Abstract
In present study, we evaluated grape seed extract (GSE) efficacy against bladder cancer and associated mechanism in two different bladder cancer cell lines T24 and HTB9. A significant inhibitory effect of GSE on cancer cell viability was observed, which was due to apoptotic cell death. Cell death events were preceded by vacuolar appearance in cytoplasm, which under electron microscopy was confirmed as swollen mitochondrial organelle and autophagosomes. Through detailed in vitro studies, we established that GSE generated oxidative stress that initiating an apoptotic response as indicated by the reversal of GSE-mediated apoptosis when the cells were pre-treated with antioxidants prior to GSE. However, parallel to a strong apoptotic cell death event, GSE also caused a pro-survival autophagic event as evidenced by tracking the dynamics of LC3-II within the cells. Since the pro-death apoptotic response was stronger than the pro-survival autophagy induction within the cells, cell eventually succumbed to cellular death after GSE exposure. Together, the findings in the present study are both novel and highly significant in establishing, for the first time, that GSE-mediated oxidative stress causes a strong programmed cell death in human bladder cancer cells, suggesting and advocating the effectiveness of this non-toxic agent against this deadly malignancy.
Keywords: bladder cancer, grape seed extract, chemoprevention, autophagy, oxidative stress
1. Introduction
Bladder cancer is marred by recurrence (Lattouf, 2009; Leppert et al., 2006; Tanaka et al., 2011), and statistics for life time risk assessments in the US indicate that 1 in 42 persons (men and women) will have bladder cancer in their life (http://seer.cancer.gov/faststats., 2013). ACS’ estimates for 2013 report ~72,570 new bladder cancer cases and 15,210 associated deaths in the US, making it the fourth most common form of cancer in men (American Cancer Society, 2013). The incidence in women is slightly less; bladder cancer being the eighth most common cancer. The probability of developing this cancer increases with age; the mean age at diagnosis being ~73 years (http://seer.cancer.gov/faststats., 2013). Importantly, among all the different types of cancers, bladder cancer shows a significantly high incidence of recurrence after treatment, and thus is recognized as the most expensive cancer to be treated depending on the costs incurred per patient basis (Lattouf, 2009; Leppert et al., 2006; Tanaka et al., 2011). Thus, the challenges associated with prevention and treatment of this recurrent malignancy will increase with time, due to better age expectancy in our population (Lattouf, 2009; Leppert et al., 2006; Tanaka et al., 2011). Due to delayed clinical manifestation, bladder cancer has been recognized, now, to be preventable, if effective measures are taken using chemoprevention strategies (Tanaka et al., 2011). Adoption of chemoprevention approach may not necessarily eliminate bladder cancer lesions; however, it could delay the on-start of progression to a clinical stage, and in turn, improve the overall life of elderly patients for whom therapeutic/clinical interventions are not ideal (Tanaka et al., 2011).
In this regard, new modalities for chemoprevention are being investigated against bladder cancer, which involve screening natural, non-toxic, dietary/non-dietary agents for their anti-bladder cancer potential (Lattouf, 2009; Leppert et al., 2006; Tanaka et al., 2011). Grape seed extract (GSE) is one such natural agent, which has shown great potential as a chemopreventive and anti-cancer agent, in pre-clinical settings, against various malignancies (Agarwal et al., 2002; Agarwal et al., 2004; Kaur et al., 2009; Kaur et al., 2006; Kaur et al., 2008; Raina et al., 2007; Tyagi et al., 2003; Velmurugan et al., 2010a; Velmurugan et al., 2010b). However, it is not known yet, whether GSE also has efficacy against bladder cancer. GSE is a complex polyphenolic mixture that comprises of dimers, trimers and other oligomers (procyanidins) of catechin (C) and epicatechin (EC) and their gallate derivatives, which together are known as procyanidins (Kaur et al., 2009a). Our research team has isolated and characterized various active constituents of GSE, mainly bioactive procyanidins (structures published in cited references), using chromatographic separation and electrospray ionization liquid chromatography spectrometry as well as LC-MS analysis and, then screened the biological efficacy of fractions against prostate cancer cells (Veluri et al., 2006; Agarwal et al., 2007; Chou et al., 2010). Gallic acid, one of the biologically active constitute of GSE, showed its efficacy against prostate cancer (Raina et al., 2008; Kaur et al., 2009b). Furthermore, completed studies by us have recently identified that procyanidin B2-3,3′-di-O-gallate (B2G2), which causes growth inhibition and apoptotic death of human prostate carcinoma cells, is a major active constituent of GSE as compared to two mono-gallate esters namely B2-3-O-gallate (B2-3G) and B2-3′-O gallate (B2-3′G) and the parent compound procyanidin B2 (Veluri et al., 2006; Agarwal et al., 2007; Chou et al., 2010).
Importantly, GSE is already marketed as a dietary supplement due to its favorable health benefits (Kaur et al., 2009a), thus any positive outcomes against bladder cancer can be of immediate translational significance. For this purpose, in the present study, we sought to establish the effect of GSE against two different urinary bladder cancer cell lines viz., T24 and HTB9, representing stage I and stage II malignancy, respectively, and to delineate associated mechanism of its efficacy.
2. Materials and Methods
2.1. Cell Culture and Treatments
Human bladder cancer T24 and HTB9 cells were cultured in DMEM and RPMI media, respectively, containing 10% FBS and 1% penicillin-streptomycin under standard culture conditions. At 50–60% confluency, cells were treated with different doses of GSE (25–100 μg/mL final concentration for treatment) in DMSO or with DMSO alone. Unless specified otherwise, DMSO concentration in medium was 0.1% (v/v). Following desired treatments, cell growth and viability was assayed as described earlier (Agarwal et al., 2003). In other experiments, following desired treatments, cell cycle distribution was analyzed by FACS analysis, utilizing the core service of the University of Colorado Cancer Center (Aurora, CO), as described earlier (Agarwal et al., 2003). For the determination of DNA fragmentation (apoptotic population), these cell pellets were subjected to Annexin V and PI staining using Annexin V Apoptosis Assay kit (Molecular Probes) and then analyzed for apoptotic death by FACS analysis. In experiments using inhibitors (anti-oxidants and 3MA), cells were pre-treated with inhibitors for 2 h followed by GSE treatment. Bafilomycin A1 (BAFA1) was added to the media in the last 4 h of GSE treatment. For autophagic flux determination, lysosomal inhibitors E64d and pepstatin A were added to the media with GSE and incubated for respective time-periods. Furthermore, total cellular lysates were prepared, their protein concentrations determined, and then analyzed by western blot (WB) using chemiluminescence ECL detection method as described previously (Agarwal et al., 2003). Some blots were multiplexed or stripped and reprobed with different antibodies including those for loading control.
