Abstract
Methylphenidate (Ritalin) is the most commonly prescribed psychoactive drug for juveniles and adolescents. Used to treat attention-deficit/hyperactivity disorder (ADHD) and for cognitive enhancement in healthy individuals, it has been regarded as a relatively safe medication for the past several decades. However, a thorough review of the literature reveals that the age-dependent activities of the drug, as well as potential developmental effects, are largely ignored. In addition, the diagnosis of ADHD is subjective, leaving open the possibility of misdiagnosis and excessive prescription of the drug. Recent studies have suggested that early life exposure of healthy rodent models to methylphenidate resulted in altered sleep/wake cycle, heightened stress reactivity, and, in fact, a dosage previously thought of as therapeutic depressed neuronal function in juvenile rats. Furthermore, juvenile rats exposed to low-dose methylphenidate displayed alterations in neural markers of plasticity, indicating that the drug might alter the basic properties of prefrontal cortical circuits. In this review of the current literature, we propose that juvenile exposure to methylphenidate may cause abnormal prefrontal function and impaired plasticity in the healthy brain, strengthening the case for developing a more thorough understanding of methylphenidate’s actions on the developing, juvenile brain, as well as better diagnostic measures for ADHD.
Keywords: Attention deficit/hyperactivity disorder (ADHD), methylphenidate, juvenile brain, cortical plasticity
Attention deficit/hyperactivity disorder (ADHD) is one of the most commonly diagnosed pediatric behavioral disorders, with current estimates ranging from 3–5% of the population globally [1, 2]. The number of children diagnosed with ADHD skyrocketed 24% between 2001 and 2010, according to a recent study published in JAMA Pediatrics [3]. Symptoms of ADHD can persist well into adulthood, and include inattentiveness, hyperactivity and impulsivity, often occurring along with other behavioral disorders or mood disorders [4, 5]. If left untreated, the effects can be devastating, preventing the affected individual from functioning successfully in a classroom or job setting, and negatively impacting social relationships [6, 7].
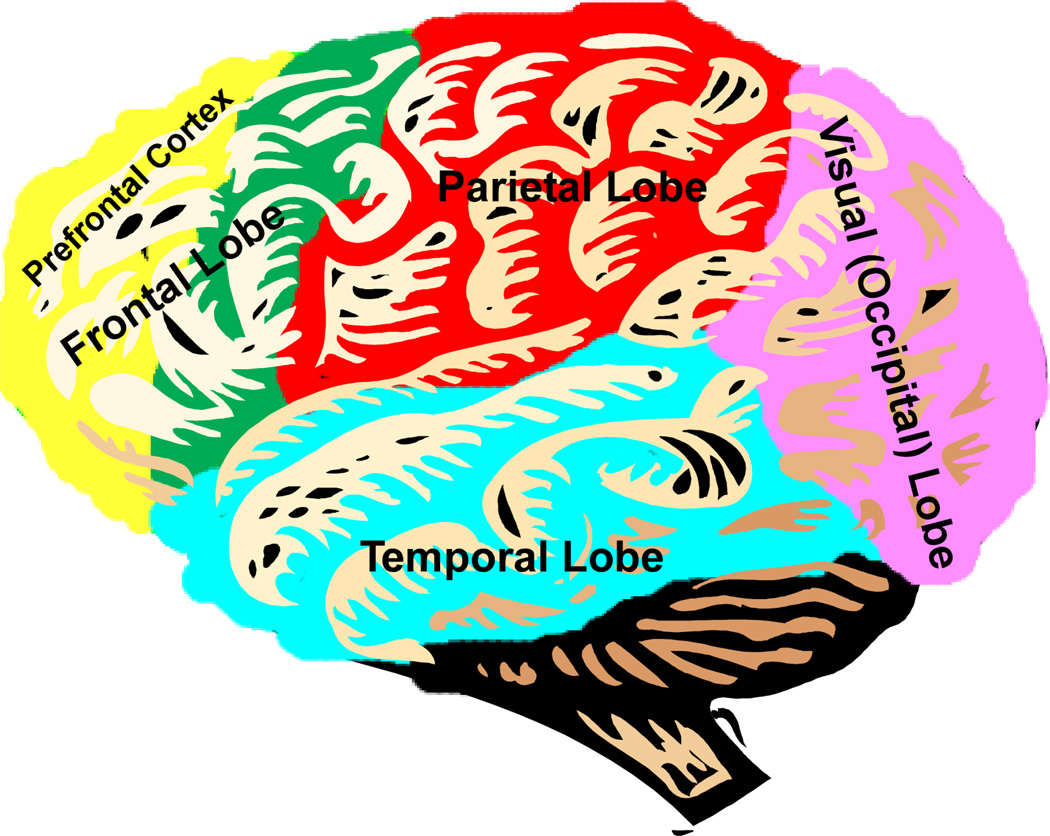
While the exact mechanisms of the disease have not been discovered, it is thought that ADHD symptoms arise from hypoactivity of the prefrontal cortex, which in turn arises from a lack of innervation from the neurotransmitter systems of dopamine and norepinephrine [8]. They are of particular importance to the proper function of the prefrontal cortex. This portion of the brain sits in the frontal-most area of the cortex, above the eyes and extending back to the temples. The prefrontal cortex is the brain's "executive control center," integrating sensory and motor information, directing attention, and planning behaviors [9–12] (Figure 1). Hypoactivity in this region results in locomotor hyperactivity, impulsivity, inability to properly direct attention, and often manifests as poor performance in school or at work [8].
Figure 1.
The prefrontal cortex (highlighted in yellow) is the brain's executive control center, involved in regulating working memory, attention, thinking, decision-making, judgment, behavioral control/inhibition, and emotion and motivation. The prefrontal cortex lies in the front-most portion of the frontal lobe (yellow and green).
