Abstract
Tumor necrosis factor-alpha (TNF-α is a major pro-inflammatory cytokine involved in systemic inflammation and the acute phase reaction. Dysregulation of TNF production has been implicated in a variety of human diseases including Fanconi anemia (FA). FA is a genomic instability syndrome characterized by progressive bone marrow failure and cancer susceptibility. The patients with FA are often found overproducing TNF-α, which may directly affect hematopoietic stem cell (HSC) function by impairing HSC survival, homing and proliferation, or indirectly change the bone marrow microenvironment critical for HSC homeostasis and function, therefore contribute to disease progression in FA. In this brief review, we discuss the link between TNF-α signaling and FA pathway with emphasis on the implication of inflammation in the pathophysiology and abnormal hematopoiesis in FA.
Fanconi anemia (FA) and the FA pathway
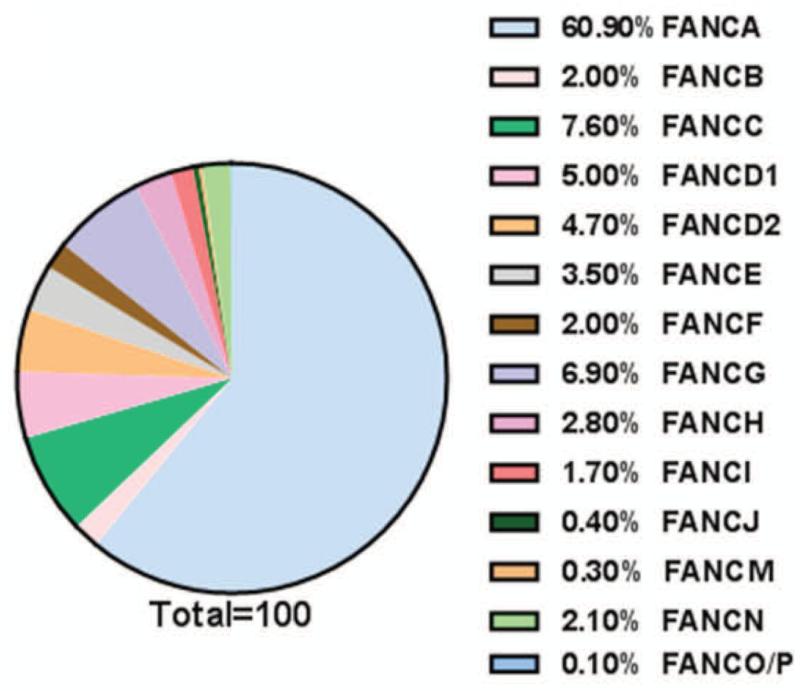
Fanconi anemia (FA) is a rare inherited disease associated with bone marrow failure (BMF), variable congenital/developmental abnormalities, and cancer susceptibility (1-3). Approximately 1,000 persons worldwide currently suffer from the disease, 10 to 20 children are born with FA in the United States each year (1). It is genetically heterogeneous, with 16 complementation groups identified thus far (4-22). The genes encoding the groups A (FANCA), B (FANCB), C (FANCC), D1 (FANCD1/BRCA2), D2 (FANCD2), E (FANCE), F (FANCF), G (FANCG), I (FANCI/KIAA1794), J (FANCJ/BRIP1), L (FANCL), M (FANCM), N (FANCN/PALB2), O (FANCO/RAD51C), P (FANCP/SLX4) and Q (FANCQ/ERCC4/XPF) proteins have been cloned (4-23, Table 1), of which FANCA, FANCG and FANCC are the most commonly mutated genes in FA populations (2-26, Fig 1). At the cellular level, FA is characterized by chromosomal instability and cross-linker sensitivity, which serves as a clinical diagnostic hallmark of FA (1, 3). Strong evidence indicates that the products of all of the FA genes function together in the FA pathway. In fact, the prominent role of the FA pathway has been implicated in DNA damage response and/or repair (DDR). (27)
Table 1. COMPLEMENTATION GROUPS AND INTERACTION PROTEINS OF FANCONI ANEMIA.
|
Subtype |
Chromosome Location |
Protein Products (kd) |
Functions |
Ref |
|---|---|---|---|---|
| A | 16q24.3 | 163 (FANCA) | FA core complex | 6 |
| B | Xp22.31 | 95 (FAAP95) | FA core complex | 13 |
| C | 9q22.3 | 63 (FANCC) | FA core complex | 5 |
| D1 | 13q12-13 | 380 (BRCA2) | RAD51 recruitment | 11 |
| D2 | 3p25.3 | 155,162 (FANCD2) | Involved in DNA damage repair | 4 |
| E | 6p21-22 | 60 (FANCE) | FA core complex | 9 |
| F | 11p15 | 42 (FANCF) | FA core complex | 8 |
| G | 9p13 | 68 (FANCG/XRCC9) | FA core complex | 7 |
| I | 15q25-16 | 140 (FANCI/KIAA1794) | Required for maintenance of chromosomal stability | 10, 18 |
| J | 17q22-q24 | 140 (FANCJ/BACH1/BRIP1) | 5′>3′ DNA helicase/ATPase | 15 |
| L | 2p16.1 | 43(FANCL/PHF9/POG) | FA core complex, FAAP43 ubiqutin ligase | 12 |
| M | 14q21.3 | 250 (FANCM) | FA core complex/ATPase/translocase | 14 |
| N | 16p12.1 | 130 (FANCN/PALB2) | Regulation of BRCA2 location | 16; 17 |
| O | 17q25.1 | 42 (FANCO/RAD51C) | Involved in HRR of DSB | 19; 20; 21 |
| P | 16p13.3 | 200 (FANCP/SLX4) | Protect genome stability | 22 |
| Q | 16p13.12 | 101 (FANCQ/ERCC4/XPF) | Required for ICL repair | 23 |
| FAAP100 | 17q25.1 | 100 | Required for D2 mono-Ub | 36 |
| FAAP24 | 19q13.11 | 24 | Required for D2 mono-Ub | 28 |
| FAAP20 | 22q12 | 20 | Required for D2 mono-Ub | 34 |
| HES1 | 3q28-q29 | 30 | FA-associated factor | 35 |
| MHF1 | 1p26.22 | 16 | FANCM-associated protein | 33 |
| MHF2 | 17q25.3 | 10 | FANCM-associated protein | 33 |
FIG. 1. Estimated percentage of FA patients in FA complementation groups.
Based on the data from references 24, 26,191,192.
Multifunctionality of FA proteins
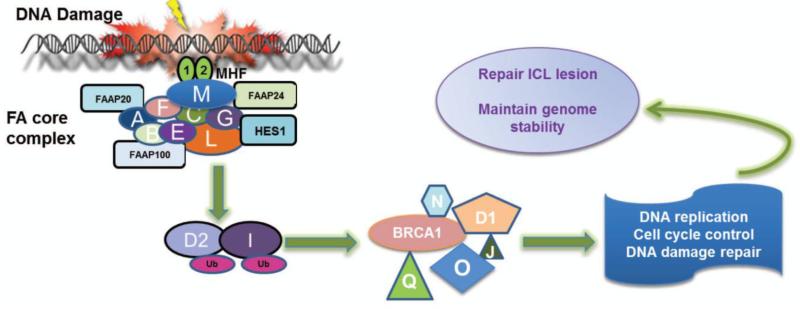
Intense studies have been focusing on biological function of FA proteins. Compelling evidence suggested that eight of the FA proteins (namely, FANCA, B, C, E, F, G, L, and M) and 6 associated factors (FAAP100, FAAP24, FAAP20 HES1, MHF1 and MHF2) form a nuclear multiprotein complex, which is required for the efficient mono-ubiquitination of downstream FANCD2/FANCI dimer in response to DNA damage or DNA replication stress (2, 8, 9, 12, 18, 28-39). After ubiquitination, FANCD2/FANCI heterodimer then recruits other downstream FA proteins including FANCD1 (which is the breast cancer protein BRCA2), and the recently identified FANCJ (BRIP1), FANCN (PALB2), FANCO (RAD51C) and another breast cancer protein, BRCA1 (40), to nuclear loci containing damaged DNA and consequently influence important cellular processes such as DNA replication, cell-cycle control, and DNA damage repair (Fig 2).
FIG. 2. Multifunctionality of FA proteins.
Eight FA proteins (FANCA, B, C, E, F, G, L and M) and 6 associated factors (FAAP20, FAAP24, FAAP100, HES1, MHF1 and MFH2) form a nuclear core complex, which acts as ubiquitin ligase. In response to DNA damage or replication stress, nuclear core complex monoubiquitinates two other FA proteins, FANCD2 and FANCI, which then recruit other downstream FA proteins FANCD1, FANCJ, FANCN, FANCO and FANCQ to damaged DNA and involved in DNA repair, cell-cycle control to repair ICL lesions and to maintain genome stability.
The FA proteins are essential for DNA interstrand cross-linking (ICL) resolution, which is a multistep DNA repair process involving nucleotide excision repair (NER), translesion synthesis (TLS) and homologous recombination (HR).(41) Indeed, cells deficient in the FA pathway exhibit hypersensitivity to DNA damage agents that induce ICL and undergo G2/M arrest as well as profound chromosomal breakage while culturing with mitomycin C (MMC) or diepoxybutane (DEB) (1, 3). Interestingly, 4 of FA genes: FANCD1 (BRCA2), FANCJ (BRIP1), FANCN (PALB2), and FANCO (RAD51C) are also breast cancer susceptibility genes, suggesting a crosstalk between the FA pathway and the breast cancer-associated (BRCA) pathway (FA/BRCA pathway, 42). To date, it is believed that the FA pathway plays important role in genome maintenance, while the FA/BRCA pathway is important during S phase of the cell cycle (43).
Hematopoietic failure and abnormal apoptotic signaling in FA
The most common clinical features of FA are hematological. A majority of children with FA invariably experience pancytopenia during the first few years of life, which is associated with stem cell loss in the hematopoietic compartment. Complications of bone marrow failure (BMF) are the major causes of morbidity and mortality of FA (1, 44-48). In addition, rapid hematopoietic cell loss forces compensatory chronic proliferation, which may lead to leukemogenesis (14, 49-51). In fact, patients with FA have higher risk of developing myelodysplasia (MDS) or acute myeloid leukemia (AML) (1-3, 29, 44, 45). In addition, secondary clonal cytogenetic abnormalities, such as 3q addition, 5q deletion and monosomy 7, are commonly occurred in children with FA who have evolved to MDS and AML after chemotherapies (52-58). It is in the context, a selective pressure of apoptosis as well as genomic instability during the BMF-MDS-AML progression may contribute to FA leukemia transformation (30, 45, 59-76). Therefore, FA has been proposed as an excellent model for studying myeloid leukemia evolution.
Surprisingly, FA murine models (such as Fanca, Fancc, Fancd2 or Fancg knockout mice) do not recapitulate the major FA clinical manifestations, such as spontaneous hematological defects or leukemia development (76-79). Those null mice only exhibit some compromised defects in response to environmental insults, including ICL and ionizing irradiation (76-80). For instance, Fanca and Fancc mouse models show normal blood count and comparable number of committed bone marrow (BM) progenitors compared to WT mice in steady state. However, sublethal dose of DNA cross-linking agent MMC, which is well-tolerant in WT mice, induces progressive decrease of all peripheral blood parameters, as well as early and committed progenitors in the mutant mice, and eventually leads to premature death within 8 weeks (76, 77). More interestingly, the phenotype of Fanca, Fancc, Fancd2 or Fancg- null mice (76, 79, 81-84) and Fanca/Fancc double knockout mice is indistinguishable (82), suggesting that FA proteins function in the same pathway and play an important role in maintaining the bone marrow HSC compartment and disruption of the FA pathway may account for the defects found in both human and mouse.
