Abstract
Background and Aims
Although an inverse association of red blood cell cis-vaccenic acid and risk of myocardial infarction has been reported, it is unclear whether cis-vaccenic acid might lower the risk of heart failure (HF) with antecedent coronary heart disease (CHD). We sought to examine the relation of plasma cis-vaccenic acid with HF with antecedent CHD.
Methods
This nested case-control study was based on 788 incident HF cases (of whom 258 cases had antecedent CHD) and 788 controls. Each control was selected using a risk set sampling technique at the time of the occurrence of the index case and matched on year of birth, age at blood collection, and race. Fatty acids were measured using gas chromatography and incident HF was self-reported on annual questionnaires and validation in a subsample using medical records.
Results
In a multivariable conditional logistic regression, the odds ratio (95% confidence interval) for HF with prior CHD were 1.0 (ref), 0.72 (0.33-1.57), 0.28 (0.12-0.67), and 0.23 (0.09-0.58) across consecutive quartiles of cis-vaccenic acid (p_trend 0.0004). Each standard deviation of cis-vaccenic acid was associated with a 41% lower risk of HF with antecedent CHD (95% CI: 17% to 59%) in a multivariable adjusted model.
Conclusions
Our data suggest that higher plasma levels of plasma cis-vaccenic acid may be associated with a lower risk of HF with antecedent CHD. Confirmation of these results in the general population including women and other ethnic groups is warranted.
Keywords: Epidemiology, Heart failure, fatty acids, risk factor, nutrition
Introduction
Heart failure (HF) is a clinical syndrome with multiple etiologies including ischemic heart disease, hypertensive heart disease, cardiomyopathy, valvular disease among others1,2. Despite progress in HF treatment, its mortality remains high3-6, underscoring the importance of novel prevention strategies. Fatty acids in the de novo lipogenesis play a role in cardiometablic disorders as adult cardiomyocytes prefer fatty acids over glucose as energy source7,8. Excess carbohydrate or alcohol can enhance endogenous de novo lipogenesis9 and byproducts of such pathway (14:0, 18:0, 16:0, 16:1n-7, 18:1 n-7) can affect the development of chronic disease. Recent data suggest that de novo lipogeneis plays a critical role in generating an endogenous ligand for peroxisome proliferator-activated receptor alpha (PPARα) in the liver10,11. Such ligand has been isolated as 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (16:0/18:1-GPC)12,10. PPARα is expressed in various tissues, but is enriched in liver, where it promotes fatty acid oxidation, lipid transport, and gluconeogenesis13. Palmitoleic acid can be desaturated to cis-vaccenic acid via stearoyl-CoA desaturase-1. Palmitic acid, palmitoleic and stearic acid (18:0) (major fatty acids from the de novo lipogeneisis) have been associated with a higher risk of diabetes14,15, hypertension16 and coronary heart disease17. Palmitoleic has also been associated with lower low-density lipoprotein cholesterol, higher high-density lipoprotein cholesterol, and lower fibrinogen18. Our group has recently reported an inverse association between red blood cell cis-vaccenic acid and the risk of myocardial infarction19. This suggests that cis-vaccenic acid may be associated with a lower risk of HF from ischemic origin (i.e., with antecedent coronary heart disease). However, no previous study has tested that hypothesis. Given the poor prognosis after HF onset, identification of factors that could lower the incidence of HF is critical for primary prevention. Hence, the current study sought to examine whether plasma phospholipid cis-vaccenic acid was associated with HF preceded by CHD. Our primary hypothesis was that cis-vaccenic acid would be associated with a lower risk of HF with antecedent CHD. Our secondary hypothesis was that plasma cis-vaccenic acid is not related to HF without antecedent CHD.
Methods
Study population
This ancillary study used a prospective nested case-control design built on the existing Physicians' Health Study, which is a completed randomized, placebo-controlled trial designed to study low-dose aspirin and beta-carotene for the primary prevention of cardiovascular disease and cancer. 20 Study base was restricted to participants that provided a blood sample at baseline (1982-1983). For each case of HF, we used a risk set technique to randomly select one control among participants who were alive and free of HF at the time of diagnosis of the index HF case. We matched each control to the index case on age at randomization (within 1 year for >95% of controls), race (white vs. non-white), year of birth (within 1 year), and time of blood collection (within 288 days). Current analyses are based on 788 pairs. Each participant signed an informed consent and the Institutional review Board at Brigham and Women's Hospital approved the study protocol.