2.2. Identification of ROS generation
Generation of intracellular and mitochondrial superoxide radical was measured using Dihydroethidium (DHE) and MitoSOX dyes, respectively. Briefly, after desired treatment, cells were incubated either with 5 M DHE or MitoSOX for 20 min at 37°C and then observed under fluorescent microscope, or harvested and analyzed immediately via FACS analysis, respectively.
2.3. Identification of Autophagy Induction
Published guidelines were followed to correctly identify autophagy induction (Bampton et al., 2005; Kimura et al., 2009; Klionsky et al., 2008; Mizushima, 2004; Mizushima and Yoshimori, 2007). For visualization of intracellular vacuoles, either Acridine orange (1 μg/mL) for 10 min or monodansylcadaverine (MDC) was applied to the cells at 50 μM for 30–45 min. Following MDC exposure, cells were incubated for 10 min with NH4Cl (to reduce lysosomes from being stained) and washed with PBS containing 10% FBS, twice, and unfixed cells were directly observed under fluorescent microscope (Hoyer-Hansen et al., 2005; Munafo and Colombo, 2001). Transmission electron microscope (TEM, FEI Technai G2 BioTwin at 80KV) was used to visualize cellular vacuoles and other cellular changes, and images were captured with Gatan First Lite digital camera (Eskelinen, 2008; Klionsky et al., 2008; Mizushima and Yoshimori, 2007; Yla-Anttila et al., 2009).
2.4. Statistical analysis
Statistical significance of differences between respective controls and treated samples were calculated by one-way ANOVA followed by a Bonferroni’s test using SigmaStat version 3.5 software (Jandel Scientific, San Rafael, CA), and both sided P values of ≤ 0.05 were considered significant. The data in most of the cases are representative of at least 3–4 independent studies with reproducible results.
3. Results
3.1. GSE Causes Growth Inhibition of Human Bladder Cancer Cell Lines
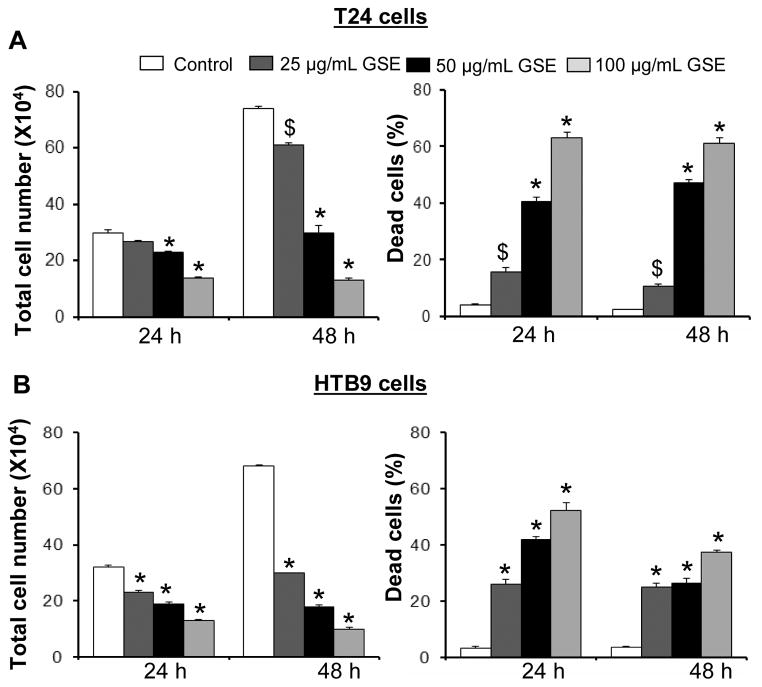
It was observed by Trypan blue dye exclusion method that treatment of bladder cancer cells with GSE not only results in growth inhibition of T24 and HTB9 cells in a dose- and time-dependent manner, but also causes an increase in dead cell population at both 24 and 48 h of treatments (Fig. 1). As shown in figure 1A (left panel), GSE at 25, 50 and 100 μg/mL doses resulted in 10–53% (P<0.001, for both 50 and 100 μg/mL doses) and 18–82% (P<0.001, for both 50 and 100 μg/mL doses) growth inhibition of T24 cells after 24 and 48 h of treatments, respectively. Concomitantly, there was an increase in the T24 dead cell population following 24 and 48 h of GSE treatments at 25, 50 and 100 μg/mL doses which accounted for ~4–16 fold and ~5–25 fold (P<0.05-P<0.001) increase in dead cell population, compared to controls, respectively (Fig. 1A, right panel). GSE also caused a significant growth inhibition in HTB9 cells ranging from 28–59% (P<0.001) and 56–85% (P<0.001) at 25, 50 and 100 μg/mL doses after 24 and 48 of treatments, respectively (Fig. 1B, left panel). In addition, GSE also increased dead cell population [~ 8–16 folds (P<0.001) and ~ 7–10 folds (P<0.001)] of HTB9 cells under identical treatment conditions at 24 and 48 h, respectively (Fig. 1B, right panel). The undetectable debris of dead cells either at higher doses or with increased exposure to GSE attributed to lower death counts for HTB9 cells in the data analysis.
Figure 1. Growth inhibitory effects of GSE against human bladder cancer cells.
GSE inhibits growth and induces death in A) T24 and B) HTB9 cells in both dose- and time- dependent manner. Cells (1.05 × 105) were plated in 60 mm dishes, treated with DMSO (control) or different doses of GSE, and after 24 and 48 h, cells were harvested and counted using Trypan Blue assay as detailed in ‘Materials and Methods’. GSE; grape seed extract, *, P<0.001; $, P<0.05.
