Abstract
Purpose
This study investigates whether sleep and stress are associated with body mass index (BMI) respectively, explores whether the combination of stress and sleep is also related to BMI, and demonstrates a thorough picture of how these associations above vary across the distribution of BMI values.
Methods
We analyze the data from 3,318 men and 6,689 women in the Philadelphia area using quantile regression (QR) to evaluate the relationships between sleep, stress, and obesity by gender.
Results
Our substantive findings include: (1) high and/or extreme stress were related to roughly an increase of 1.2 in BMI even after accounting for other covariates; (2) the pathways linking sleep and BMI differed by gender; with BMI for men increasing by 0.77–1 units with reduced sleep duration, and BMI for women declining by 0.12 unit with one units increase in sleep quality; (3) stress and sleep-related variables were confounded but there was little evidence for moderation between these two; (4) the QR results demonstrate that the association between high and/or extreme stress to BMI varied stochastically across the distribution of BMI values, with an upward trend, suggesting that stress played a more important role among adults with higher BMI (i.e., BMI > 26 for both genders); and (5) the QR plots of sleep-related variables show similar patterns, with stronger effects on BMI at the upper end of BMI distribution.
Conclusions
Our findings indicated that having sufficient and quality sleep and reducing stress may be two intervention strategies that can be promoted among adults in Philadelphia.
Keywords: Body Mass Index, quantile regression, stress, sleep, Philadelphia
Introduction
As obesity becomes a severe public health problem in the U.S., it is crucial to identify the novel and modifiable factors that are associated with body mass index (BMI), in order to control the prevalence of overweight and obesity. Previous studies were heavily focused on food consumption and certain lifestyle factors such as exercise and smoking. Whether stress and sleep are associated with BMI is underexplored, especially in the urban areas where people are more like to experience sleep disturbance and high stress [1–3].
The past decade has witnessed a growing interest in exploring the role of sleep duration in weight gain and the association with BMI. Short sleep duration has been found to be positively associated with concurrent or future obesity in both cross-sectional and longitudinal studies [4, 5]. Moreover, insomnia, poor sleep quality and short sleep have been found to be correlated with high stress [6–8], associations that hold even after controlling for competing explanations, such as physical activities or socioeconomic status. Recently, several medical studies have demonstrated that self-reported (subjective) hours of sleep are associated with increased stress level and an elevated prevalence of obesity [9, 10]. Laboratory studies, using objective measures of sleep duration, find weak, yet still significant, associations for both obesity and stress [1, 2]. One important implication of these findings is that stress and sleep may be two interrelated factors that contribute to obesity. However, little research has adequately examined the associations and this study endeavors to fill this gap.
In addition to the substantive shortcoming, a methodological weakness in the obesity literature has drawn increasing attention [11, 12]. The conventional approach either uses t-tests to address whether the mean BMI values differ by groups (usually categorized by stress level and sleep duration and quality) or models the relationships between BMI and predictors using ordinary least square (OLS) regression. It has been argued that these methods ignore the fact that “BMI is not a monotonous indicator of health since too high or too low of the level would lead to less favorable health conditions (p.487)” [11]. To more comprehensively depict the relationships between BMI and its determinants, research has begun to use quantile regression (QR) methods [13]. While relatively few obesity studies have adopted QR [11, 12, 14], the method has much potential as it can capture the heterogeneous associations specific to different populations (e.g., under- and overweight) [11]. We intend that our application of QR adds to the literature and encourages others to explore this method. In this study we use QR to understand whether (and how) the associations of stress and sleep with obesity vary across the entire distribution of BMI.
The theoretical linkages among sleep, stress and obesity are complicated but a plausible framework has been proposed [15]. On the one hand, stress has been found to be associated with the increased consumption of palatable or comfort food [16] and reduced exercise, which may directly lead to excess weight. On the other hand, stress is a determinant of impaired sleep duration and quality [17] and it has been suggested that short sleep duration and poor sleep quality affect physiological processes (increased ghrelin and decreased leptin) that lead to weight gain [5]. More importantly, among individuals with stress, smoking and alcohol consumption are common practices adopted to improve, though they are usually unsuccessful, sleep quality and duration [15, 18] and these behaviors are associated with obesity. Another plausible pathway indicates that stress may contribute to weight gain indirectly through sleep. The discussion above suggests that the relationship between stress and obesity may be moderated by sleep quality and duration. As suggested in recent research [1, 15, 19], reducing or coping with stress along with managing sleep quality and duration could be important components of strategies used for both obesity prevention and treatment.
The substantive justification for our study is based on the arguments above. After controlling for demographic, socioeconomic, and lifestyle factors, we expect that (1) short sleep duration and poor sleep quality are associated with high BMI; (2) high stress is related to high BMI; (3) the positive association between stress and BMI is moderated by sleep duration and sleep quality; and (4) the relationships of sleep and stress with BMI are heterogeneous across the distribution of BMI values. Testing these hypotheses will help us to fully describe the relationships of sleep and stress with BMI, which is a variable with heterogeneous conditional distribution [11].
Methods
Data sources
The individual-level data were collected as part of the Philadelphia Health Management Corporation’s (PHMC) 2008 Southeastern Pennsylvania Household Health Survey (HHS), the largest and most comprehensive health survey in the region. The HHS is conducted biennially and includes information on health status, health care experience and health behaviors of the residents in the Philadelphia metropolitan area [20]. PHMC uses the random-digit dial methodology from a stratified sampling frame to conduct their telephone surveys and statistical results can be generalized to the population of the five-county area cover by the survey [20]. We compared the HHS data with Census and Behavioral Risk Factor Surveillance System (BRFSS) to ensure the validity (see result section).
Our data include 10,007 adult respondents, of which 6,689 were women and 3,318 were men. The existence of the gender disparity in obesity status [21] necessitates that we analyzed the data and reported results by gender. We used SAS 9.2 to implement the analysis.
Variables
Our dependent variable was an individual’s BMI, defined as weight (in kilograms) divided by height (in squared meters). BMI has been closely related to body fat, waist circumference, and many other health outcomes [22, 23]. BMI was calculated with the participants’ self-report to two questions: “How much do you weigh without shoes?” and “How tall are you without shoes?” It has been documented that self-reported information, as in the HHS, tends to underestimate the prevalence of [24]. In obesity and sleep research, however, using these measures is not uncommon [25–27] and a recent study reported that the discrepancy between self-reported and measured BMI in the United States was small (about 3 percent) and stable in the past three decades [28]. As QR can handle extreme values or outliers effectively [29], we did not exclude respondents with high BMI.