Measurement of plasma phospholipids fatty acids
Plasma phospholipid fatty acids were measured using a method described by Cao et al.21. For the extraction of plasma phospholipid fatty acids, we mixed 0.3 mL of plasma with 0.7 volume of 0.9% saline. Lipids are extracted from plasma with a mixture of chloroform:methanol (2:1, v/v), and cholesterol, triglycerides and phospholipid subclasses were then separated on a silica thin-layer chromatography plate in a solvent mixture of petroleum ether, diethyl ether, and glacial acetic acid (80:20:1, v/v/v). The band of phospholipids was harvested for the formation of methyl esters. Fatty acid methyl esters were prepared with 1.5 mL of 14% boron trifluoride in methanol, incubated at 80°C for 90 minutes, and extracted with petroleum ether. The final product was then dissolved in heptane and injected onto a capillary Varian CP7420 100-m column with a Hewlett Packard 5890 gas chromatograph (GC) equipped with a HP6890A autosampler. We obtained adequate separation of fatty acid methylesters over a 80-min period with an initial temperature of 190°C for 25 minutes. Fatty acid methylesters were separated, identified and expressed as percent of total fatty acids. The following coefficients of variations were obtained on 30 blind duplicates: eicosapentaenoic acid = 5.1%; docosapentaenoic acid = 3.8%; docosahexaenoic acid= 4.9%; 16:0=1.1%; 16:1n7cis = 1.8%; 18:0=3.5%; and 18:1n7 = 2.9%. Cases and matching controls were sent to the laboratory in the same batch and assayed at the same time. Lastly, laboratory personnel was blinded on the case-control status of each subject to enhance validity.
Ascertainment of incident HF
HF ascertainment in the PHS was initially achieved using yearly follow-up questionnaires (except during the first year when each participant completed two questionnaires six months apart). In addition, we have previously validated self-reported HF diagnosis in the PHS using the Framingham criteria22 on supplemental questionnaires in a subsample as well as via chart review on limited number of HF23. Overall positive predictive value of self-reported HF was 90% in that subsample23.
Other variables
At baseline, each subject provided information on exercise [how often do you exercise vigorously enough to work up sweat? Possible answers included rarely/never, 1-3/month, 1/week, 2-4/week, 5-6/week, and daily]; smoking (never, former, and current smoker); and alcohol intake (rarely/never, 1-3 per month, 1 per week, 2-4/week, 5-6/week, daily, and 2+/day). Self-reported baseline weight (kg) was divided by height (meter squared) to compute body mass index. Information on comorbidity including hypertension, atrial fibrillation, hyperlipidemia, and diabetes was collected at baseline and through follow-up questionnaires. High-sensitive C-reactive protein (hsCRP) was measured using a latex-particle enhanced immunoturbidimetric assay kit (Roche Diagnostics, Indianapolis, IN 46250). The inter-assay CV was 4.5%.
Statistical analyses
As we did not assume a priori linear relation of plasma cis-vaccenic acid with HF, we initially created quartiles of cis-vacceninc acid using its distribution in the control series. We created indicator variables for modeling using the first quartile as the reference. We fitted a conditional logistic regression to estimate relative risks of HF across exposure categories. The first model adjusted for matching variables, body mass index, plasma 18:0, oleic acid, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and prevalent hypertension, coronary heart disease, atrial fibrillation, and diabetes. A full model also controlled for smoking (never, former, current smokers), vigorous physical activity (at least weekly vs. less frequent), alcohol consumption (rarely/never, <1, 1-6, and 7+ drinks per week), and docosapentaenoic acid (DPA). To calculate the p value for linear trend we used an indicator variable that treat quartiles as an ordinal variable. Of the 788 HF cases and 788 matched controls, 258 cases were preceded by CHD and were used to test the primary hypothesis. Alpha level was set at 0.05 and all tests were two-sided using SAS 9.3 for all analyses. To explore the shape of the association, we fitted restricted cubic splines with four knots at the tenth, 36.7th, 63.4th, and 90th percentile using cis-vaccenic value of 1.2 (50th percentile) as the reference in STATA/MP12.