3.2. GSE Induces Cell Cycle Arrest and Apoptotic Death in Human Bladder Cancer Cells
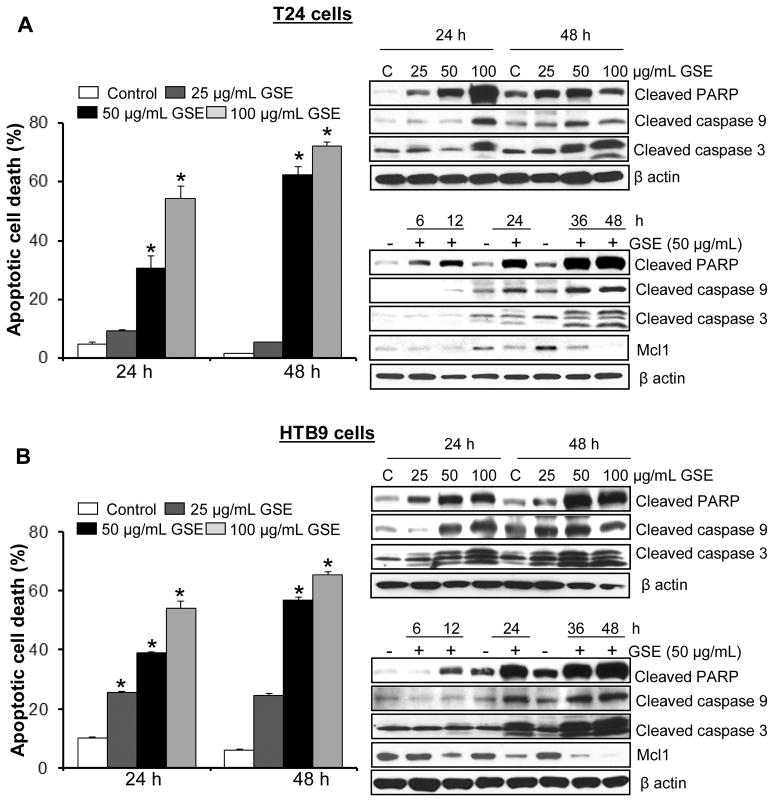
Since GSE treatment caused a significant growth inhibitory effect in T24 and HTB9 cells, we further proceeded to conduct a cell cycle distribution analysis to determine whether GSE was causing significant growth arrest in these cells. Results showed that GSE causes a significant accumulation of cells in G1-phase in cell cycle progression of T-24 cells and a strong G2-M-phase arrest in case of HTB9 cells at higher doses (data not shown). Since GSE treatment also caused a strong death of both T24 and HTB9 cells, the growth arrest by GSE was not thought to be the major cause of decreased cell number in both cancer cell lines. We next assessed the nature of GSE-induced cell death by quantitative apoptosis assay using AnnexinV/PI staining of bladder cancer cells followed by FACS analysis (Fig. 2). Compared to untreated controls, GSE doses (50–100 μg/mL) caused a strong dose- and time- dependent apoptotic cell population [~31–54% (P<0.001) and ~62–72% (P<0.001)] in T24 cells after 24 and 48 h treatments, respectively, compared to DMSO controls showing 2–4% apoptotic cells (Fig. 2A, left panel). However, a 25 μg/mL dose of GSE was unable to cause any significant apoptotic death in T24 cells. A strong apoptotic death was also caused by 25, 50 and 100 μg/mL GSE doses in HTB9 cells under the same treatment conditions (Fig. 2B, left panel), accounting for ~26–54% (P<0.001) and ~25–65% (P<0.001) apoptotic death following 24 h and 48 h treatments, respectively, compared to 6–10% in DMSO controls. Significant apoptotic death induced by GSE in these bladder cancer cells was also associated with a dose- and -time- dependent increase in the expression of apoptosis related molecules such as, cleaved caspase-3 and -9, cleaved-PARP, and a decrease in the expression of anti-apoptotic molecule Mcl-1(Fig. 2A & B, right panels). The time-dependent increase in cleaved-PARP also indicated that apoptosis was an early event; as early as 6 h in T24 cells, while in HTB9 cells it occurred around 12 h.
Figure 2. GSE induces apoptotic death in human bladder cancer cells.
A, left panel) T24 cells and B, left panel) HTB9 cells were treated with DMSO (control) or GSE for the mentioned time and dose. At the end of the treatments, both adherent and non-adherent cells were harvested and collected by trypsinization, washed with PBS and the cell pellets were stained with Annexin V- propidium iodide and processed by FACS analysis to measure apoptotic cell counts. A, right panel) T24 cells and B, right panel) HTB9 cells were subjected to respective treatments with GSE and then their cellular lysates were analyzed by Western immunobloting. Membranes were probed with cleaved caspase-3 and -9, cleaved PARP, and Mcl-1 antibodies followed by peroxidase-conjugated appropriate secondary antibody and visualized by ECL detection system. Equal protein loading was confirmed by stripping and re-probing the membranes with β-actin. All experimental procedures and statistical analysis were performed as detailed in ‘Materials and Methods’ section. GSE; grape seed extract, *, P<0.001; $, P<0.05.
3.3. GSE Causes Intense Cytoplasmic Vacuolization
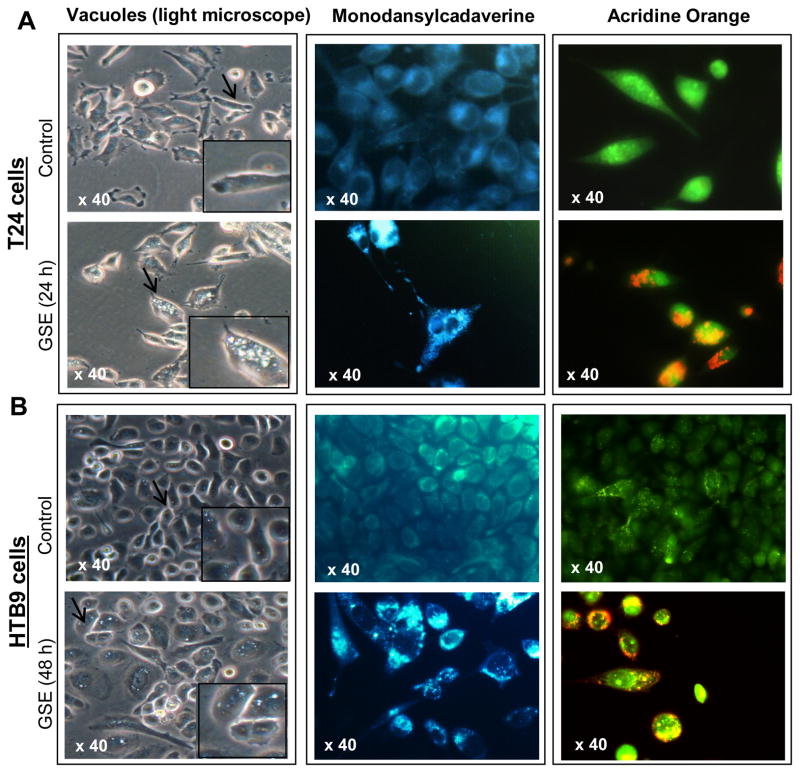
During the course of the study, we observed under the microscope that GSE treatment causes the appearance of vacuoles in the cytoplasm of these bladder cancer cells (Fig. 3). Specifically, vacuoles started to be visible from 12 h onwards in T24 cells; the vacuoles became larger and their numbers/cell increased with treatment time (Fig. 3A, left panel). In case of HTB9 cells, smaller vacuoles, than that observed in T24 cells, were visible at 24 h, which were more conspicuous after 48 h of GSE exposure (Fig. 3B, left panel).
Figure 3. Microscopic visualization of cellular morphology and autophagy induction after treatment of human bladder cancer cells with GSE.
Representative photomicrographs of A, left panel) T24 cells and B, left panel) HTB9 cells depicting intense cytoplasmic vacuolization due to GSE (50 μg/mL) treatment. A & B, middle panels) Effect of GSE on MDC incorporation in T24 and HTB9 cells, respectively. A & B, right panels) Effect of GSE on Acridine orange accumulation (punctate orange staining) in T24 and HTB9 cells, respectively.
