Abstract
In this paper, we aimed to improve the carbohydrate productivity of Spirulina platensis by generating mutants with increased carbohydrate content and growth rate. ARTP was used as a new mutagenesis tool to generate a mutant library of S. platensis with diverse phenotypes. Protocol for rapid mutation of S. platensis by 60 s treatment with helium driven ARTP and high throughput screening method of the mutants using the 96-well microplate and microplate reader was established. A mutant library of 62 mutants was then constructed and ideal mutants were selected out. The characteristics of the mutants after the mutagenesis inclined to be stable after around 9th subculture, where the total mutation frequency and positive mutation frequency in terms of specific growth rate reached 45% and 25%, respectively. The mutants in mutant library showed diverse phenotypes in terms of cell growth rate, carbohydrate content and flocculation intensity. The positive mutation frequency in terms of cellular carbohydrate content with the increase by more than 20% percent than the wild strain was 32.3%. Compared with the wild strain, the representative mutants 3-A10 and 3-B2 showed 40.3% and 78.0% increase in carbohydrate content, respectively, while the mutant 4-B3 showed 10.5% increase in specific growth rate. The carbohydrate contents of the representative mutants were stable during different subcultures, indicating high genetic stability. ARTP was demonstrated to be an effective and non-GMO mutagenesis tool to generate the mutant library for multicellular microalgae.
Introduction
Microalgae have been paid more attention because of their wide range of applications by diverse functions, such as in food, cosmetics and pharmaceutical industry [1-4]. Microalgae also can be used to treat wastewater to form biomass, thereby increasing the efficiency of microalgae productivity [1,5-9]. In recent years, microalgae as cell factories for production of biofuels and biochemicals have attracted increasing attention, due to their fixation of CO2 for reduction of the green-house gas[10]. As for biofuel production, there are two potential approaches for microalgae biotechnology: 1) harvesting lipid from the microalgae cells to produce bioiesel; 2) biofuel fermentation from the microalgae biomass capable of storing carbohydrate such as starch and glycogens, which can be used as non-edible feedstock for fermentation. Many studies have been carried out on cultivation of microalgae with the lipid content ranging from 20% to 50% to produce biodiesel[11]. Although microalgae have features such as relatively high growth rate, further efforts are required to screen or breed microalgae species with higher lipid content, efficiently harvest biomass, especially for the unicellular microalgae, establish the energy-saving lipid extraction process and develop the integrated system for economic biodiesel production [12-14]. On the other hand, using the carbohydrate-containing microalgae as the substrates for fermentation is also worth paying attention, since the saccharification of the whole microalgae biomass containing carbohydrate is relatively easily reachable for the subsequent fermentation[15-17]. For all these applications of microalgae, effective strain improvement technique is indispensable, especially non-GM (genetic modification) method for open-pond cultivation of microalgae.
Spirulina platensis is a kind of filamentous cyanobacteria with multicellular cylindrical trichomes. This microalgae has many properties such as high protein content, unique composition of fatty acids and vitamins, and high carbohydrate content [18], indicating that S. platensis can be a suitable feedstock for fermentation. Also, S. platensis can be flocculated because of its filamentous form, which makes its harvesting easier than other microalgae[19]. The carbohydrate generally make up 15-20% of the dry weight of S. platensis, which are mainly branched sugars consisting of only glucose and structurally similar glycogen [20,21]. It has been revealed that glucose content in S. platensis reach 7-8% of dry cell weight [2], which will make the hydrolysis process much easier. In the last decade, S. platensis has been commercially produced for food supplement[22]. The mature outdoor culture method will provide a platform for S. platensis application as the fermentation feedstock.
In order to make the biomass of S. platensis useful as fermentation feedstock, strain improvement for increasing the growth rate and carbohydrate content is still needed. The genetic engineering method is widely used to improve microbial performance, however it still has difficulties in modification of S. platensis[19], presumably due to the gliding motility on agar plate, lacking of the genetic modification tools and difficulty in introduction of the foreign DNA molecules[19,23,24]. Construction of a gene transfer system for S. platensis has been tried. Toyomizu et al. increased the transformation efficiency of electroporation by the optimized electric-field strength and time constant[25]. Kawata et al. applied a modified transformation strategy using a natural Tn5 transposon, transposase, and cation liposome complex by electroporation to improve the transformation efficiency[26]. However, instability of the transformants and low efficiency of foreign gene expression have been the major obstacles, so little success has been reached for efficient genetic engineering of S. platensis to improve its phenotypes.
As a non-GM mutation method, random mutagenesis has been a useful tool and widely used for generating mutants of different microorganisms. Different mutation techniques including chemical and physical mutagens have been applied to S. platensis. Singh et al. reported that after chemical mutagen (nitrosoguanidine, NTG) treatment, the isolated mutant of S. platensis cells exhibited approximately three-fold higher tolerance to metronidazole and DCMU (3,4-dichlorophenyl-1, 1-dimethylurea) as compared with wild-type strain [27], but they didn’t improve the growth of S. platensis. With the same mutagen NTG, Riccardi et al. isolated S. platensis mutants resistant to 5-fluorotryptophan, β-2-thienyl-alanine, ethionine, p-fluprophenlalanine or azetidine-2-carboxylic acid. A few of the mutants overproduce the corresponding amino acids such as proline or valine[28,29], but the highest mutation frequency is 1.2~7.1×10-6 per plated filaments. Lanfaloni et al. tried to create the mutants of S. platensis resistant to 8-azaguanine or β-2-thienyl-DL-alanine treated by 1-3 min UV irradiation followed by incubation with 50 μg N-methyl-N-nitro-N-nitrosoguanidine (MNNG), but they could not isolate an ideal mutant [30]. What is more, all the chemical mutagenesis approaches have the safety problem to the operator and environment. Therefore, a new mutagenesis tool which is rapid, effective, safe and environmental benign is needed for effective mutation of S. platensis.