Although the mechanistic details concerning the roles of FA proteins in promoting hematopoietic stem/progenitor cell (HSPC) survival or proliferation remain to be elucidated, significant evidence supports excessive apoptosis of HSPCs (59, 85), induced by stresses as a critical factor in the pathogenesis of BMF and leukemia progression in FA. Increased apoptosis due to higher level of the death receptor Fas (CD95) in FA was first reported by using CD34+ cells from children with FA (85). Conversely, overexpression of the FANCC protein suppresses apoptosis in human hematopoietic factor-dependent progenitor cell lines (61), in CD34+ cells from FA-C patients (30, 86), and in hematopoietic progenitor cells from Fancc knock-out mice (76). Subsequently, Koh and the coworkers found that FANCC modulates apoptotic responses to tumor necrosis factor-alpha (TNF-α) and Fas ligand (64). Mechanistically, the enhanced TNF-α-induced apoptosis in FANCC-deficient cells is found dependent on apoptosis signal-regulating kinase 1 (ASK1) (87), which has been considered an important kinase involved in oxidant- and TNF-α-induced apoptosis (88). FANCC also directly suppresses TNF-α production in mononuclear phagocytes by suppressing TLR8 activity, which is a key mediator in pathogen recognition and activation of innate immunity (86). Using Fancc−/− mouse model, researchers showed that inactivation of Fancc augmented interferon-gamma (IFN-γ)-induced apoptotic responses in hematopoietic cells (72) and resulted in an inability to activate casapase-3 after ionizing radiation (89). A central highly conserved domain of FANCC, which binds to and facilitates the activation of STAT1, an important signal transducer and transcription activator and mediates cellular responses to cytokines and growth factors, has been identified (69). Furthermore, loss of FANCC results in constitutively phosphorylation and increased binding affinity for double-stranded RNA (dsRNA) kinase PKR, a key effector of apoptosis to various extra and intracellular cues (71), suggesting another function of FANCC in suppressing cytokine-induced apoptosis through modulating the activity of a growth inhibitory kinase (80). In addition, elevated expression/release of TNF-related apoptosis-inducing ligand (TRAIL) at the bone marrow level has also been found in FA, which may also implicate in the pathogenesis of FA (90) and MDS.(91) FANCD2 was shown to be a target for apoptosis mediated by caspase(s)- but not proteasome-mediated pathway following DNA damage (92). These data indicate that deregulation of apoptotic responses resulted from loss of FA function in hematopoietic cells, may account at least in part for the nearly universal development of BMF in children with inactivating FA mutations (Fig 3).
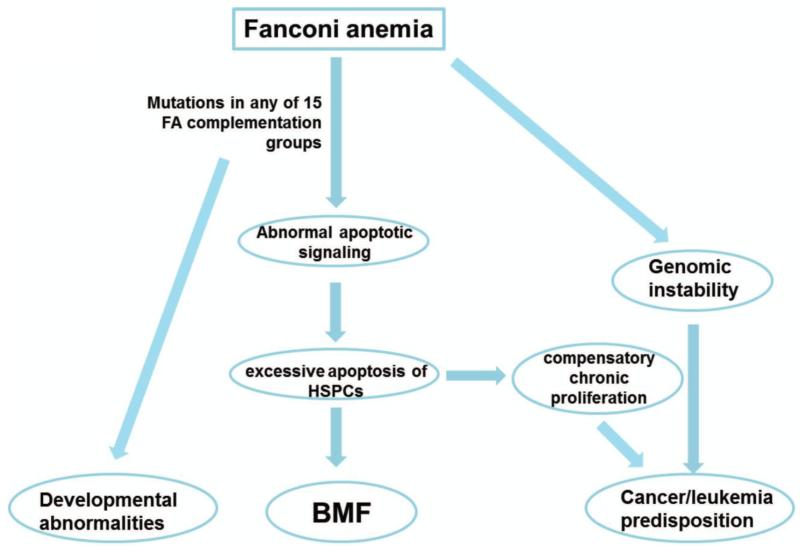
FIG. 3. Hematopoietic failure and abnormal apoptotic signaling in FA.
Mutations in any of FA genes lead to enhanced apoptotic signaling therefore cause excessive HSPC apoptosis. Compensatory chronic proliferation along with the FA genomic instability provoke leukemogenesis and results in typical phenotype such as developmental abnormalities, bone marrow failure, and cancer in FA patients. BMF = bone marrow failure
The link between pro-inflammatory cytokine TNF-α, inflammation and diseases
TNF-α is a proinflammatory cytokine produced by multiple immune and non-immune cells, including lymphocytes, mast cells, endothelial cells, fibroblasts and adipocytes. It functions in the regulation of diverse physiological cellular events, including cell proliferation, differentiation and apoptosis as well as various inflammatory processes (93, 94). TNF-α is also involved in pathological actions, such as systematic inflammation, initiation of the acute phase reaction by promoting survival/inflammatory signaling or inducing cell death, especially tumor cell necrosis and apoptosis (95, 96). In fact, dysregulation of TNF-α production has been implicated in the development of diabetes, septic shock, tumorigenesis, cardiovascular diseases, rheumatoid arthritis and inflammatory bowel disease (93). Targeting TNF-α has emerged as an established treatment for these diseases (97). With respect to abnormal hematopoiesis, it is well recognized that TNF-α is involved in many disease situations including anemia, MDS, and leukemia (49-51, 98-101).
Certain chronic inflammatory conditions provoke cell turnover coupled with carcinogen- or phagocyte-induced DNA damage, leading to transformation, therefore have been long linked to cancer. Although the molecular mechanisms by which chronic inflammatory conditions predispose the tumor formation remains unclear, it is believed that cancers are initiated by a combination of oncogenic mutational events and loss of the cellular checkpoints that prevent cell division in the presence of DNA damage. TNF-α is a well-known key mediator of cancer-associated chronic inflammation. Although TNF-α can initiate apoptotic responses (93), these pathways are frequently deactivated within tumor cells. In addition, increasing evidence suggest that in some circumstances, TNF-α can provide a survival signal for the cancer cell and thus act as a direct or indirect tumor promoter by the induction of other proinflammatory cytokines and angiogenic factors involved in cancer development (102, 103). Direct evidence for the involvement of TNF-α in malignancy comes from observations that TNF-α knockout mice are resistant to chemical carcinogenesis of the skin (104). Mechanistically, it has been shown that TNF-α mediates tumor promotion via a PKC alpha- and AP1- dependent pathway (105). Conversely, neutralization of TNF-α during the early stages of tumor promotion is sufficient to inhibit tumor formation (106). Altogether, as a central mediator of inflammation, TNF-α may serve as a molecular link between chronic inflammation and cancer development (107).
TNF-α signaling in pathophysiology
The molecular mechanisms of TNF-α functions have been intensively investigated. It is believed that the biological activities of TNF-α are mediated by two structurally related but functionally distinct receptors, designated the p55 and p75 TNF-α receptors, TNFR1 and TNFR2, respectively (93). Binding of TNF-α to the receptors initiates a complex array of signaling events in response to TNF-α receptor activation and give rise to the pleiotropic effects of TNF-α on cells (107, 108), mainly through the activation of the MAPK stress signaling cascade including JNK, p38MAPK, and ERK (108, 110, 111), as well as the NF-κB transcription factor (111-113).
The default pathway TNF-α signaling is the induction of genes involved in inflammation and cell survival. Recent studies have elucidated that the diverse biological responses mediated by TNF-α are achieved, at least in part through TNF-α triggered-activation of the Ikappa B (IκB) kinase (IKK)/NF-κB through TRAF2 and RIP1 (114, 115), and mitogen-activated protein kinase (MAPK)/AP-1 pathways through a TRAF2-dependent mechanism (116). These pathways are essential for the expression of pro-inflammatory cytokines. NF-κB activation also importantly induces negative regulators of apoptosis such as FLIPL (also known as CFLAR), BCL2 and superoxide dismutase (116). If NF-κB activation is inadequate, apoptosis, a late response to TNF-α can be mediated by the activation of caspase-8 by FADD, and lead to the accumulation of intracellular reactive oxygen (ROS), sustained Jun amino-terminal kinase (JNK) activation and mitochondrial pathways activation (117). Although JNK activation is critical for TNF-stimulated AP-1-dependent gene expression (118), the exact role of JNK in TNF-α-induced apoptosis has been controversial. For instance, some studies indicated that JNK is not essential for TNF-stimulated apoptosis (112, 119-122). In addition, JNK may antagonize TNF-stimulated apoptosis (121, 123-125). On the other hand, other studies suggest JNK activation is required for TNF-induced cell death (113, 126-130). Genetic studies using Drosophila demonstrated that JNK may mediate cell death in response to TNF-α (131-133). These studies suggest a complex relationship between JNK and TNF-stimulated cell death (111). TNF-α also induces Fas-mediated apoptosis (134-136) as well as alternative apoptosis pathway involving TNF-α receptor-associated death domain (TRADD), which cascades additional signaling molecules, including FADD, TRAF2 and RIP1 (137, 138). Collectively, the cellular response to TNF-α, in fact, represents a balance between apoptotic responses and anti-apoptotic responses (111, Fig 4).
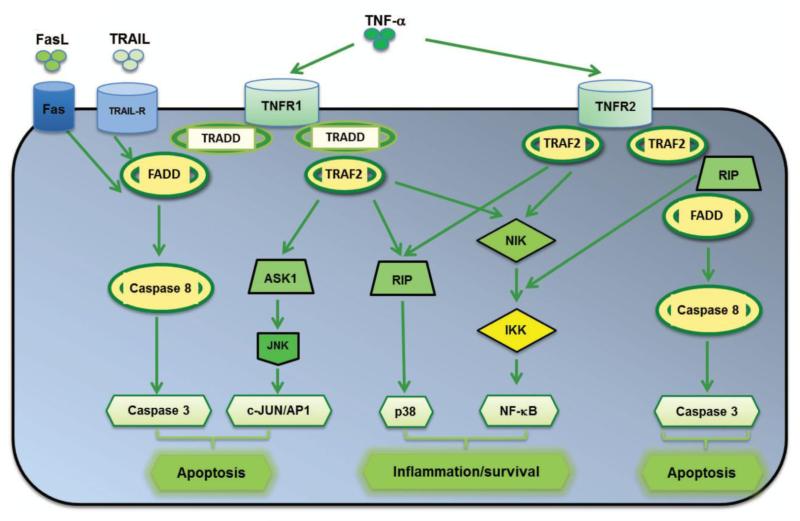
FIG. 4. TNF-α signaling in inflammation and apoptosis.
Binding of TNF-α to TNF receptor (TNFR) results in the recruitment of intracellular adapter proteins, such as TRADD (TNFR-associated death domain) and FADD (Fas-associated death domain). TRADD complex then recruits the adapter protein TRAF2 (TNFR-associated factor 2), whereas FADD stimulates the caspase cascade. NIK (NF-κB-inducing kinase), RIP (receptor-interacting protein) and ASK1 (apoptosis signal-regulating kinase 1) are known downstream signaling molecules that interact with TRAF2, therefore leads to activate multiple signal transduction pathways, including the induction of cell cycle proteins, anti-apoptotic proteins, inflammatory cytokines and chemokines; and the induction of apoptosis through activation of caspases. Binding of FasL to FAS or TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) to TRAIL-R also result in cell death through FADD.
Signal transduction triggered by TNF-α also induces an increase in intracellular ROS by its direct toxic phenomena and its effects on mitochondrial function (139-142). TNF-α-induced ROS production involves the JNK and NF-κB pathways (68, 122, 137) and influences a variety of downstream signaling events including activation of redox-sensitive kinases, alteration of intracellular calcium regulation, transcription factor activation and gene expression (143). TNF-α-induced ROS activate JNK, which in turn leads to more ROS production (137). The production of ROS by TNF-α at inflammatory sites causes DNA damage (144-150), which can further lead to TNF-α production either directly (150-153) or through activation of NF-κB (154, 155). It is believed that TNF-α-induced ROS production plays an important role in the physiopathological cellular processes.
The roles of TNF-α in HSC function and leukemic transformation in general
HSC function is regulated by microenvironment directly through cell-cell interactions or indirectly through production of cytokines (156). Many cytokines and growth factors including TNF-α are known to regulate HSC survival, homing, and proliferation (157). TNF-α plays a pivotal role in directly regulating HSC proliferation or indirectly stimulation of growth factor production and up-regulation of cytokine receptors. While TNF-α induces proliferation of the more primitive subset of progenitors, it simultaneously induces a differentiation block downstream in response to hematopoietic stress and increased demand for mature blood cells (158). Furthermore, TNF-α can elicit either a stimulatory or an inhibitory effect on the in vitro growth of hematopoietic progenitors depending on its concentration and on the cytokines microenvironment (51).