Our explanatory variables can be categorized into four groups. First, we included demographic and socioeconomic status variables. Age was measured as a continuous variable (in years) while race was operationalized using three dummy variables: White, Black, and Hispanic, with “Other” as the reference group. Poverty was converted to a dummy variable based on whether respondents’ income was below the poverty line; coded as 1 (otherwise 0). Employment was based on two dummy variables: the employed and other statuses (e.g., retired or incapable of working) with the unemployed used as the reference group. Finally, among this grouping of variables, education was classified using five ordinal levels: below high school, completed high school, some college, completed college, and post college. We generated four dummy variables with below high school serving as the reference group.
Our second grouping of variables related to lifestyle factors. Healthy diet was defined as having at least five servings of fruit and vegetables on a typical day and was coded as 1, otherwise 0. In the HHS survey, a serving of fruit or vegetables was approximately equal to a medium apple, half of a cup of peas or half of a large banana [20]. Exercise was defined as physical activities lasting for at least thirty minutes such as jogging, dancing and gardening. Data on the frequency of exercise sessions per week were collected in the HHS. This frequency of exercise variable was used to classify respondents in to three groups: no exercise (the reference group), light exercise (1 to 3 times/week), and regular exercise (> 3 times/week). Smoking has been found to negatively correlate with weight gain or BMI [30, 31]. In the HHS, those who smoked were coded as 1 (0 otherwise).
The third set of variables related to stress. The participants evaluated their stress level in the past year using a scale from 1 (no stress) to 10 (an extreme amount of stress). While we acknowledge the complexity of measuring stress, this was the only measure available in the data set. Subjective stress assessments, however, have been argued to provide what stress-related research needs [32] as it summarizes the equilibrium of stressors and individual coping ability thus reflecting day-to-day levels of stress. Due to the potential nonlinear association between stress and BMI, we categorized the stress level into five groups: stress free (reference group; 1), below average (2–3), average (4–6), above average (7–8), and high stress (9–10). Similar measure of stress has been used in the literature [33]. Four binary variables were included in the analysis.
The final grouping included sleep-related covariates. The respondents were asked to evaluate their sleep quality on a scale from 1 to 5, with 1 being “restless” and 5 being “restful.” Sleep quality was treated as a continuous variable in the following analysis. Sleep duration was derived from individuals’ answers to the question: “how many hours of sleep do you get at night?” Following a recent study [1], we categorized the hours of sleep into three groups: sleep duration fewer than 5 hours, sleep duration between 6 to 7 hours, and sleep duration greater than 7 hours. The last group was used as the reference and two dummy variables were created. As noted earlier, self-reported sleep duration was related to BMI and similar survey questions have been used to obtain sleep duration in various studies [5].
Analytic Strategy
Our analytic strategy involved two stages. First, we used OLS regression to explore if the association between stress and BMI depends on the presence of sleep duration and/or sleep quality. Controlling for demographic, socioeconomic, and lifestyle variables, we included sleep-related variables, and stress variables, respectively. In so doing, we can compare any associations with BMI to the existing literature. In addition we can use the covariates in one model to understand how they are interrelated and examine interactions between stress and sleep-related covariates [34]. The estimated coefficients of these interactions can provide evidence of whether sleep duration and quality moderate the relationship between stress and BMI.
The second stage of our analysis focused on whether the covariates listed above affect BMI differently. We employed the QR method introduced by Koenker and Bassett [13]. Whereas classic regression methods estimate conditional mean functions, QR is a method used for estimating the conditional quantile functions; where the quantiles of the conditional distribution of the dependent variable can be expressed as functions of explanatory variables [35, 36]. For a random variable Y (BMI in this study) with probability distribution function:
| [1] |
the τ th quantile of Y can be defined as:
| [2] |
where 0 < τ < 1.In the HHS, the sampling τ quantile, ξ (τ), could be expressed as the solution of the optimization problem (without any covariates):
| [3] |
where ρτ (z) = z(τ − I (z < 0)). When the explanatory variables (x) are included, the τ quantile, ξ (τ), can be rewritten as . The effects of the covariates can then be obtained by solving the function:
| [4] |
The best model from the first analytic stage was implemented with QR. The QR approach is particularly useful in obesity research as it minimizes the sum of absolute deviation under specific quantiles, enables a thorough investigation across the entire distribution of BMI (a non-monotonous health indicator), and has been proven to offer nuanced insight in the effects of predictors on BMI [11, 12, 35]. Arguably the best way to demonstrate the QR result is to generate plots across the distribution of BMI values [29] and hence we summarize the QR estimates in figures.
Results
A summary of the data used in our analyses is provided in Table 1. Overall, about 70 percent of the respondents were White, 25 percent were Black, and 5 percent were Hispanic (reflecting the population composition of the greater Philadelphia area). The mean age was 52 years old. Women were more likely to be socioeconomically disadvantaged than men. For instance, the poverty rate was 3 percent higher among women than men, the employed rate of women was 7 percent lower than that of men, and fewer women possessed a post college degree than men. As to lifestyle, in general, only four out of ten adults exercised regularly (i.e., more than 3 times per week), but it should be noted that about half of the respondents reported at least one exercise (but fewer than 3 times) per week. Ten percent of the respondents are physically inactive. Only 15 percent of the respondents consumed at least 5 servings of fruit and vegetables per day (i.e., a healthy diet) and 20 percent were smokers. The gender differences in exercise and smoking were relatively small, however, women were more likely to have healthy diet than men. To validate our data, we compared the HHS with the 2008 BRFSS data and found these two datasets were comparable with regard to the prevalence of smoking, overweight, and obesity [37]. And based on census data we are confident that the HSS data were reliable and representative of the population in the Philadelphia metropolitan area.
Table 1.