3.4. GSE Induces Autophagy in Human Bladder Cancer Cells
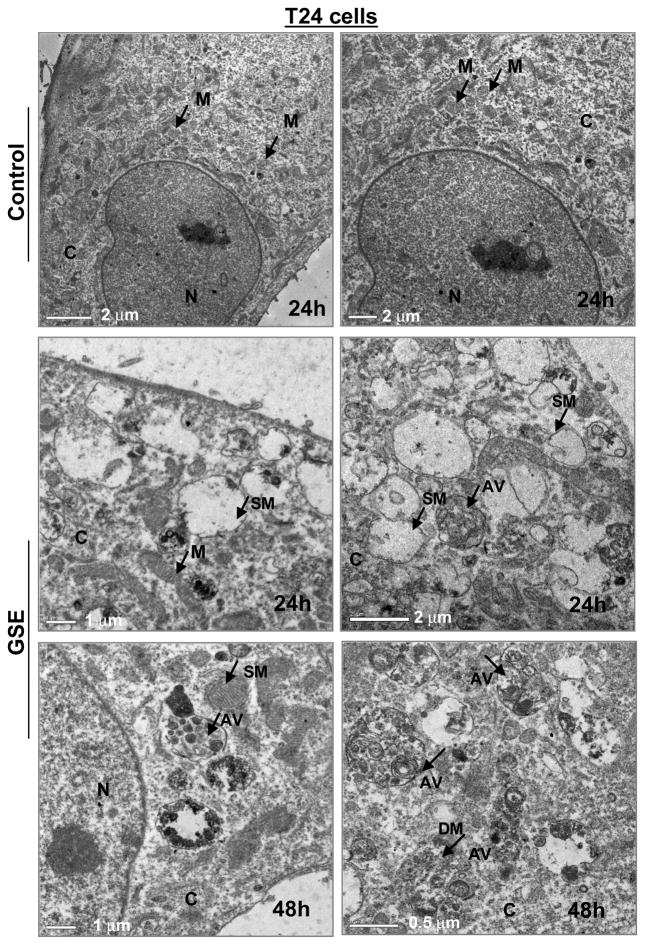
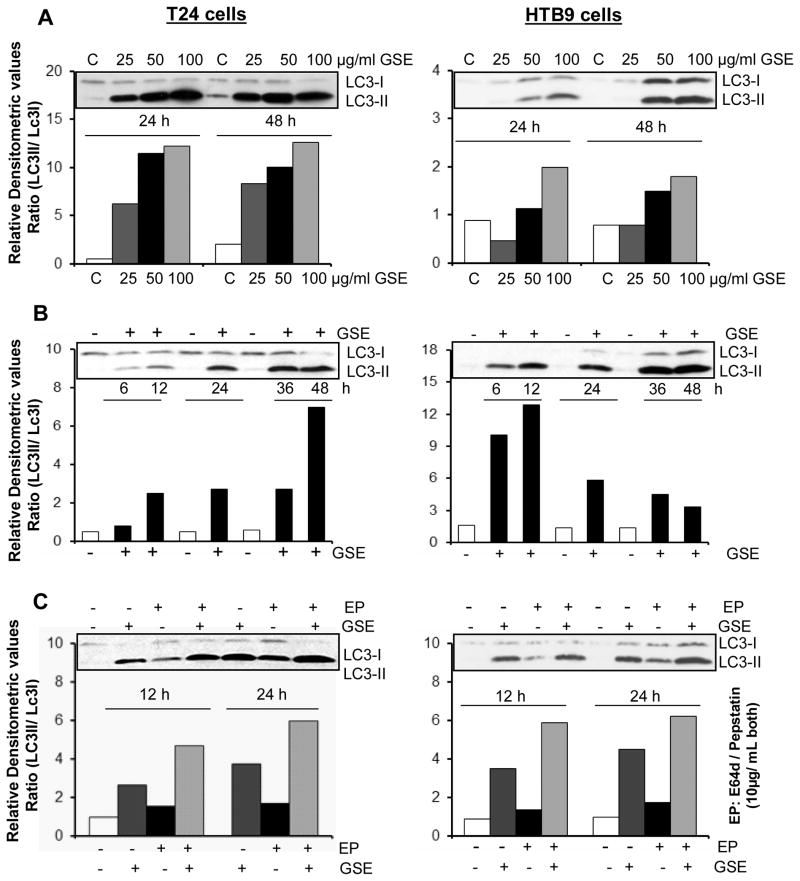
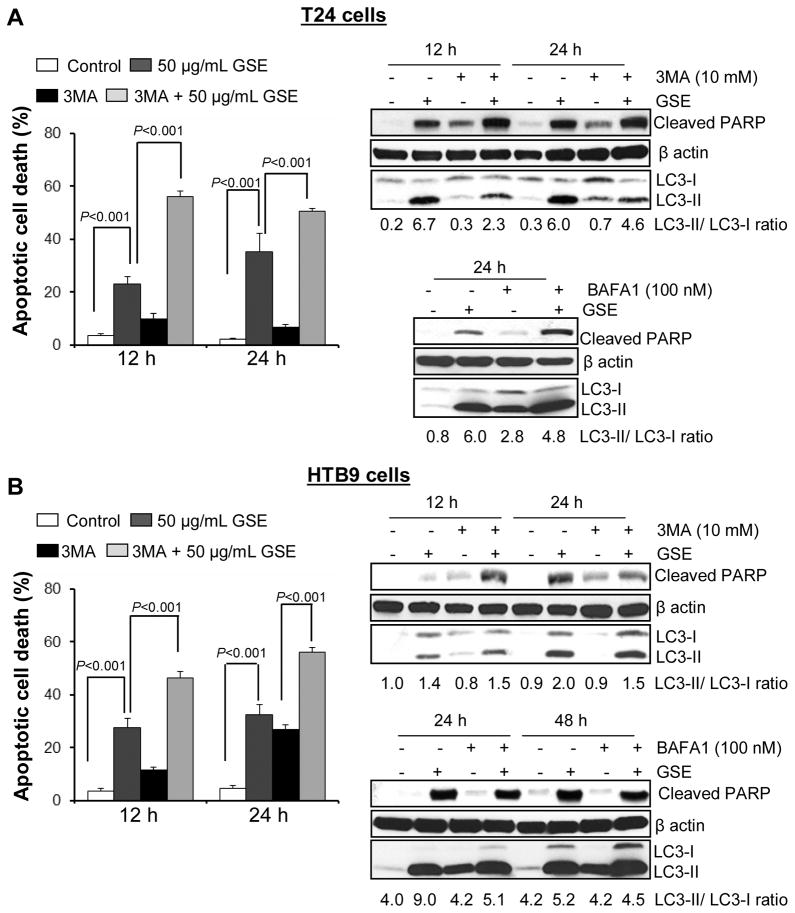
To determine whether the cytoplasmic vacuoles observed in these bladder cancer cells after GSE exposure, were due to autophagy induction, specific experiments were carried out to determine their type and origin. An important observation for autophagy induction by GSE was a significant accumulation of MDC and punctate type staining for Acridine orange (autophagolysosomal markers) in the cytoplasm and perinuclear region of GSE-treated bladder cancer cells (Fig. 3A & B). Furthermore, we examined the morphology of T24 cells by TEM [the gold-standard for determining autophagy] (Klionsky et al., 2008) to understand the type and origin of the cytoplasmic vacuoles generated after exposure to GSE in these cells. The vacuoles were either enlarged/swollen mitochondria (identified by invaginations on the vacuolar edges) or were autophagic vacuoles (with double- or multi-membranes) type, which were filled with sequestered cellular organelles and digested material (Fig. 4). The GSE induced autophagy was also indicated by a significant dose- and time-dependent increase in LC3-II/LC3-I ratio, in both these bladder cancer cell lines (Fig. 5A & B). Furthermore, it was confirmed that the induced autophagy matured to completion as the LC3II turnover markedly increased by exposure of these cells to GSE in presence of a combination of lysosomal inhibitors E64d and pepstatin A (Fig. 5C). Next we performed additional studies to determine whether the autophagy caused by GSE in bladder cancer cells is initiated in response to pro-death or pro-survival signals. Co-treatment with early autophagy inhibitor 3MA [blocks early phase of autophagy induction] significantly increased GSE-mediated apoptosis in both T24 (Fig. 6A, left panel) and HTB9 (Fig. 6B, left panel) cells. The increase in apoptosis was also corroborated by increased expression of cleaved-PARP with GSE treatments in presence of 3MA in T24 cells, at both 12 and 24 h of GSE treatment (Fig. 6A, right panel). In HTB9 cells, under similar treatment conditions, cleaved-PARP expression, though increased at 12 h time point, was later decreased at 24 h (Fig. 6B, right panel). The reason for the decrease in cleaved-PARP expression could be attributed to significant accumulation of late stage apoptotic dead cells at this time point, which thus failed to contribute to an increase in the protein expression of cleaved-PARP. In additional studies, treatment with late autophagy inhibitor BAFA1 [blocks fusion of autophagosomes with lysosomes] (Kondo and Kondo, 2006), while inhibiting last phase of autophagy, also increased GSE mediated apoptosis in T24 cells (Fig. 6A, right bottom panel), as indicated by an increase in the expression of cleaved-PARP; however, its effect on apoptosis in HTB9 cells was not evident (Fig. 6B, right bottom panel). Together, these results indicate that induction of autophagy by GSE protected the cells from more apoptotic death, and that inhibition of autophagic events increased apoptosis in these bladder cancer cells.
