Abstract
Background
Nasopharyngeal carcinoma (NPC) is a malignant neoplasm arising from the mucosal epithelium of the nasopharynx. Different races can have different etiology, presentation, and progression patterns.
Methods
Data were analyzed on NPC patients in the United States reported to the SEER (Surveillance, Epidemiology, and End Results) database between 1973 and 2009. Racial groups studied included non-Hispanic whites, Hispanic whites, blacks, Asians, and others. Patient characteristics, age-adjusted incidence and mortality rates, treatment, and five-year relative survival rates were compared across races. Stratification by stage at diagnosis and histologic type was considered. Multivariate regression was conducted to evaluate the significance of racial differences.
Results
Patient characteristics that were significantly different across races included age at diagnosis, histologic type, in situ/malignant tumors in lifetime, stage, grade, and regional nodes positive. Incidence and mortality rates were significantly different across races, with Asians having the highest rates overall and stratified by age and/or histologic type. Asians also had the highest rate of receiving radiation only. The racial differences in treatment were significant in the multivariate stratified analysis. When stratified by stage and histologic type, Asians had the best five-year survival rates. The survival experience of other races depended on stage and type. In the multivariate analysis, the racial differences were significant.
Conclusions
Analysis of the SEER data shows that racial differences exist among NPC patients in the U.S. This result can be informative to cancer epidemiologists and clinicians.
Keywords: nasopharyngeal carcinoma, racial differences, SEER
1. Introduction
Nasopharyngeal carcinoma (NPC) is a malignant neoplasm arising from the mucosal epithelium of the nasopharynx. It differs from other head and neck cancers in occurrence, causes, clinical behaviors, and treatment. It is uncommon in the U.S. and most other nations, representing less than 1 case per 100,000 in most populations [1]; it is much more common in the southern regions of China. In the Guangdong province of China, NPC occurs in about 25 cases per 100,000 people. Additionally, NPC is more common in males. While it is seen primarily in middle-aged people in Asia, a high proportion of African cases appear in children. Multiple risk factors have been suggested as associated with the risk of developing NPC, including tobacco use, alcohol consumption, geography, ancestry, Epstein-Barr virus (EBV), gender, age, diet, occupational exposures, and environmental exposures.
In 1978, the World Health Organization (WHO) classified NPC into three major histologic types. Type 1 was keratinizing squamous cell carcinoma, Type 2 was non-keratinizing carcinoma, and Type 3 was undifferentiated carcinoma. The 1991 WHO classification retained the keratinizing squamous cell carcinoma type and combined the 1978 WHO Type 2 and 3 into “non-keratinizing carcinoma”. Non-keratinizing carcinoma was then further classified as “differentiated” and “undifferentiated”. The differentiated type constituted the 1978 WHO Type 2, and the undifferentiated type constituted the 1978 WHO Type 3. The 2005 WHO classification was the same as that developed in 1991, with the exception that “basaloid squamous cell carcinoma” was added. Among U.S.-born Chinese and white individuals, keratinizing squamous cell carcinomas are more common, whereas among Chinese who reside in HongKong, Taiwan, and Macao, differentiated non-keratinizing carcinomas dominate.
Treatment of NPC depends on multiple factors, such as the stage of the cancer and the overall health condition of a patient. Commonly adopted treatments include surgery, radiation therapy, chemotherapy, targeted therapy, and others. Depending upon the stage of the cancer, some of these treatments may be combined. For many patients, a combination of radiation therapy and chemotherapy is used.
Prognosis depends on stage. According to the American Cancer Society [2], for stage 0 patients, the five-year survival rate is almost 100%. The rate drops to 80% for stage I patients and 60% for stage II patients. For patients at stage III, IVc, and IVb, the five-year survival rate is between 30% and 40%. Stage IVc patients with distant metastasis have a five-year survival rate of less than 10%. In addition, epidemiologic studies in the U.S. have identified histologic type as an independent prognostic factor [3,4]. Non-keratinizing carcinomas are generally associated with EBV positivity, which has been associated with improved NPC survival [5]. In addition, non-keratinizing carcinomas are often better controlled by ionizing radiation than keratinizing ones, and as a result, the five-year survival rate is significantly better for non-keratinizing carcinoma than keratinizing squamous cell carcinoma (51% versus 6%) [6]. Other factors that may influence prognosis include patient age and gender, lymph node metastasis, molecular risk factors, and treatment regimens.
The goal of this study is to provide a comprehensive description of racial differences among NPC patients in the U.S. in terms of patients’ characteristics, clinical-pathologic features, incidence, treatment strategy, and survival rates by analyzing the SEER database. Racial differences among cancer patients have attracted extensive attention [7,8]. However, research on NPC remains scarce. The available NPC studies may have limitations by either focusing on specific racial groups (i.e., Bhattacharyya [9] compared Chinese and whites) or specific outcomes (i.e., Ou and others [10] analyzed survival only).
2. Methods
2.1 Source population
The population-based sample was obtained from the SEER database, which contains data from 18 population-based regional and state cancer registries in the U.S. All SEER registries were included in the analysis, although not all contributed cases throughout the entire study period. NPC patients were identified from 1973 to 2009, using data published in 2011. Tumor site and histology were coded according to criteria specified by the WHO in the International Classification of Diseases for Oncology (ICD-O-3) [11]. NPC cases were identified using ICDO- 3 site code C110–C119. Histologic types were grouped using the ICD-O-3 code. Since there were very few basaloid squamous cell carcinomas, NPC patients were classified as keratinizing squamous cell carcinoma, differentiated non-keratinizing squamous cell carcinoma, undifferentiated non-keratinizing squamous cell carcinoma, and others. Squamous cell carcinoma (ICD-O codes 8070 and 8071) formed the keratinizing squamous cell carcinoma group. Large- and small-cell non-keratinizing carcinoma (ICD-O codes 8072 and 8073) formed the differentiated non-keratinizing carcinoma group. Undifferentiated, anaplastic, and lymphoepithelial carcinoma (ICD-O codes 8020, 8021, and 8082) formed the undifferentiated non-keratinizing carcinoma group. The rest were in the “others” group.