TNF-α plays important role in the disease progression of hematopoietic malignancies. The overproduced pro-inflammatory cytokines and subsequent increased oxidative stress may account for profound physiologic changes, including the development of BMF and progression to leukemia (159). For example, diseases characterized by hematopoietic insufficiency, such as aplastic anemia (AA), are often found abnormal expression of pro-inflammatory cytokines (160). In fact, overproduction of TNF-α, known to be the late effector of the damage of the HSC compartment, has been implicated in pathological conditions related to anemia, MDS, and leukemia (2, 3, 51). Autocrine production of TNF-α plays a role in the cytotoxicity of depsipeptide against a subset of leukemias (161). In addition, TNF-α induces upregulation of tumor suppressor, PTEN expression through NF-κB activation in human leukemic cells (162). TNF-α receptor 1 expression on AML blasts predicts differentiation into leukemic dendritic cells (163). Additionally, CD154 (CD40 ligand, CD40L, gp139), a co-stimulatory molecule of the TNF family, is involved in lymphoid malignancies, suggesting some therapeutic implications of CD154 interactions in lymphopoiesis (164). Furthermore, the large majority of primary hematologic tumors are resistant to TRAIL-mediated apoptosis, due to the activation of anti-apoptotic signaling pathway (such as NF-κB), overexpression of anti-apoptotic proteins (such as FLIP, Bcl-2, XIAP) or expression of TRAIL decoy receptors or reduced TRAIL-R1/-R2 expression (165). In this context, targeting TNF-α signaling pathway in the combination with chemotherapy or radiotherapy, or with proteasome or histone deacetylase or NF-κB inhibitors, hold promise for future developments in treatment of hematologic malignancies (Fig 5).
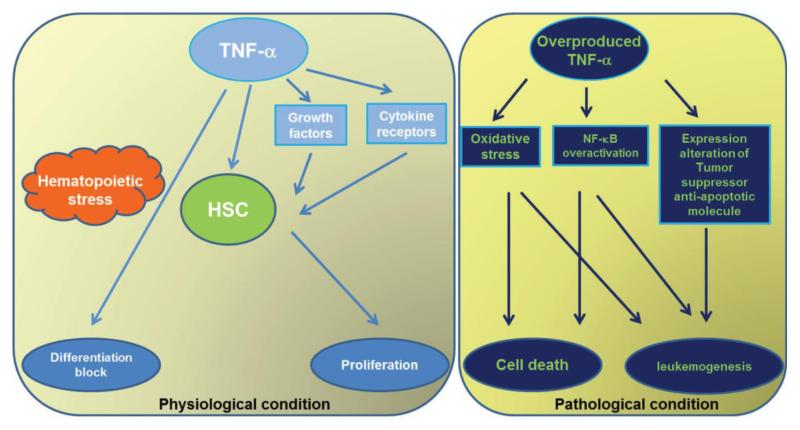
FIG. 5. Role of TNF-α in hematopoietic diseases.
Under physiological condition, TNF-α regulates hematopoietic stem cell (HSCs) function by directly promoting HSC proliferation or indirectly stimulating growth factor secretion/upregulating cytokine receptor expression. However, in the pathological circumstance, overproduced TNF-α results in oxidative stress, overactivated NF-κB and expression changes in other tumor suppressor protein/anti-apoptotic molecules, therefore leads to either cell death or leukemia evolution.
The roles of TNF-α in HSC function and leukemic transformation in FA
Elevated levels of serum, plasma or intracellular TNF-α are often found in patients with FA. The increased secretion of TNF-α along with altered production of other growth factors and cytokines, including reduced expression of interleukin-6 (IL-6) and granulocyte-macrophage colony-stimulating factor, may change the BM microenvironment by leading to factor deprivation or constant exposure to mitogenic inhibitors (159). These expression alterations may cause deregulation of cellular homeostasis and have been considered as an important pathological factor involved in the abnormal hematopoiesis In FA (49-51, 98, 166, 167). Increasing evidence indicates that progressive BMF in children with FA is resulted from excessive apoptosis in the HSC compartment (1, 3). Indeed, studies using (Fanca−/−, Fancc−/−, and Fancg−/− mouse models suggested that FA cells exhibit cytokine hypersensitivity apoptotic cues, including TNF-α (3, 47, 168) and contribute to pathogenesis of BMF in FA (169). Indeed, our previous study using Fancc−/− murine hematopoietic stem and progenitor cells showed that TNF-α overproduction results in bone marrow hypoplasia, and long-term exposure of these cells to TNF-α induces clonal evolution that leads to myelogenous leukemia (170). Consistently, ex vivo culture of Fancc−/− BM cells leads to an increase in cytogenetic abnormalities and myeloid malignancies that are associated with an acquired resistance to TNF-α, suggesting FA hematopoietic cells are prone to clonal hematopoiesis and malignancy (169, 170). A direct association of FANCD2 and NF-κB consensus sequence (κB1 site) in TNF-α promoter, which leads to the repression of its transcriptional activation, has been reported (171). This may explain, at least in part, a transcriptional mechanism for the elevated TNF-α level in FA patients and suggest that artificial modulation of TNF-α production could be a promising therapeutic approach to FA.
TNF-α-induced ROS is another pathological factor playing important roles in the disease progression of FA. Various in vitro studies and clinical observations suggest that FA patients are deficient in detoxifying superoxide anions (O2−) released by TNF-α or IFN-γ-activated phagocytes (172). Although further studies on the role of FA proteins in the regulation of TNF-α-induced ROS production remains to be elucidated, it is likely FA proteins can disrupt downstream ROS signaling by protecting chromosomal DNA from ROS attack or facilitating the repair of oxidative DNA damage. Indeed, the persistent high levels of oxidative DNA damage was observed in HSC/progenitor cells from TNF-α-injected Fancc−/− mice (172, 173), suggesting that loss of FA protein renders chromosomal DNA susceptible to ROS attack, thereby increasing oxidative DNA damage. Our previous study also suggested that major antioxidant defense genes are down-regulated in the BM cells of FA patients and that the down-regulation is selectively associated with increased oxidative DNA damage in the promoters of these antioxidant defense genes (174). In this context, FA proteins play critical role in protecting these major antioxidant defense genes from oxidative damage. Further studies indicated that TNF-α not only is a pro-apoptotic signal suppressing FA hematopoietic progenitor activity, but also promotes leukemic transformation of FA hematopoietic stem/progenitor cells evidenced by long-term exposure of Fancc−/− BM cells to TNF-α in vitro provoking the outgrowth of TNF-α-resistant cytogenetically abnormal clones which leads to acute myelogenous leukemia (AML) upon transplantation into congenic wild-type mice (170). More strikingly, the leukemic clones were TNF-α-resistant but retained their characteristic hypersensitivity to mitomycin C (MMC), and exhibited high levels of chromosomal instability. Therefore, FA disease progression to leukemia is governed not only by genetic changes intrinsic to the FA cells, but also by epigenetic and environmental factors and that TNF-α-mediated inflammation is one of the most important epigenetic and environmental factors contributing to FA leukemogenesis (Fig 6).
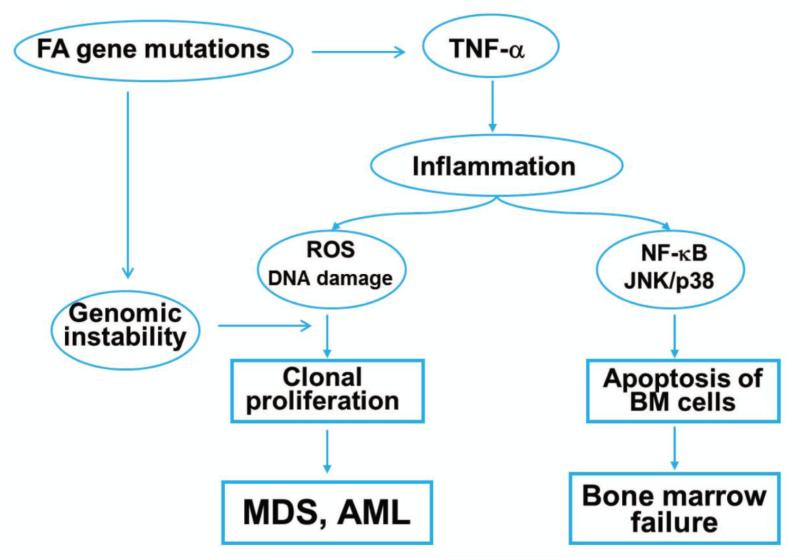
FIG. 6. The pro-inflammatory cytokine TNF-α and its potential role in FA pathophysiology.
FA deficiency results in overproduction of TNF-α. Elevated level of TNF-α leads to accumulation of inflammatory ROS and deregulated stress kinases (p38 and JNK) activation in FA marrow cells, therefore provoke premature apoptosis of marrow cells as well as clonal proliferation, eventually which lead to BMF, MDS or AML in FA patients. BMF=bone marrow failure
Therapies targeting inflammatory cytokines in treatment of human diseases including hematologic diseases
Cytokines are a large family of small proteins that function in essentially all biological processes. Abnormalities in cytokines expression, their receptors, and the signaling pathways are involved in variety of diseases, such as immune and inflammatory disorders, and cancers (93). As cytokines are potent rate-limiting extracellular molecules, therapies targeting cytokines by greater surface of interaction of receptors and antibodies with their targets have gained variable successes. For example, IL-1 blockade in the form of IL-1 receptor antagonist (IL-1Ra) is effective and approved in Rheumatoid Arthritis (RA, 175); targeting CD30/CD30L have been applied in oncology and autoimmune and inflammatory diseases (176); three interferon β preparations (Betaseron, Avonex, and Rebif) have shown efficacy in the treatment of relapsing-remitting multiple sclerosis (177).
As a major mediator of molecular cascade leading to inflammation, TNF-α plays an important role in driving the pathological process. To date, the TNF signaling pathway appears to be a valuable target in the therapy of inflammation-associated diseases. Indeed, five TNF-α-blocking biologicals, including infliximab (also known as Remicade; Centocor ortho Biotech), etanercept (enbrel; Amgen/Pfizer), adalimumab ((Humira; Abbott), golimumab (Simponi; Centocor ortho Biotech), Certolizumab pegol (Cimzia; uCB Celltech) have emerged as important agents in the treatment of many chronic inflammatory diseases, especially in cases refractory to conventional treatment modalities (95, 103, 178). TNF-α or the effects of TNF-α are also inhibited by a number of natural compounds, including curcumin (a compound present in turmeric), and catechins (in green tea). In addition, activation of cannabinoid CB1 or CB2 receptors by cannabis or Echinacea purpurea, which can be isolated from flowering plants, seems to have anti-inflammatory properties through TNF-α inhibition (179). By their ability to interfere with inflammatory processes at multiple levels, these TNF-α blockers have become invaluable tools to inhibit the inflammation-induced damage and allow recovery of the affected tissues. So far, the safety and tolerance of anti-TNF-α therapy is well established (180). Specifically, the first TNF-α blocker used in inflammatory bowel disease has been infliximab, a monoclonal chimeric IgG1 anti-TNF neutralizing antibody that is effective in adult and pediatric patients with active, luminal and perianal Crohn Disease (181, 182), and adult patients with active ulcerative colitis (183). In the context of FA, the safety and efficacy of anti-TNF-α therapy using etanercept, a recombinant protein that fuses the soluble TNF receptor 2 (TNFR2, the receptor that binds to TNF-α) to the constant end of human IgG1 antibody, has been tested (184). Although further studies to improve the clinical outcome are required, etanercept shows effect in BMF treatment in children with FA through neutralizing TNF-α (185).
However, anti-TNF-α therapy also has some drawbacks, including increased risk of infection and malignancy, and other reactions (185). Moreover, significant hematological disorders with serious complications, and in particular, profound neutropenia have been reported in patients receiving anti-TNF-α therapy (186, 187). Some of these effects are caused by the undesirable abrogation of beneficial TNF-α signaling. It is also striking that many of the mechanisms by which TNF-α enhances cancer development, including promotion of angiogenesis, leukocyte infiltration, and stimulation of other cytokines and chemokines (188). In patients with advanced cancer, TNF-α antagonists are more likely to be active in combination with other treatments (189).