Descriptive statistics of the variables used in the analysisa
| Variables | Overall (n=10,007) | Men (n=3,318) | Women (n=6,689) |
|---|---|---|---|
| BMI | 27.42 (5.99) | 27.83 (5.11) | 27.21 (6.37) |
| Socio-demographic Variables | |||
| White | 0.68 (0.47) | 0.72 (0.45) | 0.67 (0.47) |
| Black | 0.24 (0.42) | 0.20 (0.40) | 0.25 (0.43) |
| Hispanic | 0.05 (0.27) | 0.05 (0.21) | 0.05 (0.22) |
| Age | 52.00 (15.99) | 51.80 (16.02) | 52.09 (15.98) |
| Poverty | 0.08 (0.27) | 0.06 (0.23) | 0.09 (0.29) |
| Employed | 0.60 (0.49) | 0.65 (0.48) | 0.58 (0.49) |
| Other Employment Status | 0.34 (0.47) | 0.30 (0.46) | 0.36 (0.48) |
| High School | 0.32 (0.47) | 0.29 (0.45) | 0.33 (0.47) |
| Some College | 0.20 (0.40) | 0.19 (0.39) | 0.21 (0.41) |
| College | 0.25 (0.43) | 0.26 (0.44) | 0.24 (0.43) |
| Post College | 0.16 (0.37) | 0.19 (0.39) | 0.15 (0.36) |
| Lifestyle Variables | |||
| Regular Exercise | 0.39 (0.49) | 0.40 (0.49) | 0.38 (0.49) |
| Light Exercise | 0.50 (0.50) | 0.50 (0.50) | 0.50 (0.50) |
| Smoking | 0.20 (0.40) | 0.21 (0.41) | 0.20 (0.40) |
| F/V Consumption | 0.15 (0.36) | 0.10 (0.30) | 0.18 (0.38) |
| Sleep-related Variables | |||
| Sleep Quality | 3.61 (1.29) | 3.70 (1.24) | 3.56 (1.32) |
| Sleep Duration (≤ 5 hrs) | 0.20 (0.40) | 0.19 (0.39) | 0.19 (0.40) |
| Sleep Duration (6–7 hrs) | 0.27 (0.44) | 0.28 (0.45) | 0.26 (0.44) |
| Stress | |||
| Light Stress | 0.20 (0.40) | 0.24 (0.43) | 0.18 (0.39) |
| Average Stress | 0.36 (0.48) | 0.35 (0.48) | 0.36 (0.48) |
| High Stress | 0.22 (0.41) | 0.21 (0.40) | 0.23 (0.42) |
| Extreme Stress | 0.12 (0.33) | 0.08 (0.28) | 0.14 (0.35) |
The mean values were reported and the numbers in parentheses were standard deviations.
The overall self-rated sleep quality score was 3.61 (on a 1–5 scale) with a standard deviation of 1.29. Specifically, men, on average, reported better sleep quality than women, and their smaller standard deviation indicated that fewer men reported bad sleep quality than women. Overall, one-in-five respondents reported less than 5 hours sleep per night and 27 percent had a sleep duration of between 6 to 7 hours. While most of the participants had at least 7-hour sleep every night, women demonstrated a slightly higher proportion (0.55) than men (0.53). Regarding stress, only 10 percent of the adults were stress free. At the other end of the scale, 34 percent reported either high or extreme stress. Average stress was the modal category (36 percent). The gender difference in stress was more noticeable than for other covariates. For instance, only 9 percent of the women were stress free, which was lower than men. The proportion of high or extreme stress of men was 8 percent lower than that of women. Clearly, women tended to have high stress, which echoed the findings in the stress literature [38].
The OLS results by gender are summarized in Table 2 (men) and 3 (women). As the focus of this paper was on the associations of sleep-related and stress variables with BMI, the socio-demographic and lifestyle variables were included in every model as controls. The first two models reported the respective relationships of stress (Model I) and sleep-related (Model II) covariates to BMI.
Table 2.
| Variable | MODEL I | MODEL II | MODEL III | MODEL IV | MODEL V | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | 24.64(23.27,26.01) | *** | 25.30(23.93,26.67) | *** | 24.5(23.08,26.04) | *** | 24.64(23.11,26.17) | *** | 25.78(23.74,27.81) | *** |
| Socio-demographic Variables | ||||||||||
| White | 1.39(0.61, 2.16) | *** | 1.38 (0.61, 2.16) | *** | 1.24(0.46, 2.02) | *** | 1.36 (0.58, 2.14) | *** | 1.27 (0.49, 2.05) | *** |
| Black | 2.04(1.20, 2.87) | *** | 1.96(1.12, 2.79) | *** | 1.85(1.01, 2.69) | *** | 1.90(1.06, 2.74) | *** | 1.84(1.00, 2.68) | *** |
| Hispanic | 1.28 (0.26, 2.31) | ** | 1.15(0.13, 2.16) | * | 1.13(0.11, 2.15) | * | 1.20(0.18, 2.21) | * | 1.13(0.11, 2.14) | * |
| Age | 0.03(0.02, 0.04) | *** | 0.03(0.02, 0.04) | *** | 0.03(0.02, 0.04) | *** | 0.03 (0.02, 0.04) | *** | 0.03(0.02, 0.04) | *** |
| Poverty | 0.43 (−0.21,1.08) | 0.37 (−0.27, 1.02) | 0.39(−0.26, 1.04) | 0.48(−0.17, 1.13) | 0.37(−0.28, 1.02) | |||||
| Employed | 1.05 (0.38, 1.73) | ** | 0.98 (0.32, 1.65) | ** | 1.03 (0.36, 1.70) | ** | 0.91 (0.24, 1.58) | ** | 0.99(0.32, 1.66) | ** |
| Other Employment Status | 0.67(−0.08, 1.42) | 0.54(−0.20, 1.27) | 0.70(−0.05,1.44) | 0.57(−0.18, 1.32) | 0.69(−0.06, 1.44) | |||||
| High School | −0.74(−1.40, −0.08) | * | −0.69 (−1.35, −0.03) | * | −0.79 (−1.45, −0.13) | ** | −0.74 (−1.40, −0.08) | * | −0.76 (−1.42, −0.10) | * |
| Some College | −0.50 (−1.20, 0.20) | −0.50 (−1.20, 0.19) | −0.61 (−1.31, 0.09) | −0.54 (−1.24, 0.16) | −0.59 (−1.29, 0.11) | |||||
| College | −1.33(−2.03, −0.63) | *** | −1.15 (−1.85, −0.45) | *** | −1.28 (−1.98, −0.58) | *** | −1.20(−1.90, −0.49) | *** | −1.24 (−1.94, −0.54) | *** |