For the analysis of patients’ characteristics and clinical-pathologic features, SEER 9 has data for cancers diagnosed between 1973 and 2009. Among the 5,868 patients, there are 2,901 non-Hispanic whites; 212 Hispanic whites; 527 blacks; 1,945 Asians; and 232 other races. The “other” category includes American Indians, Alaska natives, others unspecified, and unknown. Features available for analysis include gender, age at diagnosis, histologic type, in situ/malignant tumors in lifetime (one primary only in lifetime, first of two or more primaries, second of two or more, third of three or more, fourth of four or more), stage (in situ, localized, regional, and distant), grade (I, II, III, and IV), tumor size, and presence of regional nodes positive. Outcome variables include incidence, treatment strategy (no treatment, surgery, radiation, radiation and surgery), mortality, and survival. For incidence, SEER 13 data, which include detailed race and incidence information for cancers diagnosed between 1992 and 2009, is analyzed. For mortality, SEER has data available from 1990 to 2009. For treatment strategy, SEER 9 has data for cancers diagnosed between 1973 and 2009. And for survival, SEER 18 has data for cancers diagnosed between 1973 and 2004 and followed up to 12/31/2009.
2.2 Statistical analysis
In the comparison of patient characteristics and clinical-pathologic features across racial groups, Chi-squared tests and ANOVA were used, and p-values were computed. The analysis was conducted using SAS version 9.2. Age-adjusted incidence and mortality rates were calculated using SEER*Stat and U.S. 2000 Census data for age-standardization. In the analysis of treatment strategy, multivariate logistic regressions were conducted, adjusting for age and gender and stratified by stage at diagnosis and histologic type. Five-year relative survival rates were calculated using SEER*Stat with an actuarial method [12]. Multivariate Cox regressions were then conducted, adjusting for age at diagnosis, gender, and treatment and stratified by stage at diagnosis and histologic type.
3. Results
3.1 Patients’ characteristics and clinical-pathologic features
Main results are shown in Table 1, and a more detailed age distribution of NPC patients is shown in Table 5 (Appendix). There are more male patients. The gender distributions are borderline different across races (p=0.081), with the “other” race category having the most male patients (75%) and the Hispanic white category having the least (66.5%). The age at diagnosis is significantly different across races. For blacks, the mean age at diagnosis is 48.3 years, compared to 58.1 years for non-Hispanic whites. The distributions of histologic types are also significantly different across races. Compared with whites and blacks, much fewer Asians and other races were diagnosed with keratinizing squamous cell carcinoma. Distributions of in situ/malignant tumors in the lifetime are also significantly different. The majority of observations are “one primary only in lifetime”, with Asians having the most (88.9%) and non-Hispanic white having the least (77.7%). Further, the stage distributions are significantly different. Non-Hispanic whites have the most localized tumors (18.3%), Asians have the most regional (72.2%), and other races have the most distant (24.9%). For the whole sample, there are 3.8%, 14.0%, 52.4%, and 29.8% grade I-IV patients, respectively. Non-Hispanic whites have more grade II (20.1%) and grade III (53.3%) patients, whereas Asians (40.9%) and other races (42.6%) have more grade IV patients. For all patients, the average tumor size is 38.1mm, and the difference is not significant across races. Asians (27.8%) and other races (23.2%) have the lowest rates of all nodes negative, non- Hispanic whites have the highest (40.0%), and the racial difference is significant. Different races also have different treatment strategies. Blacks have the highest rate of no treatment (8.2%), non-Hispanic whites have the highest rate of surgery (7.8%), Asians have the highest rate of radiation (81.1%), and Hispanic whites have the highest rate of radiation and surgery (23.8%). On average, Asians have the longest survival time, with a mean of 163.9 months, and non-Hispanic whites have the shortest survival time, with a mean of 100.4 months.
Table 1.
NPC patients’ characteristics and clinical-pathologic features for the whole cohort and different racial groups.