To take these cytokine-targeting therapies forward, more specific targeting of the pathological TNF-induced signaling without producing serious complications or even promoting cancer is urgently needed for broader applicability and improved safety. Specificity might be increased by inhibiting the soluble TNF/TNFR1 axis without affecting the often beneficial transmembrane TNF/TNFR2 signaling. These may inhibit the pathological effects of TNF and reduces the side effects thereby providing new opportunities for the treatment of those diseases in which TNFR2 inhibition is detrimental (179). In addition, targeting tumor necrosis factor apoptosis ligand (TRAIL), a type II membrane-bound ligand expressed in a broad range of tissues and exhibited a high grade of homology with the cytotoxic Fas ligand, have shown some potential therapeutic potentials with the induction of TRAIL-mediated signaling destroyed malignant cells while sparing normal cells (190). Furthermore, combinatorial therapies using TRAIL with chemotherapy or radiotherapy, or with proteasome or histone deacetylase or NF-κB inhibitors, hold promise for future developments in treatment of hematologic malignancies (163).
Summary
The pro-inflammatory cytokine, TNF-α has been considered as one important pathological factor involved in the abnormal hematopoiesis most commonly found in BMF diseases including FA, an excellent disease model for studying BMF and leukemogenesis. Although the molecular pathogenesis of FA remains to be elucidated, overproduction of TNF-α in FA most likely plays dual roles. It may act as both a death mediator and a leukemic promoter. Understanding the relationship between inflammation and FA disease progression provides a unique opportunity to mechanistically comprehend and potentially intervene in these physiologically important processes, and therefore, will yield valuable information on whether targeting the multi-step pathogenesis of TNF-α may be therapeutically useful in the treatment of BMF and the prevention of leukemia and other malignancies.
Acknowledgments
The authors are supported by NIH grants R01 HL076712 and R01 CA157537. Q.P. is supported by a Leukemia and Lymphoma Scholar award. W.D. is supported by a NIH T32 grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bagby GC. Genetic basis of Fanconi anemia. Curr. Opin. Hematol. 2003;10:68–76. doi: 10.1097/00062752-200301000-00011. [DOI] [PubMed] [Google Scholar]
- [2].Tischkowitz MD, Hodgson SV. Fanconi anaemia. J. Med. Genet. 2003;40:1–10. doi: 10.1136/jmg.40.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kennedy RD, D’Andrea AD. The Fanconi Anemia/BRCA pathway: new faces in the crowd. Genes. Dev. 2000;19:2925–2940. doi: 10.1101/gad.1370505. [DOI] [PubMed] [Google Scholar]
- [4].Timmers C, Taniguchi T, Hejna J, Reifsteck C, Lucas L, Bruun D, Thayer M, Cox B, Olson S, D’Andrea AD, Moses R, Grompe M. Positional cloning of a novel Fanconi anemia gene, FANCD2. Mol. Cell. 2001;7:241–248. doi: 10.1016/s1097-2765(01)00172-1. [DOI] [PubMed] [Google Scholar]
- [5].Strathdee CA, Gavish H, Shannon WR, Buchwald M, M Cloning of cDNAs for Fanconi’s anaemia by functional complementation. Nature. 1992;356:763–767. doi: 10.1038/356763a0. [DOI] [PubMed] [Google Scholar]
- [6].Foe JR, Rooimans MA, Bosnoyan-Collins L, Alon N, Wijker M, Parker L, Lightfoot J, Carreau M, Callen DF, Savoia A, Cheng NC, van Berkel CG, Strunk MH, Gille JJ, Pals G, Kruyt FA, Pronk JC, Arwert F, Buchwald M, Joenje H. Expression cloning of a cDNA for the major Fanconi anaemia gene. FAA. Nat. Genet. 1986;14:320–323. doi: 10.1038/ng1196-320. [DOI] [PubMed] [Google Scholar]
- [7].de Winter JP, Waisfisz Q, Rooimans MA, van Berkel CG, Bosnoyan-Collins L, Alon N, Carreau M, Bender O, Demuth I, Schindler D, Pronk JC, Arwert F, Hoehn H, Digweed M, Buchwald M, Joenje H. The Fanconi anaemia group G gene FANCG is identical with XRCC9. Nat. Genet. 1998;2:281–283. doi: 10.1038/3093. [DOI] [PubMed] [Google Scholar]
- [8].de Winter JP, Rooimans MA, van Der WL, van Berkel CG, Alon N, Bosnoyan-Collins L, de Groot J, Zhi Y, Waisfisz Q, Pronk JC, Arwert F, Mathew CG, Scheper RJ, Hoatlin ME, Buchwald M, Joenje H. The Fanconi anaemia gene FANCF encodes a novel protein with homology to ROM. Nat. Genet. 2000;24:15–16. doi: 10.1038/71626. [DOI] [PubMed] [Google Scholar]
- [9].de Winter JP, Leveille F, van Berkel CG, Rooimans MA, van Der Weel L, Steltenpool J, Demuth I, Morgan NV, Alon N, Bosnoyan-Collins L, Lightfoot J, Leegwater PA, Waisfisz Q, Komatsu K, Arwert F, Pronk JC, Mathew CG, Digweed M, Buchwald M, Joenje H. Isolation of a cDNA representing the Fanconi Anemia Complementation Group E gene. Am. J. Hum. Genet. 2000;67:1306–1308. doi: 10.1016/s0002-9297(07)62959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Joenje H, Levitus M, Waisfisz Q, D’ Andrea AD, Garcia-Higuera I, Pearson T, van Berkel CG, Rooimans MA, Morgan N, Mathew CG, Arwert F. Complementation analysis in Fanconi anemia: Assignment of the reference FA-H patient to group A. Am. J. Hum. Genet. 2000;67:759–762. doi: 10.1086/303067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Howlett NG, Taniguchi T, Olson S, Cox B, Waisfisz Q, De Die-Smulders C, Persky N, Grompe M, Joenje H, Pals G, Ikeda H, Fox EA, D’Andrea AD. Biallelic inactivation of BRCA2 in Fanconi anemia. Science. 2002;297:606–609. doi: 10.1126/science.1073834. [DOI] [PubMed] [Google Scholar]
- [12].Meetei AR, de Winter JP, Medhurst AL, Wallisch M, Waisfisz Q, van de Vrugt HJ, Oostra AB, Yan Z, Ling C, Bishop CE, Hoatlin ME, Joenje H, Wang W. A novel ubiquitin ligase is deficient in Fanconi anemia. Nat. Genet. 2003;35:65–170. doi: 10.1038/ng1241. [DOI] [PubMed] [Google Scholar]
- [13].Meetei AR, Levitus M, Xue Y, Medhurst AL, Zwaan M, Ling C, Rooimans MA, Bier P, Hoatlin M, Pals G, de Winter JP, Wang W, Joenje H. X-linked inheritance of Fanconi anemia complementation group B. Nat. Genet. 2004;36:1219–1224. doi: 10.1038/ng1458. [DOI] [PubMed] [Google Scholar]
- [14].Meetei AR, Medhurst AL, Ling C, Xue Y, Singh TR, Bier P, Steltenpool J, Stone S, Dokal I, Mathew CG, Hoatlin M, Joenje H, de Winter JP, Wang W. A human ortholog of archael DNA repair protein HEF is defective in Fanconi anemia complementation group M. Nat. Genet. 2005;37:958–963. doi: 10.1038/ng1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Levran O, Attwooll C, Henry RT, Milton KL, Neveling K, Rio P, Batish SD, Kalb R, Velleuer E, Barral S, Ott J, Petrini J, Schindler D, Hanenberg H, Auerbach AD. The BRCA1-interacting helicase BRIP1 is deficient in Fanconi anemia. Nat Genet. 2005;37:931–933. doi: 10.1038/ng1624. [DOI] [PubMed] [Google Scholar]
- [16].Xia B, Dorsman JC, Ameziane N, de Vries Y, Rooimans MA, Sheng Q, Pals G, Errami A, Gluckman E, Llera J, Wang W, Livingston DM, Joenje H, de Winter JP. Fanconi anemia is associated with a defect in the BRCA2 partner PALB2. Nat. Genet. 2006;39:159–161. doi: 10.1038/ng1942. [DOI] [PubMed] [Google Scholar]
- [17].Reid S, Schindler D, Hanenberg H, Barker K, Hanks S, Kalb R, Neveling K, Kelly P, Seal S, Freund M, Wurm M, Batish SD, Lach FP, Yetgin S, Neitzel H, Ariffin H, Tischkowitz M, Mathew CG, Auerbach AD, Rahman N. Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat. Genet. 2006;39:162–164. doi: 10.1038/ng1947. [DOI] [PubMed] [Google Scholar]
- [18].Smogorzewska A, Matsuoka S, Vinciguerra P, McDonald ER, 3rd, Hurov KE, Luo J, Ballif BA, Gygi SP, Hofmann K, D’Andrea AD, Elledge SJ. Identification of the FANCI Protein, a Monoubiquitinated FANCD2 Paralog Required for DNA Repair. Cell. 2007;129:1–13. doi: 10.1016/j.cell.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vaz F, Hanenberg H, Schuster B, Barker K, Wiek C, Erven V, Neveling K, Endt D, Kesterton I, Autore F, Fraternali F, Freund M, Hartmann L, Grimwade D, Roberts RG, R.G., Schaal H, Mohammed S, Rahman N, Schindler D, Mathew CG. Mutation of the RAD51C gene in a Fanconi anemia-like disorder. Nat Genet. 2010;42:406–409. doi: 10.1038/ng.570. [DOI] [PubMed] [Google Scholar]
- [20].Meindl A, Hellebrand H, Wiek C, Erven V, Wappenschmidt B, Niederacher D, Freund M, Lichtner P, Hartmann L, Schaal H, Ramser J, Honisch E, Kubisch C, Wichmann HE, Kast K, Deissler H, Engel C, Müller-Myhsok B, Neveling K, Kiechle M, Mathew CG, Schindler D, Schmutzler RK, Hanenberg H. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat Genet. 2010;42:410–414. doi: 10.1038/ng.569. [DOI] [PubMed] [Google Scholar]
- [21].Somyajit K, Subramanya S, Nagaraju G. RAD51C: a novel cancer susceptibility gene is linked to Fanconi anemia and breast cancer. Carcinogenesis. 2010;31(12):2031–2038. doi: 10.1093/carcin/bgq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yamamoto KN, Kobayashi S, Tsuda M, Kurumizaka H, Takata M, Kono K, Jiricny J, Takeda S, Hirota K. Involvement of SLX4 in interstrand cross-link repair is regulated by the Fanconi anemia pathway. Proc Natl Acad Sci U S A. 2011;108(16):6492–6496. doi: 10.1073/pnas.1018487108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bogliolo M, Schuster B, Stoepker C, Derkunt B, Su Y, Raams A, Trujillo JP, Minguillón J, Ramírez MJ, Pujol R, Casado JA, Baños R, Rio P, Knies K, Zúñiga S, Benítez J, Bueren JA, Jaspers NG, Schärer OD, de Winter JP, Schindler D, Surrallés J. J. Mutations in ERCC4, encoding the DNA-repair endonuclease XPF, cause Fanconi anemia. Am J Hum Genet. 2013;92(5):800–806. doi: 10.1016/j.ajhg.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].de Winter JP, Joenje H. The genetic and molecular basis of Fanconi anemia. Mutat Res. 2009;668(1-2):11–19. doi: 10.1016/j.mrfmmm.2008.11.004. [DOI] [PubMed] [Google Scholar]
- [25].Levitus M, Rooimans MA, Steltenpool J, Cool NF, Oostra AB, Mathew CG, Hoatlin ME, Waisfisz Q, Arwert F, de Winter JP, Joenje H. Heterogeneity in Fanconi anemia: evidence for two new genetic subtypes. Blood. 2004;103(7):2498–2503. doi: 10.1182/blood-2003-08-2915. [DOI] [PubMed] [Google Scholar]
- [26].Du W, Adam Z, Rani R, Zhang X, Pang Q. Oxidative stress in Fanconi anemia hematopoiesis and disease progression. Antioxid Redox Signal. 2008;10(11):1909–21. doi: 10.1089/ars.2008.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Meyer S, Neitzel H, Tönnies H. Chromosomal aberrations associated with clonal evolution and leukemic transformation in fanconi anemia: clinical and biological implications. Anemia. 2012;2012:349837. doi: 10.1155/2012/349837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Collins N, Kupfer GM. Molecular pathogenesis of Fanconi anemia. Int. J. Hematol. 2005;82(3):176–183. doi: 10.1532/IJH97.05108. [DOI] [PubMed] [Google Scholar]