Atmospheric and room temperature plasma (ARTP) is a powerful and novel physical microbial mutagenesis tool for creating mutant library of microorganisms [31-33]. ARTP driven by the radio-frequency power can be generated uniformly at atmospheric pressure without any vacuum system and the plasma can be controlled at room temperature, which is beneficial for microbial mutation. Active chemical species with the high density are the main composition of ARTP, which can penetrate the cell wall and membrane, damage DNA molecules, cause the mutation and thereby alter the metabolic networks of the target microbes[32-36]. An automated ARTP mutation breeding system has been invented by our group [32], which is compact and can be operated safely and feasibly, enabling rapid and diverse genome mutation of microbes in a non-GMO manner.
In this paper, we aimed to mutate S. platensis to generate the mutants with high carbohydrate content and high growth rate by ARTP mutation system, which are indispensable for increasing the productivity of S. platensis biomass as the fermentation feedstock. We applied the ARTP mutagenesis system to establish the mutation and a high throughput screening procedure for S. platensis to generate the mutant library. Then, S. platensis mutants with different carbohydrate contents and growth rates were selected out and examined.
Materials and Methods
1: Materials
Spirulina platensis, the wild type strain (S. platensis FACHB-904, Freshwater Algae Culture Collection of the Institute of Hydrobiology, Wuhan, China) and the mutants generated by ARTP, were routinely grown in Zarrouk medium [37] by static cultivation in an artificial climate incubator at 28 °C with white fluorescent lamp set light intensity of 49~57 μmol·m-2·s-1, with a cycle of 10 h light/14 h dark for the culture. 2. Mutation procedure of S. platensis by ARTP
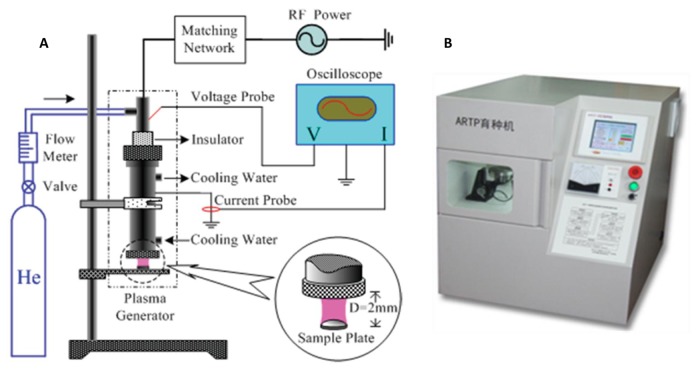
The ARTP mutation system (Figure 1) consisted of an RF(13-56 MHz) power supply subsystem, a co-axial type plasma generator, a gas supply control subsystem, and a simple plate made of stainless steel [33,35,36]. In this study, pure helium was used as the plasma working gas and the operating parameters were as follows: 1) The RF power input was 100 W, 2) the distance between the plasma torch nozzle exit and the sample plate (D) was 2 mm; 3) the temperature of the plasma jet was 25-35 °C. Under these determined conditions, the mutagenesis dosage by ARTP was dependent on the treatment period. To determine the optimal treatment period, S. platensis grown at OD560 of 0.8~1.0, which was within the log-phase, was selected to perform ARTP treatment. For the mutation, 10 μl of the culture was dipped onto the stainless steel minidisc and then exposed to ARTP jet for 5, 10, 20, 40, 60 and 80 s, respectively.
Figure 1. ARTP mutagenesis system.
Schematic diagram of ARTP (A) and outlook of the instrument (B).
After the ARTP treatment for different time, the S. platensis samples were all washed by 10-20 μl fresh broth and moved to the inverted microscope to observe the change of morphology. The S. platensis filaments were cut into short fragments because of the action of the active plasma generated by ARTP on the cell walls of S. platensis and the fragments with green color were considered to be survived. The spirals of each green S. platensis fragments were counted and the total number of spirals was calculated. The survival rate was estimated by dividing the number of the total spirals survived after ARTP treatment by the number of the total spirals before ARTP treatment and then multiplied it by 100%.
3: High throughput screening of the mutants
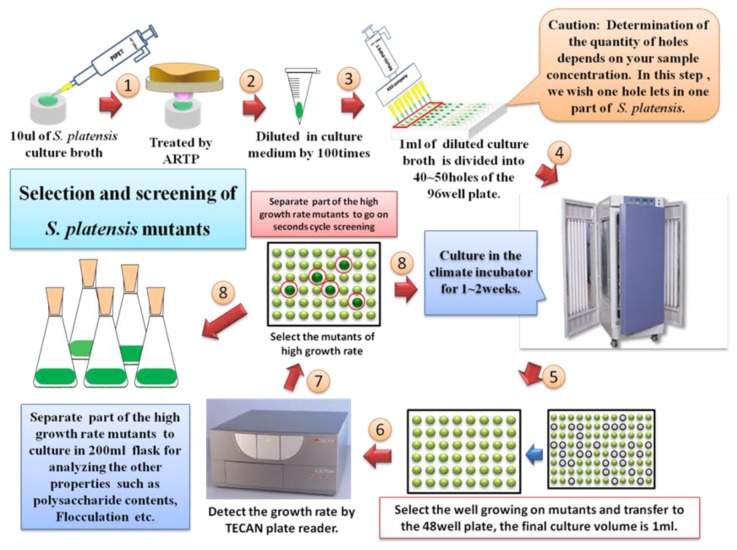
Since S. platensis is a kind of multicellular microalgae, after ARTP treatment, the long spirals or filaments were cut into short ones, the efficient separation of the short filaments by controlling the smallest spiral number into a well of microplate will ensure the efficiency to rapidly screen the mutants by aiding the microplate reader. Figure 2 shows the established high throughput screening process: 1) after ARTP treatment, ARTP-treated S. platensis was first diluted with the fresh medium by different dilution rates; 2) the diluted S. platensis solution was transferred to each well of 96-well plates to make sure that each hole contained at most one filament. This process was confirmed by a microscope (Nikon, Japan); 3) after a period of cell division and growth, some wells exhibited green color while some holes showed blank without S. platensis growing. The blank one was abandoned and the green one was picked out and transferred to 48-well plates for further screening culture. During the screening process with the 48-well plates, we detected OD560 value of each well for generating the growth curve of mutants by a microplate reader (Tecan, Switzerland). The wild strain was also cultivated in the microplate wells for comparison. After several times of subculture with the 48-well microplates where one subculture was carried out for 10 d in the artificial climate incubator, when the characteristics of the mutants became stable, all the mutants and wild strain were transferred into 50 ml flasks containing 20 ml medium for further cultivation of 10 d to detect the carbohydrate content and flocculation intensity of each strain. After establishing the mutant library, the representative mutants were chosen and cultivated in 200 ml flasks each containing 50 ml medium of two weeks to analyze the specific growth rate, flocculation intensity and carbohydrate content precisely. The genetic stability of the selected mutants was also examined by a series of subcultures for the carbohydrate content detection.