| Post College | −1.73 (−2.47, −1.00) | *** | −1.48 (−2.21, −0.76) | *** | −1.68(−2.41, −0.94) | *** | −1.58(−2.31, −0.85) | *** | −1.65(−2.37, −0.91) | *** |
| Lifestyle Variables | ||||||||||
| Regular Exercise | −0.79 (−1.36, −0.22) | ** | 0.98 (0.32, 1.65) | ** | −0.77 (−1.34, −0.20) | ** | −0.77(−1.34, −0.20) | ** | −0.73 (−1.30, −0.16) | ** |
| Light Exercise | 0.18(−0.38, 0.74) | 0.54(−0.20, 1.27) | 0.21(−0.35, 0.77) | 0.18 (−0.38, 0.74) | 0.20(−0.36, 0.76) | |||||
| Smoking | −0.72(−1.13, −0.31) | *** | −0.72 (−1.13, −0.31) | *** | −0.76(−1.17, −0.35) | *** | −0.73(−1.14, −0.32) | *** | −0.77(−1.17, − 0.36) | *** |
| Healthy Diet | 0.37(−0.15,0.89) | 0.39(−0.13,0.90) | 0.42 (−0.09, 0.94) | 0.35 (−0.17, 0.87) | 0.42(−0.10, 0.94) | |||||
| Sleep-related Variables | ||||||||||
| Sleep Quality (SQ) | −0.08(−0.22,0.06) | −0.03(−0.17, 0.12) | −0.06 (−0.21,0.08) | −0.33(−0.69, 0.04) | ||||||
| Sleep Duration (SD1) (≤ 5 hrs) | 1.01 (0.53, 1.49) | *** | 1.01 (0.53, 1.49) | *** | 1.63 (0.31,2.96) | ** | 1.09 (0.61, 1.57) | ** | ||
| Sleep Duration (SD2) (6–7 hrs) | 0.83(0.46, 1.20) | *** | 0.77(0.40, 1.14) | *** | 0.18(−1.03, 1.38) | .76(0.38, 1.13) | *** | |||
| Stress | ||||||||||
| Light Stress | 0.48 (−0.10,1.06) | 0.36 (−0.22,0.95) | 0.03 (−0.70,0.77) | −0.50(−2.48,1.49) | ||||||
| Average Stress | 0.51(−0.05,1.07) | 0.44(−0.13,1.00) | 0.34 (−0.37,1.05) | −0.34(−2.15,1.48) | ||||||
| High Stress | 1.41 (0.80,2.02) | *** | 1.19 (0.57,1.81) | *** | 1.37(0.55,2.18) | *** | −0.42(−2.28,1.45) | |||
| Extreme Stress | 0.85 (0.13,1.58) | * | 0.61 (−0.13,1.35) | 1.55 (0.52,2.59) | ** | −2.47(−4.44, −0.50) | ** | |||
| Interactions | ||||||||||
| Light Stress*SD1 | 0.17(−1.44, 1.77) | |||||||||
| Light Stress*SD2 | 1.26(−0.16, 2.67) | |||||||||
| Average Stress*SD1 | 0.19 (−1.32, 1.70) | |||||||||
| Average Stress*SD2 | 0.37(−0.98, 1.71) | |||||||||
| High Stress*SD1 | −1.61(−3.17, −0.04) | * | ||||||||
| High Stress*SD2 | 0.57(−0.86, 1.99) | |||||||||
| Extreme Stress*SD1 | −2.67(−4.41, −0.91) | ** | ||||||||
| Extreme Stress*SD2 | −0.38 (−2.24, 1.47) | |||||||||
| Light Stress*SQ | 0.20 (−0.26, 0.67) | |||||||||
| Average Stress*SQ | 0.17 (−0.25, 0.60) | |||||||||
| High Stress*SQ | 0.41(−0.05, 0.86) | |||||||||
| Extreme Stress*SQ | 0.90 (0.40, 1.40) | *** |
* = p < 0.1; ** = p < 0.05; *** = p < 0.01;
All models included socio-demographic and lifestyle variables. Model I further included stress variables, whereas Model II considered sleep-related variables. The stress and sleep-related variables were simultaneously added to Model III. Model IV and V expanded Model III by including the interactions between stress and sleep duration and sleep quality, respectively.
In Model I (see both Table 2 and 3), net of other covariates, high stress and extreme stress were positively associated with BMI for both men and women. Specifically, the BMI of the men with high stress was 1.41 higher than of stress free men. While extreme stress was also positively related to BMI, in contrast to the men without stress, the BMI of the men with extreme stress was only 0.85 higher. This association between extreme stress and BMI was intensified among women. The BMI was 1.32 higher among extremely stressed women than stress free women. A potential explanation for the gender difference here may be that men were less likely to report extreme stress.
Table 3.
| Variable | MODEL I | MODEL II | MODEL III | MODEL IV | MODEL V | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | 24.77(23.52,26.00) | *** | 26.40(25.19,27.62) | *** | 25.17(23.82,26.51) | *** | 25.21(23.81,26.61) | *** | 26.21(24.23,28.19) | *** |
| Socio-demographic Variables | ||||||||||
| White | 0.62(−0.10, 1.34) | 0.69 (−0.03, 1.42) | 0.67 (−0.05, 1.40) | *** | 0.64(−0.08, 1.37) | 0.68(−0.04, 1.41) | ||||
| Black | 3.73(2.97, 4.49) | *** | 3.71(2.95, 4.47) | *** | 3.74(2.98, 4.50) | *** | 3.73(2.97, 4.49) | *** | 3.75(2.99, 4.51) | *** |
| Hispanic | 2.39(1.50, 3.27) | *** | 2.43(1.54, 3.31) | *** | 2.46(1.57, 3.35) | *** | 2.41(1.52, 3.30) | *** | 2.47(1.58, 3.36) | *** |
| Age | 0.04 (0.03, 0.05) | *** | 0.04(0.03, 0.05) | *** | 0.04 (0.03, 0.05) | *** | 0.04(0.03, 0.05) | *** | 0.04(0.03, 0.05) | *** |
| Poverty | 0.82 (0.39, 1.25) | *** | 0.94(0.50, 1.37) | *** | 0.78 (0.34, 1.22) | 0.80(0.36, 1.23) | *** | 0.78(0.35, 1.22) | *** | |
| Employed | 0.13 (−0.40, 0.66) | 0.002 (−0.53,0.54) | 0.16(−0.38, 0.69) | 0.15(−0.39, 0.68) | 0.16(−0.38, 0.69) | |||||
| Other Employment Status | −0.06(−0.61, 0.50) | −0.19(−0.75, 0.37) | −0.03(−0.59, 0.54) | −0.02(−0.59, 0.54) | −0.02(−0.58, 0.54) | |||||
| High School | −0.62 (−1.17, −0.07) | * | −0.63 (−1.19, −0.08) | * | −0.58 (−1.14, −0.02) | * | −0.62(−1.18, −0.06) | * | −0.58(−1.14, −0.02) | * |