- [29].D’Andrea AD. The Fanconi road to cancer. Genes. Dev. 2003;17(16):1933–1936. doi: 10.1101/gad.1128303. [DOI] [PubMed] [Google Scholar]
- [30].Walsh CE, Nienhuis AW, Samulski RJ, Brown MG, Miller JL, Young NS, Liu JM. Phenotypic correction of Fanconi anemia in human hematopoietic cells with a recombinant adeno-associated virus vector. J. Clin. Invest. 1994;94(4):1440–1448. doi: 10.1172/JCI117481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ciccia A, Ling C, Coulthard R, Yan Z, Xue Y, Meetei AR, Laghmani el. H., Joenje H, McDonald N, de Winter JP, Wang W, West SC. Identification of FAAP24, a Fanconi anemia core complex protein that interacts with FANCM. Mol. Cell. 2007;25(3):331–343. doi: 10.1016/j.molcel.2007.01.003. [DOI] [PubMed] [Google Scholar]
- [32].Horejŝí Z, Collis SJ, Boulton SJ. FANCM-FAAP24 and HCLK2: roles in ATR signalling and the Fanconi anemia pathway. Cell. Cycle. 2009;8(8):1133–1137. doi: 10.4161/cc.8.8.8204. [DOI] [PubMed] [Google Scholar]
- [33].Collis SJ, Ciccia A, Deans AJ, Horejsí Z, Martin JS, Maslen SL, Skehel JM, Elledge SJ, West SC, Boulton SJ. FANCM and FAAP24 function in ATR-mediated checkpoint signaling independently of the Fanconi anemia core complex. Mol. Cell. 2008;32(3):313–324. doi: 10.1016/j.molcel.2008.10.014. [DOI] [PubMed] [Google Scholar]
- [34].Schwab RA, Blackford AN, Niedzwiedz W. ATR activation and replication fork restart are defective in FANCM-deficient cells. EMBO. J. 2010;29(4):806–818. doi: 10.1038/emboj.2009.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Luke-Glaser S, Luke B, Grossi S, Constantinou A. FANCM regulates DNA chain elongation and is stabilized by S-phase checkpoint signaling. EMBO. J. 2010;29(4):795–805. doi: 10.1038/emboj.2009.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Singh TR, Saro D, Ali AM, Zheng XF, Du CH, Killen MW, Sachpatzidis A, Wahengbam K, Pierce AJ, Xiong Y, Sung P, Meetei AR. MHF1-MHF2, a histone-fold-containing protein complex, participates in the Fanconi anemia pathway via FANCM. Mol. Cell. 2010;37(6):879–886. doi: 10.1016/j.molcel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ali AM, Pradhan A, Singh TR, Du C, Li J, Wahengbam K, Grassman E, Auerbach AD, pang Q, Meetei AR. FAAP20: a novel ubiquitin-binding FA nuclear core-complex protein required for functional integrity of the FA-BRCA DNA repair pathway. Blood. 2012;119(14):3285–94. doi: 10.1182/blood-2011-10-385963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tremblay CS, Huang FF, Habi O, Huard CC, Godin C, Lévesque G, Carreau M. HES1 is a novel interactor of the Fanconi anemia core complex. Blood. 2008;112(5):2062–70. doi: 10.1182/blood-2008-04-152710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ling C, Ishiai M, Ali AM, Medhurst AL, Neveling K, Kalb R, Yan Z, Xue Y, Oostra AB, Auerbach AD, Hoatlin ME, Schindler D, Joenje H, de Winter JP, Takata M, Meetei AR, Wang W. FAAP100 is essential for activation of the Fanconi anemia-associated DNA damage response pathway. EMBO. J. 2007;26(8):2104–14. doi: 10.1038/sj.emboj.7601666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].D’Andrea AD. Susceptibility pathways in Fanconi’s anemia and breast cancer. N. Engl. J. Med. 2010;362(20):1909–19. doi: 10.1056/NEJMra0809889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Moldovan GL, D’Andrea AD. How the fanconi anemia pathway guards the genome. Annu. Rev. Genet. 2009;43:223–249. doi: 10.1146/annurev-genet-102108-134222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Levy-Lahad E. Fanconi anemia and breast cancer susceptibility meet again. Nat. Genet. 2010;42(5):368–369. doi: 10.1038/ng0510-368. [DOI] [PubMed] [Google Scholar]
- [43].Haitjema A, Brandt BW, Ameziane N, May P, Heringa J, de Winter JP, Joenje H, Dorsman JC. A Protein Prioritization Approach Tailored for the FA/BRCA Pathway. Plos. One. 2013;8(4):e62017. doi: 10.1371/journal.pone.0062017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Buchwald M, Moustacchi E. Is Fanconi anemia caused by a defect in the processing of DNA damage? Mutat. Res. 1998;408(2):75–90. doi: 10.1016/s0921-8777(98)00024-x. [DOI] [PubMed] [Google Scholar]
- [45].Fagerlie SR, Diaz J, Christianson TA, McCartan K, Keeble W, Faulkner GR, Bagby GC. Functional correction of FA-C cells with FANCC suppresses the expression of interferon gamma-inducible genes. Blood. 2001;97(10):3017–3024. doi: 10.1182/blood.v97.10.3017. [DOI] [PubMed] [Google Scholar]
- [46].Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, Hanenberg H, Auerbach AD. A 20-year perspective on the International Fanconi Anemia Registry (IFAR) Blood. 2003;101(4):1249–1256. doi: 10.1182/blood-2002-07-2170. [DOI] [PubMed] [Google Scholar]
- [47].Lensch MW, Rathbun RK, Olson SB, Jones GR, Bagby GC., Jr. Selective pressure as an essential force in molecular evolution of myeloid leukemic clones: a view from the window of Fanconi anemia. Leukemia. 1999;13(11):1784–1789. doi: 10.1038/sj.leu.2401586. [DOI] [PubMed] [Google Scholar]
- [48].Liu JM. Gene transfer for the eventual treatment of Fanconi’s anemia. Semin. Hematol. 1998;35(2):168–179. [PubMed] [Google Scholar]
- [49].Schultz JC, Shahidi NT. Tumor necrosis factor-alpha overproduction in Fanconi’s anemia. Am. J. Hematol. 1993;42(2):196–201. doi: 10.1002/ajh.2830420211. [DOI] [PubMed] [Google Scholar]
- [50].Rosselli F, Sanceau J, Gluckman E, Wietzerbin J, Moustacchi E. Abnormal lymphokine production: a novel feature of the genetic disease Fanconi anemia. II. In vitro and in vivo spontaneous overproduction of tumor necrosis factor alpha. Blood. 1994;83(5):1216–1225. [PubMed] [Google Scholar]
- [51].Dufour C, Corcione A, Svahn J, Haupt R, Poggi V, Béka’ssy AN, Scimè R, Pistorio A, Pistoia V. TNF-alpha and IFN-gamma are overexpressed in the bone marrow of Fanconi anemia patients and TNF-alpha suppresses erythropoiesis in vitro. Blood. 2003;102(6):2053–2059. doi: 10.1182/blood-2003-01-0114. [DOI] [PubMed] [Google Scholar]
- [52].Freie BW, Ciccone SL, Li X, Plett PA, Orschell CM, Srour EF, Hanenberg H, Schindler D, Lee SH, Clapp DW. A role for the Fanconi anemia C protein in maintaining the DNA damage-induced G2 checkpoint. J. Biol. Chem. 2004;279(49):50986–50993. doi: 10.1074/jbc.M407160200. 2004. [DOI] [PubMed] [Google Scholar]
- [53].Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030–9040. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- [54].Futaki M, Igarashi T, Watanabe S, Kajigaya S, Tatsuguchi A, Wang J, Liu JM. The FANCG Fanconi anemia protein interacts with CYP2E1: possible role in protection against oxidative DNA damage. Carcinogenesis. 2002;23(1):67–72. doi: 10.1093/carcin/23.1.67. [DOI] [PubMed] [Google Scholar]
- [55].Giaccia AJ, Kastan MB. The complexity of p53 modulation: emerging patterns from divergent signals. Genes. 1998;12:2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- [56].Luna-Fineman S, Shannon KM, Lange BJ. Childhood monosomy 7: epidemiology, biology, and mechanistic implications. Blood. 1995;85:1985–1989. [PubMed] [Google Scholar]
- [57].Rubin CM, Arthur DC, Woods WG, Lange BJ, Nowell PC, Rowley JD, Nachman J, Bostrom B, Baum ES, Suarez CR, Shah NR, Morgan E, Mauer HS, McKenzie SE, Larson RA, Le Beau MM. Therapy-related myelodysplastic syndrome and acute myeloid leukemia in children: correlation between chromosomal abnormalities and prior therapy. Blood. 1991;78:2982–2988. [PubMed] [Google Scholar]
- [58].West RR, Stafford DA, White AD, Bowen DT, Padua RA. Cytogenetic abnormalities in the myelodysplastic syndromes and occupational or environmental exposure. Blood. 2000;95:2093–2097. [PubMed] [Google Scholar]
- [59].Cumming RC, Liu JM, Youssoufian H, Buchwald M. Suppression of apoptosis in hematopoietic factor-dependent progenitor cell lines by expression of the FAC gene. Blood. 1996;88(12):4558–4567. [PubMed] [Google Scholar]
- [60].Cumming RC, Lightfoot J, Beard K, Youssoufian H, O’Brien PJ, Buchwald M. Fanconi anemia group C protein prevents apoptosis in hematopoietic cells through redox regulation of GSTP1. Nat. Med. 2001;7(7):814–820. doi: 10.1038/89937. [DOI] [PubMed] [Google Scholar]
- [61].Haneline LS, Broxmeyer HE, Cooper S, Hangoc G, Carreau M, Buchwald M, Clapp DW. Multiple inhibitory cytokines induce deregulated progenitor growth and apoptosis in hematopoietic cells from Fac−/− mice. Blood. 1998;91(11):4092–4098. [PubMed] [Google Scholar]
- [62].Haneline LS, Gobbett TA, Ramani R, Carreau M, Buchwald M, Yoder MC, Clapp DW. Loss of FancC function results in decreased hematopoietic stem cell repopulating ability. Blood. 1999;94(1):1–8. [PubMed] [Google Scholar]
- [63].Haneline LS, Li X, Ciccone SL, Hong P, Yang Y, Broxmeyer HE, Lee SH, Orazi A, Srour EF, Clapp DW. Retroviral-mediated expression of recombinant Fancc enhances the repopulating ability of Fancc−/− hematopoietic stem cells and decreases the risk of clonal evolution. Blood. 2003;101(4):1299–1307. doi: 10.1182/blood-2002-08-2404. [DOI] [PubMed] [Google Scholar]
- [64].Koh PS, Hughes GC, Faulkner GR, Keeble WW, Bagby GC. The Fanconi anemia group C gene product modulates apoptotic responses to tumor necrosis factor-alpha and Fas ligand but does not suppress expression of receptors of the tumor necrosis factor receptor superfamily. Exp. Hematol. 1999;27(1):1–8. doi: 10.1016/s0301-472x(98)00064-2. [DOI] [PubMed] [Google Scholar]
- [65].Li X, Yang Y, Yuan J, Hong P, Freie B, Orazi A, Haneline LS, Clapp DW. Continuous in vivo infusion of interferon-gamma (IFN-gamma) preferentially reduces myeloid progenitor numbers and enhances engraftment of syngeneic wild-type cells in Fancc−/− mice. Blood. 2004;104(4):1204–1209. doi: 10.1182/blood-2004-03-1094. [DOI] [PubMed] [Google Scholar]
- [66].Li Y, Youssoufian H. MxA overexpression reveals a common genetic link in four Fanconi anemia complementation groups. J. Clin. Invest. 1997;100(11):2873–80. doi: 10.1172/JCI119836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Maciejewski JP, Risitano A. Hematopoietic stem cells in aplastic anemia. Arch. Med. Res. 2003;34(6):520–527. doi: 10.1016/j.arcmed.2003.09.009. [DOI] [PubMed] [Google Scholar]
- [68].Nakata S, Matsumura I, Tanaka H, Ezoe S, Satoh Y, Ishikawa J, Era T, Kanakura Y. NF-kappa B family proteins proteins participate in multiple steps of hematopoiesis through elimination of reactive oxygen species. J. Biol. Chem. 2004;279:55578–55586. doi: 10.1074/jbc.M408238200. [DOI] [PubMed] [Google Scholar]
