Abstract
The tumor microenvironment mediates induction of the immunosuppressive programmed death-1 (PD-1) pathway, targeted interventions against which can help restore antitumor immunity. To gain insight into these responses, we studied the interaction between PD-1 expressed on T cells and its ligands (PD-1:PD-L1, PD-1:PD-L2, and PD-L1:B7.1) expressed on other cells in the tumor microenvironment, using a syngeneic orthotopic mouse model of epithelial ovarian cancer (ID8). Exhaustion of tumor-infiltrating lymphocytes (TILs) correlated with expression of PD-1 ligands by tumor cells and tumor-derived myeloid cells, including macrophages (TAM), dendritic cells (DC) and myeloid-derived suppressor cells (MDSC). When combined with GVAX or FVAX vaccination (consisting of irradiated ID8 cells expressing GM-CSF or FLT3 ligand) and co-stimulation by agonistic α4-1BB or TLR 9 ligand, antibody mediated blockade of PD-1 or PD-L1 triggered rejection of ID8 tumors in 75% of tumor-bearing mice. This therapeutic effect was associated with increased proliferation and function of tumor antigen-specific effector CD8+ T cells, inhibition of suppressive T regulatory cells (Tregs) and MDSC, upregulation of effector T cell signaling molecules and generation of T memory precursor cells. Overall, PD-1/PD-L1 blockade enhanced the amplitude of tumor immunity by reprogramming suppressive and stimulatory signals that yielded more powerful cancer control.
Introduction
At the time of diagnosis, over 75% of patients with ovarian cancer present with advanced stage III or IV disease (1–2). Despite appropriate surgery and receiving highly effective first-line chemotherapy, ~70% of patients with advanced-stage disease who achieve remission eventually relapse (1–2). Thus, there is an immediate need for therapeutic targets for treating ovarian cancer (3). Our group and others have reported that tumor-infiltrating T lymphocytes (TILs) with anti-tumor potential exist in cancer patients (4–7). Studies in a primary co-culture system showed that TILs from many ovarian cancer patients secrete low to intermediate levels of IFN-γ and limited proliferation in response to cognate peptides (unpublished observation). The programmed cell death 1 (PD-1) is an inhibitory surface receptor expressed by T cells, B cells, natural killer T cells, monocytes, and DCs, but not by resting T cells. PD-1 binds two ligands, programmed cell death ligand 1 (PD-L1) and PD-L2, also called B7-H1 and B7-DC, respectively (8–9). Tumors can employ the PD-1 inhibitory pathway to silence the immune system (8). The expression of PD-L1 in tumors is inversely correlated with survival of patients (10–11). This indicates that although anti-tumor immunity is elicited against ovarian cancer, it is counterbalanced by immunosuppressive factors.
In ovarian tumors, myeloid cells are one of the major determinants of immune suppression. These include tumor-associated macrophages (TAMs), immature/tolerogenic DCs, and myeloid-derived suppressor cells (MDSCs) (12–21). In addition, CD4+CD25+Foxp3+ T regulatory cells (Tregs) play a critical role in the control of anti-tumor immune responses, relying on PD-1, PD-L1 or cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) to perform these functions (22–27). Most studies describe mechanisms for the accumulation of these immunosuppresive myeloid cells or Tregs. In this study, we demonstrate a cross-regulation among these cell types using the ID8 syngeneic mouse model of epithelial ovarian cancer. We provide evidence that T cell dysfunction can be reversed by targeting the PD-1 pathway simultaneously in all these cell types. We found that expansion of ovarian antigen-specific CD8+ TILs was dependent on the amount of PD-L1 signaling by tumor cells, tumor-derived myeloid cells and Tregs. Furthermore, combining PD-1 blockade with a single dose of GVAX or FVAX vaccination resulted in enhanced clonal expansion of antigen-specific CD8+ T cells and tumor control. Finally, we observed a further boost of CD8+ T cell function when PD-L1 blockade was combined with both vaccination and α4-1BB co-stimulation. Overall, our study shows that αPD-L1 blockade therapy greatly synergizes with other immunotherapy modalities.
Methods
Mice and tumor lines
All experiments were performed using protocols approved by the University of Pennsylvania Laboratory Animal Resources (ULAR) policies. A mouse ovarian epithelial papillary serous adenocarcinoma cell line (ID8) was obtained from Dr. K. F. Roby, University of Kansas Medical Center (28). Development of ID8 cells expressing murine GM-CSF (ID8-GVAX) or Flt3-ligand (ID8-FVAX) was based on methods described previously (29).
Blocking and agonistic antibodies
Rat anti-mouse PD-1 (29F.1A12, at IgG2a, k), PD-L1 (10F.9G2, rat IgG2b, k), PD-L2 (3.2, mouse IgG1), and isotype control antibodies were used (7). The rat anti-mouse 4-1BB antibody from BioXcell was also used.
Tumor experiments
C57BL6 mice (n=12) were given an intraperitoneal (i.p) injection containing 5 × 106 ID8 cells. Two hundred micrograms of rat α-mouse PD-1, -PD-L1, -PD-L2 antibodies, 100 µg of α-CTLA-4 (clone 9D9) or isotype control antibodies were administered i.p. 4 weeks after tumor inoculation 5 times on alternate days. For co-stimulation 200 µg of α4-1BB antibody (BioXcell) was given once along with first aforementioned dose of blocking antibodies. In vaccination experiments, 106 irradiated (150Gy) gene-modified ID8-GVAX or ID8-FVAX cells were given by i.p. once, 3 weeks after tumor inoculation. Mice were monitored by their weight gain twice a week. Mice weighing >35 gm as a result of tumor growth and/or ascites fluid accumulation were euthanized.
Methods are detailed in the supplementary section.
Results
Lack of T cell infiltration in ovarian tumors is acquired
We have previously reported that, at the steady state, established ID8 tumors are poorly infiltrated by T cells (33) and are thus considered intrinsically non-immunogenic, mimicking many human ovarian cancers lacking TILs (6). In this orthotopic model of ovarian cancer, gross metastatic i.p. nodules appear at approximately 28 days, and tumor and ascites rapidly accumulate leading to 100% death at 55–60 days (Fig. 1a. We investigated the changes in the frequency of TILs in ovarian tumors, ascites, and spleens of mice from early (~28 days) to late times of tumor growth (~52 days). Interestingly, CD3+ (CD8+ and CD4+) T cells infiltrating during the early tumors were almost completely absent in advanced tumors (Fig. 1b).
Figure 1. Accumulation of myeloid and T cell populations in ID8 tumor.