| Total (n=5,868) |
Non- Hispanic white (n=2,901) |
Hispanic white (n=212) |
Black (n=527) |
Asian (n=1,945) |
Other (n=232) |
P | |
|---|---|---|---|---|---|---|---|
| Gender | 0.081 | ||||||
| Male | 3983 (68.5) | 1954 (67.4) | 141 (66.5) | 374 (71.0) | 1340 (68.9) | 174 (75.0) | |
| Female | 1834 (31.5) | 947 (32.6) | 71 (33.5) | 153 (29.0) | 605 (31.1) | 58 (25.0) | |
| Age at diagnosis | 54.7±16.6 | 58.1±16.9 | 52.2±19.2 | 48.3±18.2 | 51.9±14.5 | 51.1±14.6 | <0.001 |
| Histologic type | <0.001 | ||||||
| Keratinizing squamous cell carcinoma | 2456 (42.2) | 1520 (52.4) | 89 (42.0) | 226 (42.9) | 549 (28.2) | 72 (31.0) | |
| Differentiated non-keratinizing carcinoma | 664 (11.4) | 208 (7.2) | 24 (11.3) | 54 (10.2) | 333 (17.1) | 45 (19.4) | |
| Undifferentiated non-keratinizing carcinoma | 1172 (20.1) | 412 (14.2) | 33 (15.6) | 106 (20.1) | 552 (28.4) | 69 (29.7) | |
| Others | 1525 (26.2) | 761 (26.2) | 66 (31.1) | 141 (26.8) | 511 (26.3) | 46 (19.8) | |
| In situ/Malignant tumors in lifetime | <0.001 | ||||||
| One primary only in lifetime | 4793 (82.4) | 2255 (77.7) | 179 (84.4) | 428 (81.2) | 1729 (88.9) | 202 (87.1) | |
| First of two or more primaries | 537 (9.2) | 304 (10.5) | 14 (6.6) | 50 (9.5) | 152 (7.8) | 17 (7.3) | |
| Second of two or more primaries | 409 (7.0) | 285 (9.8) | 13 (6.1) | 41 (7.8) | 58 (3.0) | 12 (5.2) | |
| Third of three or more primaries | 66 (1.1) | 49 (1.7) | 6 (2.8) | 6 (1.1) | 4 (0.2) | 1 (0.4) | |
| Forth of four or more primaries | 12 (0.2) | 8 (0.3) | 0 (0) | 2 (0.4) | 2 (0.1) | 0 (0) | |
| Stage | <0.001 | ||||||
| In situ | 9 (0.2) | 7 (0.3) | 0 (0) | 2 (0.5) | 0 (0) | 0 (0) | |
| Localized | 671 (15.9) | 389 (18.3) | 26 (17.7) | 47 (12.3) | 195 (13.9) | 14 (8.3) | |
| Regional | 2764 (65.4) | 1294 (60.9) | 101 (68.7) | 243 (63.8) | 1013 (72.2) | 113 (66.9) | |
| Distant | 782 (18.5) | 435 (20.5) | 20 (13.6) | 89 (23.4) | 196 (14.0) | 42 (24.9) | |
| Grade | <0.001 | ||||||
| Grade • • | 154 (3.8) | 126 (6.4) | 4 (2.7) | 7 (2.1) | 16 (1.2) | 1 (0.6) | |
| Grade • • | 563 (14.0) | 394 (20.1) | 25 (16.8) | 57 (16.7) | 75 (5.4) | 12 (6.8) | |
| Grade • • | 2104 (52.4) | 1045 (53.3) | 73 (49.0) | 168 (49.3) | 730 (52.5) | 88 (50.0) | |
| Grade • • | 1196 (29.8) | 396 (20.2) | 47 (31.5) | 109 (32.0) | 569 (40.9) | 75 (42.6) | |
| Tumor size (mm) | 38.1±41.5 | 39.9±55.1 | 40.0±20.5 | 42.0±22.0 | 35.1±29.5 | 38.0±22.6 | 0.205 |
| Regional nodes positive | <0.001 | ||||||
| All nodes negative | 1826 (34.0) | 1045 (40.0) | 57 (30.2) | 157 (31.6) | 516 (27.8) | 51 (23.2) | |
| One or more nodes positive | 3549 (66.0) | 1566 (60.0) | 132 (69.8) | 340 (68.4) | 1342 (72.2) | 169 (76.8) | |
| Treatment | <0.001 | ||||||
| No treatment | 323 (5.8) | 178 (6.5) | 11 (5.4) | 41 (8.2) | 77 (4.1) | 16 (7.1) | |
| Surgery | 293 (5.3) | 211 (7.8) | 15 (7.4) | 31 (6.2) | 30 (1.6) | 6 (2.7) | |
| Radiation | 3883 (70.0) | 1726 (63.4) | 128 (63.4) | 314 (63.1) | 1542 (81.1) | 173 (76.5) | |
| Radiation & Surgery | 1050 (18.9) | 607 (22.3) | 48 (23.8) | 112 (22.5) | 252 (13.3) | 31 (13.7) | |
| Survival time (month) | 124.2±2.4 | 100.4±2.9 | 122.3±12.1 | 111.6±7.0 | 163.9±4.7 | 117.4±11.1 | <0.001 |
Cancers diagnosed 1973–2009 in the SEER 9 database. For a continuous variable, mean ± standard deviation, and for a categorical variable, count (percentage).
3.2 Incidence
For all age groups combined, the incidence rate is 0.74 per 100,000 person-years (Table 2). For the three major age groups, the incidence rates are 0.20, 1.31, and 1.75, respectively. More detailed age-adjusted incidence rates are provided in Table 6 (Appendix). Overall, Asians have the highest incidence rate (3.03, 95% CI 2.91–3.16), followed by other races (2.19, 95% CI 1.84–2.58). Hispanic whites have the lowest incidence rate (0.38, 95% CI 0.34–0.43). For the three major age groups separately, the incidence rate ranking remains the same. For each histologic type separately, Asians have the highest incidence rates (0.71, 0.53, 0.81, and 0.99, respectively), followed by other races and blacks.
Table 2.
Age-adjusted NPC incidence and mortality rates per 100,000 person-years for the whole cohort and different racial groups, stratified by age at diagnosis and histologic type.