| Some College | −0.85 (−1.44, −0.27) | ** | −0.87 (−1.46, −0.28) | ** | −0.82(−1.41, −0.23) | ** | −0.86(−1.45, −0.26) | ** | −0.81(−1.40, −0.22) | ** |
| College | −1.99(−2.58, −1.39) | *** | −2.09(−2.69, −1.48) | *** | −1.95(−2.55, −1.34) | *** | −1.98(−2.59, −1.37) | *** | −1.94(−2.55, −1.33) | *** |
| Post College | −2.23 (−2.87, −1.60) | *** | −2.18(−2.83, −1.53) | *** | −2.15(−2.80, −1.50) | *** | −2.19(−2.84, −1.54) | *** | −2.14(−2.79, −1.49) | *** |
| Lifestyle Variables | ||||||||||
| Regular Exercise | −1.47 (−1.91, −1.03) | *** | −1.51 (−1.95, −1.07) | *** | −1.42 (−1.87, −0.98) | *** | −1.41(−1.86, −0.97) | *** | −1.43(−1.87, −0.98) | *** |
| Light Exercise | −0.55 (−0.98, −0.13) | ** | −0.62 (−1.05, −0.19) | ** | −0.53 (−0.96, −0.10) | ** | −0.52(−0.95, −0.09) | ** | −0.53(−0.97, −0.10) | ** |
| Smoking | −0.50(−0.83, −0.17) | ** | −0.44(−0.77, −0.10) | ** | −0.58 (−0.91, −0.25) | *** | −0.59(−0.93, −0.26) | *** | −0.58(−0.91, −0.24) | *** |
| Healthy Diet | 0.39(0.045, 0.73) | * | 0.38(0.03, 0.72) | * | 0.38 (0.04, 0.73) | * | 0.39(0.05, 0.74) | ** | 0.39(0.04, 0.73) | * |
| Sleep-related Variables | ||||||||||
| Sleep Quality (SQ) | −0.17(−0.26, −0.08) | *** | −0.12(−0.23, −0.01) | ** | −0.12(−0.21, −0.02) | ** | −0.15(−0.46, 0.15) | |||
| Sleep Duration (SD1) (≤ 5 hrs) | 0.25(−0.07, 0.57) | 0.12(−0.26, 0.50) | 0.20(−0.99, 1.39) | 0.12(−0.20, 0.44) | ||||||
| Sleep Duration (SD2) (6–7 hrs) | 0.02(−0.23, 0.27) | −0.01(−0.31, 0.29) | −0.49(−1.49, 0.51) | −0.01(−0.26, 0.24) | ||||||
| Stress | ||||||||||
| Light Stress | −0.20(−0.66, 0.26) | −0.19(−0.66, 0.27) | −0.17(−0.73, 0.39) | −0.08(−165, 1.50) | ||||||
| Average Stress | −0.05(−0.48, 0.39) | −0.05(−0.49, 0.38) | −0.22(−0.76, 0.32) | −0.22(−1.67, 1.23) | ||||||
| High Stress | 0.41(−0.04, 0.87) | 0.36(−0.10, 0.83) | 0.34(−0.23, 0.91) | 0.10(−1.37, 1.57) | ||||||
| Extreme Stress | 1.32(0.83, 1.80) | *** | 1.21(0.72, 1.71) | *** | 1.06(0.41, 1.72) | *** | 1.03(−0.43, 2.50) | |||
| Interactions | ||||||||||
| Light Stress*SD1 | −0.19(−1.95, 1.57) | |||||||||
| Light Stress*SD2 | −1.00(−2.45, 0.44) | |||||||||
| Average Stress*SD1 | 0.38(−1.21, 1.99) | |||||||||
| Average Stress*SD2 | 0.01(−1.31, 1.34) | |||||||||
| High Stress*SD1 | −0.23(−1.84, 1.38) | |||||||||
| High Stress*SD2 | 0.73(−0.66, 2.12) | |||||||||
| Extreme Stress*SD1 | 0.53(−1.13, 2.18) | |||||||||
| Extreme Stress*SD2 | 0.24(−1.25, 1.73) | |||||||||
| Light Stress*SQ | 0.33(−0.12, 0.78) | |||||||||
| Average Stress*SQ | 0.22(−0.19, 0.63) | |||||||||
| High Stress*SQ | 0.31(−0.11, 0.74) | |||||||||
| Extreme Stress*SQ | 0.30(−0.13, 0.73) |
* = p < 0.1; ** = p < 0.05; *** = p < 0.01;
All models included socio-demographic and lifestyle variables. Model I further included stress variables, whereas Model II considered sleep-related variables. The stress and sleep-related variables were simultaneously added to Model III. Model IV and V expanded Model III by including the interactions between stress and sleep duration and sleep quality, respectively.
Second, when only taking sleep-related variables into account (Model II), better sleep quality was related to lower BMI and this relationship held for only women. In general, a unit increase in sleep quality was associated with a BMI decrease of 0.17 for women. While the association between sleep quality and BMI was negative among men, it was not statistically significant. In addition, sleep duration was a determinant of BMI among men. On average, men whose sleep duration was less than 5 hours (or between 6 and 7 hours) had a BMI that was 1.01 (or 0.83) higher than those slept 7 or more hours. While the estimated associations of sleep duration with BMI among women followed our expectation, they did not reach statistical significance.
To further explore the intertwined associations between sleep, stress and BMI, we included all the variables in Model III. In contrast to Model I and II, the most visible change in Model III was that extreme stress was not a significant predictor of BMI among men (see Table 2). Sleep duration between 6 and 7 hours (SD2), however, was still associated with BMI and the magnitude of its effect was changed. Coupled with the fact that the impact of high stress on BMI dropped more than 15 percent from Model I to Model III ((1.41–1.19)/1.41), for men, it appeared that stress was confounded with SD2. On the other hand, the inclusion of stress decreased the effect of sleep quality on BMI among women (see Table 3). Specifically, the impact of sleep quality on BMI was reduced by almost 30 percent from Model II to Model III, and the influence of extreme stress dropped by 8 percent. These findings suggest that BMI among women was sensitive to sleep quality and BMI among men to sleep duration.