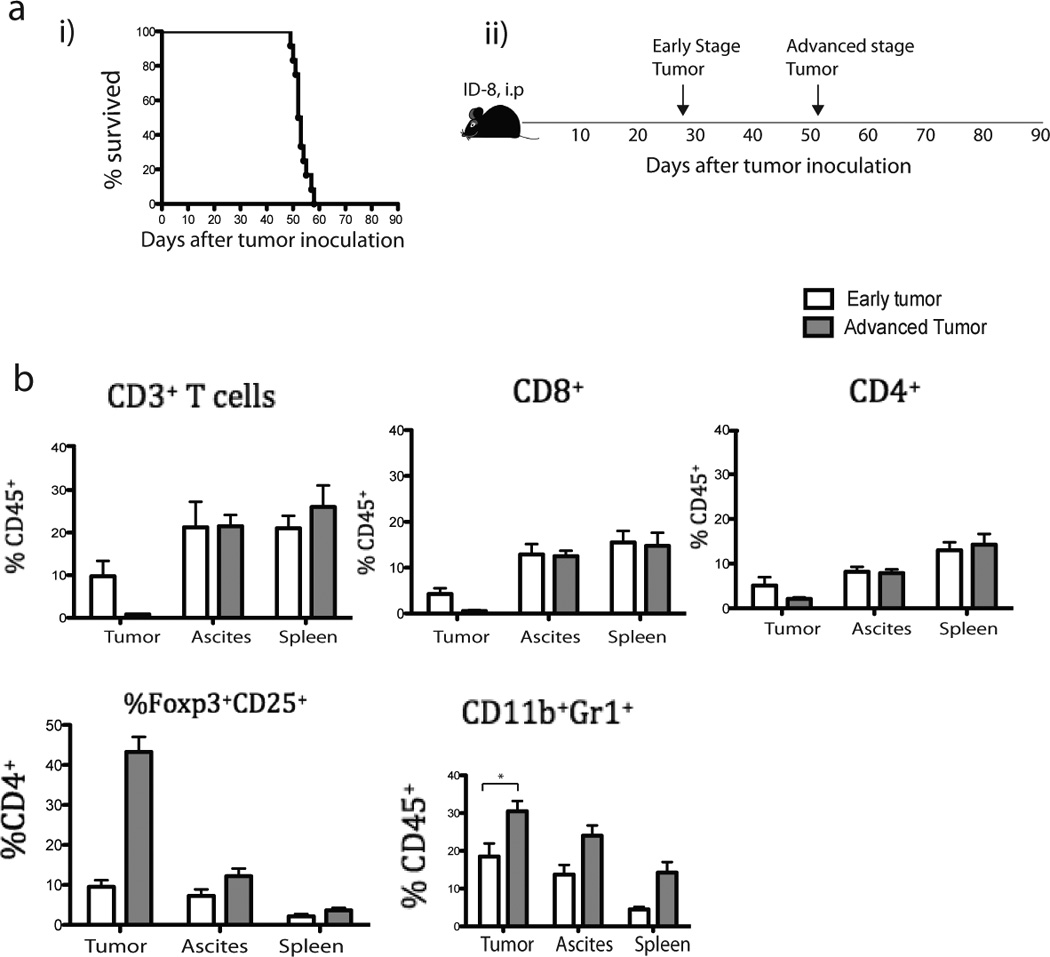
(a) Six to 8 weeks-old mice (n=12) were inoculated i.p. with 5 × 106 ID8 tumor cells. The survival (i) and scheme (ii) are shown. (b) T (CD3+, CD8+, and CD4+) cells, Treg (CD4+CD25+Foxp3+) cells, and MDSC (CD11b+Gr1+) from tumor, ascites, spleen of ID8 tumor-bearing mice were isolated and counted (using 0.4% trypan blue stain) from 6 mice during early (4–5 weeks after tumor inoculation) or rest of the 6 mice during advanced (7–8 weeks after tumor inoculation) tumors. All cell subtypes were CD45+ gated. Bars represent mean ± SEM.
Interestingly, a high percent of T cells in advanced tumors were Tregs (34), while there were no detectable CD8+ T cells left and the depletion of T cells was restricted to tumors. Parallel to the observed disappearance of CD3+ TILs and the increase in Treg cells, we found a significant increase in MDSCs (CD11b+Gr-1+, p=0.024) (Fig. 1b; Supplementary Fig. 1) and TAMs (CD11b+F4/80+, p=0.048) in tumors, but no significant changes in CD11chi DCs (p=0.10) (not shown). Thus, lack of immunogenicity in established ID8 tumors appears to be acquired rather than intrinsic.
PD-1 ligands are present in the microenvironment of ovarian tumors
Previous immunohistochemical studies have reported upregulation of PD-L1 in human ovarian cancers (10). Here we observed high levels of PD-L1 and moderate levels of PD-L2 on ID8 tumor cells, as well as macrophages, DCs and MDSCs derived from the same tumors (Fig. 2a–b; Supplementary Fig. 2a). Similarly, myeloid cells derived from ascites showed moderate expression of high levels of PD-L1 and PD-L2, however, spleens showed only low level expression of PD-L1, but not PD-L2. Next, we found that PD-1 was expressed by matched CD8+ T cells in tumor at both early and later stages (Fig. 2c; Supplementary Fig. 2b), but PD-1 expression was not detected in other organs except PD-1dim CD8+ T cells in the liver at early stages of tumor. Thus T cells specifically upregulate PD-1 in the tumor microenvironment where they are likely to receive inhibitory signals.
Figure 2. Expression of PD-L1, PD-L2 and PD-1 in ID8 mice.
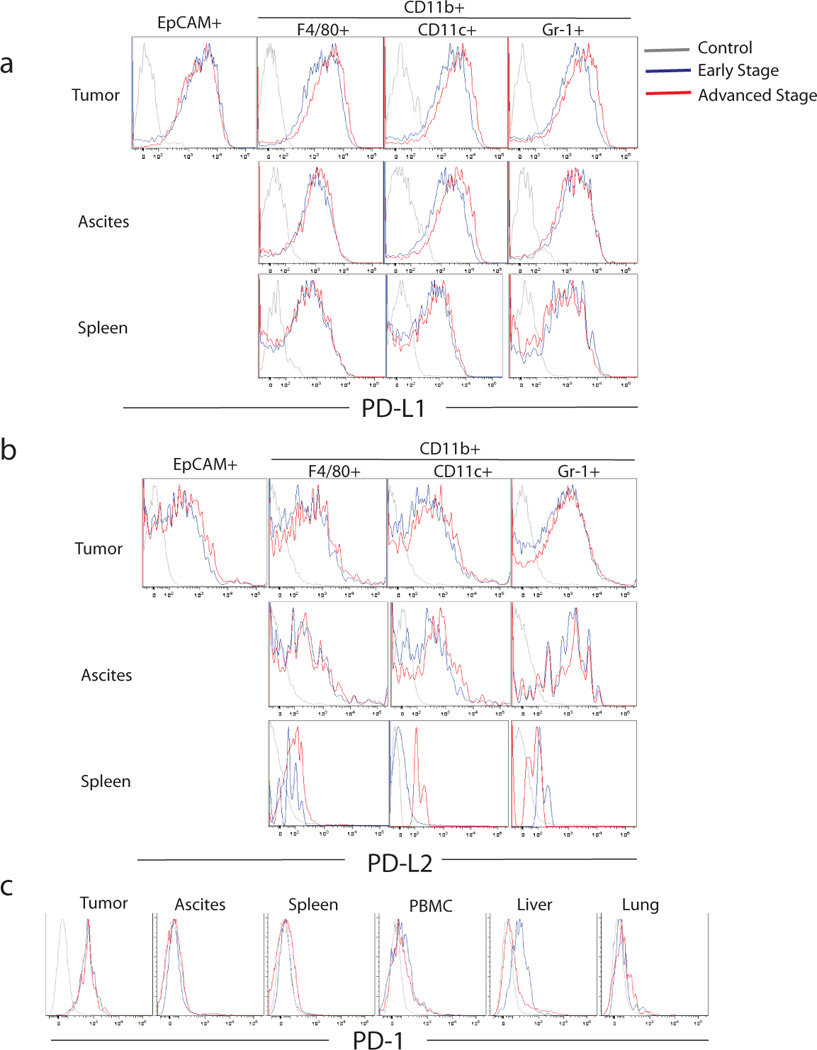
Mice were inoculated i.p. with 5×106 ID8 tumor cells (n=12), and their tumor, ascites, spleen, liver, lung and blood were harvested from half of the mice at early and the other half at advanced tumor. Tumor cells (EpCAM+), macrophages (CD45+CD11B+F4/80+), DCs (CD45+CD11c+) and MDSCs (CD45+CD11B+Gr1+) were isolated from the tumor, ascites and spleen. Histograms show PD-L1 (a) and PD-L2 (b) expression by ID-8 tumor cells as well as macrophages, DCs and MDSCs from tumor, ascites and spleen of ID-8 tumor-bearing mice. (c) PD-1 expression on CD8+ T cells from tumor, ascites, spleen, blood, liver and lung of ID-8 tumor-bearing mice. Results are from one of the 3 experiments.