- [69].Pang Q, Keeble W, Christianson TA, Faulkner GR, Bagby GC. FANCC interacts with Hsp70 to protect hematopoietic cells from IFN-gamma/TNF-alpha-mediated cytotoxicity. EMBO J. 2001;20(16):4478–4489. doi: 10.1093/emboj/20.16.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Pang Q, Christianson TA, Keeble W, Diaz J, Faulkner GR, Reifsteck C, Olson S, Bagby GC. The Fanconi anemia complementation group C gene product: structural evidence of multifunctionality. Blood. 2001;98(5):1392–1401. doi: 10.1182/blood.v98.5.1392. [DOI] [PubMed] [Google Scholar]
- [71].Pang Q, Christianson TA, Keeble W, Koretsky T, Bagby GC. The anti-apoptotic function of Hsp70 in the interferon-inducible double-stranded RNA-dependent protein kinase-mediated death signaling pathway requires the Fanconi anemia protein, FANCC. J. Biol. Chem. 2002;277(51):49638–49643. doi: 10.1074/jbc.M209386200. [DOI] [PubMed] [Google Scholar]
- [72].Rathbun RK, Faulkner GR, Ostroski MH, Christianson TA, Hughes G, Jones G, Cahn R, Maziarz R, Royle G, Keeble W, Heinrich MC, Grompe M, Tower PA, Bagby GC. Inactivation of the Fanconi anemia group C gene augments interferon-gamma-induced apoptotic responses in hematopoietic cells. Blood. 1997;90(3):974–985. [PubMed] [Google Scholar]
- [73].Rathbun RK, Christianson TA, Faulkner GR, Jones G, Keeble W, O’Dwyer M, Bagby GC. Interferon-gamma-induced apoptotic responses of Fanconi anemia group C hematopoietic progenitor cells involve caspase 8-dependent activation of caspase 3 family members. Blood. 2000;96(13):4204–4011. [PubMed] [Google Scholar]
- [74].Si Y, Ciccone S, Yang FC, Yuan J, Zeng D, Chen S, van de Vrugt HJ, Critser J, Arwert F, Haneline LS, Clapp DW. Continuous in vivo infusion of interferon-gamma (IFN-gamma) enhances engraftment of syngeneic wild-type cells in Fanca−/− and Fancg−/− mice. Blood. 2006;108(13):4283–4287. doi: 10.1182/blood-2006-03-007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wang J, Otsuki T, Youssoufian H, Foe JL, Kim S, Devetten M, Yu J, Li Y, Dunn D, Liu JM. Overexpression of the fanconi anemia group C gene (FAC) protects hematopoietic progenitors from death induced by Fas-mediated apoptosis. Cancer. Res. 1998;58(16):3538–3541. [PubMed] [Google Scholar]
- [76].Whitney MA, Royle G, Low MJ, Kelly MA, Axthelm MK, Reifsteck C, Olson S, Braun RE, Heinrich MC, Rathbun RK, Bagby GC, Grompe M. Germ cell defects and hematopoietic hypersensitivity to gamma-interferon in mice with a targeted disruption of the Fanconi anemia C gene. Blood. 1996;88(1):49–58. [PubMed] [Google Scholar]
- [77].Cheng NC, van de Vrugt HJ, van der Valk MA, Oostra AB, Krimpenfort P, de Vries Y, Joenje H, Berns A, Arwert F. Mice with a targeted disruption of the Fanconi anemia homolog Fanca. Hum. Mol. Genet. 2000;9(12):1805–1811. doi: 10.1093/hmg/9.12.1805. [DOI] [PubMed] [Google Scholar]
- [78].Wong JC, Buchwald M. Disease model: Fanconi anemia. Trends. Mol. Med. 2002;8(3):139–142. doi: 10.1016/s1471-4914(01)02262-6. [DOI] [PubMed] [Google Scholar]
- [79].Yang Y, Kuang Y, Montes De Oca R, Hays T, Moreau L, Lu N, Seed B, D’Andrea AD. Targeted disruption of the murine Fanconi anemia gene, Fancg/Xrcc9. Blood. 2001;98(12):3435–3440. doi: 10.1182/blood.v98.12.3435. [DOI] [PubMed] [Google Scholar]
- [80].Pang Q, Fagerlie S, Christianson TA, Keeble W, Faulkner G, Diaz J, Rathbun RK, Bagby GC. The Fanconi anemia protein FANCC binds to and facilitates the activation of STAT1 by gamma interferon and hematopoietic growth factors. Mol. Cell. Biol. 2000;20(13):4724–4735. doi: 10.1128/mcb.20.13.4724-4735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Chen M, Tomkins DJ, Auerbach W, McKerlie C, Youssoufian H, Liu L, Gan O, Carreau M, Auerbach A, Groves T, Guidos CJ, Freedman MH, Cross J, Percy DH, Dick JE, Joyner AL, Buchwald M. Inactivation of Fac in mice produces inducible chromosomal instability and reduced fertility reminiscent of Fanconi anaemia. Nat. Genet. 1996;12:448–451. doi: 10.1038/ng0496-448. [DOI] [PubMed] [Google Scholar]
- [82].Noll M, Battaile KP, Bateman R, Lax TP, Rathbun K, Reifsteck C, Bagby G, Finegold M, Olson S, Grompe M. Fanconi anemia group A and C double-mutant mice: functional evidence for a multi-protein Fanconi anemia complex. Exp. Hematol. 2002;30(7):679–688. doi: 10.1016/s0301-472x(02)00838-x. [DOI] [PubMed] [Google Scholar]
- [83].Río P, Segovia JC, Hanenberg H, Casado JA, Martínez J, Göttsche K, Cheng NC, Van de Vrugt HJ, Arwert F, Joenje H, Bueren HA. In vitro phenotypic correction of hematopoietic progenitors from Fanconi anemia group A knockout mice. Blood. 2002;100(6):2032–2039. [PubMed] [Google Scholar]
- [84].Koomen M, Cheng NC, van de Vrugt HJ, Godthelp BC, van der Valk MA, Oostra AB, Zdzienicka MZ, Joenje H, Arwert F. Reduced fertility and hypersensitivity to mitomycin C characterize Fancg/Xrcc9 null mice. Hum. Mol. Genet. 2002;11(3):273–281. doi: 10.1093/hmg/11.3.273. [DOI] [PubMed] [Google Scholar]
- [85].Otsuki T, Nagakura S, Wang J, Bloom M, Grompe M, Liu JM. Tumor necrosis factor-alpha and CD95 ligation suppress erythropoiesis in Fanconi anemia C gene knockout mice. J. Cell. Physiol. 1999;179(1):79–86. doi: 10.1002/(SICI)1097-4652(199904)179:1<79::AID-JCP10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- [86].Vanderwerf SM, Svahn J, Olson S, Rathbun RK, Harrington C, Yates J, Keeble W, Anderson DC, Anur P, Pereira NF, Pilonetto DV, Pasquini R, Bagby GC. TLR8-dependent TNF-(alpha) overexpression in Fanconi anemia group C cells. Blood. 2009;114(26):5290–5298. doi: 10.1182/blood-2009-05-222414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Nijangi-Cishehsaraei K, Saadatzadeh MR, Werne A, McKenzie KA, Kapur R, Ichijo H, Haneline LS. Enhanced TNF-alpha-induced apoptosis in Fanconi anemia type C-deficient cells is dependent on apoptosis signal-regulating kinase 1. Blood. 2005;106(13):4124–4130. doi: 10.1182/blood-2005-05-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275(5296):90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- [89].Guillouf C, Vit JP, Rosselli F. Loss of the Fanconi anemia group C protein activity results in an inability to activate caspase-3 after ionizing radiation. Biochimie. 2000;82(1):51–58. doi: 10.1016/s0300-9084(00)00359-x. [DOI] [PubMed] [Google Scholar]
- [90].Pigullo S, Ferretti E, Lanciotti M, Bruschi M, Candiano G, Svahn J, Haneline L, Dufour C, Pistoia V, Corcione A. Human Fanconi A cells are susceptible to TRAIL-induced apoptosis. Br. J. Haematol. 2007;136(2):315–318. doi: 10.1111/j.1365-2141.2006.06432.x. [DOI] [PubMed] [Google Scholar]
- [91].Campioni D, Secchiero P, Corallini F, Melloni E, Capitani S, Lanza F, Zauli G. Evidence for a role of TNF-related apoptosis-inducing ligand (TRAIL) in the anemia of myelodysplastic syndromes. Am. J. Pathol. 2005;166(2):557–563. doi: 10.1016/S0002-9440(10)62277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Park SJ, Beck BD, Saadatzadeh MR, Haneline LS, Clapp DW, Lee SH. Fanconi anemia D2 protein is an apoptotic target mediated by caspases. J. Cell. Biochem. 2011;112(9):2383–2391. doi: 10.1002/jcb.23161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Liu ZG. Molecular mechanism of TNF signaling and beyond. Cell. Res. 2005;15(1):24–27. doi: 10.1038/sj.cr.7290259. [DOI] [PubMed] [Google Scholar]
- [94].Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N. Engl. J. Med. 1996;334(26):1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- [95].Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov. 2013;12(2):147–168. doi: 10.1038/nrd3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Chu WM. Tumor necrosis factor. Cancer. Lett. 2013;328(2):222–225. doi: 10.1016/j.canlet.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wiedmann MW, Mössner J, Baerwald C, Pierer M. TNF alpha inhibition as treatment modality for certain rheumatologic and gastrointestinal diseases. Endocr. Metab. Immune. Disord. Drug. Targets. 2009;9(3):295–314. doi: 10.2174/187153009789044347. [DOI] [PubMed] [Google Scholar]
- [98].Rosselli F, Sanceau J, Wietzerbin J, Moustacchi E. Abnormal lymphokine production: a novel feature of the genetic disease Fanconi anemia. I. Involvement of interleukin-6. Hum. Genet. 1992;89:42–48. doi: 10.1007/BF00207040. [DOI] [PubMed] [Google Scholar]
- [99].Young W, Mahboubi K, Haider A, Li I, Ferreri NR. Cyclooxygenase-2 is required for tumor necrosis factor-alpha- and angiotensin II-mediated proliferation of vascular smooth muscle cells. Circ. Res. 2000;86(8):906–914. doi: 10.1161/01.res.86.8.906. [DOI] [PubMed] [Google Scholar]
- [100].Oster W, Cicco NA, Klein H, Hirano T, Kishimoto T, Lindemann A, Mertelsmann RH, Herrmann F. Participation of the cytokines interleukin 6, tumor necrosis factor-alpha, and interleukin 1-beta secreted by acute myelogenous leukemia blasts in autocrine and paracrine leukemia growth control. J. Clin. Invest. 1989;84(2):451–457. doi: 10.1172/JCI114186. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [101].Kitagawa M, Saito I, Kuwata T, Yoshida S, Yamguchi S, Takahashi M, Tanizawa T, Kamiyama R, Hirokawa K. Overexpression of tumor necrosis factor (TNF)-alpha and interferon (IFN)-gamma by bone marrow cells from patients with myelodysplastic syndromes. Leukemia. 1997;11(12):2049–2054. doi: 10.1038/sj.leu.2400844. [DOI] [PubMed] [Google Scholar]
- [102].Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann. N. Y. Acad. Sci. 2006;1091:151–169. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- [103].Kopf M, Bachmann MF, Marsland BJ. Averting inflammation by targeting the cytokine environment. Nat. Rev. Drug. Discov. 2010;9(9):703–718. doi: 10.1038/nrd2805. [DOI] [PubMed] [Google Scholar]
- [104].Arnott CH, Scott KA, Moore RJ, Robinson SC, Thompson RG, Balkwill FR. Expression of both TNF-alpha receptor subtypes is essential for optimal skin tumour development. Oncogene. 2004;23(10):1902–1910. doi: 10.1038/sj.onc.1207317. [DOI] [PubMed] [Google Scholar]
- [105].Arnott CH, Scott KA, Moore RJ, Hewer A, Philips DH, Parker P, Balkwill FR, Owens DM. Tumour necrosis factor-alpha mediates tumour promotion via a PKC alpha- and AP-1-dependent pathway. Oncogene. 2002;21(31):4728–4738. doi: 10.1038/sj.onc.1205588. [DOI] [PubMed] [Google Scholar]
- [106].Scott KA, Moore RJ, Arnott CH, East N, Thompson RG, Scallon BJ, Shealy DJ, Balkwill FR. An anti-tumor necrosis factor-alpha antibody inhibits the development of experimental skin tumors. Mol. Cancer. Ther. 2003;2(5):445–451. [PubMed] [Google Scholar]
- [107].Federico A, Filippelli A, Falciani M, Tuccillo C, Tiso A, Floreani A, Naccarato R, Rossi F, Del Vecchio Blanco C, Loguercio C. Platelet aggregation is affected by nitrosothiols in patients with chronic hepatitis: in vivo and in vitro studies. World. J. Gastroenterol. 2007;13(27):3677–3683. doi: 10.3748/wjg.v13.i27.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends. Cell. Biol. 2001;11(9):372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- [109].Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104(4):487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- [110].Liu ZG. Adding facets to TNF signaling. The JNK angle. Mol. Cell. 2003;12(4):795–796. doi: 10.1016/s1097-2765(03)00399-x. [DOI] [PubMed] [Google Scholar]