Figure 4. Visualization of autophagy induction under transmission electron microscopy (TEM) in T24 cells after GSE treatment.
All experimental procedures were performed as detailed in ‘Materials and Methods’ section. N, nucleus; C, cytoplasm; M, mitochondria; SM, swollen mitochondria; AV, autophagic vacuoles; DM, digested material. GSE (GSE dose 50 μg/mL).
Figure 5. Effect of GSE on autophagy induction in human bladder cancer cells.
A) Dose-dependent, B) Time-dependent effect of GSE on the expression levels of LC3-I and LC3-II. C) Effect of GSE on the turn over of LC3-II after co-treatment with lysosomal inhibitors (E64d and pepstatin A). Equal protein loading was confirmed by stripping and re-probing the membranes with β-actin. All experimental procedures and statistical analysis were performed as detailed in ‘Materials and Methods’ section. GSE (GSE dose 50 μg/mL)
Figure 6. Effect of autophagy inhibitors on GSE-induced apoptotic cell death in human bladder cancer cells.
A–B, left panels) Effect of co-treatment with early autophagy inhibitor 3MA on apoptotic induction by GSE in T24 cells and HTB9 cells, respectively. A–B, right upper panels) Effect of co-treatment with early autophagy inhibitor 3MA and GSE on cleaved-PARP expression in T24 cells and HTB9 cells, respectively. A–B, right lower panels) Effect of co-treatment with late autophagy inhibitor BAFA1 on cleaved-PARP expression in T24 cells and HTB9 cells, respectively. Equal protein loading was confirmed by stripping and re-probing the membranes with β-actin. All experimental procedures and statistical analysis were performed as detailed in ‘Materials and Methods’ section. GSE (GSE dose 50 μg/mL).
3.5. GSE causes ROS generation in Human Bladder Cancer Cells
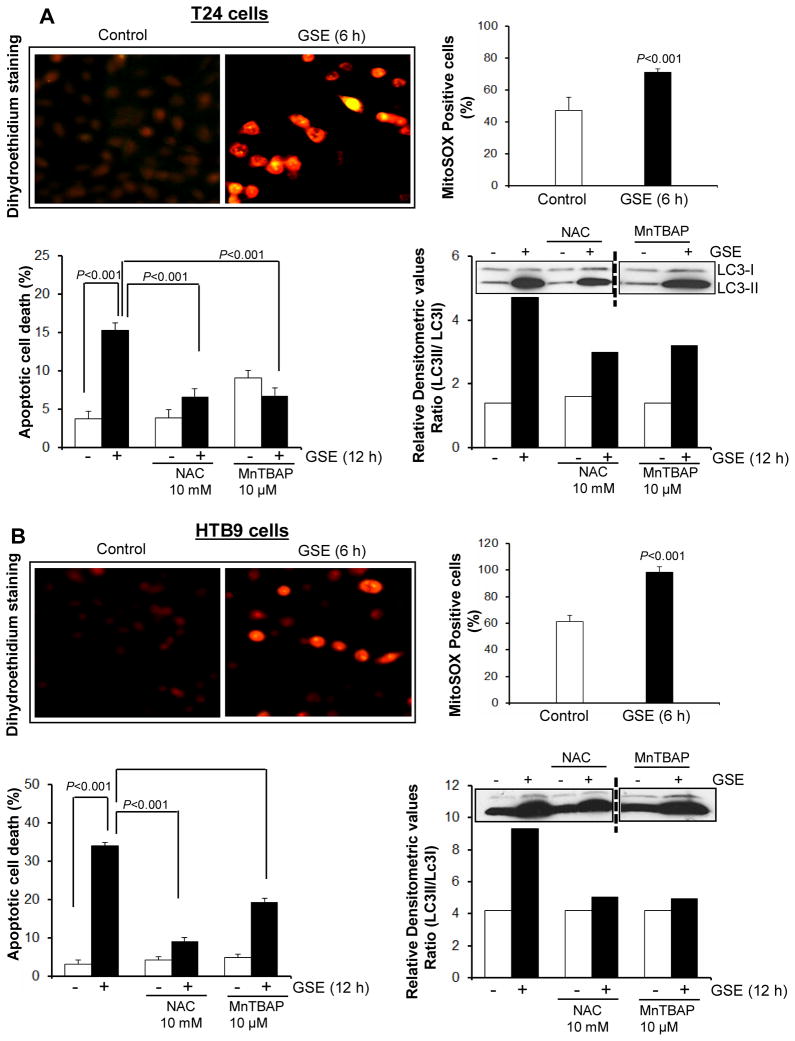
To determine whether GSE causes oxidative stress in these bladder cancer cells, we stained the cells with DHE dye, which undergoes oxidization in presence of superoxide radical to yield bright red fluorescence. Epifluorescence studies indicated that in DMSO control cells, while there was an evidence of red nuclear ethidium-fluorescence, its intensity was not as strong and significant as observed in GSE-treated cells (both T24 and HTB9), indicating the generation of superoxide radical in the presence of GSE (Fig. 7A & B, left upper panels). Furthermore, FACS analysis also revealed a time-dependant increase in MitoSOX-fluorescence intensity in GSE-treated cells, indicative of mitochondrial superoxide generation (Fig. 7A & B, right upper panels). Studies using the DCFH2-DA dye, for H2O2 detection, did not show any increase in fluorescence after GSE exposure (data not shown), indicating that ROS generated was specifically of the superoxide radical type.