PD-1 or PD-L1, but not PD-L2, blockade therapy cause regression of ovarian tumors
We next tested whether the blockade of PD-1 could reverse the decline of T cell immunity and promote tumor rejection in mice. We also sought to compare the relative contribution of PD-L1 versus PD-L2. We inoculated C57BL/6 mice i.p. with ID8 tumor cells and then administered α-PD-1, α-PD-L1 or α-PD-L2 antibodies starting on day 28 (Fig. 3a left). Treatment with α-PD-1 or α-PD-L1 antibodies resulted in tumor rejection in 25% (3/12) of the mice, as indicated by normalized mouse weights after treatment (weight gain is due to ascites fluid accumulation and is an accurate surrogate of tumor growth in this model), and long-term survival. On the contrary, α-PD-L2 antibody did not reject tumors. To test whether PD-1-mediated TIL suppression was active at even earlier stages of tumor development, we treated mice with α-PD-L1 starting on day 21. PD-L1 blockade resulted in tumor rejection in 60% (7/12) of the mice (Fig. 3a right). Thus, the PD-1 pathway is highly relevant, and is active very early in the process of establishment of ovarian tumors.
Figure 3. PD-1 or PD-L1 blockade causes regression of ID8 tumor.
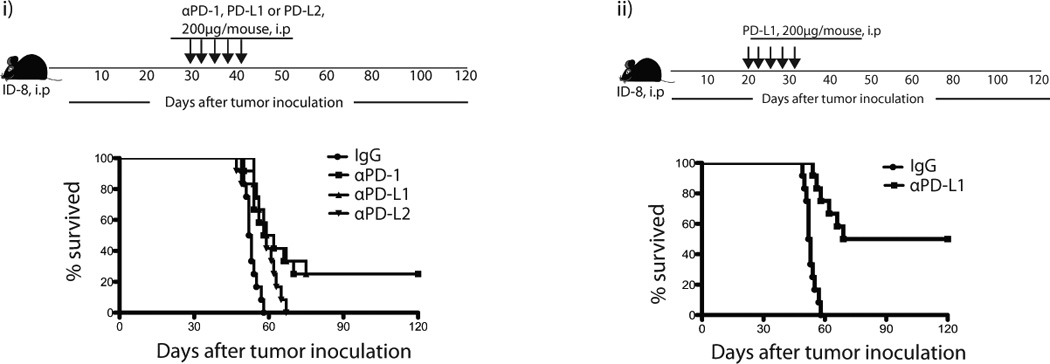
Mice were inoculated i.p. with 5 × 106 ID8 tumor cells (n=12). These mice were injected either therapeutically (starting at 30 days after tumor inoculation) or prophylactically (starting at 20 days after tumor inoculation) 5 times i.p. with αPD-1 (200ug), αPD-L1 (200ug), or αPD-L2 (200ug) blocking antibodies on alternate days either alone or in combination as indicated. The scheme and survival of therapeutic (i) and prophylactic models (ii) are shown. Results are from one of the 3 representative experiments.
PD-1 or PD-L1, but not PD-L2, blockade therapy prevent immune decline in the tumor microenvironment
Next, we tested whether PD-1 blockade was associated with reversal of immune decline in tumors and enhanced TIL infiltration. We chose the more advanced treatment schedule (starting on day 28), as above. A week after completion of the treatment (day ~45), residual peritoneal tumor deposits were resected and analyzed. The frequency of CD8+ and CD4+ T cells (as well as total CD45+ leukocytes) was markedly increased following α-PD-1 and α-PD-L1 administration. Interestingly, in spite of the lack of tumor response, there was an increase in CD8+ and total CD45+ cells also following α-PD-L2 (Fig. 4a). Importantly, PD-1 and PD-L1 blockade significantly reduced Tregs and thereby increased the CD8+ to Treg and CD4+ to Treg cell ratios within the tumor (Fig. 4b). On the contrary, no such decrease in Tregs was seen after α-PD-L2 treatment, and thus there was no significant change in the CD8+ or CD4+ to Treg ratios.
Figure 4. PD-1 or PD-L1 blockade increases immune activation of TILs in ID8 tumor.
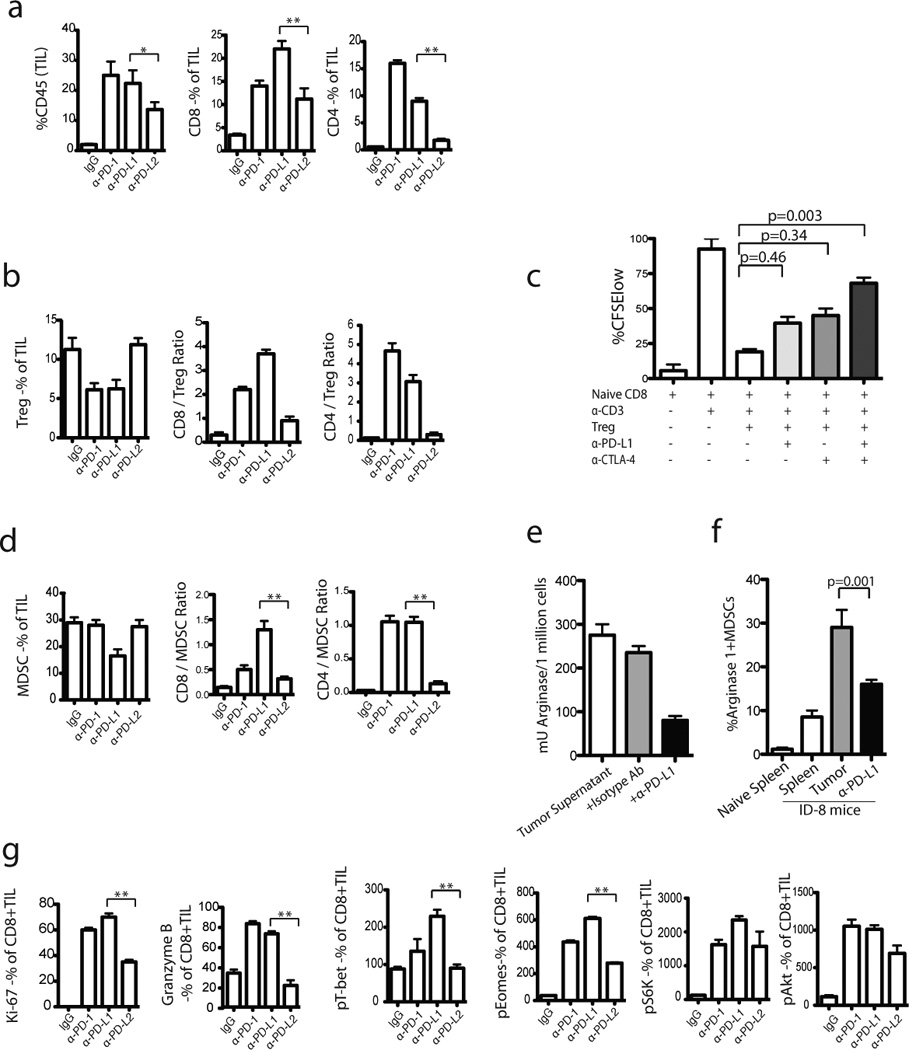
One week following completion of blockade treatment, the TILs were harvested from regressing tumors and stained with various markers. Percentages of CD8+ and CD4+ TIL (CD45+) infiltration of total leucocytes (a) and the ratio of CD8+ T cells to Tregs (b) are shown in treated versus untreated groups. (c) Blocking PD-1/PD-L1 interaction reduced Treg-mediated suppression of CD8+ T cells in vitro. CFSE-labeled CD8+ T cells were co-cultured with syngeneic, αCD3-loaded DCs with or without Tregs and αPD-L1 or αCTLA-4 as indicated. CD8+ T cells and stimulator APCs were obtained from naive B6 mice. Tregs were obtained from ID8-tumor bearing mice. Treg-mediated suppression of proliferation of naïve CD8+ T cells was noticed. Results from one of 3 experiments are shown. (d) The ratio of CD8+ T cells to MDSCs are shown. (e) CD8+ TILs from αPD-L1 treated mice were stained with arginase-1 (8C9 clone from Santa Cruz) and analyzed by flow cytometry. The CD11b+arginase-1+ MDSCs within CD45+ TILs are shown. (f) Tumor-dervived MDSCs were plated at 1×106/well in 24-well plates and stimulated with equal amount of tumor supernatants (from ID8 cells). Following stimulation, cells were added with αPD-L1 and then arginase I was analyzed after 24 h following washing with PBS and lysis buffer. (g) Percentage of Ki67+ and Granzyme B+ as well as MFI of pT-bet, pEomes, pS6, and pAkt expression by CD8+ TILs are shown. The results are the sum of three independent experiments with 8–10 mice per group.