Figure 2. The experimental protocol for high throughput screening of the S. platensis mutants generated by ARTP.
4: Analysis
4.1: Biomass specific growth rate and mutation frequency
The biomass concentration was determined by measuring the OD560 value of the culture at 560nm with a microplate reader (Tecan, Switzerland) using the 48-wells plates. Specific growth rate (μ) was calculated by following Eq. (1)[24].
| (1) |
where, x 1 and x 2 were the biomass concentration in terms of dry cell weight at time intervals t 1 and t 2. Biomass concentration of dry cell weight was calculated by following Eq. (2)
| (2) |
where, x and y were biomass concentration (g/L) and OD560 value, respectively, which was obtained from the relationship between dry cell weight and OD560 value.
The mutant whose growth rate was higher or lower than that of the wild strain by 20% was defined as the an effective mutant, and the percentage of the effective mutants in the mutant library was defined as mutation frequency. The mutant whose growth rate was higher than that of the wild type by 20% was defined as a positive mutant, and the percentage of the positive mutants in the library was defined as positive mutation frequency.
4.2: Carbohydrate content
After biomass culture was collected by centrifugation at 13000×g for 10 min, 6 N HCl solution of same volume was used to break up the cell walls of S. platensis and hydrolyze the intracellular carbohydrate at 95°C for 10 min, then 6 N NaOH of the same volume were added to neutralize the solution. The formed reduced sugar was detected by the DNS methods for calculating the carbohydrate content [38].
4.3: Chlorophyll assay
The chlorophyll(Chl) content was measured according to the method proposed by Lee [39] and Chen [40]. The 5 ml S. platensis spirals were collected by centrifugation at 13000×g for 10 min. The collected cells were washed 3 times by 0.9% NaCl solution, followed by the addition of 5 ml of 90% methanol solution, and then sonication. The extracted solution was measured at 665 and 650nm by UV-spectrophotometer, respectively, and the Chl content (mg/L) was evaluated according to Eq. (3) [39,40]
| (3) |
4.4: Flocculation intensity
For detection of the flocculation of S. platensis, optical density of the supernatant (AS) and mixture (AM) of the culture broth were detected at 560nm by UV-spectrophotometer (GE, New York, USA). The degree of flocculation intensity (F) was calculated by Eq. (4).
| (4) |
Results
1: Mutation procedure setup and assessment
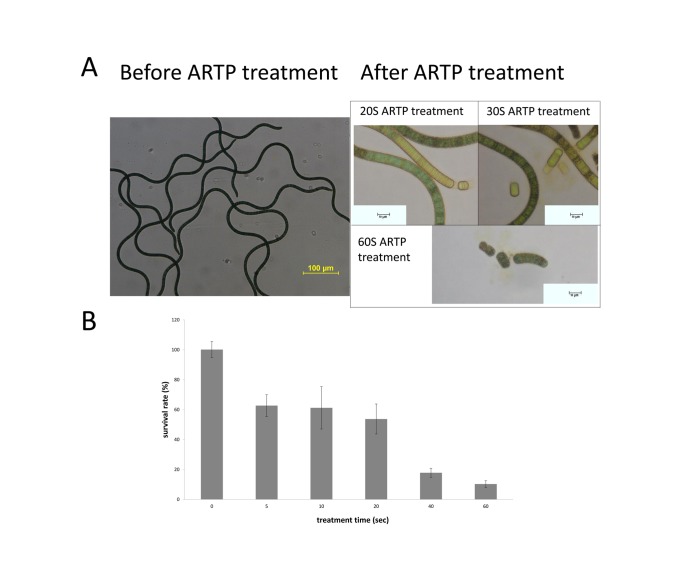
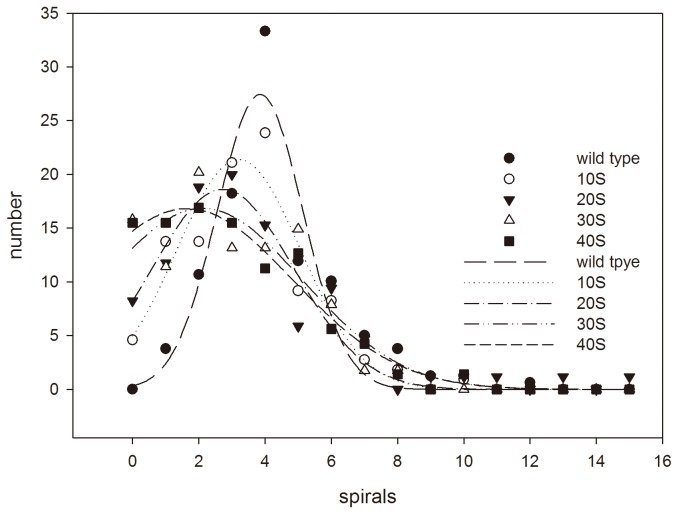
In this study, S. platensis was mutated by the new mutation tool of ARTP (Figure 1). For generating a mutant library, the ARTP mutagenesis condition was firstly determined by detecting the survival rate and morphology change of S. platensis. The survival rate decreased to 10.28% when the treatment time was 60 s (Figure 3B). Meanwhile, morphology was also an important feature of S. platensis, as shown in Figure 3A, after the ARTP treatment, the long spirals of S. platensis were cut into short ones, the broken pieces of S. platensis were presented as healthy green color when the treatment time was short, while with the increase of the treatment time, the spirals became fewer. When the treatment time exceeded 60 seconds, S. platensis spirals were all broken into small debris. Figure 4 shows the change in spiral number distribution of S. platensis before and after ARTP treatment for different treatment time by regressing with Gaussian distribution. The peak of spiral numbers of wild S. platensis without ARTP treatment was about 3.9, while after 40 seconds of ARTP treatment, the peak for the spiral numbers decreased to 1.6. When the treatment time was longer, the peak value of the spirals became lower. This result demonstrated that the ARTP treatment could cause breakage of the filaments of S. platensis.