Methylphenidate (Ritalin©; MPH) is currently the most commonly prescribed medication for the treatment of ADHD [13]. Methylphenidate is a psychostimulant, related to amphetamine, cocaine, and caffeine, and exerts its effects by blocking the transporters that reuptake dopamine and norepinephrine into the presynaptic neuron following their release; thus, it increases the levels or prolongs the availability of these neurotransmitters in the synapses to exert effects on postsynaptic neurons [14–16]. In optimal amounts, dopamine acts via the D1-like receptors, and norepinephrine via the α2A receptors to increase signal-to-noise ratio in the prefrontal cortex; thus enhancing the flow of information and neuronal communication [9, 17]. Excessive levels of dopamine and norepinephrine begin to activate dopamine D2-class receptors and noradrenergic α2 and β receptors, leading to weakening of the signal-to-noise ratio, and activation of neurons that may not be involved in the current task [9]. However, a recent study suggested that prefrontal cortex-dependent processes display differential sensitivity to the cognition-enhancing actions of psychostimulants that are linked to the differential involvement of α1- versus α2-receptors in these processes [18].
A large proportion of the literature on mechanisms of action of methylphenidate and dose-response curves comes from studies performed on normal human and animal subjects [18–22]. At first thought, this may seem like a glaring weakness - how could studying the actions of a drug on a normal population represent its effects on a diseased subset? It is important to note that reduced hyperactivity and impulsivity in stimulant-treated ADHD patients were not “paradoxical” effects, but in fact also occurred in healthy individuals given the same doses [23, 24]. The ability of low-dose stimulants to increase sustained attention, improve response inhibition and decrease locomotor activity is not unique to patients with ADHD. All of these actions are observed in non-ADHD humans and normal animals [25–27]. Additionally, low-dose MPH exerts similar actions on PFC neuronal activity (assessed by functional MRI) in a go/no-go task in both ADHD patients and normal control subjects [26]. Thus, the therapeutic actions of stimulants used to treat ADHD are not unique to ADHD patients nor do they necessarily reflect pathology within catecholaminergic systems in ADHD. Similar evidence exists to validate investigations of MPH actions in normal, experimental animals. More recent MPH studies in both humans and rats have found that low doses of MPH that correspond to those given to ADHD patients in the clinic appear to enhance prefrontal-dependent functions and cognition in much the same way in healthy humans and rats as they do in ADHD patients and disease model rat strains [1, 25, 28–34]. Therefore, study of the behavioral and physiological actions of clinically and behaviorally relevant doses of MPH in normal animals provides an opportunity to explore the role of catecholamine transmitters in prefrontal cortical function and attentional processes as they relate to the dynamic operation of brain circuits under normal and abnormal (e.g. ADHD) conditions.
This fact, along with the lack of reliable animal models of ADHD, led to the acceptance of stimulant study in healthy human subjects and in normal animals. Studying methylphenidate’s effects in healthy individuals can provide valuable insights into the drug’s mechanisms of action, as well as potentially highlight differences between ADHD-diseased and healthy brains. Higher doses (doses greater than those given to treat ADHD) increase locomotor activity and impair attention and performance on prefrontal cortex-dependent cognitive tasks; however, lower doses (those equivalent to the range given to ADHD patients) improve cognitive performance and reduce locomotor activity in healthy individuals [18, 19, 25, 35]. Likewise, lower doses of methylphenidate (0.25–1 mg/kg, intraperitoneal, i.p.) in normal adult rats resulted in increased performance on attention tasks along with no effect on locomotor activity, while higher doses impaired performance and induced hyperactivity; doses beyond 10 mg/kg resulted in "stereotypies" (repetitive, fine motor movements similar to the tics seen in disorders like Tourette's syndrome) [25, 36, 37]. Why might a medication that is reportedly a stimulant result in increased focus and attention while reducing locomotor hyperactivity? The answer lies in the regulation of dopamine and norepinephrine. Low, cognition enhancing doses of MPH have been shown to cause increases in these neurotransmitters selectively in prefrontal cortex, resulting in a heightening of executive functions without altering locomotion or physical activity levels (as would be seen if striatum were activated by the drug) [38]. However, higher doses of MPH (above 5–10mg/kg) have been shown to indiscriminately activate other cortical regions, resulting in an overshoot of dopamine and norepinephrine, depressing neuronal function in prefrontal cortex and inducing hyperactivity and stereotypies [39, 40]. The inverted U-curve of dopamine/norepinephrine actions on prefrontal function has been well illustrated [18, 21, 41–44]. Specifically, low, cognition-enhancing doses of MPH purportedly induce only slight increase of these neurotransmitters, optimizing function and placing the levels at the “peak” of that curve. However, when dosages of MPH are further increased, the levels surpass the peak and fall along the right hand of the curve, where over-excitation and stimulation of cAMP pathways begin to cause hyperactivity, impulsivity, over-stimulation and distractibility. Levels of dopamine and norepinephrine in a normal, healthy brain are not static; they may fall at the optimal peak for that individual, slightly less, or slightly more than optimal. There is currently no method for determining the optimal levels of these neurotransmitters in each individual; thus, predicting how psychostimulants will act is mainly guess-work. It is also possible that, although many studies found no overt cognitive differences between the effects of low-dose MPH on normal individuals and ADHD patients, molecular or cellular differences that cannot be detected in humans with our current technology might exist. Thus, one must examine the research on MPH as a cognitive enhancer and studies using normal individuals with caution and a critical mind.
Whatever the actual neuronal consequences of its usage may be, MPH is a hot-topic in research today, and is increasingly studied and accepted as a cognitive enhancer. MPH is frequently sold on the “black market” at schools and universities, and is used/abused more and more by graduate and medical students, military personnel, doctors and lawyers – any high-stress position where cognitive ability is highly valued [45, 46]. This frequent off-label usage stresses the need for a deeper understanding of its actions and effects on the brain.