We also noticed PD-L1 surface expression on Tregs (Supplementary Fig. 1). To understand whether PD-1-PD-L1 interactions contribute directly to the inhibitory function of tumor Tregs, CD4+CD25+ Tregs isolated from ID8 tumors were incubated with responder spleen CD8+ T cells stimulated by immobilized α-CD3 antibody, in the presence or absence of PD-L1 blocking antibody. T cell proliferation was assessed by CFSE dilution. Whereas Tregs suppressed responder cell proliferation, α-PD-L1 attenuated the ability of Tregs to suppress CD8+ T cell proliferation (Fig. 4c, p<0.05; Supplementary Fig. 3). Thus, PD-L1 blockade not only reduced the relative frequency of Tregs in ID8 tumors, but also attenuates the suppressive function of tumor-derived Tregs.
MDSCs were also decreased specifically by PD-L1 blockade, which thereby increased the ratio of T cells to MDSCs within the tumor (Fig. 4d). MDSCs suppress effector T cells through the production of arginase-I (40, 44). Interestingly, we detected a significantly lower number of arginase-I positive MDSC in ID8 tumors after treatment with α-PD-L1 (Fig. 4e). To assess whether arginase-I is involved in the mechanisms through which PD-L1+ MDSCs suppress TILs in ID8 tumors, CD11b+ Gr1+ isolated from ID8 tumors were further cultured with ID8 tumor cell supernatants in the presence or absence of α-PD-L1 antibody. Arginase catalyzes the conversion of arginine to ornithine and urea in the urea cycle. Arginase activity was evaluated by measuring the urea concentration in the media. We found that PD-L1 blockade decreased the level and activity of arginase-I (Fig. 4f; p=0.0003). Thus, PD-L1 blockade not only reduced the relative frequency of CD11b+ Gr1+ cells, but also reduced their suppressive phenotype by reducing arginase-I activity, which appears to be dependent in part on PD-1-PD-L1 interactions.
The above data indicate that PD-1 or PD-L1 blockade significantly attenuated the number and function of Tregs and/or MDSC. We thus tested whether the above changes were associated with activation of CD8+ TILs. We detected significant upregulation of Ki-67 and intracellular granzyme B, as well increased levels of phosphorylated transcription factors, T-bet, Eomes and S6 kinase, and Akt, supporting the notion that effective PD-1 blockade activated function and survival pathways in CD8+ TILs (35–36) (Fig. 4g; Supplementary Fig. 4).
Vaccination synergizes with PD-1 blockade to prevent immune decline in tumors
We theorized that vaccine administered in combination with PD-1 blockade could enhance the efficacy of PD-1 blockade in preventing immune decline in tumors. Previous studies have described a therapeutic efficacy of B16 autologous melanoma tumor vaccines expressing either granulocyte macrophage colony-stimulating factor (GM-CSF) or FMS like tyrosine kinase 3 ligand (Flt3-ligand), when combined with the blocking CTLA-4 (29, 37–40). We transduced ID8 whole tumor cells to express either GM-CSF (ID8-GVAX, Fig. 5a) or Flt3L (ID8-FVAX, Fig. 5b) and used radiated cells as vaccine in combination with PD-1, PD-L1, or PD-L2 blockade. Mice received weekly vaccine, starting 3 weeks after tumor inoculation. A week later, mice were treated with PD-1 blocking antibodies 5 times on alternate days. Although GVAX alone had no effect on tumor growth or mouse survival, addition of GVAX doubled the effect of checkpoint blockade on mouse survival. Fifty percent of mice that received GVAX plus PD-1 or PD-L1 blockade rejected their tumors, while antibodies alone resulted in 25% tumor rejection (Fig. 5a left). By contrast, α-PD-L2 did not show any positive interaction with vaccination. Similar results were obtained with FVAX (Fig. 5b left). Interestingly, the positive interaction between vaccine and checkpoint blockade required full development of the vaccine effect, and this was achieved by increasing the time interval between vaccination and checkpoint blockade to one week (data not shown).
Figure 5. Synergistic effect of PD-1 blockade, GVAX or FVAX vaccination, and α4-1BB costimulation on ovarian ID8 tumor rejection.
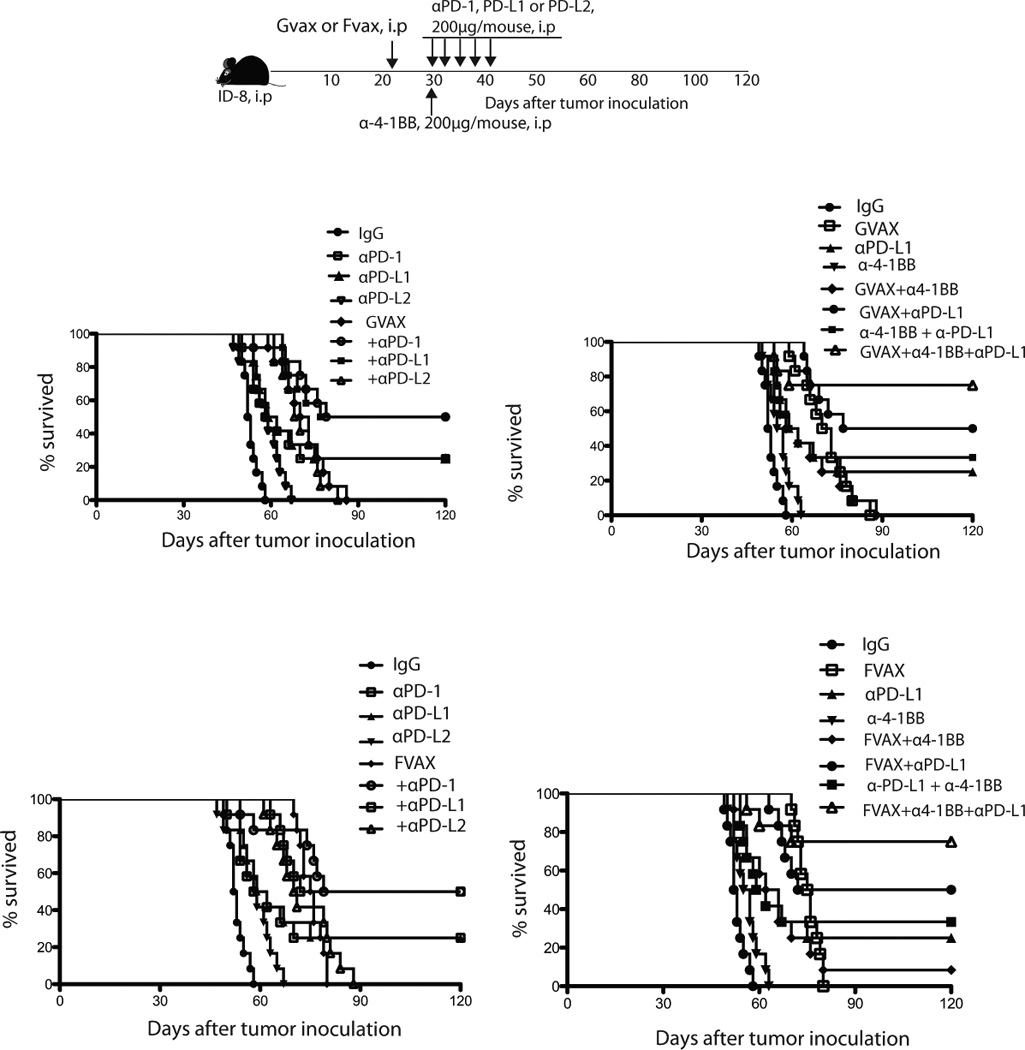
(a) Three weeks after ID8 tumor inoculation, the mice were given i.p with 2 × 106 irradiated (150Gy) GVAX (top) or FVAX (bottom) once. A week later mice were injected 5 times i.p. with αPD-1 (200ug), αPD-L1 (200ug), αPD-L2 (200ug) on alternate days or a single dose 200ug of α-4-1BB either alone or in combination as indicated. Results are from one of the 3 experiments.