Figure 3. Change of S. platensis after treatment of ARTP.
Change of morphology (A) and survival rate (B) of S. platensis during ARTP treatment (n=3).
Figure 4. Change in the number distribution of S. platensis spirals with increase of ARTP mutagenesis dosage.
In this figure, the length of S. platensis was calculated by spiral numbers in one filament. The number distribution of spirals during the ARTP treatment was regressed by Gaussian distribution, X0: peak, R: Regression, SEE:Standard Error of Estimate (Table 3).
Table 3. Values in Gaussian distribution of Figure. 4.
| Time | 0 | 10S | 20S | 30S | 40S |
|---|---|---|---|---|---|
| Xo | 3.8502 | 3.2050 | 2.7308 | 2.1184 | 1.6447 |
| R | 0.9429 | 0.9661 | 0.9619 | 0.9521 | 0.9882 |
| SEE | 3.2454 | 2.2099 | 2.0365 | 2.3971 | 1.1435 |
2: Mutant screening and quality assessment of the mutant library
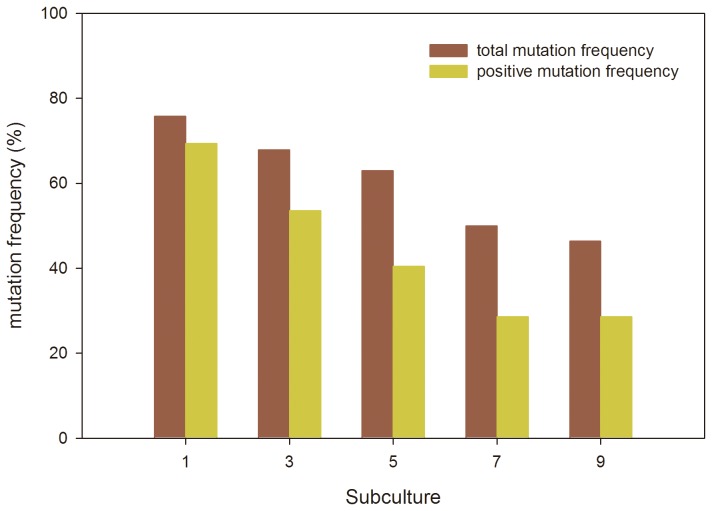
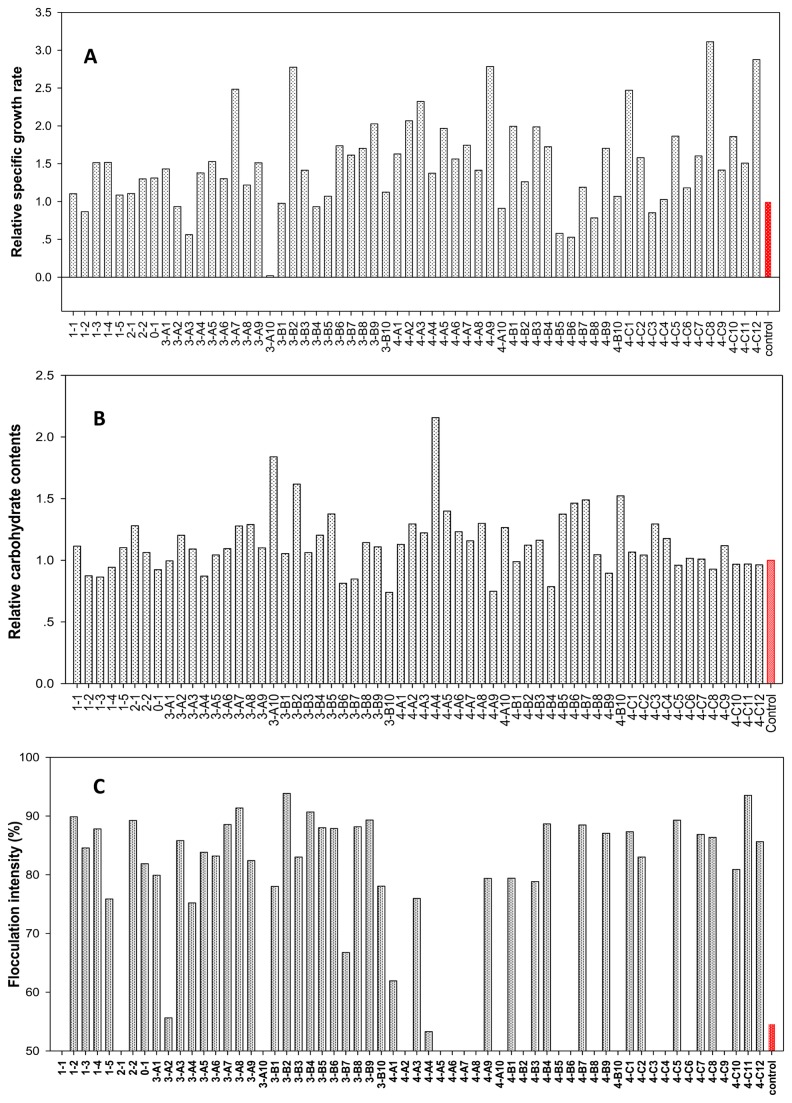
After the ARTP mutagenesis for S. platensis, 62 mutants of S. platensis were obtained from the 96-well microplate after 10 days culture. These 62 mutants were transferred to 48-well plates for subsequent subcultures of 10 days. The specific growth rate of the mutants and wild strain at different subcultures were listed in Supporting Information (Figure S1). As to the first subculture, we got a 72.6% positive mutation frequency based on the growth rate. During the subsequent subculture, some mutants, such as 4-A5, 4-B8, 4-B10 and 4-C7, were presumably unstable and recovered, causing the change in the mutant distribution with the subculture. While some mutants, such as 1-3, 3-B9, 3-A10 and 4-C11,showed stability with high growth rate. Figure 5 showed the change in mutation frequency in terms of specific growth rate with the subcultures, indicating that the mutation frequency was trended to be stable after 9th subculture, with 45% total mutation frequency and 25% positive mutation frequency in terms of specific growth rate (Figure 5). After 10th subculture, the specific growth rate (Figure 6A), carbohydrate content and the flocculation intensity of the mutants were evaluated and compared with the wild strain in 50 ml flask culture of 10 days. In the mutant library, 32.3% of the mutants showed increase in carbohydrate content by over 20% compared with wild type (Figure 6B), and 12.9% of the mutants exhibited the increase in flocculation intensity by more than 100% compared with wild type (Figure 6C).