- [111].Varfolomeev EE, Ashkenazi A. Tumor necrosis factor: an apoptosis JuNKie? Cell. 2004;116(4):491–497. doi: 10.1016/s0092-8674(04)00166-7. [DOI] [PubMed] [Google Scholar]
- [112].Wang CY, Cusack JC, Jr., Liu R, Baldwin AS., Jr. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat. Med. 1999;5(4):412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- [113].Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;2001;414(6861):313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- [114].Yeh WC, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pompa JL, Ferrick D, Hum B, Iscove N, Ohashi P, Rothe M, Goeddel DV, Mak TW. Early lethality, functional NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7(5):715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- [115].Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8(3):297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- [116].Balkwill F. Tumour necrosis factor and cancer. Nat. Rev. Cancer. 2009;9(5):361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- [117].Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, El-Deiry WS, Lowe SW, Goeddel DV, Mak TW. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279(5358):1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- [118].Ventura JJ, Kennedy NJ, Lamb JA, Flavell RA, Davis RJ. c-Jun NH(2)-terminal kinase is essential for the regulation of AP-1 by tumor necrosis factor. Mol. Cell. Biol. 2003;23(8):2871–2882. doi: 10.1128/MCB.23.8.2871-2882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87(3):565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- [120].Natoli G, Costanzo A, Ianni A, Templeton DJ, Woodgett JR, Balsano C, Levrero M. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science. 1997;275(5297):200–203. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- [121].Lamb JA, Ventura JJ, Hess P, Flavell RA, Davis RJ. JunD mediates survival signaling by the JNK signal transduction pathway. Mol. Cell. 2003;11:1479–1789. doi: 10.1016/s1097-2765(03)00203-x. [DOI] [PubMed] [Google Scholar]
- [122].Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, Nakano H. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO. J. 2003;22(15):3898–3909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Lee SY, Reichlin A, Santana A, Sokol KA, Nussenzweig MC, Choi Y. TRAF2 is essential for JNK but not NF-kappaB activation and regulates lymphocyte proliferation and survival. Immunity. 1997;7(5):703–713. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- [124].Roulston A, Reinhard C, Amiri P, Williams LT. Early activation of c-Jun N-terminal kinase and p38 kinase regulate cell survival in response to tumor necrosis factor alpha. J. Biol. Chem. 1998;273(17):10232–10239. doi: 10.1074/jbc.273.17.10232. [DOI] [PubMed] [Google Scholar]
- [125].Reuther-Madrid JY, Kashatus D, Chen S, Li X, Westwick J, Davis RJ, Earp HS, Wang CY, Baldwin AS., Jr. The p65/RelA subunit of NF-kappaB suppresses the sustained, antiapoptotic activity of Jun kinase induced by tumor necrosis factor. Mol. Cell. Biol. 2002;22(23):8175–8183. doi: 10.1128/MCB.22.23.8175-8183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Guo YL, Kang B, Williamson JR. Inhibition of the expression of mitogen-activated protein phosphatase-1 potentiates apoptosis induced by tumor necrosis factor-alpha in rat mesangial cells. J. Biol. Chem. 1998;273(17):10362–10366. doi: 10.1074/jbc.273.17.10362. [DOI] [PubMed] [Google Scholar]
- [127].De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signaling. Nature. 2001;414(6861):308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- [128].Tang F, Tang G, Xiang J, Dai Q, Rosner MR, Lin A. The absence of NF-kappaB-mediated inhibition of c-Jun N-terminal kinase activation contributes to tumor necrosis factor alpha-induced apoptosis. Mol. Cell. Biol. 2002;2002;22(24):8571–8579. doi: 10.1128/MCB.22.24.8571-8579.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell. 2003;115(1):61–70. doi: 10.1016/s0092-8674(03)00757-8. [DOI] [PubMed] [Google Scholar]
- [130].Papa S, Zazzeroni F, Bubici C, Jayawardena S, Alvarez K, Matsuda S, Nguyen DU, Pham CG, Nelsbach AH, Melis T, De Smaele E, Tang WJ, D’Adamio L, Franzoso G. Gadd45 beta mediates the NF-kappa B suppression of JNK signaling by targeting MKK7/JNKK2. Nat. Cell. Biol. 2004;6(2):146–153. doi: 10.1038/ncb1093. [DOI] [PubMed] [Google Scholar]
- [131].Igaki T, Kanda H, Yamamoto-Goto Y, Kanuka H, Kuranaga E, Aigaki T, Miura M. Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. EMBO. J. 2002;21(12):3009–3018. doi: 10.1093/emboj/cdf306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Moreno E, Yan M, Basler K. Evolution of TNF signaling mechanisms: JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Curr. Biol. 2002;12(14):1263–1268. doi: 10.1016/s0960-9822(02)00954-5. [DOI] [PubMed] [Google Scholar]
- [133].Kauppila S, Maaty WS, Chen P, Tomar RS, Eby MT, Chapo J, Chew S, Rathore N, Zachariah S, Sinha SK, Abrams JM, Chaudhary PM. Eiger and its receptor, Wengen, comprise a TNF-like system in Drosophila. Oncogene. 2003;22(31):4860–4867. doi: 10.1038/sj.onc.1206715. [DOI] [PubMed] [Google Scholar]
- [134].Maciejewski J, Selleri C, Anderson S, Young NS. Fas antigen expression on CD34+ human marrow cells is induced by interferon gamma and tumor necrosis factor alpha and potentiates cytokine-mediated hematopoietic suppression in vitro. Blood. 1995;85(11):3183–3190. [PubMed] [Google Scholar]
- [135].Selleri C, Sato T, Del Vecchio L, Luciano L, Barrett AJ, Rotoli B, Young NS, Maciejewski JP. Involvement of Fas-mediated apoptosis in the inhibitory effects of interferon-alpha in chronic myelogenous leukemia. Blood. 1997;89:957–964. [PubMed] [Google Scholar]
- [136].Young NS, Barrett AJ. The treatment of severe acquired aplastic anemia. Blood. 1995;85(12):3367–3377. [PubMed] [Google Scholar]
- [137].Ventura JJ, Cogswell P, Flavell RA, Baldwin AS, Jr., Davis RJ. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes. Dev. 2004;18(23):2905–15. doi: 10.1101/gad.1223004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296(5573):1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- [139].Jamaluddin M, Wang S, Boldogh I, Tian B, Brasier AR. TNF-alpha-induced NF-kappaB/RelA Ser(276) phosphorylation and enhanceosome formation is mediated by an ROS-dependent PKAc pathway. Cell. Signal. 2007;19(7):1419–1433. doi: 10.1016/j.cellsig.2007.01.020. [DOI] [PubMed] [Google Scholar]
- [140].Moe GW, Marin-Garcia J, Konig A, Goldenthal M, Lu X, Feng Q. In vivo TNF-alpha inhibition ameliorates cardiac mitochondrial dysfunction, oxidative stress, and apoptosis in experimental heart failure. Am. J. Physiol. Heart. Circ. Physiol. 2004;287(4):H1813–1820. doi: 10.1152/ajpheart.00036.2004. [DOI] [PubMed] [Google Scholar]
- [141].Kimura H, Sawada T, Oshima S, Kozawa K, Ishioka T, Kato M. Toxicity and roles of reactive oxygen species. Curr. Drug. Targets. Inflamm. Allergy. 2005;4(4):489–95. doi: 10.2174/1568010054526287. [DOI] [PubMed] [Google Scholar]
- [142].Suematsu N, Tsutsui H, Wen J, Kang D, Ikeuchi M, Ide T, Hayashidani S, Shiomi T, Kubota T, Hamasaki N, Takeshita A. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation. 2003;107(10):1418–1423. doi: 10.1161/01.cir.0000055318.09997.1f. [DOI] [PubMed] [Google Scholar]
- [143].Hancock WW, Muller WA, Cotran RS. Interleukin 2 receptors are expressed by alveolar macrophages during pulmonary sarcoidosis and are inducible by lymphokine treatment of normal human lung macrophages, blood monocytes, and monocyte cell lines. J. Immunol. 1987;138(1):185–191. [PubMed] [Google Scholar]
- [144].Aggarwal BB, Shishodia S, Ashikawa K, Bharti AC. The role of TNF and its family members in inflammation and cancer: lessons from gene deletion. Curr. Drug. Targets. Inflamm. Allergy. 2002;1(4):327–341. doi: 10.2174/1568010023344571. [DOI] [PubMed] [Google Scholar]
- [145].Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell. Death. Differ. 2003;10(1):45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- [146].Wheelhouse NM, Chan YS, Gillies SE, Caldwell H, Ross JA, Harrison DJ, Prost S. TNF-alpha induced DNA damage in primary murine hepatocytes. Int. J. Mol. Med. 2003;12(6):889–894. [PubMed] [Google Scholar]
- [147].Jialal I, Devaraj S, Venugopal SK. Oxidative stress, inflammation, and diabetic vasculopathies: the role of alpha tocopherol therapy. Free. Radic. Res. 2002;36(12):1331–1336. doi: 10.1080/1071576021000038531. [DOI] [PubMed] [Google Scholar]
- [148].Nathan I, Dizdaroglu M, Bernstein L, Junker U, Lee C, Muegge K, Durum SK. Induction of oxidative DNA damage in u937 cells by TNF or anti-Fas stimulation. Cytokine. 2000;12(7):881–887. doi: 10.1006/cyto.1999.0638. [DOI] [PubMed] [Google Scholar]
- [149].Park YM, Han MY, Blackburn RV, Lee YJ. Overexpression of HSP25 reduces the level of TNF alpha-induced oxidative DNA damage biomarker, 8-hydroxy-2′-deoxyguanosine, in L929 cells. J. Cell. Physiol. 1998;174(1):27–34. doi: 10.1002/(SICI)1097-4652(199801)174:1<27::AID-JCP4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- [150].Nagakawa Y, Williams GM, Zheng Q, Tsuchida A, Aoki T, Montgomery RA, Klein AS, Sun Z. Oxidative mitochondrial DNA damage and deletion in hepatocytes of rejecting liver allografts in rats: role of TNF-alpha. Hepatology. 2005;42(1):208–215. doi: 10.1002/hep.20755. [DOI] [PubMed] [Google Scholar]
- [151].Yarosh DB, Boumakis S, Brown AB, Canning MT, Galvin JW, Both DM, Kraus E, O’Connor A, Brown DA. Measurement of UVB-Induced DNA damage and its consequences in models of immunosuppression. Methods. 2002;28(1):55–62. doi: 10.1016/s1046-2023(02)00209-8. [DOI] [PubMed] [Google Scholar]
- [152].Park JO, Lopez CA, Gupta VK, Brown CK, Mauceri HJ, Darga TE, Manan A, Hellman S, Posner MC, Kufe DW, Weichselbaum RR. Transcriptional control of viral gene therapy by cisplatin. J. Clin. Invest. 2002;110(3):403–410. doi: 10.1172/JCI15548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Calveley VL, Khan MA, Yeung IW, Vandyk J, Hill RP. Partial volume rat lung irradiation: temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. Int J Radiat Biol. 2005;81(12):887–899. doi: 10.1080/09553000600568002. [DOI] [PubMed] [Google Scholar]
- [154].Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003;22(37):5885–5896. doi: 10.1038/sj.onc.1206701. [DOI] [PubMed] [Google Scholar]