Co-stimulatory signals further augment the combination of vaccine plus PD-L1 blockade
The above results indicate that PD-1 or PD-L1 blockade can prevent immune decline in tumors and that additional of vaccine further enhances this effect. Next we hypothesized that adding a costimulatory signal, 4-1BB (CD137), a tumor necrosis factor receptor gene family member (41–45), could further boost TILs and enhance the effects of checkpoint blockade and vaccination. Mice were vaccinated with GVAX or FVAX starting 3 weeks after tumor inoculation, and a week later, we administered 5 doses of αPD-L1 on alternate days plus a single dose of α4-1BB (200 µg) antibody with the first dose of αPD-L1 (Fig. 5a right). Administration of the α4-1BB antibody alone had no impact on tumor growth or survival. Furthermore, addition of α4-1BB antibody to PD-L1 blockade had moderate impact on survival (Fig. 5a right). Remarkably, 75% of mice receiving GVAX followed by αPD-L1 plus α4-1BB antibodies rejected their tumors. Similar results were obtained with FVAX (Fig. 5b right).
In tumors, costimulatory signals could be provided by properly activated antigen-presenting cells, which express the 4-1BB ligand as well as other important costimulatory ligands. Hypothesizing that similar therapeutic benefit as above could be obtained through T cell costimulation by TLR-activated APCs, we used an agonistic murine TLR9 ligand, CpG 1668 (46). Mice were treated with 5 doses of CpG 1668 i.p. (5 µg/mouse) in combination with αPD-L1 antibody (Supplementary Fig. 5). Similar to α4-1BB, we found significant therapeutic benefit by adding CpG. Importantly, the effect from this combination was seen only when CpG was given in combination with αPD-L1 antibody but not if CpG was given earlier, in combination with the vaccine, indicating that CpG did not contribute at enhancing T cell priming. Thus, the therapeutic PD-L1 blockade has the potential to induce significant tumor rejection, which can be maximized if PD-L1 blockade is preceded by tumor vaccination, and accompanied by costimulation.
Combination therapy reprograms the tumor immune microenvironment
We next asked what were the changes in the tumor microenvironment induced by the ineffective vaccines GVAX and FVAX. Interestingly, vaccine alone increased significantly the frequency of TILs, especially CD8+ cells (p<0.01) (Fig. 5a and Supplementary Fig. 7a). Because vaccines proved ineffective, we asked whether this was associated with an increase in immunosuppressive populations. Importantly, we noticed that both GVAX and FVAX failed to reduce the frequency of Tregs in tumors. Moreover, GVAX increased the frequency of the MDSC population in tumor leukocytes (Fig. 6c). On the other hand, FVAX increased the frequency of plasmacytoid DC (pDCs) levels in tumors (Supplementary Fig. 6), which have been shown to have immunosuppressive properties (47). Thus, vaccine alone increased TILs but also immunosuppressive cells.
Figure 6. Synergistic effect of PD-1 blockade, GVAX vaccination, and α4-1BB costimulation on immune activation.
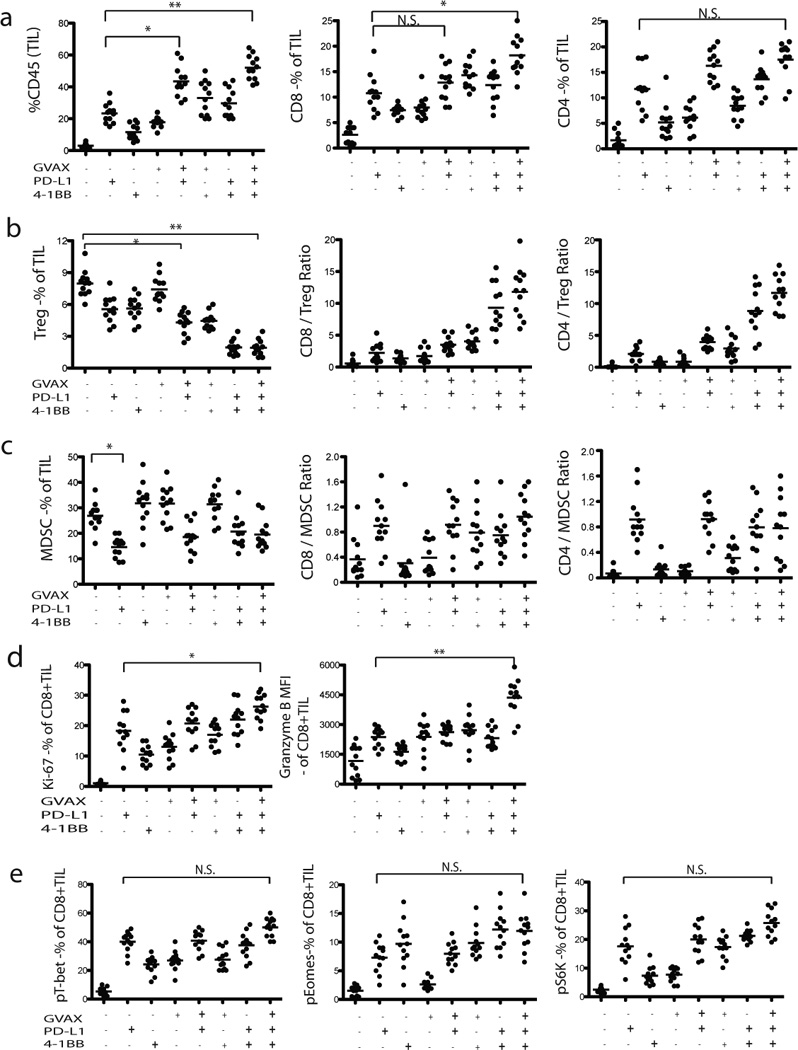
TILs from regressing tumors of ID8 mice were harvested a week following completion of treatment and stained for various markers. Percentages of CD8+ and CD4+ TIL (CD45+) infiltration of total leucocytes are shown in treated versus untreated groups. The ratio of CD8+ T cells to Tregs and MDSC are also shown. Percentages of Ki67+CD8+ and MFI of Granzyme B+CD8+ TIL (CD45+) infiltration of total leukocytes are shown in treated versus untreated groups. Mean intensity of pT-bet, pEomes, and pS6 expression by CD8+ TILs is shown. Results from one of the 3 experiments are shown. Bars represent mean ± SEM. *P ≤ 0.05; **P ≤ 0.01; ***P < 0.001.