Figure 5. Change in mutation frequency in terms of specific growth rate during subcultures.
Figure 6. Characteristics of mutants in the mutant library.
Comparison of the relative specific growth rate (A), relative carbohydrate content (B) and flocculation intensity(C) of the mutants and the wild strain of S. platensis after the 10th subculture.
3: Analysis of the representative mutants
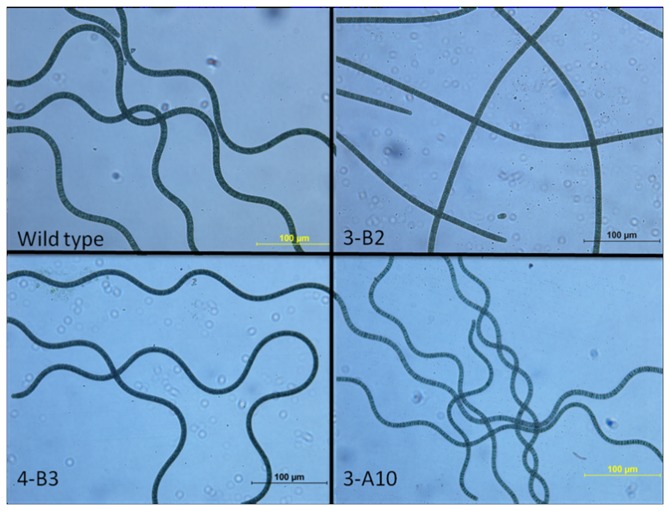
From the mutant library after 10th subculture, 3 representative mutants were selected to analyze their characteristics in 200 mL flask culture of two weeks: 3-A10, with the slower initial growth rate but higher carbohydrate content and no flocculation intensity; 3-B2, with higher carbohydrate content, higher growth rate and improved flocculation intensity; and 4-B3, with higher growth rate, carbohydrate content similar to wild strain and higher flocculation intensity. Table 1 listed the characteristics of these three mutants and comparison with the wild strain after 10th subculture. The mutant of 4-B3 showed the highest growth rate of 0.093 g/(L×d), 3-B2 showed flocculation intensity of 92.5%, and carbohydrate content of 33.1%, which was increased by 78.0% than the wild type. Moreover, if the carbohydrate content was assumed to be constant during the culture, from the data of specific growth rate and the carbohydrate content, the carbohydrate productivity could be estimated. Mutant of 3-B2 also showed the highest carbohydrate productivity of 26.0 mg/(L×d) which was 65.6% higher than the wild type (Table 1). It was also found that chlorophyll content was higher in the mutants with improved growth rate (Table 1). Figure 7 showed the morphology of the 3 mutants and the wild strain. The mutant of 3-B2 had the big change from the original spiral of the wild strain to linear filament. The stability of carbohydrate content of different mutants and wild strain (control) during different subcultures was shown in Table 2, indicating high genetic stability.
Table 1. Characteristics of the mutants and the wild strain (control) (n=3).
| Growth rate (g/L×d) | Flocculation (%) | Carbohydrates content (g/g) | Carbohydrates productivity (mg/L×d ) | Chlorophyll (mg/g) | |
|---|---|---|---|---|---|
| Control | 0.0842±0.018 | 63.6±6.13 | 0.186±0.045 | 15.7±3.8 | 3.53±0.069 |
| 3-A10 | 0.0583±0.016 | 46.1±3.65 | 0.261±0.051 | 15.2±3.0 | 1.07±0.008 |
| 3-B2 | 0.0786±0.017 | 92.3±9.33 | 0.331±0.049 | 26.0±3.9 | 3.51±0.040 |
| 4-B3 | 0.0930±0.025 | 40.7±1.83 | 0.131±0.046 | 12.2±4.3 | 4.52±0.446 |
Figure 7. Comparison of morphology of the representative mutants and the wild strain of S. platensis.
Table 2. Stability of carbohydrate content (g/g) of different mutants and wild strain (control) during different subcultures (n=3).
| subculture | 11 | 15 | 20 |
|---|---|---|---|
| Control | 0.177±0.022 | 0.184±0.012 | 0.186±0.011 |
| 3-A10 | 0.265±0.021 | 0.249±0.032 | 0.261±0.025 |
| 3-B2 | 0.333±0.027 | 0.327±0.024 | 0.331±0.014 |
| 4-B3 | 0.134±0.020 | 0.141±0.026 | 0.131±0.031 |
Discussion
In this study, we successfully applied a novel ARTP mutagenesis tool for the first time to mutate S. platensis spirals for construction of a mutant library with altered carbohydrate content, growth rate and flocculation intensity. Since S. platensis is a multicellular microorganism, single cell might be more feasible for ARTP mutagenesis. Actually, we tried to make single cells from S. platensis spirals and we succeeded in making single cells by sonication. However, it was quite difficult for the separated single cells to survive, and after overnight recovering culture, only few cells survived, making them impossible to be mutated by ARTP treatment. Therefore, in this study, S. platensis spirals were used directly for ARTP mutagenesis. The ARTP mutagenesis has been succeeded in mutation breeding of many kinds microbes [32,35,41-43]. As shown in this study, the long spirals of S. platensis were cut into shorter ones, indicating the breakage effect of ARTP on S. platensis, which proved the direct interaction of the plasma and S. platensis. The ARTP driven by pure helium contains a large amount of activated chemical species[32,33,36,44,45], which can penetrate the cell wall and cell membrane, and then damage DNA and proteins, thereby creating mutation via damaging the genome in the cells [33]. At the determined RF power input and D (the distance between the plasma torch nozzle exit and the sample plate), ARTP radiation time is proportional to the plasma dosage, i.e., mutagenesis dosage. When the ARTP treatment period was more than 60 seconds, the cells of S. platensis were all dead (data not shown). The possible reasons might be that, after the long period of ARTP treatment, single cells from the spirals of S. platensis were released, presumably leading to the difficulty in recovery, and/or apart from the plasma action, when the spirals were exposed to the helium gas flow for long time, the water of the medium would be brought away to make cells dried, causing the death of S. platensis.