Figure 7. Effect of GSE on reactive oxygen species (ROS) generation and its consequences on biological events in human bladder cancer cells.
A–B, left upper panels) Effect of GSE on intracellular superoxide radical generation as measured using Dihydroethidium (DHE) dye. A–B, right upper panels) Effect of GSE on mitochondrial superoxide radical generation as measured using FACS analysis after MitoSOX dye staining. A–B, left lower panels) Effect of co-treatment of antioxidants on apoptotic induction by GSE. A–B, right lower panels) Effect of co-treatment of antioxidants on expression levels of LC3-I and LC3-II in the presence of GSE. All experimental procedures and statistical analysis were performed as detailed in ‘Materials and Methods’ section. GSE (GSE dose 50 μg/mL).
3.6. GSE-caused Oxidative Stress Serves as a Trigger for Apoptosis and Autophagy in Human Bladder Cancer Cells
In order to confirm that the oxidative stress was generated well before any other morphological and/or biological changes occurred in these bladder cancer cells after GSE exposure, and that the oxidative stress generated served as a trigger for both apoptotic and autophagic responses, we pre-treated bladder cancer cells with anti-oxidants prior to GSE exposure. Specifically, to determine whether ROS generation was involved in the apoptotic response initiated after GSE treatment, cells were pretreated with ROS scavengers N-acetyl cysteine (NAC) and metalloporphyrin: Mn(III)tetrakis (4-Benzoic acid) porphyrin chloride (MnTBAP) and the effect on apoptotic cell death mediated by GSE was determined. The results indicated that indeed ROS generation served as a trigger for apoptosis as indicated by significant reversal of GSE-caused apoptotic death by these anti-oxidants (Fig. 7A & B, left bottom panels). Interestingly, GSE exposure in presence of these ROS scavengers also decreased autophagic events in these bladder cancer cells as indicated by a decrease in LC3-II/LC3-I ratio (Fig. 7A & B, right bottom panels).
4. Discussion
The discovery and development of agents, especially natural, non-toxic, anti-cancer and chemopreventive agents, which target growth and development of bladder cancer cells, might provide opportunities in bladder cancer management. (Lattouf, 2009; Leppert et al., 2006; Tanaka et al., 2011). Pre-clinical studies by us and others have identified GSE as one such ant-cancer and chemopreventive agent, which due to its pleiotropic mechanisms, has the ability to inhibit proliferation and induce apoptosis in various human cancer cells (Kaur et al., 2009a). Results of the present study, however, for the first time, identified that GSE causes a strong growth inhibition of human bladder cancer cell lines T24 and HTB9, which is accompanied by a strong and significant apoptotic death. Importantly, growth inhibition was not observed in normal cells (NHEK and NCM460) even at higher doses of GSE (Shrotriya et al., 2012; Derry et al., 2013). Interestingly, the apoptotic death events in bladder cancer cells were preceded by vacuolar appearance in the cytoplasm. We, therefore, explored the possibility of GSE also inducing autophagic events in the cytoplasm; which resulted in the appearance of vacuoles within the bladder cancer cells. Specific experiments to explore this possible biological event, employing MDC and Acridine orange dye accumulation in the cells, indicated towards the possibility that GSE might be causing autophagy in these bladder cancer cells. Tracking the dynamics of LC3-II within the cells, together with electron microscopy assessment further confirmed that indeed GSE was causing autophagy induction in conjunction with apoptosis in these cells. Whether this autophagic response was being initiated in response to pro-death or pro-survival signal was, however, not clear. Use of early and late autophagy inhibitors during GSE exposure increased the apoptotic population of cells, confirming that autophagic response was being generated in these cancer cells as a pro-survival mechanism, and thus, when autophagic initiation or its completion was interfered, cells were shifting more towards apoptosis.
Another possible explanation that could be attributed to the appearance of vacuoles was that the cells were under oxidative stress after GSE treatment, which in turn was causing enlargement of cellular organelles, especially mitochondria, reflected in terms of vacuolar appearance. Additional studies exploring this possible phenomenon revealed that GSE was indeed causing ROS generation (both cytoplasmic and mitochondrial) in these cells. Since oxidative stress occurred well before any other morphological changes, it was evident that oxidative stress was not a result but preceded apoptotic cell death. The oxidative stress generated was in fact initiating an apoptotic response as indicated by a reversal of GSE mediated apoptosis when the cells were pre-treated with ROS scavengers prior to GSE exposure. Since apoptosis and autophagy can occur simultaneously or independent of each other in a cellular system, we further explored the connection between the two events in these cancer cells (Gozuacik and Kimchi, 2004; Kondo and Kondo, 2006; Reggiori and Klionsky, 2005; Thorburn, 2008). Interestingly, pretreatment of bladder cancer cells with ROS scavengers not only decreased apoptosis in these cancer cells but was able to decrease the autophagic response generated in these cells during the exposure to GSE. Together, these results indicated that GSE caused oxidative stress in bladder cancer cells, which initiated a strong apoptotic response in these cells; however, parallel to such a significant apoptotic cell death event, it also caused a pro-survival autophagic event (Gozuacik and Kimchi, 2004; Kondo and Kondo, 2006; Reggiori and Klionsky, 2005; Thorburn, 2008). Since the pro-death apoptotic response was stronger than the pro-survival autophagy induction within the cells, cell eventually succumbed to cellular death after GSE exposure.