It is not only the issue of healthy vs. ADHD-diseased brains that clouds our understanding of MPH’s actions - the vast majority of animal research has been conducted on mature rats, whereas the drug is most commonly given to children. These studies conducted in adult animals have provided great insights into the actions and potential side effects of MPH. However, because MPH is mostly used to treat children with ADHD, abused by teenagers as learning drugs, and the prefrontal cortex is still developing during this time, it is particularly important to investigate the drug’s effects on juvenile and adolescent brain systems. It is not hard to imagine that the adult brain may react differently to MPH treatment than would a juvenile, still developing brain [6]. The prefrontal cortex does not finish development until young adulthood; in humans this falls around the end of the second decade or early third decade of life [47–50]. This late prefrontal cortical development is of particular relevance to the development of ADHD [51]; levels of norepinephrine and dopamine surge and wane during maturation of the prefrontal cortex, and disruptions to the developmental flow may be responsible for some of the symptoms [52]. This development and remodeling of the neurotransmitter systems implicated in ADHD reveals a period of vulnerability during which insults to the system might change the developmental course and cause lasting alterations in the neurotransmitter levels.
In fact, a small, recently emerging body of research has begun to demonstrate the differential actions of MPH on juvenile versus adult animals. These studies have found that juvenile treatment with fairly low doses of methylphenidate resulted in disruption of the sleep/wake cycle and heightened anxiety responses even into adulthood [53, 54]. There have also been conflicting studies examining sensitization and vulnerability to drug abuse in children or adolescent rodents exposed to methylphenidate early in life, although more recent studies seem to agree that early life treatment with therapeutic doses protects against drug abuse and resolves complications of ADHD [55].
Despite the increasing usage of young rats in MPH research, no attempt has been made to determine if there is a difference in the rate of metabolism of MPH between juvenile rats and adult rats - given the higher basal metabolic and growth rates of juvenile rats, it would stand to reason that differences in the speed of metabolism and breakdown of pharmaceuticals such as MPH would be evident. While no study has yet examined the specific metabolism of MPH in juvenile rats, it should be noted that adolescent rats (PD40-45) that received 10 mg/kg i.p. of MPH displayed increased brain glucose metabolism across most brain regions [56]. This dosage is far beyond the clinical range (0.5–2 mg/kg i.p.), but it does suggest that MPH increases brain metabolic rate in juvenile rats. Therefore, if metabolic rates differ between juvenile and adult rats, the dose-response curve and therapeutic range might differ as well. Given the inconsistencies of current juvenile rat MPH studies, and the wide range of doses used in these studies, it is imperative to determine a proper therapeutic range for juvenile rats following both injected and orally-administered MPH, as well as to determine if early life exposure has the potential to cause lasting alterations in basic function of the prefrontal cortex.
One important unique property of the prefrontal cortex is its high level of plasticity, allowing for executive functions like working memory and active decision-making; this plasticity may be a product of the slow maturation of this region [51, 57, 58]. Plasticity in the brain refers to the ability of the circuits to weaken or strengthen neuronal pathways based on the environment and the individual's experiences; it is the brain’s flexibility. It is plasticity that allows us to learn, remember, and also to forget past lessons that are no longer applicable in the present. When we are young, all areas of the brain are highly plastic, allowing the circuitry involved in motor movements and sensory input to form; however, once adulthood is reached, the cortex becomes more rigid. The prefrontal cortex, however, retains that high level of plasticity throughout life, especially during the juvenile and adolescent period, and it is thought that this is necessary for working memory, behavioral inhibition, judgment, and decision-making [51]. The plasticity, at least in part, is thought to be due to a unique composition of NMDA receptors, a major receptor type involved in excitatory glutamatergic synaptic transmission [59–62]. NMDA receptors all contain two NR1 subunits, and also contain either NR2A or NR2B; NR2B conveys slower kinetics to the channel, allowing it to remain partially open during multiple stimulations [61]. This results in summation of responses and the continuation of neural activity briefly after input has stopped [62]. Without this unique feature, we would not be able to hold information in our minds during attention/working memory tasks, like remembering a phone number long enough to write it down, or walking into another room to fetch something [63]. It is unclear whether treatment with MPH during adolescence will alter the developmental course of the prefrontal cortex. However, it is apparent that developmental age does play a critical role in determining the effects of drug administration on prefrontal neurons. Based on this review of the current literature, we hypothesize that MPH within the reportedly therapeutic range will induce distinct biochemical and physiological changes in the developing brain. Specifically, treatment with MPH will result in significant changes in neuronal excitability, synaptic transmission and plasticity in the prefrontal neurons in juvenile rats. We have designed the following experiments to test this intriguing hypothesis.