For construction of S. platensis mutant library, a high throughput screening method for the mutants is of importance. The gliding growth properties and slow growth of S. platensis on the solid medium cause the difficulty in using the colony formation for mutant screening from the ARTP-treated spirals[24]. In this study, we successfully established a dilution method using 96-well microplates combined with the microscope observation to put at most one filament into each well of the microplates and to screen the mutants with microplate reader after the ARTP mutagenesis of multicellular S. platensis (Figure 2). For the multicellular S. platensis, sonication to generate the fragmented filaments of S. platensis prior to mutagenesis is reportedly helpful to improve mutagenesis [24]. After ARTP treatment, the filaments of S. platensis were significantly cut into short fragments (Figure 3), presumably contributing to the high mutation frequency. However, the short spirals were still multicellular, which might contain different DNA damage degree and different DNA damage locations for the different cells in a spiral and/or different spirals, probably causing the mutants with different phenotypes. Therefore, controlling the number of the shortly cut filaments in one well of microplates to minimum value by dilution was effective to screening of the mutants after ARTP treatment on S. platensis. Moreover, during the subculture of 10 days of the mutants using 48-well microoplates for screening, some of them would be enriched. The percentage of mutants with higher or lower growth rate changed as the subculture times increased, so the characteristics of the mutants showed difference between different subcultures before reaching the stable state (Figure S1 in Supporting Material and Figure 5). In fact, after more than the 9th subculture, the specific growth rates of the mutants became stable (Figure 5).
In addition, some mutants showed the morphology changes. Previous studies showed that the pitch and diameter of the spirals had significant relation with the photosynthesis activity and biomass growth [24,46]. Among the mutant library, morphology of the mutant 3-B2 was changed from spiral to near linear shape. Wang and Zhao reported that the morphogenesis of S. platensis filaments from spiral to linear shape was due to DNA mutation [47]. Wu et al. found that UV broke the spiral structure and suggested that, over long-time radiation, adaptation of S. platensis to solar UVR could bring about changes in the spiral structure from rather loose helix to a very compressed helix[48]. In this study, the mutant 3-B2 changed its shape just after 3~4 subcultures after the ARTP mutation, and the change of spirals from spiral shape to linear shape might be related to the highest flocculation intensity of the mutant 3-B2 (Table 1). This result also suggested that, ARTP treatment caused the genome mutation of S. platensis.
In this research, we selected 3 representative mutants with different phenotypes, 3-A10, 3-B2 and 4-B3. The high genetic stability in terms of carbohydrate content of these representative mutants was confirmed by subcultures (Table 2). Compared with the wild strain, the mutant of 3-B2 with similar growth rate, higher flocculation intensity, higher carbohydrate content (33.1%) and higher carbohydrate productivity (26.0 mg/L×d ), which was 65.6% higher than the wild type, would increase the productivity of S. platensis biomass as the fermentation feedstock for the further application. The different phenotypes among the 3 mutants also demonstrated the efficiency of ARTP mutagenesis method implying that ARTP mutagenesis could also be used to generate the mutants of S. platensis with tolerance to CO2 and salt or other factors , which is undergoing now. Moreover, the undergoing genome sequencing and comparative analysis of these 3 mutants and wild strain showed that the metabolic networks of carbon fixation and metabolites were changed after mutagenesis (data not shown), and this study will elucidate the genome mutation mechanism by ARTP mutagenesis, which are responsible for these different phenotypes, thus helpful to guide the further strain modification to create ideal mutants.
Supporting Information
Comparison of relative specific growth rates of the mutants generated by ARTP mutagenesis and wild strain of S. platensis after different subculture. Relative specific growth rates of 1st(A) ,2nd(B) ,5th(C) and 9th(D) subculture.
(DOCX)
Funding Statement
This work was supported by JSTCREAST of Japan, Tsinghua University Initiative Scientific Research Program (2011Z01019) and China postdoctoral science foundation (No.20100480280). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Markou G, Georgakakis D (2011) Cultivation of filamentous cyanobacteria (blue-green algae) in agro-industrial wastes and wastewaters: A review. Appl Energ 88: 3389-3401. doi: 10.1016/j.apenergy.2010.12.042. [DOI] [Google Scholar]
- 2. Ortega-Calvo J, Mazuelos C, Hermosin B, Saiz-Jimenez C (1993) Chemical composition of Spirulina and eukaryotic algae food products marketed in Spain. J Appl Phycol 5: 425-435. doi: 10.1007/BF02182735. [DOI] [Google Scholar]
- 3. Harun R, Singh M, Forde GM, Danquah MK (2010) Bioprocess engineering of microalgae to produce a variety of consumer products. Renewable Sustain Energ Rev 14: 1037-1047. doi: 10.1016/j.rser.2009.11.004. [DOI] [Google Scholar]