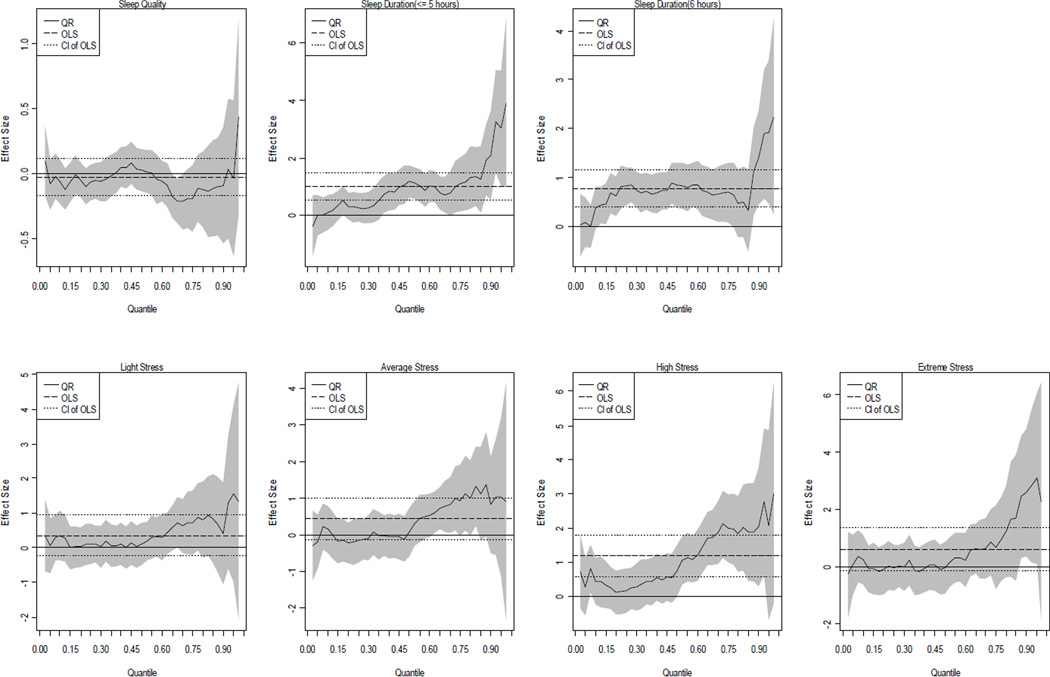
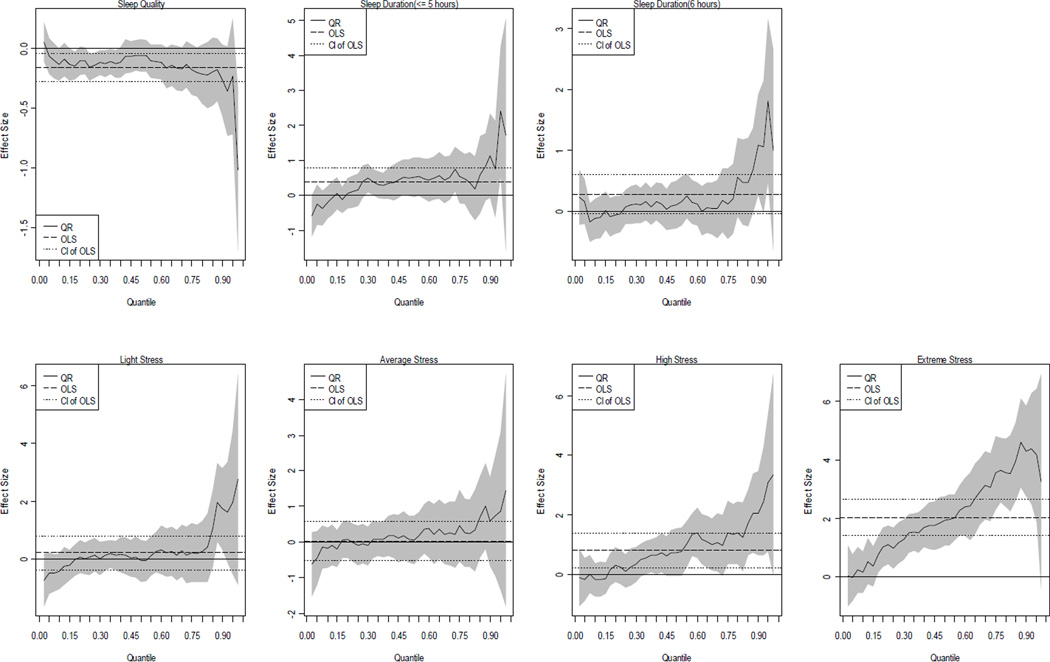
Next, the interactions between sleep-related and stress variables were included (see Model IV and V in Tables 2 and 3). If the interactions were significant this would provide evidence to suggest that the association between stress and BMI depended on sleep-related covariates. According to Models IV and V, little evidence for the expected moderation was found. It is important to note that most of the interactions in Model IV and V were not significant, suggesting that their inclusion likely over-fits the data. That is, among the five models, Model III is the one we regarded as the best model. We summarize Model III QR results in Figure 1 (men) and 2 (women), respectively.
Figure 1.
The QR plots of the associations of sleep quality, duration and stress with BMI among men (based on Model III)
Figure 2.
The QR plots of the associations of sleep quality, duration and stress with BMI among women (based on Model III)
For the purpose of brevity, we focused on the impacts of sleep-related and stress covariates on BMI. Each figure includes 7 graphs, with labels depicting variables of primary interest. The y-axis represents the effect size of the variable and the x-axis indicates the quantile of the BMI distribution. The long-dashed horizontal lines in each block of the figures represent the OLS estimates and the short-dashed horizontal lines capture the 90 percent confidence interval of the OLS estimates. The solid varying line captures the profile of the QR estimates and their confidence interval is shown in the grey-shaded areas. It should be reemphasized that all the quantiles reported below were based upon BMI values not on the values of other covariates.
For men (Figure 1), while the OLS estimate suggested that the effect of sleeping less than 5 hours (SD1) on BMI among men was significantly different from 0, the QR plot revealed how the relationship varied across the BMI distribution. The effects of SD1 were significant above the 0.35 quantiles (35th percentile, BMI >25.31), and increased dramatically after 85th percentile (BMI>31.95). In addition, the effects of SD2 were relatively stable between the 15th (BMI=23.11) to the 75th (BMI=30.13) percentiles but the effects soar after the 85th percentile. The plots show that the marginal effect of sleep duration was not constant, and sleep duration played an important role in determining BMI for all but the lower quantiles (τ <0.15). That is, sleep duration was a factor more important for men with high BMI than those with low BMI. Without using QR approach, the comprehensive picture of the relationships between sleep duration and BMI would be unknown. Moreover, echoing the results of Table 2, high stress was positively associated with BMI and the plots provide further insight into the association. Indeed, high stress was significant only between the 50th (BMI=26.87) and 95th (BMI=35.94) percentiles.
With respect to women (Figure 2), the QR plot indicated that the negative association between sleep quality and BMI was not driven by middle quantiles (40th to 60th percentiles). Instead, women in the lower quantiles seemed to be affected by sleep quality more than others, especially among those with BMI less than 24.54 (40th percentile). Although the QR estimates suggested that the association between sleep quality and BMI became stronger at the high quantiles (τ>0.85 where BMI=32.31), this was not statistically significant (even if we used the alpha level at 0.1). The QR estimates of high stress was positive and significant for upper quantiles (τ>0.5 where BMI=25.74) and increased with τ. More importantly, the relationship between extreme stress and BMI followed an upward trend starting from the 15th percentile (BMI=21.44) and throughout the rest of the BMI distribution. These findings not only provided evidence that stress and sleep quality are crucial in understanding obesity, but also elaborated on how these relationships vary stochastically across the distribution of BMI values among women, which to the best of our knowledge has not been investigated.
Discussion and Conclusions
Recent development in obesity research has shifted the focus toward an exploration of potential new determinants of BMI. Though stress and sleep-related variables have been found to be associated with obesity [1, 2], little prior research has employed sophisticated statistical techniques to control potential confounders and hence validated these associations. This study fully explored the relationships between stress, sleep, and obesity and demonstrated how these associations vary across the BMI distribution in an urban setting.