Together the findings in the present study are both novel and highly significant in establishing, for the first time, that GSE causes a strong programmed cell death in human bladder cancer cells, suggesting and advocating the effectiveness of this non-toxic agent against this deadly malignancy. Clinical study conducted on hypercholesterolemic patients showed that GSE reduced oxidized LDL, a known biomarker of cardiovascular disease (Bagchi et al., 2003); moreover, GSE is in Phase 1 clinical trial in breast cancer patients. Considering that GSE consumption is safe (Kaur et al., 2009a) and its active component epicatechin have high bioavailability (172 ng/ml) in plasma within 2 h of 1–2 g of GSE ingestion in humans (Prasain et al., 2009; Eich et al., 2012), GSE efficacy should be studied in detail in preclinical in vivo models of human bladder cancer.
Highlights.
For the first time, we showed a strong anti-cancer efficacy of grape seed extract (GSE) in human bladder cancer cells.
Mechanistic studies showed that GSE causes generation of superoxide radical in human bladder cancer cells.
GSE-caused oxidative stress leads to autophagy and a strong apoptotic cell death in human bladder cancer cells.
Acknowledgments
Grant support: Supported by RO1 grants AT003623, CA91883 and CA102514. The sponsors have no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Abbreviations
- GSE
grape seed extract
- TEM
transmission electron microscopy
- ROS
reactive oxygen species
- DHE
Dihydroethidium
- MDC
monodansylcadaverine
- NAC
N-acetyl cysteine
- MnTBAP
metalloporphyrin: Mn(III)tetrakis (4-Benzoic acid) porphyrin chloride
- BAFA1
Bafilomycin A1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Cancer Society. Cancer Facts and Figures. Atlanta, Ga: American Cancer Society; 2013. Available online Last accessed March 13 2013. [Google Scholar]
- Agarwal C, Singh RP, Agarwal R. Grape seed extract induces apoptotic death of human prostate carcinoma DU145 cells via caspases activation accompanied by dissipation of mitochondrial membrane potential and cytochrome c release. Carcinogenesis. 2002;23:1869–1876. doi: 10.1093/carcin/23.11.1869. [DOI] [PubMed] [Google Scholar]
- Agarwal C, Singh RP, Dhanalakshmi S, Agarwal R. Anti-angiogenic efficacy of grape seed extract in endothelial cells. Oncology reports. 2004;11:681–685. [PubMed] [Google Scholar]
- Agarwal C, Veluri R, Kaur M, Chou SC, Thompson JA, Agarwal R. Fractionation of high molecular weight tannins in grape seed extract and identification of procyanidin B2-3,3′-di-O-gallate as a major active constituent causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis. 2007;7:1478–1484. doi: 10.1093/carcin/bgm045. [DOI] [PubMed] [Google Scholar]
- Agarwal C, Singh RP, Dhanalakshmi S, Tyagi AK, Tecklenburg M, Sclafani RA, Agarwal R. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene. 2003;22:8271–8282. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- Bampton ET, Goemans CG, Niranjan D, Mizushima N, Tolkovsky AM. The dynamics of autophagy visualized in live cells: from autophagosome formation to fusion with endo/lysosomes. Autophagy. 2005;1:23–36. doi: 10.4161/auto.1.1.1495. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Sen CK, Ray SD, Das DK, Bagchi M, Preuss HG, Vinson JA. Molecular mechanisms of cardioprotection by a novel grape seed proanthocyanidin extract. Mutat Res. 2003;523–524:87–97. doi: 10.1016/s0027-5107(02)00324-x. [DOI] [PubMed] [Google Scholar]
- Chou SC, Kaur M, Thompson JA, Agarwal R, Agarwal C. Influence of gallate esterification on the activity of procyanidin B2 in androgen-dependent human prostate carcinoma LNCaP cells. Pharm Res. 2010;4:619–627. doi: 10.1007/s11095-009-0037-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry M, Raina K, Agarwal R, Agarwal C. Differential effects of grape seed extract against human colorectal cancer cell lines: The intricate role of death receptors and mitochondria. Cancer Lett. 2012 doi: 10.1016/j.canlet.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eich N, Schneider E, Cuomo J, Rabovsky A, Vita JA, Palmisano J, Holbrook M. Bioavailability of epicatechin after consumption of grape seed extract in humans. Experimental Biology Meeting; Washington, DC. 2012. p. abstr. [Google Scholar]
- Eskelinen EL. To be or not to be? Examples of incorrect identification of autophagic compartments in conventional transmission electron microscopy of mammalian cells. Autophagy. 2008;4:257–260. doi: 10.4161/auto.5179. [DOI] [PubMed] [Google Scholar]
- Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- Hoyer-Hansen M, Bastholm L, Mathiasen IS, Elling F, Jaattela M. Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death. Cell Death Differ. 2005;12:1297–1309. doi: 10.1038/sj.cdd.4401651. [DOI] [PubMed] [Google Scholar]
- Surveillance Research Program. National Cancer Institute; 2013. [Accessed on 4-27-2013]. Fast Stats: An interactive tool for access to SEER cancer statistics. http://seer.cancer.gov/faststats. [Google Scholar]
- Kaur M, Agarwal C, Agarwal R. Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. J Nutr. 2009a;139:1806S–1812S. doi: 10.3945/jn.109.106864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M, Agarwal R, Agarwal C. Grape seed extract induces anoikis and caspase-mediated apoptosis in human prostate carcinoma LNCaP cells: possible role of ataxia telangiectasia mutated-p53 activation. Molecular cancer therapeutics. 2006;5:1265–1274. doi: 10.1158/1535-7163.MCT-06-0014. [DOI] [PubMed] [Google Scholar]