- [155].Hur GM, Lewis J, Yang Q, Lin Y, Nakano H, Nedospasov S, Liu ZG. The death domain kinase RIP has an essential role in DNA damage-induced NF-kappa B activation. Genes. Dev. 2003;17(7):873–882. doi: 10.1101/gad.1062403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Reya T. Regulation of hematopoietic stem cell self-renewal. Recent. Prog. Horm. Res. 2003;58:283–295. doi: 10.1210/rp.58.1.283. [DOI] [PubMed] [Google Scholar]
- [157].Rezzoug F, Huang Y, Tanner MK, Wysoczynski M, Schanie CL, Chilton PM, Ratajczak MZ, Fugier-Vivier IJ, Ildstad ST. TNF-alpha is critical to facilitate hemopoietic stem cell engraftment and function. J. Immunol. 2008;180(1):49–57. doi: 10.4049/jimmunol.180.1.49. [DOI] [PubMed] [Google Scholar]
- [158].Harrison DE, Lerner CP. Most primitive hematopoietic stem cells are stimulated to cycle rapidly after treatment with 5-fluorouracil. Blood. 1991;78(5):1237–1240. [PubMed] [Google Scholar]
- [159].Du W, Adam Z, Rani R, Zhang X, Pang Q. Oxidative stress in Fanconi anemia hematopoiesis and disease progression. Antioxid. Redox. Signal. 2008;10(11):1909–1921. doi: 10.1089/ars.2008.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Winkler T, Hong SG, Decker JE, Morgan MJ, Wu C, Hughes WM, 5th, Yang Y, Wangsa D, Padilla-Nash HM, Ried T, Young NS, Dunbar CE, Calado RT. Defective telomere elongation and hematopoiesis from telomerase-mutant aplastic anemia iPSCs. J. Clin. Invest. 2013;123(5):1952–1963. doi: 10.1172/JCI67146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Sutheesophon K, Nishimura N, Kobayashi Y, Furukawa Y, Kawano M, Itoh K, Kano Y, Ishii H, Furukawa Y. Involvement of the tumor necrosis factor (TNF)/TNF receptor system in leukemic cell apoptosis induced by histone deacetylase inhibitor depsipeptide (FK228) J. Cell. Physiol. 2005;203(2):387–397. doi: 10.1002/jcp.20235. [DOI] [PubMed] [Google Scholar]
- [162].Lee YR, Yu HN, Noh EM, Youn HJ, Song EK, Han MK, Park CS, Kim BS, Park YS, Park BK, Lee SH, Kim JS. TNF-alpha upregulates PTEN via NF-kappaB signaling pathways in human leukemic cells. Exp. Mol. Med. 2007;39(1):121, 127. doi: 10.1038/emm.2007.14. [DOI] [PubMed] [Google Scholar]
- [163].Houtenbos I, Westers TM, de Gruijl TD, Scheper RJ, Ossenkoppele GJ, van de Loosdrecht AA. TNF-alpha receptor 1 expression on acute myeloid leukemic blasts predicts differentiation into leukemic dendritic cells. Leukemia. 2004;18(6):1149–1153. doi: 10.1038/sj.leu.2403359. [DOI] [PubMed] [Google Scholar]
- [164].Seijkens T, Engel D, Tjwa M, Lutgens E. The role of CD154 in haematopoietic development. Thromb. Haemost. 2010;104(4):693–701. doi: 10.1160/TH10-03-0174. [DOI] [PubMed] [Google Scholar]
- [165].Testa U. TRAIL/TRAIL-R in hematologic malignancies. J. Cell. Biochem. 2010;110(1):21–34. doi: 10.1002/jcb.22549. [DOI] [PubMed] [Google Scholar]
- [166].de Cremoux P, Gluckman E, Podgorniak MP, Menier C, Thierry D, Calvo F, Socié G. Decreased IL-1 beta and TNF alpha secretion in long-term bone marrow culture supernatant from Fanconi’s anaemia patients. Eur. J. Haematol. 1996;57(3):202–207. doi: 10.1111/j.1600-0609.1996.tb01364.x. [DOI] [PubMed] [Google Scholar]
- [167].Stark R, Andre C, Thierry D, Cherel M, Galibert F, Gluckman E. The expression of cytokine and cytokine receptor genes in long-term bone marrow culture in congenital and acquired bone marrow hypoplasias. Br. J. Haematol. 1993;83(4):560–566. doi: 10.1111/j.1365-2141.1993.tb04691.x. [DOI] [PubMed] [Google Scholar]
- [168].Bijangi-Cishehsaraei K, Saadatzadeh MR, Werne A, McKenzie KA, Kapur R, Ichijo H, Haneline LS. Enhanced TNF-alpha-induced apoptosis in Fanconi anemia type C-deficient cells is dependent on apoptosis signal-regulating kinase 1. Blood. 2005;106(13):4124–4130. doi: 10.1182/blood-2005-05-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Li X, Le Beau MM, Ciccone S, Yang FC, Freje B, Chen S, Yuan J, Hong P, Orazi A, Haneline LS, Clapp DW. Ex vivo culture of Fancc−/− stem/progenitor cells predisposes cells to undergo apoptosis, and surviving stem/progenitor cells display cytogenetic abnormalities and an increased risk of malignancy. Blood. 2005;105(9):3465–3471. doi: 10.1182/blood-2004-06-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Li J, Sejas DP, Zhang X, Qiu Y, Nattamai KJ, Rani R, Rathbun KR, Geiger H, Williams DA, Bagby GC, Pang Q. TNF-alpha induces leukemic clonal evolution ex vivo in Fanconi anemia group C murine stem cells. J. Clin. Invest. 2007;117(11):3283–3295. doi: 10.1172/JCI31772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Matsushita N, Endo Y, Sato K, Kurumizaka H, Yamashita T, Takata M, Yanagi S. Direct inhibition of TNF-α promoter activity by Fanconi anemia protein FANCD2. PLoS. One. 2011;6(8):e23324. doi: 10.1371/journal.pone.0023324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Pagano G, Talamanca AA, Castello G, Pallardó FV, Zatterale A, Degan P. Oxidative stress in Fanconi anaemia: from cells and molecules towards prospects in clinical management. Biol. Chem. 2012;393(1-2):11–21. doi: 10.1515/BC-2011-227. [DOI] [PubMed] [Google Scholar]
- [173].Zhang X, Sejas DP, Qiu Y, Williams DA, Pang Q. Inflammatory ROS promote and cooperate with the Fanconi anemia mutation for hematopoietic senescence. J. Cell. Sci. 2007;120:1572–1583. doi: 10.1242/jcs.003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Du W, Rani R, Sipple J, Schick J, Myers KC, Mehta P, Andreassen PR, Davies SM, Pang Q. The FA pathway counteracts oxidative stress through selective protection of antioxidant defense gene promoters. Blood. 2012;119(18):4142–4151. doi: 10.1182/blood-2011-09-381970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Furst DE, Breedveld FC, Kalden JR, Smolen JS, Burmester GR, Bijlsma JW, Dougados M, Emery P, Keystone EC, Klareskog L, Mease PJ. Updated consensus statement on biological agents, specifically tumour necrosis factor {alpha} (TNF{alpha}) blocking agents and interleukin-1 receptor antagonist (IL-1ra), for the treatment of rheumatic diseases. Ann. Rheum. Dis. 2005;64(Suppl 4):iv2–14. doi: 10.1136/ard.2005.044941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Oflazoglu E, Grewal IS, Gerber H. Targeting CD30/CD30L in oncology and autoimmune and inflammatory diseases. Adv. Exp. Med. Biol. 2009;647:174–185. doi: 10.1007/978-0-387-89520-8_12. [DOI] [PubMed] [Google Scholar]
- [177].Arnason BG. Immunologic therapy of multiple sclerosis. Annu. Rev. Med. 1999;50:291–302. doi: 10.1146/annurev.med.50.1.291. [DOI] [PubMed] [Google Scholar]
- [178].Bessissow T, Renard M, Hoffman I, Vermeire S, Rutgeerts P, Van Assche G. Review article: non-malignant haematological complications of anti-tumour necrosis factor alpha therapy. Aliment. Pharmacol. Ther. 2012;36:312–323. doi: 10.1111/j.1365-2036.2012.05189.x. [DOI] [PubMed] [Google Scholar]
- [179].Harvey BS, Nicotra LL, Vu M, Smid SD. Cannabinoid CB2 receptor activation attenuates cytokine-evoked mucosal damage in a human colonic explant model without changing epithelial permeability. Cytokine. 2013:S1043–4666. doi: 10.1016/j.cyto.2013.04.032. [DOI] [PubMed] [Google Scholar]
- [180].Van Hauwermeiren F, Vandenbroucke RE, Libert C. Treatment of TNF mediated diseases by selective inhibition of soluble TNF or TNFR1. Cytokine. Growth. Factor. Rev. 2011;22(5-6):311–319. doi: 10.1016/j.cytogfr.2011.09.004. [DOI] [PubMed] [Google Scholar]
- [181].Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N. Engl. J. Med. 1997;337(15):1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- [182].Moriconi F, Malik IA, Amanzada A, Blaschke M, Raddatz D, Khan S, Ramadori G. The anti-TNF-α antibody infliximab indirectly regulates PECAM-1 gene expression in two models of in vitro blood cell activation. Lab. Invest. 2012;92(2):166–177. doi: 10.1038/labinvest.2011.160. [DOI] [PubMed] [Google Scholar]
- [183].Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005;353(23):2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- [184].Mehta PA, Svahn J, Davies SM, Pang Q, Harris R, Ghezzi P, Lanza T, Ferretti E, Barabino P, Mueller R, Dufour C. Etanercept treatment in Fanconi anaemia; combined US and Italian experience. Br. J. Haematol. 2012;158(6):809–811. doi: 10.1111/j.1365-2141.2012.09250.x. [DOI] [PubMed] [Google Scholar]
- [185].Miehsler W, Novacek G, Wenzl H, Vogelsang H, Knoflach P, Kaser A, Dejaco C, Petritsch W, Kapitan M, Maier H, Graninger W, Tilg H, Reinisch W. Austrian Society of Gastroenterology and Hepatology. A decade of infliximab: The Austrian evidence based consensus on the safe use of infliximab in inflammatory bowel disease. J. Crohns. Colitis. 2010;4(3):221–256. doi: 10.1016/j.crohns.2009.12.001. [DOI] [PubMed] [Google Scholar]
- [186].Furst DE, Schiff MH, Fleischmann RM, Strand V, Birbara CA, Compagnone D, Fischkoff SA, Chartash EK. Adalimumab, a fully human anti-tumor necrosis factor-alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis) J. Rheumatol. 2003;30(12):2563–2571. [PubMed] [Google Scholar]
- [187].Ang HT, Helfhhott S. Do the clinical responses and complications following etanercept or infliximab therapy predict similar outcomes with the other tumor necrosis factor-alpha antagonists in patients with rheumatoid arthritis? J. Rheumatol. 2003;30(11):2315–2318. [PubMed] [Google Scholar]
- [188].O’Connor PM, Lapointe TK, Beck PL, Buret AG. Mechanisms by which inflammation may increase intestinal cancer risk in inflammatory bowel disease. Inflamm. Bowel. Dis. 2010;16(8):1411–20. doi: 10.1002/ibd.21217. [DOI] [PubMed] [Google Scholar]
- [189].Stone JH, Holbrook JT, Marriott MA, Tibbs AK, Sejismundo LP, Min YI, Specks U, Merkel PA, Spiera R, Davis JC, Clair EW, McCune WJ, Ytterberg SR, Allen NB, Hoffman GS. Wegener’s Granulomatosis Etanercept Trial Research Group. Solid malignancies among patients in the Wegener’s Granulomatosis Etanercept Trial. Arthritis. Rheum. 2006;54(5):1608–18. doi: 10.1002/art.21869. [DOI] [PubMed] [Google Scholar]
- [190].Boehrer S, Nowak D, Hoelzer D, Mitrou PS, Chow KU. The molecular biology of TRAIL-mediated signaling and its potential therapeutic exploitation in hematopoietic malignancies. Curr. Med. Chem. 2006;13(18):2091–2100. doi: 10.2174/092986706777935294. [DOI] [PubMed] [Google Scholar]
- [191].Tischkowitz MD, Hodgson SV. Fanconi anaemia. J Med Genet. 2003;40(1):1–10. doi: 10.1136/jmg.40.1.1. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Levitus M, Rooimans MA, Steltenpool J, Cool NF, Oostra AB, Mathew CG, Hoatlin ME, Waisfisz Q, Arwert R, de Winter JP, Joenje H. Heterogeneity in Fanconi anemia: evidence for 2 new genetic subtypes. Blood. 2004;103(7):2498–503. doi: 10.1182/blood-2003-08-2915. 2004. [DOI] [PubMed] [Google Scholar]