| Non-Hispanic white |
Hispanic white | Black | Asian | Other | Total | |
|---|---|---|---|---|---|---|
| Incidence | ||||||
| All ages | 0.40 (0.38–0.42) | 0.38 (0.34–0.43) | 0.65 (0.59–0.71) | 3.03 (2.91–3.16) | 2.19 (1.84–2.58) | 0.74 (0.72–0.76) |
| <40 years | 0.09 (0.08–0.11) | 0.09 (0.07–0.11) | 0.21 (0.18–0.26) | 0.86 (0.78–0.95) | 0.37 (0.24–0.55) | 0.20 (0.19–0.22) |
| 40–64 years | 0.64 (0.59–0.68) | 0.62 (0.52–0.72) | 1.05 (0.92–1.20) | 6.03 (5.72–6.36) | 3.46 (2.78–4.27) | 1.31 (1.26–1.36) |
| 65+ years | 1.23 (1.14–1.32) | 1.16 (0.91–1.46) | 1.62 (1.32–1.98) | 5.61 (5.10–6.15) | 7.31 (5.29–9.85) | 1.75 (1.66–1.85) |
| Keratinizing squamous cell carcinoma | 0.18 (0.17–0.19) | 0.14 (0.11–0.17) | 0.26 (0.22–0.30) | 0.71 (0.65–0.77) | 0.46 (0.31–0.65) | 0.25 (0.24–0.26) |
| Differentiated non-keratinizing carcinoma | 0.05 (0.04–0.05) | 0.04 (0.03–0.06) | 0.09 (0.07–0.12) | 0.53 (0.47–0.58) | 0.41 (0.26–0.60) | 0.11 (0.10–0.12) |
| Undifferentiated non-keratinizing carcinoma | 0.06 (0.05–0.07) | 0.07 (0.05–0.09) | 0.11 (0.09–0.14) | 0.81 (0.75–0.88) | 0.54 (0.37–0.75) | 0.16 (0.15–0.17) |
| Others | 0.11 (0.10–0.12) | 0.14 (0.11–0.17) | 0.19 (0.16–0.23) | 0.99 (0.92–1.06) | 0.78 (0.58–1.03) | 0.23 (0.22–0.24) |
| Mortality | ||||||
| All ages | 0.19 (0.19–0.19) | 0.16 (0.15–0.18) | 0.32 (0.31–0.34) | 1.07 (1.02–1.12) | 0.39 (0.32–0.46) | 0.24 (0.23–0.24) |
| <40 years | 0.02 (0.02–0.02) | 0.02 (0.01–0.02) | 0.07 (0.06–0.07) | 0.14 (0.12–0.16) | 0.03 (0.02–0.06) | 0.03 (0.03–0.03) |
| 40–64 years | 0.27 (0.26–0.27) | 0.21 (0.18–0.24) | 0.51 (0.48–0.54) | 1.94 (1.83–2.05) | 0.52 (0.41–0.66) | 0.36 (0.35–0.37) |
| 65+ years | 0.78 (0.76–0.80) | 0.71 (0.62–0.80) | 1.02 (0.94–1.10) | 3.14 (2.88–3.41) | 1.68 (1.26–2.20) | 0.87 (0.85–0.89) |
Diagnoses in the period of 1992–2009, and deaths in the period of 1990–2009 in the SEER 13 database. In each cell, estimated rate (95% CI). Rates were age-adjusted using the U.S. 2000 Census population.
3.3 Treatment strategy
Among the 5,549 patients, 323 had no treatment; 293 had surgery; 3,883 had radiation; and 1,050 had both surgery and radiation. As shown in Table 3, for localized tumors, Asians had a much higher rate of radiation (85.9%) compared to 61.2% for non-Hispanic whites, 57.7% for Hispanic whites, 70.2% for blacks, and 61.5% for other races. For regional tumors, Asians and other races had high rates of radiation (86.0% and 81.4%, respectively). For distant tumors, Asians (83.7%) and other races (80.5%) had the highest rates of radiation, with Hispanic whites having the lowest (55.0%). In the stratified multivariate logistic regression, racial differences are found to be significant (Table 3; Detailed regression results are provided in Table 7 in the Appendix). When stratified by histologic type, for keratinizing squamous cell carcinoma, Asians had the highest rate of radiation (83.1%), followed by other races (76.8%). Non-Hispanic whites and blacks had high rates of radiation and surgery (19.8% and 19.0%, respectively). For differentiated non-keratinizing squamous cell carcinoma, Asians (83.8%) and other races (79.5%) had even higher rates of radiation. The other three racial groups had higher rates of radiation and surgery (23.4%, 25.0%, and 25.9%, respectively). For undifferentiated non-keratinizing squamous cell carcinoma and others, the observed patterns are similar. In the stratified multivariate logistic regression, racial differences are significant (Table 3; Detailed results are provided in Table 7 in the Appendix).
Table 3.
Treatment strategy for different racial groups, stratified by stage at diagnosis and histologic type.
| Non- Hispanic white (n=2,015) |
Hispanic white (n=142) |
Black (n=362) |
Asian (n=1,376) |
Other (n=167) |
P | ||
|---|---|---|---|---|---|---|---|
| Stage at diagnosis | |||||||
| Localized | No treatment | 4 (1.1) | 2 (7.7) | 1 (2.1) | 3 (1.6) | 2 (15.4) | <0.001 |
| Surgery | 49 (13.4) | 4 (15.4) | 9 (19.1) | 1 (0.5) | 2 (15.4) | ||
| Radiation | 224 (61.2) | 15 (57.7) | 33 (70.2) | 165 (85.9) | 8 (61.5) | ||
| Radiation & Surgery | 89 (24.3) | 5 (19.2) | 4 (8.5) | 23 (12.0) | 1 (7.7) | ||
| Regional | No treatment | 50 (4.0) | 3 (3.1) | 14 (6.0) | 18 (1.8) | 4 (3.5) | <0.001 |