Results
The average age was 58.8±8.0 years. The mean plasma phospholipid cis-vaccenic acid (percentage of total fatty acids) was lower in cases (1.39±0.23) than in controls (1.42±0.21), Wilcoxon sign rank test p=0.02. Cis-vaccenic acid was associated with older age, lower body mass index, higher concentration of oleic acid, DPA and DHA, lower prevalence of diabetes, hypertension, CHD, and current smoking and a high prevalence of vigorous exercise (Table 1). Plasma cis-vaccenic acid was inversely associated with HF preceded by CHD (p for trend 0.004, Table 2); each standard deviation higher cis-vaccenic acid was associated with a 41% lower risk of HF with antecedent CHD (95% CI: 17% o 59%) in a fully adjusted model, Table 2. Using cubic splines to fit the data showed evidence for an inverse association (Fig. 1). In the control series, plasma cis-vaccenic acid was positively correlated with DHA, DPA, adiponectin, palmitoleic acid, and inversely related to body mass index, 18:0, and C-reactive protein (Table 3).
Table 1. Characteristics of 1576 male physicians by quartiles of plasma cis-vaccenic acid*.
| Quartiles of plasma phospholipid cis-vaccenic acid | |||||
|---|---|---|---|---|---|
| Characteristics | Q1 (low) 1.17[0.08-1.29] (N=441) | Q2 1.35[1.30-1.41] (N=398) | Q3 1.47[1.42-1.53] (N=342) | Q4 (high) 1.67[1.54-2.24] (N=395) | P value |
| Age (y) | 57.6±7.9 | 58.6±7.8 | 59.3±8.0 | 60.1±8.3 | <0.0001 |
| Body mass index (kg/m2) | 26.0±3.0 | 25.5±3.1 | 24.6±2.7 | 24.4±2.5 | <0.0001 |
| Plasma cis 16:1 n-7† | 0.32±0.13 | 0.31±0.11 | 0.32±0.14 | 0.35±0.19 | 0.0002 |
| Plasma DPA† | 0.80±0.21 | 0.84±0.24 | 0.87±0.28 | 0.86±0.23 | 0.0002 |
| Plasma EPA† | 0.79±0.37 | 0.74±0.37 | 0.74±0.42 | 0.75±0.56 | 0.19 |
| Plasma DHA† | 2.92±0.88 | 3.00±0.92 | 3.05±0.91 | 3.25±1.12 | <0.0001 |
| Plasma 18:0† | 14.3±1.2 | 14.0±1.2 | 13.7±1.1 | 13.5±1.2 | <0.0001 |
| Plasma oleic acid (18:1)† | 7.72±1.02 | 7.77±0.98 | 7.82±0.92 | 8.12±1.21 | <0.0001 |
| C-reactive protein (mg/dl) | 3.0±5.2 | 2.6±5.2 | 2.4±5.9 | 2.1±3.3 | 0.005 |
| Prevalent diabetes (%) | 9.5 | 4.8 | 3.5 | 3.8 | 0.0003 |
| Prevalent CHD (%)‡ | 19.3 | 15.6 | 12.0 | 13.2 | 0.02 |
| Atrial fibrillation (%) | 4.1 | 4.5 | 4.4 | 2.8 | 0.58 |
| Hypertension (%) | 37.1 | 31.7 | 26.8 | 27.8 | 0.006 |
| Current smoking (%) | 15.4 | 8.3 | 10.9 | 9.9 | 0.008 |
| Never smokers (%) | 43.5 | 45.8 | 41.9 | 49.1 | 0.22 |
| Current alcohol intake (%) | 85.0 | 81.8 | 85.0 | 84.3 | 0.57 |
| Vigorous exercise (%) | 70.5 | 71.8 | 79.1 | 74.3 | 0.04 |
|
| |||||
Data are presented as means ± standard deviation or percentages. Few participants had missing data on hypertension (n=10), smoking (n=2), alcohol use (n=6), and physical activity (n=10).
Plasma phospholipid fatty acids expressed as percentage of total plasma phospholipids (DPA: docosapentaenoic acid; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid)
CHD: coronary heart disease (myocardial infarction, coronary angioplasty or bypass surgery)
Table 2. Odds ratios for heart failure with antecedent coronary heart disease (CHD) by quartiles or per standard deviation increase of plasma phospholipid cis-vaccenic acids in the Physicians' Health Study*.