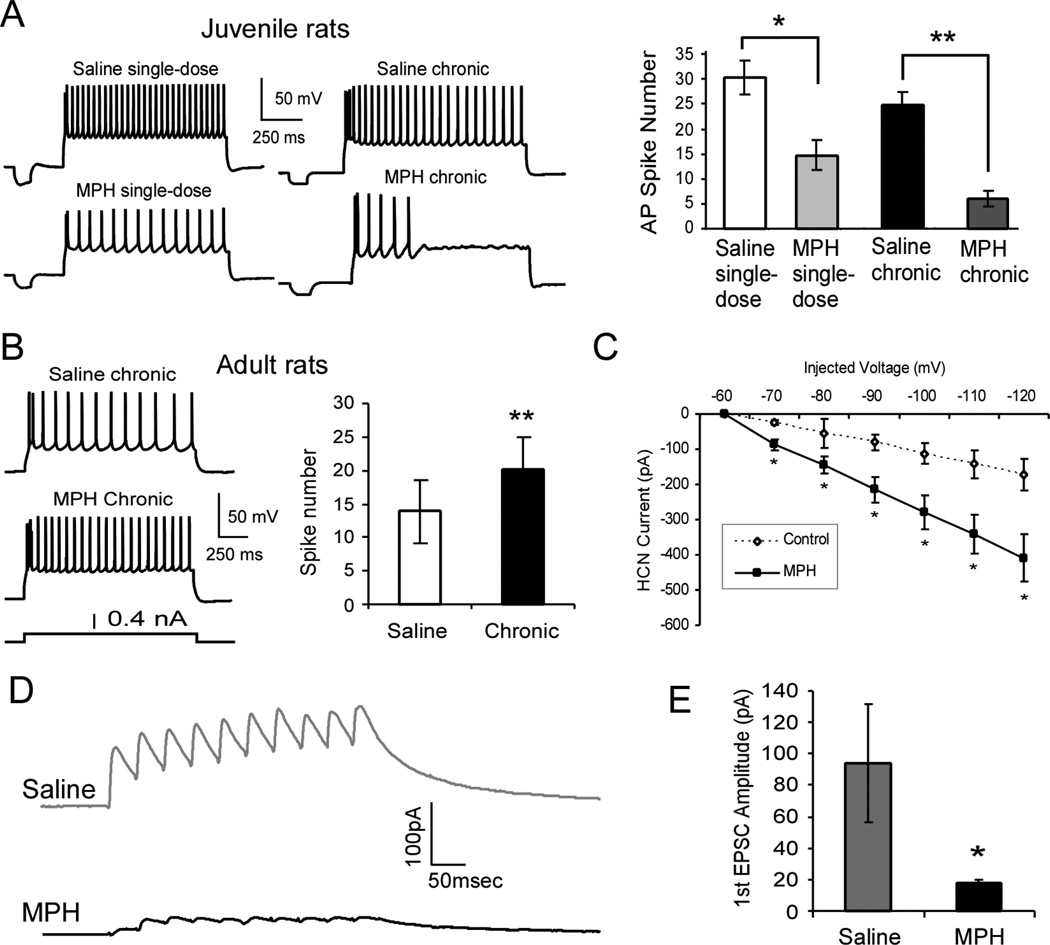
First, as we recently reported, we found that treatment with MPH resulted in significant reduction in both excitability and synaptic transmission in the juvenile rat prefrontal cortical neurons [64] (Figure 2A to 2C). This reduction in excitability may seem counterintuitive, as the drug has previously been found to increase cortical excitability at low doses; it is this increase in neuronal activity that is thought of as a therapeutic effect [38]. The reason for these discrepancies is unknown, but it would appear that juvenile rat prefrontal neurons are supersensitive to MPH and that the observed effects are age-dependent. The juvenile results were opposite to those seen in adult rats, in which prefrontal neurons are excited by low-dose stimulant treatment [35, 65] or by systemic administration of 2 mg/kg MPH [66]. Taken together, these results argue for an age-dependent action of MPH on prefrontal neurons. However, how this cellular depression occurs, and what effects it may have on circuitry level function remain unclear and further study on this issue is warranted.
Figure 2.
Age-dependent effects of 1 mg/kg MPH on the neuronal excitability in layer 5 pyramidal neurons in rat prefrontal cortex. A, Excitability of layer 5 pyramidal neurons in juvenile (postnatal day 15–25) rat prefrontal cortex is suppressed by MPH treatment. Treatment with 1 mg/kg i.p. MPH significantly reduces the excitability (spike numbers) of layer 5 pyramidal neurons in juvenile rat prefrontal cortex. As shown in these representative sweeps, fewer action potentials were observed in single-dose-treated rats than control animals and even fewer in chronic-treated animals. Right panel, summary histogram shows the significant decrease of spike numbers in single-dose (n = 11, p = 0.019) compared with single-dose saline control (n = 18), and even fewer in chronic MPH-treated rats (n = 18, p = 4.58 × 10−5) compared to a chronic saline control (n = 12). B, In contrast, neurons from adult rats (postnatal day 90–100) exhibited an increase in excitability following 1 mg/kg MPH treatment (n = 6; p = 0.0006) when compared to saline control (n= 6). C, Examination of the cAMP-HCN channel-mediated Ih current reveals an increase in Ih current amplitude concurrent with decreased neuronal excitability following 1 mg/kg MPH treatment (n = 10) when compared to saline controls (n = 7). I/V graph reveals significant increase of Ih current in MPH-treated neurons from −70 mV to −120 mV injected voltage (treatment by sweep interaction, p = 0.0003, f = 5.3, df = 5,75; treatment effect, p = 0.0182, f = 7, df = 1, 15; injected voltage effect, p < 0.0001, f = 27.4, df = 5, 75). D, Representative traces of NMDA receptor-mediated EPSCs recorded at +60 mV in the presence of picrotoxin (50 µM) and DNQX (20 µM) in response to a 10-pulse 20-Hz train of stimuli from saline-treated controls (n = 7) and MPH-treated neurons (n = 7). E, The amplitude of the first EPSC was significantly reduced in MPH-treated neurons compared to saline controls (*p < 0.05).