- Kaur M, Mandair R, Agarwal R, Agarwal C. Grape seed extract induces cell cycle arrest and apoptosis in human colon carcinoma cells. Nutrition and cancer. 2008;60(Suppl 1):2–11. doi: 10.1080/01635580802381295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M, Velmurugan B, Rajamanickam S, Agarwal R, Agarwal C. Gallic acid, an active constituent of grape seed extract, exhibits anti-proliferative, pro-apoptotic and anti-tumorigenic effects against prostate carcinoma xenograft growth in nude mice. Pharm Res. 2009b;9:2133–2140. doi: 10.1007/s11095-009-9926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Fujita N, Noda T, Yoshimori T. Monitoring autophagy in mammalian cultured cells through the dynamics of LC3. Methods Enzymol. 2009;452:1–12. doi: 10.1016/S0076-6879(08)03601-X. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clave C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di Sano F, Dice JF, Difiglia M, Dinesh-Kumar S, Distelhorst CW, Djavaheri-Mergny M, Dorsey FC, Droge W, Dron M, Dunn WA, Jr, Duszenko M, Eissa NT, Elazar Z, Esclatine A, Eskelinen EL, Fesus L, Finley KD, Fuentes JM, Fueyo J, Fujisaki K, Galliot B, Gao FB, Gewirtz DA, Gibson SB, Gohla A, Goldberg AL, Gonzalez R, Gonzalez-Estevez C, Gorski S, Gottlieb RA, Haussinger D, He YW, Heidenreich K, Hill JA, Hoyer-Hansen M, Hu X, Huang WP, Iwasaki A, Jaattela M, Jackson WT, Jiang X, Jin S, Johansen T, Jung JU, Kadowaki M, Kang C, Kelekar A, Kessel DH, Kiel JA, Kim HP, Kimchi A, Kinsella TJ, Kiselyov K, Kitamoto K, Knecht E, Komatsu M, Kominami E, Kondo S, Kovacs AL, Kroemer G, Kuan CY, Kumar R, Kundu M, Landry J, Laporte M, Le W, Lei HY, Lenardo MJ, Levine B, Lieberman A, Lim KL, Lin FC, Liou W, Liu LF, Lopez-Berestein G, Lopez-Otin C, Lu B, Macleod KF, Malorni W, Martinet W, Matsuoka K, Mautner J, Meijer AJ, Melendez A, Michels P, Miotto G, Mistiaen WP, Mizushima N, Mograbi B, Monastyrska I, Moore MN, Moreira PI, Moriyasu Y, Motyl T, Munz C, Murphy LO, Naqvi NI, Neufeld TP, Nishino I, Nixon RA, Noda T, Nurnberg B, Ogawa M, Oleinick NL, Olsen LJ, Ozpolat B, Paglin S, Palmer GE, Papassideri I, Parkes M, Perlmutter DH, Perry G, Piacentini M, Pinkas-Kramarski R, Prescott M, Proikas-Cezanne T, Raben N, Rami A, Reggiori F, Rohrer B, Rubinsztein DC, Ryan KM, Sadoshima J, Sakagami H, Sakai Y, Sandri M, Sasakawa C, Sass M, Schneider C, Seglen PO, Seleverstov O, Settleman J, Shacka JJ, Shapiro IM, Sibirny A, Silva-Zacarin EC, Simon HU, Simone C, Simonsen A, Smith MA, Spanel-Borowski K, Srinivas V, Steeves M, Stenmark H, Stromhaug PE, Subauste CS, Sugimoto S, Sulzer D, Suzuki T, Swanson MS, Tabas I, Takeshita F, Talbot NJ, Talloczy Z, Tanaka K, Tanaka K, Tanida I, Taylor GS, Taylor JP, Terman A, Tettamanti G, Thompson CB, Thumm M, Tolkovsky AM, Tooze SA, Truant R, Tumanovska LV, Uchiyama Y, Ueno T, Uzcategui NL, van der Klei I, Vaquero EC, Vellai T, Vogel MW, Wang HG, Webster P, Wiley JW, Xi Z, Xiao G, Yahalom J, Yang JM, Yap G, Yin XM, Yoshimori T, Yu L, Yue Z, Yuzaki M, Zabirnyk O, Zheng X, Zhu X, Deter RL. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Kondo S. Autophagy and cancer therapy. Autophagy. 2006;2:85–90. doi: 10.4161/auto.2.2.2463. [DOI] [PubMed] [Google Scholar]
- Lattouf JB. Chemoprevention in bladder cancer: What’s new? Canadian Urological Association journal = Journal de l’Association des urologues du Canada. 2009;3:S184–187. doi: 10.5489/cuaj.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert JT, Shvarts O, Kawaoka K, Lieberman R, Belldegrun AS, Pantuck AJ. Prevention of bladder cancer: a review. European urology. 2006;49:226–234. doi: 10.1016/j.eururo.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Methods for monitoring autophagy. Int J Biochem Cell Biol. 2004;36:2491–2502. doi: 10.1016/j.biocel.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- Munafo DB, Colombo MI. A novel assay to study autophagy: regulation of autophagosome vacuole size by amino acid deprivation. J Cell Sci. 2001;114:3619–3629. doi: 10.1242/jcs.114.20.3619. [DOI] [PubMed] [Google Scholar]
- Prasain JK, Peng N, Dai Y, Moore R, Arabshahi A, Wilson L, Barnes S, Michael Wyss J, Kim H, Watts RL. Liquid chromatography tandem mass spectrometry identification of proanthocyanidins in rat plasma after oral administration of grape seed extract. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2009;16:233–243. doi: 10.1016/j.phymed.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina K, Singh RP, Agarwal R, Agarwal C. Oral grape seed extract inhibits prostate tumor growth and progression in TRAMP mice. Cancer research. 2007;67:5976–5982. doi: 10.1158/0008-5472.CAN-07-0295. [DOI] [PubMed] [Google Scholar]
- Raina K, Rajamanickam S, Deep G, Singh M, Agarwal R, Agarwal C. Chemopreventive effects of oral gallic acid feeding on tumor growth and progression in TRAMP mice. Mol Cancer Ther. 2008;5:1258–1267. doi: 10.1158/1535-7163.MCT-07-2220. [DOI] [PubMed] [Google Scholar]
- Reggiori F, Klionsky DJ. Autophagosomes: biogenesis from scratch? Curr Opin Cell Biol. 2005;17:415–422. doi: 10.1016/j.ceb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Shrotriya S, Deep G, Gu M, Kaur M, Jain AK, Inturi S, Agarwal R, Agarwal C. Generation of reactive oxygen species by grape seed extract causes irreparable DNA damage leading to G2/M arrest and apoptosis selectively in head and neck squamous cell carcinoma cells. Carcinogenesis. 2012;4:848–858. doi: 10.1093/carcin/bgs019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Miyazawa K, Tsukamoto T, Kuno T, Suzuki K. Pathobiology and chemoprevention of bladder cancer. Journal of oncology. 2011;2011:528353. doi: 10.1155/2011/528353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn A. Apoptosis and autophagy: regulatory connections between two supposedly different processes. Apoptosis. 2008;13:1–9. doi: 10.1007/s10495-007-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi A, Agarwal R, Agarwal C. Grape seed extract inhibits EGF-induced and constitutively active mitogenic signaling but activates JNK in human prostate carcinoma DU145 cells: possible role in antiproliferation and apoptosis. Oncogene. 2003;22:1302–1316. doi: 10.1038/sj.onc.1206265. [DOI] [PubMed] [Google Scholar]
- Velmurugan B, Singh RP, Agarwal R, Agarwal C. Dietary-feeding of grape seed extract prevents azoxymethane-induced colonic aberrant crypt foci formation in fischer 344 rats. Molecular carcinogenesis. 2010a;49:641–652. doi: 10.1002/mc.20643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velmurugan B, Singh RP, Kaul N, Agarwal R, Agarwal C. Dietary feeding of grape seed extract prevents intestinal tumorigenesis in APCmin/+ mice. Neoplasia. 2010b;12:95–102. doi: 10.1593/neo.91718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yla-Anttila P, Vihinen H, Jokitalo E, Eskelinen EL. Monitoring autophagy by electron microscopy in Mammalian cells. Methods Enzymol. 2009;452:143–164. doi: 10.1016/S0076-6879(08)03610-0. [DOI] [PubMed] [Google Scholar]