| Surgery | 64 (5.1) | 4 (4.2) | 13 (5.5) | 15 (1.5) | 2 (1.8) | ||
| Radiation | 853 (68.2) | 63 (65.6) | 150 (63.8) | 860 (86.0) | 92 (81.4) | ||
| Radiation & Surgery | 283 (22.6) | 26 (27.1) | 58 (24.7) | 107 (10.7) | 15 (13.3) | ||
| Distant | No treatment | 29 (7.4) | 0 | 6 (7.7) | 3 (1.6) | 3 (7.3) | <0.001 |
| Surgery | 35 (8.9) | 4 (20.0) | 3 (3.8) | 6 (3.3) | 1 (2.4) | ||
| Radiation | 253 (64.4) | 11 (55.0) | 61 (78.2) | 154 (83.7) | 33 (80.5) | ||
| Radiation & Surgery | 76 (19.3) | 5 (25.0) | 8 (10.3) | 21 (11.4) | 4 (9.8) | ||
| Histologic type | |||||||
| Keratinizing squamous cell carcinoma | No treatment | 70 (4.9) | 4 (4.7) | 21 (9.7) | 14 (2.6) | 6 (8.7) | <0.001 |
| Surgery | 104 (7.2) | 6 (7.1) | 12 (5.6) | 8 (1.5) | 1 (1.4) | ||
| Radiation | 978 (68.1) | 60 (70.6) | 142 (65.7) | 443 (83.1) | 53 (76.8) | ||
| Radiation & Surgery | 285 (19.8) | 15 (17.6) | 41 (19.0) | 68 (12.8) | 9 (13.0) | ||
| Differentiated non-keratinizing carcinoma | No treatment | 9 (4.4) | 2 (8.3) | 0 (0) | 12 (3.7) | 3 (6.8) | <0.001 |
| Surgery | 10 (4.9) | 1 (4.2) | 0 (0) | 6 (1.8) | 2 (4.5) | ||
| Radiation | 138 (67.3) | 15 (62.5) | 40 (74.1) | 275 (83.8) | 35 (79.5) | ||
| Radiation & Surgery | 48 (23.4) | 6 (25.0) | 14 (25.9) | 35 (10.7) | 4 (9.1) | ||
| Undifferentiated non-keratinizing carcinoma | No treatment | 19 (4.9) | 1 (3.0) | 4 (4.1) | 22 (4.0) | 3 (4.3) | <0.001 |
| Surgery | 13 (3.3) | 1 (3.0) | 2 (2.1) | 6 (1.1) | 1 (1.4) | ||
| Radiation | 252 (64.8) | 18 (54.5) | 62 (63.9) | 435 (79.7) | 53 (76.8) | ||
| Radiation & Surgery | 105 (27.0) | 13 (39.4) | 29 (29.9) | 83 (15.2) | 12 (17.4) | ||
| Others | No treatment | 80 (11.6) | 4 (6.7) | 16 (12.2) | 29 (5.9) | 4 (9.1) | <0.001 |
| Surgery | 84 (12.2) | 7 (11.7) | 17 (13.0) | 10 (2.0) | 2 (4.5) | ||
| Radiation | 358 (51.8) | 35 (58.3) | 70 (53.4) | 389 (78.7) | 32 (72.7) | ||
| Radiation & Surgery | 169 (24.5) | 14 (23.3) | 28 (21.4) | 66 (13.4) | 6 (13.6) |
Cancers diagnosed in the period of 1973–2009 in the SEER 9 database. In each cell, count (percentage). P-values were generated from multivariate logistic regression.
3.4 Mortality and survival
For the whole sample, the mortality rate is 0.24 (Table 2). For the three major age groups, the mortality rates are 0.03, 0.36, and 0.87, respectively. For all ages, Asians had the highest mortality rate (1.07, 95% CI 1.02–1.12), followed by other races (0.39, 95% CI 0.32–0.46) and blacks (0.32, 95% CI 0.31–0.34). For the 40–64 and 65+ age groups, the rankings are the same as all ages combined. For the below-40 age group, blacks had a higher mortality rate (0.07, 95% CI 0.06–0.07) than other races (0.03, 95% CI 0.02–0.06).
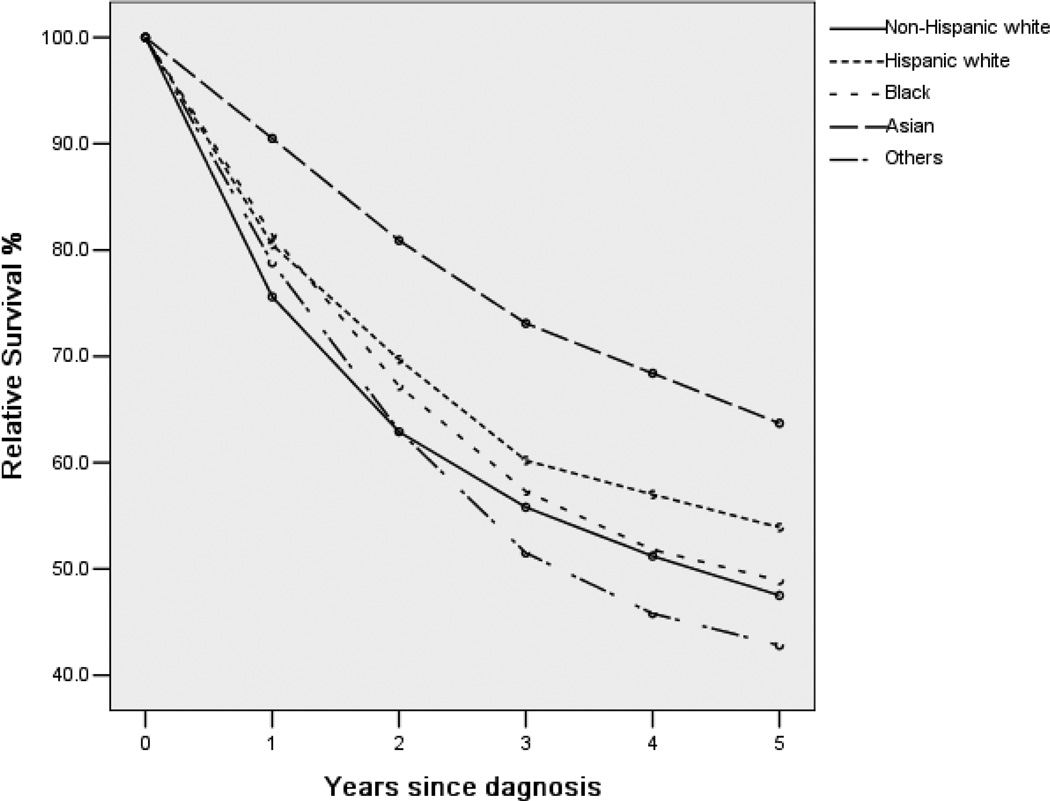
Figure 1 shows the unadjusted survival rate for five years. Asians had better survival rates at all times, whereas the survival curves of other racial groups may cross. The five-year relative survival rates, stratified by stage at diagnosis and histologic type, are shown in Table 4. For localized tumors, Asian patients had the highest five-year survival rate (83.5%, 95% CI 77.8– 87.8%), followed by other races (81.0%), Hispanic whites (74.5%), blacks (62.6%), and non-Hispanic whites (61.6%). For regional tumors, Asians still had the highest survival rate, followed by Hispanic whites. For distant tumors, Asians had the best five-year survival rate (34.1%), with other races having the lowest (15.8%). For all three stages, the multivariate Cox regression suggests significant racial differences. Detailed results are shown in Table 8 in the Appendix. When stratified by histologic type, Asian keratinizing squamous cell carcinoma patients had the best survival rate (57.1%), with other races having the worst (32.7%). For differentiated nonkeratinizing squamous cell carcinoma, blacks had the second-best (58.9%), with other races still having the worst (45.2%). For undifferentiated non-keratinizing squamous cell carcinoma, non- Hispanic whites had the second-best survival rate (65.9%). For other histologic types, Hispanic whites had the second-best survival rate (57.8%). In the stratified multivariate Cox regression, racial differences are significant. Detailed Cox regression results are shown in Table 9 (Appendix).