In order to understand the potential effects of MPH on cortical plasticity in the juvenile brain, one must look beyond the dopamine/norepinephrine systems, to the glutamate system, which is mostly involved in regulating plasticity via AMPA and NMDA receptors. The dopaminergic and glutamatergic systems are intricately interlinked via signaling pathways as well as physical interactions [67]. NMDARs control acute plasticity by regulating trafficking of AMPARs to the postsynaptic sites - AMPARs are associated with excitability of neurons and parameters such as spike timing and hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. During development, NR2B-containing subunits are replaced by NR2A-containing ones in the NMDA receptors, raising the threshold for LTP but lowering for LTD, making learning more selective [68]. A high percentage of NR2B-containing NMDA receptors lowers the threshold for LTP, and therefore increases the rate of learning and long-term memory formation, while a high percentage of NR2A-containing receptors lowers the threshold for LTD, increasing extinction and decreasing formation of long-term memories of the current situation [68]. Since NR2B levels should remain high in prefrontal cortex throughout life [62], any substance that deceases the levels of NR2B would likely cause a dampening of this region’s innate plasticity. A recent study by our lab indicated that excessive levels of dopamine indiscriminately activate the D2-like receptors, which result in a reduction in NMDAR trafficking via disruption of cAMP-independent glycogen synthase kinase 3 beta (GSK-3β) signaling pathway [69]. Furthermore, amphetamine has been shown to cause selective internalization and degradation of NR2B subunits, with no effect on NR2A levels [70]. We also found that when juvenile rats were treated with a single (1 mg/kg, i.p.) dose of methylphenidate, the levels of NR2B protein, as well as NR1, in the prefrontal cortex was markedly reduced, while NR2A levels did not change [64]. The behavioral and functional consequences of such a specific decrease in NR1 and NR2B levels are striking and potentially devastating to proper prefrontal cortical function. The prefrontal cortex is unique among cortical regions in that it does not experience a significant alteration in the NR2A/B ratio during development [62]. Thus, selectively decreasing the levels of NR2B-containing NMDA receptors in the prefrontal cortex might remove this region’s unique plasticity, rendering the prefrontal cortex similar to other cortical regions and potentially altering or impairing executive function. Indeed, following juvenile rat treatment with a single dose of 1 mg/kg MPH, our lab saw a significant decrease in NMDA receptor-mediated excitatory postsynaptic currents in response to 20-Hz train stimulation (Figure 2D and E). Furthermore, we also observed a shifting of long-term potentiation in the MPH-treated juvenile rat brain towards potentiation, with long-term potentiation significantly enhanced, while long-term depression was reduced [71]. These results indicate that treatment of the juvenile brain with clinically-relevant, low doses of MPH may alter plasticity of the prefrontal cortex. How might these changes manifest behaviorally? It is as of yet unclear; however, one hypothesis is that MPH may affect the integration of incoming signals and thus impair memory function in the juvenile brain. But the question is how? The prefrontal cortex’s uniquely high levels of NR2B throughout life impart the ability of the neurons to summate responses to incoming stimuli, resulting in the short-term potentiation of neural activity necessary for working memory; thus, decreasing the levels of NR2B in prefrontal cortex leads to reduction in the summation, which should impair working memory [62, 63, 71]. However, long-term potentiation is enhanced upon treatment with MPH [71]. Long-term potentiation was found to be enhanced following juvenile treatment with MPH. The exact roles of NR2A versus NR2B receptor subunits in long-term potentiation regulation in the prefrontal cortex are not well understood, but it is currently believed that the direction of plasticity in prefrontal cortex (potentiation or depression) is dependent on the ration of NR2A/NR2B, rather than exact levels of each subunit [59, 60, 72–74]. Thus, reducing NR2B levels without altering NR2A levels, as was seen following juvenile MPH treatment, was enough to alter the direction of long-term plasticity in the prefrontal cortex [71]. The behavioral ramifications of altering long-term potentiation and depression in the prefrontal cortex are unclear, as it is not known exactly what long-term potentiation is representing in this region. However, it might be hypothesized that, if short-term potentiation is a marker of working memory, then long-term potentiation might be a marker of sustained attention and longer-term memory consolidation. Thus, perhaps treatment of the healthy juvenile brain with these low doses of MPH results in impaired working memory and behavioral flexibility, but enhanced sustained attention and long-term memory? If this is the case, it could indicate that MPH-treated children who do not in fact have ADHD would appear successfully treated in a class-room setting – these children would be paying attention to the teacher, less hyperactive and learning might improve; however, stringent testing of their behavioral flexibility and working memory might reveal deficits that could affect other areas of their lives.
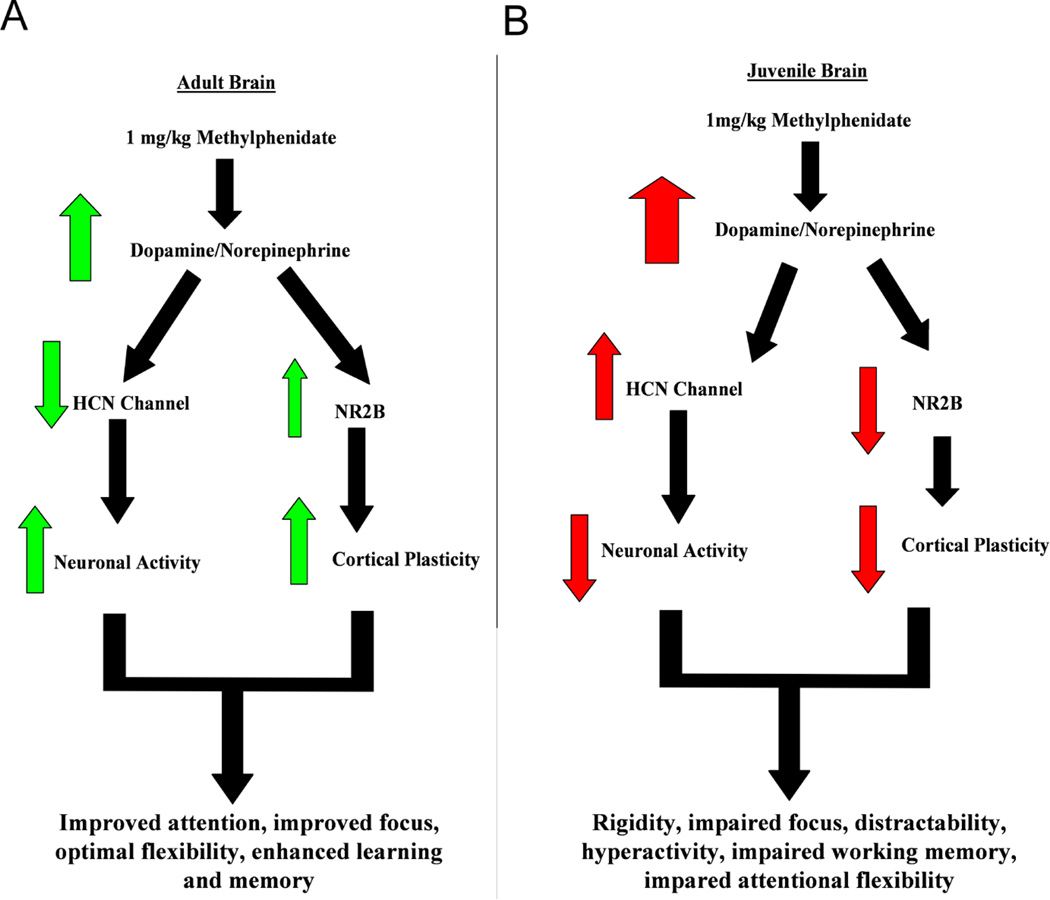
Overall, these recent studies suggest that MPH may affect the integration of incoming signals and thus impair working memory function in the juvenile brain. A flowchart demonstrating the differential actions of methylphenidate on juvenile and adult normal brain systems is shown in Figure 3.