Mice treated with GVAX or FVAX plus αPD-L1 showed significantly increased infiltration of total inflammatory CD45+ cells and T cells in tumors compared to mice treated with αPD-L1 (p<0.01) or vaccine (p<0.001) alone. Among them, CD8+ T cells were more abundant than CD4+ T cells (Fig. 6a and Supplementary Fig. 7a). When PD-L1 blockade was combined with GVAX or FVAX, there was a moderate reduction in Treg as well as MDSC levels in the tumor (Figs. 6b&6c; and Supplementary Figs. 7b&7c). The addition of α4-1BB to αPD-L1 increased significantly TILs relative to each agent alone, both CD8+ and CD4+. In addition, it markedly reduced Treg cells relative to each agent alone. Consequently, the combination of α4-1BB plus αPD-L1 resulted in a marked increase in the CD8/Treg and CD4/Treg ratios, superior than each antibody alone. The combination of αPD-L1 and α4-1BB with GVAX or FVAX yielded the highest frequency in TILs, both CD8+ and CD4+. Furthermore, the addition of vaccine did not increase Tregs when αPD-L1 and α4-1BB were combined with GVAX or FVAX, thus resulting in the highest CD8/Treg and CD4/Treg ratios seen in this study. The triple combination also yielded the highest frequency of Ki67+ CD8+ T cells compared to that seen with the other combinations (Figs. 6d and Supplementary Fig. 7d). Combination treatment (either GVAX or FVAX plus α4-1BB and αPD-L1) also increased granzyme B expression, suggesting an enhancement in cytolytic potential of effector T cells (Figs. 6d and Supplementary Fig. 7d). Increased phosphorylation of T-bet and Eomes was induced by αPD-L1 antibody alone, and addition of α4-1BB to αPD-L1 produced only moderate further increases (Fig. 6e and 7e). Thus, the PD-L1 blockade induced TIL activation, and the combination of GVAX (or FVAX) and α4-1BB treatment resulted in a further stimulation of the proliferative and cytolytic capability of TILs. Taken together, these changes could result in a more permissive environment for immune-mediated tumor rejection.
Figure 7. In vivo PD-L1 blockade increases ovarian tumor antigen (FR-α) specific inflammatory cytokine production by ID8 TILs.
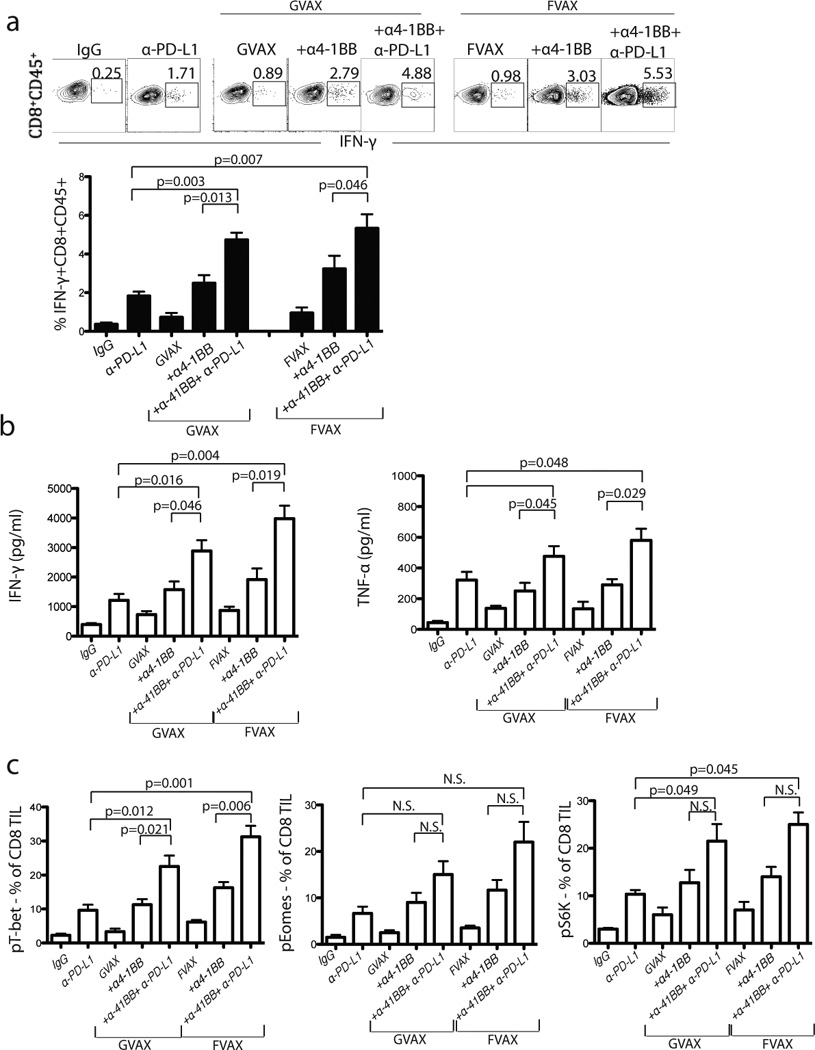
(a) Representative data and summary showing percentage of IFN-γ production by CD8+ TILs from treated mice as indicated. Following treatment with GVAX or FVAX and/or in vivo αPD-L1 and α-4-1BB antibody treatment, the TILs were harvested and stimulated ex vivo with FR-α peptide for 5 h at 37°C in the presence of brefeldin-A. For intracellular staining, permeabilized cells were stained for IFN-γ or phosphorylated form of transcription factors (T-bet, eomes and S6 kinase). (b) A week after completion of treatment, 2 × 105 CD8+ T cells from 5–10 pooled tumor draining mediastinal lymph nodes were re-stimulated with 1 × 105 FR-α peptide pulsed DCs for 36–48 h. Cytokine (IFN-γ and TNF-α) production from culture supernatant was measured following in vitro culture using cytokine bead array (CBA) kit. (c) Flow cytometry analysis showing percentage of phosphorylated (p) T-bet, pEomes and pS6K expression by CD8+ TIL from treated mice. All analyses were performed using CD8+CD45+ TIL from treated mice. N.S. not significant.
Combination therapy results in maximal stimulation of tumor-reactive CD8+ TILs
Given the significant changes in the tumor microenvironment, we next tested whether the functional capacity of tumor-reactive CD8+ TILs was increased following combination therapy. Tumors from the various treatment groups were freshly dissociated enzymatically, and total leukocytes isolated from these tumors were seeded in primary mixed co-cultures and stimulated with Kb-restricted peptides of folate receptor-α or mesothelin, two known ovarian tumor antigens expressed by ID8 cells. TIL cultures derived from mice treated with GVAX or FVAX showed minimally increased IFN-γ+ and TNF-α+ CD8+ TIL when stimulated with the FR-α (SSGHNECPV) and mesothelin (LSIFKHKL and LIWIPALL) peptides (Fig. 7a & b; and Supplementary Fig. 8). TIL cultures derived from mice treated with αPD-L1 alone showed modestly increased IFN-γ+ and TNF-α+ CD8+ TIL when stimulated with the peptides. TIL cultures derived from mice treated with GVAX (or FVAX) plus αPD-L1 (not shown) or α4-1BB showed further increased IFN-γ+ and TNF-α+ CD8+ TIL when stimulated with the peptides. However, when mice were treated with αPD-L1 combined with GVAX (or FVAX) and α4-1BB, TILs exhibited the highest numbers of IFN-γ+ and TNF-α+ CD8+ T cells ex vivo (Fig. 7a & b). Importantly, the increase in IFN-γ production by TILs specific to FR-α and mesothelin peptides appeared to be correlated with a decrease in tumor load (Figs. 3 & 5). The functional status of CD8+ TILs was corroborated by testing the phosphorylation status of key transcription factors. Triple combination therapy showed significant increases in pT-bet and pS6K levels, although only modest increase in pEomes (Fig. 7c).