Substantively, we found that high and/or extreme stress was related to high BMI but the pathways linking sleep and BMI differed by gender. Specifically, man’s BMI increased with a decrease in sleep duration. Men sleeping more than 7 hours per night were found to have the lowest BMI, ceteris paribus. Women with better sleep quality had lower BMI. On average, one unit increase in sleep quality was associated with a BMI decrease of 0.12. The findings here not only advanced the literature by showing the gender difference, but also provided evidence supporting our first two hypotheses – high BMI is associated with short sleep duration, poor sleep quality, and high stress. However, as to the third hypothesis, we found little evidence to bolster the statement that high BMI is a result of the combination of high stress and poor sleep quality or short sleep duration. In the QR analysis the results vividly demonstrated that the relationships of high and/or extreme stress to BMI stochastically varied across the distribution of BMI values and showed an upward trend, especially for women. That is, stress played a more important role among adults with high BMI values. The QR plots of sleep-related variables showed the similar patterns and that their impacts on BMI increased dramatically at the higher end of BMI distribution. These trends confirmed the fourth hypothesis that the associations of sleep and stress with BMI are not as stable as the traditional analytic approach indicated across the distribution of BMI values.
While the results did not support the hypothesis that sleep may moderate the relationship between stress and BMI, our findings did suggest that sleep and stress were two seemingly independent predictors for BMI. This parallels the finding of several earlier studies [9, 10, 26, 27]. Our study is significant because it has revealed the heterogeneous associations among sleep duration, sleep quality, stress and BMI using QR and identified the gender-specific pathways that link sleep to BMI. Though several obesity studies have employed QR as the analytic tool [11, 12], sleep duration and sleep quality have not been discussed simultaneously along with stress and their roles have not been explored by gender in previous studies.
What are the implications that can be drawn on our findings in the urban setting? First, following Taheri [19], the associations we observed suggest that reductions in BMI among men will require more sleep, ideally at least 7 hours of sleep per night, and while among women it is sleep quality that matters. For instance, it has been demonstrated that learning Tai Chi and taking cognitive-behavioral therapy can improve sleep quality and sleep outcomes [39, 40]. Second, our findings imply that improvements in the ability to cope with stress may contribute to obesity prevention. Talking to close friends or seeking spiritual support may help reduce stress, which is positively related to BMI. Third, based on the QR results, obesity prevention policies could be better targeted to specific groups. With a clearer understanding of how the risk factors affect BMI, the prevention policies could be more cost-effective. For example, helping men to increase sleep duration and women to improve sleep quality may effectively reduce BMI.
This study has several limitations. First, our analyses were based on adult self-report data. Though it has been argued that subjective measures of stress and sleep duration are superior and better connected to wellbeing [2, 32], more efforts to explore the associations between subjective measures and obesity are still desirable. Second, it should be cautious to generalize the findings to other areas because the data were collected only in the Philadelphia metropolitan area. Third, the findings of this study cannot be used to derive causality among stress, sleep and obesity. As suggested [15, 17], more efforts are warranted to investigate the complex associations between stress, sleep, and BMI. Fourth, as the data used in this study are self-reported and cross-sectional, two sources of endogeneity that may lead to inconsistent coefficient estimates should be noted—i.e., measurement errors and unclear causal relationships among stress, sleep and obesity. A common approach to endogeneity is to include an instrumental variable in the analysis. Though the analytic framework of instrumental variable quantile regression (IVQR) has been proposed [41–43], no software programs are readily available. Furthermore, the extant literature has not identified candidate instrumental variables that may fit the IVQR analysis of stress, sleep and BMI. Future QR research should thus pay attention to endogeneity and possible IV strategies.
Obesity has become an important public health issue in the world, especially in the developed and developing countries [44]. A recent study projected that if the current trend of obesity continues, by 2030 over 1 billion people worldwide will have become obese [45]. To limit the effects of this epidemic, more and more interdisciplinary obesity research has tried to identify novel risk factors associated with obesity and has adopted advanced methods to better depict the associations between BMI and its determinants. Though this study has shed some new light in this area, future work should explore the determinants beyond individual level and analyze them with appropriate designs, measures and analytic tools [46, 47].
Contributor Information
Tse-Chuan Yang, Department of Biobehavioral Health, The Social Science Research Institute, The Pennsylvania State University, 803 Oswald Tower, University Park, PA 16802, USA. Fax:+1-814-865-3746, Telephone: +1-814-865-5553, tuy111@psu.edu
Stephen A. Matthews, Department of Sociology, The Pennsylvania State University, 601 Oswald Tower, University Park, PA 16802, USA
Vivian Y.J. Chen, Department of Statistics, Tamkang University, 151 Ying-Chuan Rd, Tamsui, Taipei, Taiwan 251
References
- 1.Vgontzas AN, et al. Obesity and Self-Reported Short Sleep Duration: A Marker of Sleep Complaints and Chronic Psychosocial Stress. Sleep Medicine Clinics. 2009;4(1):65–75. [Google Scholar]
- 2.Vgontzas AN, et al. Short sleep duration and obesity: the role of emotional stress and sleep disturbances. International Journal of Obesity. 2008;32(5):801–809. doi: 10.1038/ijo.2008.4. [DOI] [PubMed] [Google Scholar]
- 3.Schulz A, et al. Do Neighborhood Economic Characteristics, Racial Composition, and Residential Stability Predict Perceptions of Stress Associated with the Physical and Social Environment? Findings from a Multilevel Analysis in Detroit. Journal of Urban Health. 2008;85(5):642–661. doi: 10.1007/s11524-008-9288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawada T, Inagaki H, Suzuki S. Sleep duration and body mass index. Sleep Medicine. 2008;9(7):808–808. doi: 10.1016/j.sleep.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Patel SR, Hu FB. Short sleep duration and weight gain: A systematic review. Obesity. 2008;16(3):643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fortunato VJ, Harsh J. Stress and sleep quality: The moderating role of negative affectivity. Personality and Individual Differences. 2006;41(5):825–836. [Google Scholar]
- 7.Knudsen HK, Ducharme LJ, Roman PM. Job stress and poor sleep quality: Data from an American sample of full-time workers. Social Science & Medicine. 2007;64(10):1997–2007. doi: 10.1016/j.socscimed.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Utsugi M, et al. Relationships of occupational stress to insomnia and short sleep in Japanese workers. Sleep. 2005;28(6):728–735. doi: 10.1093/sleep/28.6.728. [DOI] [PubMed] [Google Scholar]
