large proton polarizability caused by collective proton fluctuation. Biophys J. 1995;68:104–110. doi: 10.1016/S0006-3495(95)80164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theg SM, Chiang G, Dilley RA. Protons in the thylakoid membrane-sequestered domains can directly pass through the coupling factor during ATP synthesis in flashing light. J Biol Chem. 1988;263:673–681. [PubMed] [Google Scholar]
- Panfoli I, Ravera S, Bruschi M, Candiano G, Morelli A. Proteomics unravels the exportability of mitochondrial respiratory chains. Expert Rev Proteomics. 2011;8:231–239. doi: 10.1586/epr.11.1. [DOI] [PubMed] [Google Scholar]
- Arakaki N, Nagao T, Niki R, Toyofuku A, Tanaka H, Kuramoto Y, et al. Possible role of cell surface H+ -ATP synthase in the extracellular ATP synthesis and proliferation of human umbilical vein endothelial cells. Mol Cancer Res. 2003;1:931–939. [PubMed] [Google Scholar]
- Panfoli I, Musante L, Bachi A. Proteomic analysis of the retinal rod outer segment disks. J Proteome Res. 2008;7:2654–2669. doi: 10.1021/pr7006939. [DOI] [PubMed] [Google Scholar]
- Panfoli I, Calzia D, Bianchini P, Ravera S, Diaspro A, Candiano G, et al. Evidence for aerobic metabolism in retinal rod outer segment disks. Int J Biochem Cell Biol. 2009;41:2555–2565. doi: 10.1016/j.biocel.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Mangiullo R, Gnoni A, Leone A, Gnoni GV, Papa S, Zanotti F. Structural and functional characterization of F(o)F(1)-ATP synthase on the extracellular surface of rat hepatocytes. Biochim Biophys Acta. 2008;1777:1326–1335. doi: 10.1016/j.bbabio.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Ravera S, Panfoli I, Calzia D, Aluigi MG, Bianchini P, Diaspro A, et al. Evidence for aerobic ATP synthesis in isolated myelin vesicles. Int J Biochem Cell Biol. 2009;41:1581–1591. doi: 10.1016/j.biocel.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Harris JJ, Attwell D. Is myelin a mitochondrion. J Cereb Blood Flow Metab. 2012;33:33–36. doi: 10.1038/jcbfm.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger AL, Wadkins CL, Cooper C, Devlin TM, Gamble JL., Jr Oxidative phosphorylation. Science. 1958;128:450–456. doi: 10.1126/science.128.3322.450. [DOI] [PubMed] [Google Scholar]