Discussion
In this study, we demonstrate a critically important role of the PD-1 pathway in establishing an immune privilege site in the ovarian cancer microenvironment. Although tumors were infiltrated by T cells in very early stages of tumor orthotopic inoculation, a progressive reduction was noted in the frequency of CD8+ cells and the CD8/Treg cell ratio, until very few TILs were finally found in advanced tumors. Most of these were Tregs. The role of Tregs in mediating immune suppression and tumor growth has been previously established in ovarian cancer (24, 34). A parallel increase in MDSC and TAMs completed the picture of a well-organized tumor immune suppressive environment. These findings are important in two ways. First, they indicate that lack of tumor immunogenicity is not intrinsic, but rather acquired, at least in ID8 ovarian tumors. Second, we found that such progressive establishment of immune suppression can be reversed by blocking the PD-1 pathway. Thus, PD-1 besides mediating TIL exhaustion, can facilitate a profound depletion of TILs. Importantly, this phenomenon was mediated by PD-L1, since PD-L1 blockade produced similar results, but not PD-L2.
PD-1 blockade restored the immune decline and led to expansion of TILs. An increase in the number of CD4+ and CD8+ cells was associated with increased CD8/Treg and CD4/Treg ratios. This was associated with a moderate clinical response, along with molecular evidence of functional activation of TILs. Reversal of decline was time-sensitive, since PD-1 blockade was more effective in earlier times, when more TILs were present in tumor microenvironment. Moreover, the therapeutic effect of blocking PD-1/PD-L1 correlated with increased numbers of polyfunctional ovarian tumor-antigen (mouse folate receptor-α and mesothelin) specific CD8+ T cells. Our study also provides further insight that tumor antigen-specific CD8+ T cells upregulate the key effector and memory precursor signaling molecules (T-bet, Eomesodermin, Akt, and S6 kinase) up on PD-1/PD-L1 blockade. This is in agreement with recent studies that described inhibition of signaling pathways by PD-1/PD-L1 mediated T cell exhaustion (48–53).
Given this observation, we theorized that expanding the pool of tumor reactive TILs through a whole tumor antigen vaccine (GVAX or FVAX) could be beneficial in more advanced time points of the disease. We found that in fact, although ineffective on its own, vaccine could double the efficacy of PD-L1 blockade. Importantly, vaccine inefficiency was not due to its inability to expand tumor reactive TILs. Rather, increase in TILs was accompanied by a similar (compensatory) increase in Treg and other immunospuppressive populations (such as MDSC or pDCs). Thus failure of vaccines was due to their failure to reprogram the tumor microenvironment, which continued to take advantage of homeostatic anti-inflammatory mechanisms such as Treg, MDSC and/or pDCs. Importantly, PD-L1 blockade again was able to break these homeostatic mechanisms, to establish tumor rejection. In addition, we found administration of agonistic 4-1BB stimulation and α-PD-L1 after vaccination produced maximal expansion of TILs and tumor rejection. We acknowledge that the dose of 4-1BB antibody (200ug) used was high for human translation and that in humans, 4-1BB agonistic Abs have resulted in liver toxicity (54). However, we believe that our studies still provide proof of principle for adding costimulation to checkpoint blockade as a beneficial therapeutic combination. Interestingly, similar benefit was also obtained by administering CpG, a TLR9 agonist, intraperitoneally.
In this model PD-L1 blockade was very effective while PD-L2 blockade was ineffective. This effect is likely due to the high expression level of PD-L1 in ID8 tumor cells as well as tumor-derived myeloid cells. Although αPD-L2 blockade resulted in moderately increased levels of TIL infiltration (CD45+, CD8+), proliferation (Ki67+CD8+) and signaling molecules (Eomes, Akt, and pS6 kinase), Treg and MDSC levels were not reduced after αPD-L2 blockade. In this regard, we previously noticed low level expression of PD-L2 in other types of tumor models (23). Hence the lack of response to αPD-L2 blockade may be due to low level expression of PD-L2, lacking sufficient endogenous response upon in vivo blocking PD-L2. Although our studies provide strong evidence of a role of PD-L1 in the syngeneic ovarian model, they do not allow us to discern the relative contribution of PD-L1 expressed by tumor cells versus tumor stroma or infiltrating leukocytes. Future studies using PD-L1 KO mice and bone marrow chimeras will be required to clarify this point. PD-L1 blockade overcame the upregulation of MDSCs and pDCs by GVAX and FVAX, respectively. Previous studies reported that FVAX was more effective in combination with blockade therapy than GVAX (35). Our study used different clones of rat anti-mouse PD-1 (RMP1-30), and rat anti-mouse PD-L1 (10F.9G2) compared to Bioxcell antibodies, αPD-1 (RMP1-14), αPD-L1 (9G2) used in their study which may have accounted for eliminating the differences previously observed between the two vaccine preparations.
Our findings have important implications in understanding the immunobiology of ovarian cancer and developing rational therapies. We have previously described the classification of ovarian cancers in tumors with and tumors without intraepithelial TILs (4). The present findings suggest that tumors lacing intraepithelial TILs may have a very active PD-L1 mediated suppression, leading to depletion of TILs. This would be in agreement with observations by Hamanishi et al. reporting that increased expression of PD-L1 (but not PD-L2) is associated with poor survival in patients with advanced ovarian cancer, similar to the lack of intraepithelial TILs (10). Our finding of high frequency of Treg cells in tumors lacking TILs is consistent with an association of Treg cells with poor outcome in human ovarian cancer (4) and with the observation that in these tumors we found increased expression of vascular endothelial growth factor (4), which also correlates with Treg cell preponderance in the tumor microenvironment (34). Based on the present findings, human ovarian cancer lacking intraepithelial TILs should not lack intrinsic immunogenicity, consistent with the notion that most advanced ovarian cancers express known tumor-associated antigens and harbor tons of non-synonymous mutations, which could give rise to immunogenic epitopes. Rather, powerful immunosuppressive factors would eliminate TILs, suggesting that reversal of these factors would restore immune recognition and attack. PD-L1 blockade could be an important step towards reversing such immunosuppressive environment, but additional maneuvers are likely required to optimize tumor immune attack.
In the present study, addition of a whole tumor antigen vaccine significantly enhanced the ability of PD-L1 blockade. Importantly, just as in human patients with ovarian cancer treated with GVAX (38, 55), vaccine alone was ineffective in the mouse, but was quite beneficial in association with checkpoint blockade. We found that the reason whole tumor vaccines were ineffective was because they elicited a significant activation of tumor microenvironment homeostatic mechanisms, with increased MDSC or pDCs and no decrease in Treg cells. Importantly, PD-L1 blockade abrogated this rebound increase in immunosuppressive cells and depleted Treg cells, resulting in clinical benefit. Concomitant costimulation, through an agonistic antibody or CpG, further enhanced this benefit. Whole tumor antigens have been proposed as better alternatives than molecularly defined vaccines and can be effective in inducing antitumor immune responses in patients with ovarian cancer (56). The increased availability of immunomodulatory antibodies could provide a new role for whole tumor antigen vaccines according to our findings.
Supplementary Material
Acknowledgements
We thank Dr. J. Allison and Dr. M. Curran (MD Anderson Cancer Center, Houston, Texas) for GM-CSF and Flt3-ligand plasmids, Dr. R. Mittler (Emory Vaccine Center, Emory University, Atlanta) for rat anti-mouse 4-1BB Ab for initial experiments, and our lab members, Mr. T. Garrabrant and Mr. J. Leferovich. This work was supported by NIH CA083638 (GC), Pilot (JD), and NIH-P01 AI056299 (GF) grants.
Abbreviations
- ID8
ovarian cancer model
- TIL
tumor-infiltrating lymphocytes
- PD-1
programmed death-1
- PD-L
programmed death-1 ligands
- TAM
tumor associated macrophages
- DC
dendritic cells
- MDSC
myeloid derived suppressor cells
- Tregs
Foxp3+CD4+CD25+T cells
- ID8-GVAX or ID8-FVAX
ID8 tumor cells transduced to express GM-CSF or Flt3-ligand
Footnotes
Disclosures. G.J. Freeman draws royalties from patents regarding PD-1.