Figure 3.
Flowchart explaining the proposed age-dependent actions of methylphenidate. A, in the adult brain, MPH causes moderate increase of dopamine and norepinephrine, leading to deactivation of HCN channels and thus increased neuronal activity on one hand, and enhancing NR2B levels through modulation of signal-to-noise ratio to result in increased cortical plasticity on the other hand. These results are beneficial to attentional performance and memory enhancement. B, However, the juvenile brain is supersensitive to MPH, the same dose of MPH causes excessive levels of dopamine and norepinephrine, leading to activation of HCN channels and decreased levels of NR2B-containing NMDA receptors. These opposite effects result in decreased neuronal activity and impaired cortical plasticity, which, consequently, causes a depression and reduced attention and memory.
In addition, while it is currently unclear whether the selective decrease in NR2B protein levels indicates a deleterious side effect of treatment, or suggests that ADHD pathology may involve an alteration (presumably an increase) in NR2B levels, what is apparent is that we need to develop stronger diagnostic and treatment measures for ADHD. Currently the diagnosis based on the Diagnostic and Statistical Manual, Version V is subjective, and leaves room for erroneous diagnosis and treatment [75]. Findings such as those presented in the above studies stress the need for the development of more stringent, effective diagnostic and treatment measures that will help to minimize the number of persons needing psychostimulant medication. Furthermore, our studies suggest that children treated erroneously with MPH may be at risk for abnormal prefrontal function and behavioral plasticity; however, further studies are needed to determine the exact ramifications of the neuronal changes we uncovered following juvenile treatment of healthy rats with clinically-relevant doses of MPH. Our results also suggest that, if the alterations in NMDA receptor composition and plasticity do not turn out to be detrimental to behavioral function, then they likely represent a therapeutic action of the drug. If this is the case, perhaps ADHD is a disorder of glutamate dysfunction, and medications that affect NMDA receptors could provide a future avenue of drug development?
Whether the pharmaceutical companies may develop future ADHD treatments targeting the glutamatergic system, or our findings on NMDAR dysfunction indicate a potentially therapeutic side effect for individuals with ADHD, the fact that even a very low dose of methylphenidate can have such striking effects on developing brains should raise alarm. These age-dependent sensitivities may not only apply to psychostimulants, but to other classes of psychoactive drugs, such as antidepressants and antipsychotics. The take-home message is that, whatever the drug being considered, one must play an active role in researching and understanding the medication, the disease it is prescribed for, and other non-pharmaceutical treatment possibilities. Only then will we, as a society, be able to rest assured that we are not improving children's sustained attention at the expense of other vital cognitive functions.
Acknowledgements
This work is supported by NIH R01MH085666 to W.J. Gao.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
We affirm that the content of this manuscript is not under consideration for publication elsewhere. Figure 2 was derived from two recent publications in the Biological Psychiatry and Neurobiology of Learning and Memory, which we have obtained permissions from the Elsevier Publisher (see the attached permissions). Both authors have fulfilled all conditions required for authorship and have approved the submission. Institutional review board approval is not required for this project. The authors declare no financial disclosure and conflict of interests.
References
- 1.Swanson J, Baler RD, Volkow ND. Understanding the effects of stimulant medications on cognition in individuals with attention-deficit hyperactivity disorder: a decade of progress. Neuropsychopharmacology. 2011;36(1):207–226. doi: 10.1038/npp.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair J, et al. Clinical review: evidence-based diagnosis and treatment of ADHD in children. Missouri Medicine. 2006;103(6):617–621. [PubMed] [Google Scholar]
- 3.Getahun D, et al. Recent Trends in Childhood Attention-Deficit/Hyperactivity Disorder. JAMA Pediatr. 2013:1–7. doi: 10.1001/2013.jamapediatrics.401. [DOI] [PubMed] [Google Scholar]
- 4.Heal DJ, Cheetham SC, Smith SL. The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety. Neuropharmacology. 2009;57(7–8):608–618. doi: 10.1016/j.neuropharm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Hazell P. Review of attention-deficit/hyperactivity disorder comorbid with oppositional defiant disorder. Australas Psychiatry. 2010;18(6):556–559. doi: 10.3109/10398562.2010.498049. [DOI] [PubMed] [Google Scholar]
- 6.Urban KR, Gao WJ. Evolution of the study of methylphenidate and its actions on the adult versus juvenile brain. J Atten Disord. 2012;24:24. doi: 10.1177/1087054712455504. [DOI] [PubMed] [Google Scholar]
- 7.Barkley RA, et al. Young adult follow-up of hyperactive children: antisocial activities and drug use. Journal of Child Psychology & Psychiatry. 2004;45(2):195–211. doi: 10.1111/j.1469-7610.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 8.Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. Journal of Clinical Psychiatry. 2006;67(Suppl 8):7–12. [PubMed] [Google Scholar]
- 9.Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57(11):1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14(3):477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 11.Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28(7):771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Challman TD, Lipsky JJ. Methylphenidate: its pharmacology and uses. Mayo Clin Proc. 2000;75(7):711–721. doi: 10.4065/75.7.711. [DOI] [PubMed] [Google Scholar]