Figure 1.
Relative survival up to five years for different racial groups. Cancers diagnosed in the period of 1973–2004, and followed up to 12/31/2009.
Table 4.
Five-year relative survival rates for different racial groups, stratified by stage at diagnosis and histologic type.
| Non- Hispanic white (n=4013) |
Hispanic white (n=535) |
Black (n=937) |
Asian (n=3381) |
Other (n=148) |
P-value | |
|---|---|---|---|---|---|---|
| Stage at diagnosis | ||||||
| Localized | 61.6 (55.9–66.7) | 74.5 (53.1–87.2) | 62.6 (46.8–75.0) | 83.5 (77.8–87.8) | 81.0 (18.5–97.4) | <0.001 |
| Regional | 50.6 (48.0–53.2) | 51.6 (44.1–58.6) | 46.5 (41.0–51.8) | 64.0 (61.5–66.4) | 43.9 (30.7–56.3) | <0.001 |
| Distant | 22.4 (18.6–26.5) | 30.3 (17.8–43.7) | 30.3 (21.7–39.4) | 34.1 (28.6–39.6) | 15.8 (4.9– 32.2) | <0.001 |
| Histologic type | ||||||
| Keratinizing squamous cell carcinoma | 39.3 (36.9–41.7) | 43.4 (34.8–51.7) | 34.8 (29.3–40.3) | 57.1 (53.5–60.6) | 32.7 (15.4–51.3) | <0.001 |
| Differentiated non-keratinizing carcinoma | 57.8 (51.4–63.7) | 55.3 (39.1–68.8) | 58.9 (46.8–69.2) | 64.0 (59.2–68.4) | 45.2 (23.7–64.5) | 0.002 |
| Undifferentiated non-keratinizing carcinoma | 65.9 (61.2–70.1) | 62.8 (51.2–72.4) | 65.6 (57.1–72.8) | 71.5 (68.1–74.6) | 47.8 (27.4–65.7) | <0.001 |
| Others | 49.5 (45.9–53.0) | 57.8 (48.8–65.8) | 52.3 (44.9–59.1) | 62.2 (58.5–65.6) | 44.3 (28.5–59.0) | <0.001 |
Cancers diagnosed in the period of 1973–2004, and followed up to 12/31/2009 in the SEER 18 database. In each cell, estimated rate (95% CI). P-values were generated from multivariate Cox models.
4. Discussion
4.1 Main findings and possible interpretations
NPC remains a challenging malignancy of the head and neck. Some published studies investigated racial differences for a small number of racial groups or a small number of outcome variables. In this study, we have analyzed SEER data, comprehensively compared non-Hispanic whites, Hispanic whites, blacks, Asians, and other races, and found that for NPC patients in the U.S., significant racial differences exist in terms of patients’ characteristics, clinical-pathologic features, incidence, treatment, mortality, and survival. Such results can be informative to cancer epidemiologists, clinicians, and policy-makers.
Racial differences in NPC patient characteristics have been studied in the literature [13,14], although in a less systematic manner. Our analysis suggests that the distributions of age at diagnosis, histologic type, stage, grade, and number of regional nodes positive are significantly different across racial groups. The findings are mostly consistent with those in the literature [15,16,17]. The observed differences may reflect the complex interactions of genetic makeup, viral agents, dietary exposures to chemical carcinogens, occupational exposures, and environmental exposures in NPC patients [18].
Racial differences in incidence have been studied in a few publications. Richey and others limited their study to patients age 30 or younger, analyzed SEER data from 1973 to 2002, and found that blacks had the highest incidence rate [19]. Our analysis includes a much wider range of age and race distributions and generates different results. For the general population, it is commonly agreed that Asians have the highest incidence rate. Multiple factors may have contributed to the racial difference in incidence. Specifically, genetic predispositions may make Asians more susceptible. For example, two recent genome-wide association studies suggested the HLA (human leukocyte antigens) region as an NPC risk locus for Cantonese and Taiwanese populations [20,21]. A few other genetic changes have been linked with the risk of developing NPC, including loci 4p15.1–4q12, 3p21.31–21.2, and genes ITGA9 and RAD51L1. However, the associations between those genetic changes and race have not been established. The high incidence rate in Asians can also be attributed to environmental risk factors particularly the high intake of preserved or fermented food, which contains high levels of nitrosamines as well as bacterial mutagens, direct genotoxins, and EBV-reactivating substances. In addition, the common use of Chinese medicinal herbs by Asians may contribute either by reactivating EBV or through a direct promoting effect on EBV-transformed cells. For other races, the observed racial differences may also be linked with differences in alcohol and tobacco usage [22].