- 4. Pangestuti R, Kim S-K (2011) Biological activities and health benefit effects of natural pigments derived from marine algae. J Funct Foods 3: 255-266. doi: 10.1016/j.jff.2011.07.001. [DOI] [Google Scholar]
- 5. Vasconcelos VM, Martins J, Peixe L (2011) Unraveling Cyanobacteria Ecology in Wastewater Treatment Plants (WWTP). Microb Ecol 62: 241-256. doi: 10.3354/ame01461. PubMed: 21287346. [DOI] [PubMed] [Google Scholar]
- 6. Logan C, Sims R (2011) Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol Adv 29: 686-702. doi: 10.1016/j.biotechadv.2011.05.015. PubMed: 21664266. [DOI] [PubMed] [Google Scholar]
- 7. Pittman JK, Dean AP, Osundeko O (2011) The potential of sustainable algal biofuel production using wastewater resources. Bioresour Technol 102: 17-25. doi: 10.1016/j.biortech.2010.06.035. PubMed: 20594826. [DOI] [PubMed] [Google Scholar]
- 8. Chinnasamy S, Bhatnagar A, Hunt RW, Das KC (2010) Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour Technol 101: 3097-3105. doi: 10.1016/j.biortech.2009.12.026. PubMed: 20053551. [DOI] [PubMed] [Google Scholar]
- 9. Rawat I, Ranjith Kumar R, Mutanda T, Bux F (2011) Dual role of microalgae: Phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl Energ 88: 3411-3424. doi: 10.1016/j.apenergy.2010.11.025. [DOI] [Google Scholar]
- 10. Bezerra RP, Montoya EYO, Sato S, Perego P, de Carvalho JCM et al. (2011) Effects of light intensity and dilution rate on the semicontinuous cultivation of Arthrospira (Spirulina) platensis. A kinetic Monod-type approach. Bioresour Technol 102: 3215-3219. doi: 10.1016/j.biortech.2010.11.009. PubMed: 21111613. [DOI] [PubMed] [Google Scholar]
- 11. Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M et al. (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54: 621-639. doi: 10.1111/j.1365-313X.2008.03492.x. PubMed: 18476868. [DOI] [PubMed] [Google Scholar]
- 12. Brennan L, Owende P (2010) Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renewable Sustain Energ Rev 14: 557-577. doi: 10.1016/j.rser.2009.10.009. [DOI] [Google Scholar]
- 13. Amaro HM, Guedes AC, Malcata FX (2011) Advances and perspectives in using microalgae to produce biodiesel. Appl Energ 88: 3402-3410. doi: 10.1016/j.apenergy.2010.12.014. [DOI] [Google Scholar]
- 14. Xu L, Brilman DWF, Withag JAM, Brem G, Kersten S (2011) Assessment of a dry and a wet route for the production of biofuels from microalgae: Energy balance analysis. Bioresour Technol 102: 5113-5122. doi: 10.1016/j.biortech.2011.01.066. PubMed: 21334889. [DOI] [PubMed] [Google Scholar]
- 15. Matsumoto M, Yokouchi H, Suzuki N, Ohata H, Matsunaga T (2003) Saccharification of marine microalgae using marine bacteria for ethanol production. Appl Biochem Biotechnol 105: 247-254. doi: 10.1385/ABAB:105:1-3:247. PubMed: 12721490. [DOI] [PubMed] [Google Scholar]
- 16. Hirayama S, Ueda R, Ogushi Y, Hirano A, Samejima Y et al. (1998) Ethanol production from carbon dioxide by fermentative microalgae. In: Yamaguchi T, T Inui MAKISY. Studies in Surface Science and Catalysis: Elsevier; pp. 657-660. [Google Scholar]
- 17. Harun R, Danquah MK, Forde GM (2010) Microalgal biomass as a fermentation feedstock for bioethanol production. J Chem Technol Biotechnol 85: 199-203. [Google Scholar]
- 18. Mohapatra TM, Singh RK, Tiwari SP, Rai AK (2011) Cyanobacteria: an emerging source for drug discovery. J Antibiot 64: 401-412. doi: 10.1038/ja.2011.21. PubMed: 21468079. [DOI] [PubMed] [Google Scholar]
- 19. Borowitzka MA, Borowitzka LJ, editors (1988) Micro-algal biotechnology. Cambridge: Cambridge University Press. 395 pp. [Google Scholar]
- 20. Ciferri O (1983) Spirulina, the edible microorganism. Microbiol Rev 47: 551-578. PubMed: 6420655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aikawa S, Izumi Y, Matsuda F, Hasunuma T, Chang J-S et al. (2012) Synergistic enhancement of glycogen production in Arthrospira platensis by optimization of light intensity and nitrate supply. Bioresour Technol 108: 211-215. doi: 10.1016/j.biortech.2012.01.004. PubMed: 22277210. [DOI] [PubMed] [Google Scholar]
- 22. Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101: 87-96. doi: 10.1263/jbb.101.87. PubMed: 16569602. [DOI] [PubMed] [Google Scholar]
- 23. Kawamura M, Sakakibara M, Watanabe T, Kita K, Hiraoka N et al. (1986) A New Restriction Endonuclease from Spirulina-Platensis. Nucleic Acids Res 14: 1985-1989. doi: 10.1093/nar/14.5.1985. PubMed: 3008081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vonshak A (1997) Spirulina platensis, physiology, cell-biology and biotechnology. London: Taylor & Francis Ltd. [Google Scholar]
- 25. Toyomizu M, Suzuki K, Kawata Y, Kojima H, Akiba Y (2001) Effective transformation of the cyanobacterium Spirulina platensis using electroporation. J Appl Phycol 13: 209-214. doi: 10.1023/A:1011182613761. [DOI] [Google Scholar]
- 26. Kawata Y, Si Yano, Kojima H, Toyomizu M (2004) Transformation of Spirulina platensis Strain. (Arthrospira SP PCC C1: 9438) with Tn5 Transposase–Transposon DNA–Cation Liposome Complex. Marine Biotechnology 6: 355-363 [DOI] [PubMed] [Google Scholar]
- 27. Singh DP, Singh N (1997) Isolation and characterization of a metronidazole tolerant mutant of the cyanobacterium Spirulina platensis exhibiting multiple stress tolerance. World J Microbiol Biotechnol 13: 179-183. doi: 10.1023/A:1018537730287. [DOI] [Google Scholar]