- 14.Berridge CW, Devilbiss DM. Psychostimulants as cognitive enhancers: the prefrontal cortex, catecholamines, and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69(12):e101–e111. doi: 10.1016/j.biopsych.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yano M, Steiner H. Methylphenidate and cocaine: the same effects on gene regulation? Trends in Pharmacological Sciences. 2007;28(11):588–596. doi: 10.1016/j.tips.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Kuczenski R, Segal DS. Stimulant actions in rodents: implications for attention-deficit/hyperactivity disorder treatment and potential substance abuse. Biol Psychiatry. 2005;57(11):1391–1396. doi: 10.1016/j.biopsych.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 17.Arnsten AF, Scahill L, Findling RL. alpha2-Adrenergic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: emerging concepts from new data. J Child Adolesc Psychopharmacol. 2007;17(4):393–406. doi: 10.1089/cap.2006.0098. [DOI] [PubMed] [Google Scholar]
- 18.Berridge CW, et al. Differential sensitivity to psychostimulants across prefrontal cognitive tasks: differential involvement of noradrenergic alpha-1 and alpha-2 receptors. Biol Psychiatry. 2012;71(5):467–473. doi: 10.1016/j.biopsych.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behav Brain Funct. 2005;1(1):2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clatworthy PL, et al. Dopamine Release in Dissociable Striatal Subregions Predicts the Different Effects of Oral Methylphenidate on Reversal Learning and Spatial Working Memory. J. Neurosci. 2009;29(15):4690–4696. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gamo NJ, Wang M, Arnsten AF. Methylphenidate and atomoxetine enhance prefrontal function through alpha2-adrenergic and dopamine D1 receptors. J Am Acad Child Adolesc Psychiatry. 2010;49(10):1011–1023. doi: 10.1016/j.jaac.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spencer RC, Klein RM, Berridge CW. Psychostimulants act within the prefrontal cortex to improve cognitive function. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rapoport JBM, Zahn T, Weingartner H, Ludlow C, Mikkelsen E. Dextroamphetamine: cognitive and behavioral effects in normal prepubertal boys. Science. 1978;199:560–563. doi: 10.1126/science.341313. [DOI] [PubMed] [Google Scholar]
- 24.Rapoport JLBMS, Weingartner H, Zahn TP, Ludlow C, Mikkelsen EJ. Dextroamphetamine- its cognitive and behavioral effects in normal and hyperactive boys and normal men. Arch Gen Psychiatry. 1980;37:933–943. doi: 10.1001/archpsyc.1980.01780210091010. [DOI] [PubMed] [Google Scholar]
- 25.Mehta MASBJ, Mavaddat N, Pickard JD, Robbins TW. Comparative psychopharmacology of methylpyhenidate and related drugs in human volunteers, patients with ADHD, and experimental animals. In: MVA Solanto AFT, Castellanos FX, editors. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. New York: Oxford University Press; 2001. pp. 303–331. [Google Scholar]
- 26.Vaidya CJ, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci U S A. 1998;95(24):14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22(16):7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volkow ND, et al. Dopamine Transporter Occupancies in the Human Brain Induced by Therapeutic Doses of Oral Methylphenidate. Am J Psychiatry. 1998;155(10):1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- 29.Volkow ND, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21(2):RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volkow ND, et al. Mechanism of action of methylphenidate: insights from PET imaging studies. J Atten Disord. 2002;6(Suppl 1):S31–S43. doi: 10.1177/070674370200601s05. [DOI] [PubMed] [Google Scholar]
- 31.Agay N, et al. Non-specific effects of methylphenidate (Ritalin) on cognitive ability and decision-making of ADHD and healthy adults. Psychopharmacology (Berlin) 2010;210(4):511–519. doi: 10.1007/s00213-010-1853-4. [DOI] [PubMed] [Google Scholar]
- 32.Askenasy EPTKH, Yang PB, Dafny N. Methylphenidate (Ritalin): Behavioral Studies in the Rat. International Journal of Neuroscience. 2007;117:757–794. doi: 10.1080/00207450600910176. [DOI] [PubMed] [Google Scholar]
- 33.Dow-Edwards DL, Weedon JC, Hellmann E. Methylphenidate improves performance on the radial arm maze in periadolescent rats. Neurotoxicol Teratol. 2008;30(5):419–427. doi: 10.1016/j.ntt.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linssen AM, et al. Methylphenidate produces selective enhancement of declarative memory consolidation in healthy volunteers. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berridge CW, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60(10):1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 36.Kuczenski R, Segal DS. Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. J Pharmacol Exp Ther. 2001;296(3):876–883. [PubMed] [Google Scholar]
- 37.Gray JD, et al. Methylphenidate administration to juvenile rats alters brain areas involved in cognition, motivated behaviors, appetite, and stress. J. Neurosci. 2007;27(27):7196–7207. doi: 10.1523/JNEUROSCI.0109-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berridge CWDDM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biological Psychiatry. 2006;60(10):1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 39.Gaytan O, et al. Sensitization to locomotor effects of methylphenidate in the rat. Life Sci. 1997;61(8):PL101–PL107. doi: 10.1016/s0024-3205(97)00598-5. [DOI] [PubMed] [Google Scholar]
- 40.Gaytan O, et al. Dose response characteristics of methylphenidate on different indices of rats' locomotor activity at the beginning of the dark cycle. Brain Res. 1996;727(1–2):13–21. doi: 10.1016/0006-8993(96)00296-x. [DOI] [PubMed] [Google Scholar]
- 41.Levy F. Dopamine vs noradrenaline: inverted-U effects and ADHD theories. Aust N Z J Psychiatry. 2009;43(2):101–108. doi: 10.1080/00048670802607238. [DOI] [PubMed] [Google Scholar]