NPC has been traditionally treated with full course radiotherapy [23]. It has been suggested that stage 1 and 2 NPCs are treated with radiation alone, while stage 3 and 4 NPCs are treated by concurrent chemotherapy and radiation. In the past few years, there is strong evidence for using concurrent chemotherapy and radiation in advanced-stage NPC [23]. Surgery is often reserved for recurrent local and regional diseases. The aforementioned treatment recommendations are independent of race. In SEER, chemotherapy is not a treatment option, which may affect the results in Table 3. Shavers and Brown suggested that cancer treatment strategies may depend on race after adjusting for disease characteristics, and such dependence can, to some degree, be explained by behavior differences across races and by racial disparities [24]. Such dependence, however, cannot be proved or disproved using SEER data. More comprehensive data is needed to fully interpret the treatment differences.
The survival of NPC patients has been studied in a few publications. In our analysis, the survival advantage of Asians is observed for all stages and histologic types. In comparison, Richey and others analyzed two- and five-year relative survival rates and found little variation across racial groups [19]. Bhattacharyya observed that overall survival was better for Chinese compared to whites, and disease-specific survival was similar between these racial groups [9]. Ou and others found that Chinese had a significantly better survival rate than whites [10], and similar observations have been made in independent studies [23]. In the literature, there is limited research on the cause of racial differences in survival. Lee and Ko suggested that the higher survival rate in Asians may be partly attributed to the more favorable histology often seen in this group [25]. However, in our analysis, the survival advantage of Asians is observed for all histologic types. Genetic factors may contribute to the racial differences. Genes in the EGFR pathway have been linked with poor prognosis in NPC patients [26], and genetic association studies have shown that the Sp1-216T haplotype has a significantly higher distribution in blacks (29.3%) and whites (34.1%) than Asians (8.7%) [27]. A few other genetic changes have also been suggested [27]. In the literature, lifestyle, treatment regimen, EBV, age, stage, type, and gender have been suggested as associated with survival rates [28,29], but there is still a lack of consensus. In our analysis, after accounting for treatment, age, stage, type, and gender, racial differences still exist. Lifestyle variables and EBV are not available in SEER and thus cannot be accounted for.
4.2 Limitations
The SEER database is used because it is the largest cancer registry in the U.S. However, it has limitations. With multiple sites, errors may arise in tumor classification and staging. However, we do not expect a series of systematic errors correlated with ethnicity. Furthermore, data collected in SEER may not be comprehensive enough. For example, there is a lack of measurement on EBV, genetic factors, and lifestyle, which may contribute to NPC. In addition, the ethnicity and geographical origin information is not detailed enough. In analysis, we treated Asians as one ethnicity, following published studies [8]. In Table 10 (Appendix), we compared the five-year survival rate of Chinese against non-Chinese Asians and found differences. In China, the development and progression of NPC differ significantly between the southeast region (especially the Guangdong province) and the rest of the country. However, in SEER, for the Asian patients in the U.S., the geographical origin information is not available. In treatment analysis, the use of chemotherapy is not accounted for, as SEER data have generally been captured from hospital records, and chemotherapy is often given outside of the hospital setting. In addition, treatment selection can be affected by many other factors, including insurance status, socioeconomic status, treatment availability, and others. Such information is not available in SEER. Also, all patients are from the U.S. It is not clear whether the results hold for other nations.
4.3 Summary
Analysis of the SEER data shows that significant racial differences exist among NPC patients in the U.S. in terms of patient characteristics, incidence and mortality rates, treatment, and survival. The exact causes of such differences remain to be found. Despite several limitations, the identified racial differences can be informative to NPC epidemiologists and clinicians.
Acknowledgements
We thank the associate editor and four reviewers for careful review and insightful comments, which have led to a significant improvement of this article.
Funding
This study was supported by awards CA142774 and CA165923 from NCI/NIH and a Yale Cancer Center pilot grant. The funders had no role in study design, data collection and analysis, or manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
None declared.
Contributor Information
Yu Wang, Email: abyuer@gmail.com.
Yawei Zhang, Email: yawei.zhang@yale.edu.
Shuangge Ma, Email: shuangge.ma@yale.edu.