- 28. Riccardi G, Sora S, Ciferri O (1981) PRODUCTION OF AMINO-ACIDS BY ANALOG-RESISTANT MUTANTS OF THE CYANOBACTERIUM SPIRULINA-PLATENSIS. J Bacteriol 147: 1002-1007. PubMed: 6792182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Riccardi G, De Rossi E, Milano A, De Felice M (1988) Mutants of Spirulina platensis resistant to valine inhibition. FEMS Microbiol Lett 49: 19-23. doi: 10.1016/0378-1097(88)90095-X. [DOI] [Google Scholar]
- 30. Lanfaloni L, Trinei M, Russo M, Gualerzi CO (1991) Mutagenesis of the Cyanobacterium Spirulina-Platensis by Uv and Nitrosoguanidine Treatment. FEMS Microbiol Lett 83: 85-90. doi: 10.1111/j.1574-6968.1991.tb04394.x. [DOI] [PubMed] [Google Scholar]
- 31. Wang LY, Huang ZL, Li G, Zhao HX, Xing XH et al. (2010) Novel mutation breeding method for Streptomyces avermitilis using an atmospheric pressure glow discharge plasma. J Appl Microbiol 108: 851-858. doi: 10.1111/j.1365-2672.2009.04483.x. PubMed: 19735332. [DOI] [PubMed] [Google Scholar]
- 32. He-Ping L, Zhi-Bin W, Nan G, Pei-Si L, Hao W et al. (2012) Studies on the Physical Characteristics of the Radio-Frequency Atmospheric-Pressure Glow Discharge Plasmas for the Genome Mutation of Methylosinus trichosporium. Plasmas Science IEEE Transactions On 40: 2853-2860. doi: 10.1109/TPS.2012.2213274. [DOI] [Google Scholar]
- 33. Li G, Li H-P, Wang L-Y, Wang S, Zhao H-X et al. (2008) Genetic effects of radio-frequency, atmospheric-pressure glow discharges with helium. Appl Phys Lett 92: 221504. doi: 10.1063/1.2938692. [DOI] [Google Scholar]
- 34. Li HP, Li G, Wang LY, Wang S, Zhao HX et al. (2008) Genetic effects of radio-frequency, atmospheric-pressure glow discharges with helium. Appl Phys Lett 92. [Google Scholar]
- 35. Wang LY, Huang ZL, Li G, Zhao HX, Xing XH et al. (2010) Novel mutation breeding method for Streptomyces avermitilis using an atmospheric pressure glow discharge plasma. J Appl Microbiol 108: 851-858. doi: 10.1111/j.1365-2672.2009.04483.x. PubMed: 19735332. [DOI] [PubMed] [Google Scholar]
- 36. Li H-P, Sun W-T, Wang H-B, Li G, Bao C-Y (2007) Electrical Features of Radio-frequency, Atmospheric-pressure, Bare-metallic-electrode Glow Discharges. Plasma Chem Plasma Process 27: 529-545. doi: 10.1007/s11090-007-9079-x. [DOI] [Google Scholar]
- 37. C Z; (1966)Contribution a l’etude d’ une cyanophycee. Influence de diverse facteurs physiques et chimiques sur la croissance et la photosynthese de Spirulina maxima (Setch et Gardner) Geitler [PhD thesis]. Paris: University of Paris; . 4-5 p [Google Scholar]
- 38. Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31: 426-428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 39. Lee Y-K (2004) Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmind A. Ames, IA: Blackwell Publishing House Science Ltd. [Google Scholar]
- 40. Chen H-B, Wu J-Y, Wang C-F, Fu C-C, Shieh C-J et al. (2010) Modeling on chlorophyll a and phycocyanin production by Spirulina platensis under various light-emitting diodes. Biochem Eng J 53: 52-56. doi: 10.1016/j.bej.2010.09.004. [DOI] [Google Scholar]
- 41. Jin L, Fang M, Zhang C, Jiang P, Ge N et al. (2011) Operating conditions for the rapid mutation of the oleaginous yeast by atmospheric and room temperature plasmas and the characteristics of the mutants. Chin J Biotechnol 27: 461-467. [PubMed] [Google Scholar]
- 42. Lu Y, Wang L, Ma K, Li G, Zhang C et al. (2011) Characteristics of hydrogen production of an Enterobacter aerogenes mutant generated by a new atmospheric and room temperature plasma (ARTP). Biochem Eng J 55: 17-22. doi: 10.1016/j.bej.2011.02.020. [DOI] [Google Scholar]
- 43. Hua X, Wang J, Wu Z, Zhang H, Li H et al. (2010) A salt tolerant Enterobacter cloacae mutant for bioaugmentation of petroleum- and salt-contaminated soil. Biochem Eng J 49: 201-206. doi: 10.1016/j.bej.2009.12.014. [DOI] [Google Scholar]
- 44. Li H-P, Li G, Sun W-T, Wang S, Bao C-Y et al. (2008) Radio-Frequency, Atmospheric-Pressure Glow Discharges: Producing Methods, Characteristics and Applications in Bio-Medical Fields. AIP Conf Proc 982: 584-591. doi: 10.1063/1.2897862. [DOI] [Google Scholar]
- 45. Li H-P, Wang L-Y, Li G, Jin L-H, Le P-S et al. (2011) Manipulation of Lipase Activity by the Helium Radio-Frequency, Atmospheric-Pressure Glow Discharge Plasma Jet. Plasma Processes Polym 8: 224-229. doi: 10.1002/ppap.201000035. [DOI] [Google Scholar]
- 46. Gao K, Ma Z (2008) Photosynthesis and growth of Arthrospira (Spirulina) platensis (Cyanophyta) in response to solar UV radiation, with special reference to its minor variant. Environ Exp Bot 63: 123-129. doi: 10.1016/j.envexpbot.2007.10.031. [DOI] [Google Scholar]
- 47. Wang ZP, Zhao Y (2005) MORPHOLOGICAL REVERSION OF Spirulina (Arthrospira) PLATENSIS (CYANOPHYTA): FROM LINEAR TO HELICAL1. J Phycol 41: 622-628. doi: 10.1111/j.1529-8817.2005.00087.x. [DOI] [Google Scholar]
- 48. Wu H, Gao K, Ma Z, Watanabe T (2005) Effects of solar ultraviolet radiation on biomass production and pigment contents of Spirulina platensis in commercial operations under sunny and cloudy weather conditions. Fish Sci 71: 454-456. doi: 10.1111/j.1444-2906.2005.00984.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of relative specific growth rates of the mutants generated by ARTP mutagenesis and wild strain of S. platensis after different subculture. Relative specific growth rates of 1st(A) ,2nd(B) ,5th(C) and 9th(D) subculture.
(DOCX)