- 42.Vijayraghavan S, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10(3):376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 43.Devilbiss DM, Waterhouse BD. The effects of tonic locus ceruleus output on sensory-evoked responses of ventral posterior medial thalamic and barrel field cortical neurons in the awake rat. J Neurosci. 2004;24(48):10773–10785. doi: 10.1523/JNEUROSCI.1573-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arnsten T. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franke AG, et al. Non-medical use of prescription stimulants and illicit use of stimulants for cognitive enhancement in pupils and students in Germany. Pharmacopsychiatry. 2011;44(2):60–66. doi: 10.1055/s-0030-1268417. [DOI] [PubMed] [Google Scholar]
- 46.Goodman R. Cognitive enhancement, cheating, and accomplishment. Kennedy Inst Ethics J. 2010;20(2):145–160. doi: 10.1353/ken.0.0309. [DOI] [PubMed] [Google Scholar]
- 47.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31(4):183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9(4):267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- 49.Kolb B, et al. Experience and the developing prefrontal cortex. Proceedings of the National Academy of Sciences. 2012;109(Supplement 2):17186–17193. doi: 10.1073/pnas.1121251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casey BJJRM, Hare TA. The Adolescent Brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao W-J, et al. The Unique Properties of the Prefrontal Cortex and Mental Illness, in When Things Go Wrong – Diseases and Disorders of the Human Brain. In: Mantamadiotis T, editor. InTech. 2012. pp. 3–26. [Google Scholar]
- 52.Kanitz E, et al. Age-related changes in corticosteroid receptor expression and monoamine neurotransmitter concentrations in various brain regions of postnatal pigs. J Neurosci Res. 2011;89(7):1134–1141. doi: 10.1002/jnr.22621. [DOI] [PubMed] [Google Scholar]
- 53.Algahim MF, et al. Repetitive ritalin treatment modulates the diurnal activity pattern of young SD male rats. Central Nervous System Agents in Medicinal Chemistry. 2010;10(3):247–257. doi: 10.2174/1871524911006030247. [DOI] [PubMed] [Google Scholar]
- 54.Lee MJ, et al. Does repetitive Ritalin injection produce long-term effects on SD female adolescent rats? Neuropharmacology. 2009;57(3):201–207. doi: 10.1016/j.neuropharm.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Andersen SL, et al. Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nature Neuroscience. 2002;5(1):13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- 56.Torres-Reveron A, Weedon J, Dow-Edwards DL. Methylphenidate response in prenatal cocaine-exposed rats: a behavioral and brain functional study. Brain Res. 2010;1337:74–84. doi: 10.1016/j.brainres.2010.03.112. [DOI] [PubMed] [Google Scholar]
- 57.Jernigan TL, et al. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114(Pt 5):2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- 58.Kuboshima-Amemori S, Sawaguchi T. Plasticity of the primate prefrontal cortex. Neuroscientist. 2007;13(3):229–240. doi: 10.1177/1073858406298554. [DOI] [PubMed] [Google Scholar]
- 59.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14(6):383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 60.Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55(7):1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11(3):327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 62.Wang H, et al. A specialized NMDA receptor function in layer 5 recurrent microcircuitry of the adult rat prefrontal cortex. Proc Natl Acad Sci U S A. 2008;105(43):16791–16796. doi: 10.1073/pnas.0804318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang M, et al. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron. 2013;77(4):736–749. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Urban KR, Waterhouse BD, Gao WJ. Distinct age-dependent effects of methylphenidate on developing and adult prefrontal neurons. Biol Psychiatry. 2012;72(10):880–888. doi: 10.1016/j.biopsych.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Devilbiss DM, Berridge CW. Cognition-enhancing doses of methylphenidate preferentially increase prefrontal cortex neuronal responsiveness. Biol Psychiatry. 2008;64(7):626–635. doi: 10.1016/j.biopsych.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waterhouse BD, Agster KL, Calrk BD. Society for Neuroscience. Washington, DC: 2008. The effects of methylphenidate on sensory signal processing in the rodent lateral geniculate nucleus. Astract Program No. 161.5. [Google Scholar]
- 67.Cepeda C, Levine MS. Where do you think you are going? The NMDA-D1 receptor trap. Sci. STKE. 2006;2006(333):pe20–pe24. doi: 10.1126/stke.3332006pe20. [DOI] [PubMed] [Google Scholar]
- 68.Kopp C, Longordo F, Luthi A. Experience-dependent changes in NMDA receptor composition at mature central synapses. Neuropharmacology. 2007;53(1):1–9. doi: 10.1016/j.neuropharm.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 69.Li YC, et al. Activation of glycogen synthase kinase-3 beta is required for hyperdopamine and D2 receptor-mediated inhibition of synaptic NMDA receptor function in the rat prefrontal cortex. J Neurosci. 2009;29(49):15551–15563. doi: 10.1523/JNEUROSCI.3336-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mao LM, et al. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci. 2009;12(5):602–610. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Urban KR, Li YC, Gao WJ. Treatment with a clinically-relevant dose of methylphenidate alters NMDA receptor composition and synaptic plasticity in the juvenile rat prefrontal cortex. Neurobiol Learn Mem. 2012;101:65–74. doi: 10.1016/j.nlm.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Foster KA, et al. Distinct roles of NR2A and NR2B cytoplasmic tails in long-term potentiation. J Neurosci. 2010;30(7):2676–2685. doi: 10.1523/JNEUROSCI.4022-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Massey PVJBE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu Z, et al. Metaplastic regulation of long-term potentiation/long-term depression threshold by activity-dependent changes of NR2A/NR2B ratio. J Neurosci. 2009;29(27):8764–8773. doi: 10.1523/JNEUROSCI.1014-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghanizadeh A. Psychometric analysis of the new ADHD DSM-V derived symptoms. BMC Psychiatry. 2012;12:21. doi: 10.1186/1471-244X-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]