References
- 1.Initiative for Vaccine Research (IVR): Viral cancers. World Health Organization; Retrieved on 10/02/2012. [Google Scholar]
- 2.American Cancer Society: How is nasopharyngeal cancer staged? http://www.cancer.org/cancer/nasopharyngealcancer/detailedguide/nasopharyngeal-cancer-staging. [Google Scholar]
- 3.Levine PH, Connelly RR, Easton JM. Demographic patterns for nasopharyngeal carcinoma in the United States. Int J Cancer. 1980;26:741–748. doi: 10.1002/ijc.2910260607. [DOI] [PubMed] [Google Scholar]
- 4.Burt RD, Vaughan TL, Mcknight B. Descriptive epidemiology and survival analysis of nasopharyngeal carcinoma in the United States. Int J Cancer. 1992;52:549–556. doi: 10.1002/ijc.2910520409. [DOI] [PubMed] [Google Scholar]
- 5.Shi W, Pataki I, MacMillan C, Pintilie M, Payne D, O'Sullivan B, et al. Molecular pathology parameters in human nasopharyngeal carcinoma. Cancer. 2002;94:1997–2006. doi: 10.1002/cncr.0679. [DOI] [PubMed] [Google Scholar]
- 6.Reddy SP, Raslan WF, Gooneratne S, Kathuria S, Marks JE. Prognostic significance of keratinization in nasopharyngeal carcinoma. Am J Otolaryngol. 2005;16:103–108. doi: 10.1016/0196-0709(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 7.Pacheco JM, Gao F, Bumb C, Ellis MJ, Ma CX. Racial differences in outcomes of triplenegative breast cancer. Breast Cancer Research and Treatment. 2013;138:281–289. doi: 10.1007/s10549-012-2397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moran MS, Gonsalves L, Goss DM, Ma S. Breast cancers in U.S. residing Indian-Pakistani versus non-Hispanic White women: comparative analysis of clinical-pathologic features, treatment, and survival. Breast Cancer Research and Treatment. 2011;128:543–551. doi: 10.1007/s10549-011-1362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharyya N. The impact of race on survival in nasopharyngeal carcinoma: a matched analysis. American Journal of Otolaryngology. 2004;25:94–97. doi: 10.1016/j.amjoto.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Ou SHI, Zell JA, Ziogas A, Anton-Culver H. Epidemiology of nasopharyngeal carcinoma in the United States: improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Annals of Oncology. 2007;18:29–35. doi: 10.1093/annonc/mdl320. [DOI] [PubMed] [Google Scholar]
- 11.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. International Classification of Disease for Oncology. 3rd edition. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 12.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. NCI Monograph. 1961;6:101–121. [PubMed] [Google Scholar]
- 13.Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chinese Journal of Cancer. 2011;30:114–119. doi: 10.5732/cjc.010.10377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kataki AC, Simons MJ, Das AK, Sharma K, Mehra NK. Nasopharyngeal carcinoma in the Northeastern states of India. Chinese Journal of Cancer. 2011;30:106–113. doi: 10.5732/cjc.010.10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marks JE, Phillips JL, Menck HR. The national cancer data base report on the relationship of race and national origin to the histology of nasopharyngeal carcinoma. Cancer. 1998;83:582–588. doi: 10.1002/(sici)1097-0142(19980801)83:3<582::aid-cncr29>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 16.August M, Dodson DB, Nastri A, Chuang SK. Nasopharyngeal carcinoma: clinical assessment and review of 176 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:205–214. doi: 10.1067/moe.2001.110698. [DOI] [PubMed] [Google Scholar]
- 17.Erkal H, Serin M, Akmak AC. Nasopharyngeal carcinomas: analysis of patient, tumor and treatment characteristics determining outcome. Radiother Oncol. 2001;61:247–256. doi: 10.1016/s0167-8140(01)00448-0. [DOI] [PubMed] [Google Scholar]
- 18.Ung A, Chen CJ, Levine PH, Cheng YJ, Brinton LA, Chen IH, et al. Familial and sporadic cases of nasopharyngeal carcinoma in Taiwan. Anticancer Res. 1999;19:661–665. [PubMed] [Google Scholar]
- 19.Richey LM, Olshan AF, George J, Shores CG, Zanation AM, Cannon T, et al. Incidence and survival rates for young blacks with nasopharyngeal carcinoma in the United States. Arch Otolaryngol Head Neck Surg. 2006;132:1035–1040. doi: 10.1001/archotol.132.10.1035. [DOI] [PubMed] [Google Scholar]
- 20.Bei JX, Li Y, Jia WH, Feng BJ, Zhou G, Chen LZ, et al. A genome-wide association study of nasopharyngeal carcinoma identifies three new susceptibility loci. Nat Genet. 2010;42:599–603. doi: 10.1038/ng.601. [DOI] [PubMed] [Google Scholar]
- 21.Tse KP, Su WH, Chang KP, Tsang NM, Yu CJ, Tang P, et al. Genome-wide association study reveals multiple nasopharyngeal carcinoma-associated loci within the HLA region at chromosome 6p21.3. Am J Hum Genet. 2009;85:194–203. doi: 10.1016/j.ajhg.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaughan TL, Shapiro JA, Burt RD, Swanson GM, Berwick M, Lynch CF, et al. Nasopharyngeal cancer in a low-risk population: defining risk factors by histological type. Cancer Epidemiology, Biomarkers and Prevention. 1996;5:587–593. [PubMed] [Google Scholar]
- 23.Her C. Nasopharyngeal cancer and the southeast Asian patient. American Family Physician. 2001;63:1776–1782. [PubMed] [Google Scholar]
- 24.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. JNCI. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 25.Lee JT, Ko CY. Has survival improved for nasopharyngeal carcinoma in the United States? Otolaryngol Head Neck Surg. 2005;132:303–308. doi: 10.1016/j.otohns.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Ma BB, Poon TC, To KF, Zee B, Mo FK, Chan CM, et al. Prognostic significance of tumor angiogenesis, Ki 67 p53 oncoprotein, epidermal growth factor receptor and HER2 receptor protein expression in undifferentiated nasopharyngeal carcinoma - a prospective study. Head Neck. 2003;25:864–872. doi: 10.1002/hed.10307. [DOI] [PubMed] [Google Scholar]
- 27.Liu W, Innocenti F, Wu MH, Desai AA, Dolan ME, Cook EH, Jr, et al. A functional common polymorphism in a Sp1 recognition site of the epidermal growth factor receptor gene promoter. Cancer Res. 2005;65:46–53. [PubMed] [Google Scholar]
- 28.Tao Q, Chan AT. Nasopharyngeal carcinoma: molecular pathogenesis and therapeutic developments. Expert Rev Mol Med. 2007;9:1–24. doi: 10.1017/S1462399407000312. [DOI] [PubMed] [Google Scholar]
- 29.Lee AW, Poon YF, Foo W, Law SC, Cheung FK, Chan DK, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976–1985: overall survival and patterns of failure. Int J Radiat Onco Biol Phys. 1992;23:261–270. doi: 10.1016/0360-3016(92)90740-9. [DOI] [PubMed] [Google Scholar]