- 9.Gangwisch JE, et al. Inadequate sleep as a risk factor for obesity: Analyses of the NHANES I. Sleep. 2005;28(10):1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 10.Kohatsu ND, et al. Sleep duration and body mass index in a rural population. Archives of Internal Medicine. 2006;166(16):1701–1705. doi: 10.1001/archinte.166.16.1701. [DOI] [PubMed] [Google Scholar]
- 11.Chen CM, Chang CK, Yeh CY. A quantile regression approach to re-investigate the relationship between sleep duration and body mass index in Taiwan. International Journal of Public Health. 2012;57:485–493. doi: 10.1007/s00038-011-0239-7. [DOI] [PubMed] [Google Scholar]
- 12.Belasco E, et al. Using quantile regression to measure the differential impact of economic and demographic variables on obesity. Journal of Health Behavior and Public Health. 2012;2(2):35–45. [Google Scholar]
- 13.Koenker R, Bassett G. Quantile regression. Econometrica. 1978;46(1):33–50. [Google Scholar]
- 14.Li Y, Graubard BI, Korn EL. Application of nonparametric quantile regression to body mass index percentile curves from survey data. Statistics in Medicine. 2010;29(5):558–572. doi: 10.1002/sim.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vgontzas AN, Bixler EO. Short sleep and obesity: are poor sleep, chronic stress, and unhealthy behaviors the link? Sleep. 2008;31(9):1203. [PMC free article] [PubMed] [Google Scholar]
- 16.Dallman MF, et al. Chronic stress and obesity: a new view of "comfort food". Proceedings of the National Academy of Sciences. 2003;100(20):11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ãkerstedt T. Psychosocial stress and impaired sleep. Scandinavian Journal of Work, Environment & Health. 2006;32(6):493–501. [PubMed] [Google Scholar]
- 18.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. Jama-Journal of the American Medical Association. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 19.Taheri S. The link between short sleep duration and obesity: we should recommend more sleep to prevent obesity. Archives of Disease in Childhood. 2006;91(11):881–884. doi: 10.1136/adc.2005.093013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.PHMC. The 2008 Household Health Survey. 2008 [Google Scholar]
- 21.Wang Y, Beydoun MA. The obesity epidemic in the United States - Gender, age, socioeconomic, Racial/Ethnic, and geographic characteristics: A systematic review and meta-regression analysis. Epidemiologic Reviews. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 22.Lawlor DA, et al. Reverse causality and confounding and the associations of overweight and obesity with mortality. Obesity. 2006;14:2294–2304. doi: 10.1038/oby.2006.269. [DOI] [PubMed] [Google Scholar]
- 23.Pi-Sunyer FX. The obesity epidemic: Pathophysiology and consequences of obesity. Obesity Research. 2002;10:97S–104S. doi: 10.1038/oby.2002.202. [DOI] [PubMed] [Google Scholar]
- 24.Flegal KM, et al. Prevalence and trends in obesity among US adults, 1999–2000. Jama-Journal of the American Medical Association. 2002;288(14):1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 25.Kripke DF, et al. Mortality associated with sleep duration and insomnia. Archives of General Psychiatry. 2002;59(2):131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 26.Singh M, et al. The association between obesity and short sleep duration: a populationbased study. Journal of Clinical Sleep Medicine. 2005;1(4):357–363. [PubMed] [Google Scholar]
- 27.Tamakoshi A, Ohno Y. Self-reported sleep duration as a predictor of all-cause mortality: Results from the JACC Study, Japan. Sleep. 2004;27(1):51–54. [PubMed] [Google Scholar]
- 28.Gorber SC, Tremblay MS. The Bias in Self-reported Obesity From 1976 to 2005: A Canada-US Comparison. Obesity. 18(2):354–361. doi: 10.1038/oby.2009.206. [DOI] [PubMed] [Google Scholar]
- 29.Hao L, Naiman DQ. Quantitative Applications in the Social Sciences. Thousand Oaks, CA: Sage Publication; 2007. Quantile Regression. [Google Scholar]
- 30.Chou SY, Grossman M, Saffer H. An economic analysis of adult obesity: results from the Behavioral Risk Factor Surveillance System. Journal of Health Economics. 2004;23(3):565–587. doi: 10.1016/j.jhealeco.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Froom P, et al. Smoking cessation and body mass index of occupationally active men: The Israeli CORDIS study. American Journal of Public Health. 1999;89(5):718–722. doi: 10.2105/ajph.89.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazarus R. Theory-based stress measurement. Psychological Inquiry. 1990;1:3–13. [Google Scholar]
- 33.Matthews SA, Yang T-C. Exploring the rold of built and social neighborhood environments in moderating stress and health. Annals of Behavioral Medicine. 2010;39:171–183. doi: 10.1007/s12160-010-9175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller D, Judd C, Yzerbyt V. When moderation is mediated and mediation is moderated. Journal of Personality and Social Psychology. 2005;89(6):852–863. doi: 10.1037/0022-3514.89.6.852. [DOI] [PubMed] [Google Scholar]
- 35.Koenker R, Hallock KF. Quantile regression. Journal of Economic Perspectives. 2001;15(4):143–156. [Google Scholar]
- 36.Koenker R, Dorey V. A REMARK ON ALGORITHM AS-229 - COMPUTING DUAL REGRESSION QUANTILES AND REGRESSION RANK SCORES. Applied Statistics-Journal of the Royal Statistical Society Series C. 1994;43(2):410–414. [Google Scholar]
- 37.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Data. Department of Health and Human Services; 2008. [Google Scholar]
- 38.Shields M. Stress, health and the benefit of social support. Health Reports. 2004;15:9–38. [PubMed] [Google Scholar]
- 39.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+years of age. Health Psychology. 2006;25(1):3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 40.Li FZ, et al. Tai chi and self-rated quality of sleep and daytime sleepiness in older adults: A randomized controlled trial. Journal of the American Geriatrics Society. 2004;52(6):892–900. doi: 10.1111/j.1532-5415.2004.52255.x. [DOI] [PubMed] [Google Scholar]
- 41.Chernozhukov V, Hansen C. Instrumental variable quantile regression: A robust inference approach. Journal of Econometrics. 2008;142(1):379–398. [Google Scholar]
- 42.Chernozhukov V, Hansen C, Jansson M. Inference approaches for instrumental variable quantile regression. Economics Letters. 2007;95(2):272–277. [Google Scholar]
- 43.Chernozhukov V, Hansen C. Instrumental quantile regression inference for structural and treatment effect models. Journal of Econometrics. 2006;132(2):491–525. [Google Scholar]
- 44.Popkin BM. The world is fat. Scientific American. 2007;297(3):88–95. doi: 10.1038/scientificamerican0907-88. [DOI] [PubMed] [Google Scholar]
- 45.Kelly T, et al. Global burden of obesity in 2005 and projections to 2030. International Journal of Obesity. 2008;32(9):1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 46.Matthews SA, Moudon AV, Daniel M. Work Group II: Using Geographic Information Systems for Enhancing Research Relevant to Policy on Diet, Physical Activity, and Weight. American Journal of Preventive Medicine. 2009;36(4):S171–S176. doi: 10.1016/j.amepre.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 47.Oakes JM, Masse LC, Messer LC. Work Group III: Methodologic Issues in Research on the Food and Physical Activity Environments Addressing Data Complexity. American Journal of Preventive Medicine. 2009;36(4):S177–S181. doi: 10.1016/j.amepre.2009.01.015. [DOI] [PubMed] [Google Scholar]