References
- 1.Bukowski RM, Ozols RF, Markman M. The management of recurrent ovarian cancer. Semin Oncol. 2007;34:S1–S15. doi: 10.1053/j.seminoncol.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Ozols RF. Challenges for chemotherapy in ovarian cancer. Ann Oncol. 2006;17(Suppl 5):v181–v187. doi: 10.1093/annonc/mdj978. [DOI] [PubMed] [Google Scholar]
- 3.Chu CS, Kim SH, June CH, Coukos G. Immunotherapy opportunities in ovarian cancer. Expert Rev Anticancer Ther. 2008;8:243–257. doi: 10.1586/14737140.8.2.243. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 5.Krempski J, Karyampudi L, Behrens MD, Erskine CL, Hartmann L, Dong H, Goode EL, Kalli KR, Knutson KL. Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer. J Immunol. 2011;186(12):6905–6913. doi: 10.4049/jimmunol.1100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leao IC, Ganesan P, Armstrong TD, Jaffee EM. Effective depletion of regulatory T cells allows the recruitment of mesothelin-specific CD8 T cells to the antitumor immune response against a mesothelin-expressing mouse pancreatic adenocarcinoma. Clin Transl Sci. 2008;1(3):228–239. doi: 10.1111/j.1752-8062.2008.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang B, Kuroiwa JM, He LZ, Charalambous A, Keler T, Steinman RM. The human cancer antigen mesothelin is more efficiently presented to the mouse immune system when targeted to the DEC-205/CD205 receptor on dendritic cells. Ann N Y Acad Sci. 2009;174:6–17. doi: 10.1111/j.1749-6632.2009.04933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuzaki J, Gnjatic S, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci USA. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilke CM, Kryczek I, Zou W. Antigen-presenting cell (APC) subsets in ovarian cancer. Int Rev Immunol. 2011;30:120–126. doi: 10.3109/08830185.2011.567362. [DOI] [PubMed] [Google Scholar]
- 13.Molon B, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu T, et al. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest. 2011;121:4015–4029. doi: 10.1172/JCI45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinha P, Clements VK, Miller S, Ostrand-Rosenberg S. Tumor immunity: a balancing act between T cell activation, macrophage activation and tumor-induced immune suppression. Cancer Immunol Immunother. 2005;54(11):1137–1142. doi: 10.1007/s00262-005-0703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 18.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010;16:348–353. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- 19.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 20.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–266. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 21.Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, Cubillos-Ruiz JR, Jacobs AC, Gonzalez JL, Weaver J, Fiering S, Conejo-Garcia JR. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med. 2012;209(3):495–506. doi: 10.1084/jem.20111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual Blockade of PD-1 and CTLA-4 Combined with Tumor Vaccine Effectively Restores T Cell Rejection Function in Tumors. Cancer Res. 2013;73(12):3591–3603. doi: 10.1158/0008-5472.CAN-12-4100. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Q, et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood. 2010;116:2484–2493. doi: 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, et al. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol. 2009;21:1065–1077. doi: 10.1093/intimm/dxp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 28.Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, Persons DL, Smith PG, Terranova PF. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21(4):585–591. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 29.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krutzik PO, Clutter MR, Nolan GP. Coordinate analysis of murine immune cell surface markers and intracellular phosphoproteins by flow cytometry. J Immunol. 2005;175:2357–2365. doi: 10.4049/jimmunol.175.4.2357. [DOI] [PubMed] [Google Scholar]
- 31.Irish JM, Kotecha N, Nolan GP. Mapping normal and cancer cell signalling networks: towards single-cell proteomics. Nat Rev Cancer. 2006;6:146–155. doi: 10.1038/nrc1804. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Yu Y, Yang S, Zeng B, Zhang Z, Jiao G, Zhang Y, Cai L, Yang R. Regulation of arginase I activity and expression by both PD-1 and CTLA-4 on the myeloid-derived suppressor cells. Cancer Immunol Immunother. 2009;58(5):687–697. doi: 10.1007/s00262-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, Katsaros D, O'Brien-Jenkins A, Gimotty PA, Coukos G. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14(1):28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 34.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475(7355):226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Y, Ju S, Chen E, Dai S, Li C, Morel P, Liu L, Zhang X, Lu B. T-bet and eomesodermin are required for T cell-mediated antitumor immune responses. J Immunol. 2010;185(6):3174–3183. doi: 10.4049/jimmunol.1000749. [DOI] [PubMed] [Google Scholar]
- 36.Curran MA, Geiger TL, Montalvo W, Kim M, Reiner SL, Al-Shamkhani A, Sun JC, Allison JP. Systemic 4-1BB activation induces a novel T cell phenotype driven by high expression of Eomesodermin. J Exp Med. 2013;210(4):743–755. doi: 10.1084/jem.20121190. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA-4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J. Clin. Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dranoff G. GM-CSF-secreting melanoma vaccines. Oncogene. 2003;22:3188–3192. doi: 10.1038/sj.onc.1206459. [DOI] [PubMed] [Google Scholar]
- 39.Mach N, et al. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]
- 40.Kingston D, Schmid MA, Onai N, Obata-Onai A, Baumjohann D, Manz MG. The concerted action of GM-CSF and Flt3-ligand on in vivo dendritic cell homeostasis. Blood. 2009;114(4):835–843. doi: 10.1182/blood-2009-02-206318. [DOI] [PubMed] [Google Scholar]
- 41.Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen CP, Pearson TC, Ledbetter JA, Aruffo A, Mittler RS. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev. 2009;229:192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 43.Kocak E, Lute K, Chang X, May KF, Jr, Exten KR, et al. Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res. 2006;66:7276–7284. doi: 10.1158/0008-5472.CAN-05-2128. [DOI] [PubMed] [Google Scholar]
- 44.Vezys V, Penaloza-MacMaster P, Barber DL, Ha SJ, Konieczny B, Freeman GJ, Mittler RS, Ahmed R. 4-1BB signaling synergizes with programmed death ligand 1 blockade to augment CD8 T cell responses during chronic viral infection. J Immunol. 2011;187(4):1634–1642. doi: 10.4049/jimmunol.1100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Curran MA, Kim M, Montalvo W, Al-Shamkhani A, Allison JP. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One. 2011;6(4):e19499. doi: 10.1371/journal.pone.0019499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCluskie MJ, Krieg AM. Enhancement of infectious disease vaccines through TLR9-dependent recognition of CpG DNA. Curr. Top. Microbiol. Immunol. 2006;311:155–178. doi: 10.1007/3-540-32636-7_6. [DOI] [PubMed] [Google Scholar]
- 47.Labidi-Galy SI, Sisirak V, Meeus P, Gobert M, Treilleux I, Bajard A, et al. Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer. Cancer Res. 2011;71:5423–5434. doi: 10.1158/0008-5472.CAN-11-0367. [DOI] [PubMed] [Google Scholar]
- 48.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chemnitz JM, et al. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 50.Wherry EJ, Ha S-J, Kaech SM, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Quigly M, Pereyra F, Nilsson B, et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med. 2010;16:1147–1151. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamphorst AO, Ahmed R. Manipulating the PD-1 pathway to improve immunity. Curr Opin Immunol. 2013 Apr 9; doi: 10.1016/j.coi.2013.03.003. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei F, Zhong S, Ma Z, Kong H, Medvec A, Ahmed R, Freeman GJ, Krogsgaard M, Riley JL. Strength of PD-1 signaling differentially affects T-cell effector functions. Proc Natl Acad Sci USA. 2013 Apr 22; doi: 10.1073/pnas.1305394110. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin Oncol. 2010;37(5):508–516. doi: 10.1053/j.seminoncol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Hodi FS, Butler M, Oble DA, Wang L, Noguchi T, Sato E, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kandalaft LE, Powell DJ, Jr, Chiang CL, Tanyi J, Kim S, Bosch M, Montone K, Mick R, Levine BL, Torigian DA, June CH, Coukos G. Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. Oncoimmunology. 2013 Jan 1;2(1):e22664. doi: 10.4161/onci.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