| Odds ratio (95% CI) for heart failure with antecedent CHD | |||
|---|---|---|---|
|
|
|||
| Plasma cis-vaccenic acid quartiles [Range] | Cases | Model 1† | Model 2‡ |
| Q1 (low) 1.17[0.08-1.29] | 97 | 1.0 | 1.0 |
| Q2 1.35[1.30-1.41] | 74 | 0.75 (0.37-1.52) | 0.72 (0.33-1.57) |
| Q3 1.47[1.42-1.53] | 43 | 0.30 (0.13-0.68) | 0.28 (0.12-0.67) |
| Q4(high) 1.67[1.54-2.24] | 44 | 0.25 (0.11-0.59) | 0.23 (0.09-0.58) |
| P for linear trend | 0.0003 | 0.0004 | |
| Per SD (0.21%) higher plasma cis-vaccenic acid | 258 | 0.57 (0.41-0.79) | 0.59 (0.41-0.83) |
SD=standard deviation
Model 1 adjusted for matching variables plus body mass index, prevalent hypertension, coronary heart disease, atrial fibrillation, and diabetes, and plasma phospholipid 18:0, palmitoleic acid, oleic acid, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA).
Model 2 adjusted for variables in model 2 plus smoking (never, former, current smokers), vigorous physical activity (at least weekly vs. less frequent), alcohol consumption (rarely/never, <1, 1-6, and 7+ drinks per week), oleic acid, and marine omega-3 fatty acids (EPA. DHA, and DPA)
Figure 1. Cubic splines showing association of cis-vaccenic acid with HF with antecedent CHD.
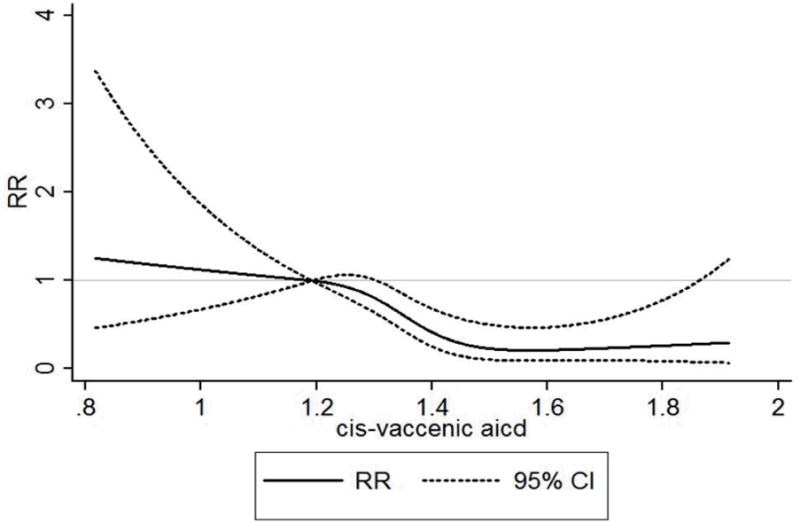
Solid dark line represents the relative risk and dotted lines represent 95% confidence intervals. Model adjusted for matching factors, body mass index, prevalent hypertension, atrial fibrillation, and diabetes; plasma phospholipid18:0, smoking (never, former, and current smokers), vigorous physical activity (at least weekly vs. less frequently), alcohol consumption (rarely/never, <1, 1-6, and 7+ drinks per week); and marine omega-3 fatty acids (EPA, DHA, and DPA).
Table 3. Spearman correlation coefficients (p value) and partial R-squared of predictors of plasma cis vaccenic acids in 788 controls.
| Variables | Spearman Correlation coefficient (p value) | Partial R-square |
|---|---|---|
| Plasma phospholipid DHA* | 0.18 (<0.0001) | 6.5% |
| Plasma phospholipid EPA* | 0.03 (0.4) | 3.1% |
| Plasma phospholipid 16:1n-7* | 0.15 (<0.0001) | 3.0% |
| Plasma phospolipid 18:0* | -0.23 (<0.0001) | 2.9% |
| Plasma log(adiponectin) (ug/ml) | 0.15 (<0.0001) | 1.0% |
| Plasma phospholipid DPA* | 0.11 (0.002) | 0.9% |
| Body mass index (kg/m2) | -0.16 (<0.0001) | 0.8% |
| Log-hsCRP (mg/dl) | -0.15 (<0.0001) | 0.8% |
| Age (y) | 0.07 (0.04) | 0.2% |
Fatty acids are expressed as percentage of total plasma phospholipid fatty acids. EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid; DPA: docosapentaenoic acid; hsCRP=high-sensitive C-reactive protein.
Discussion
In this prospective nested case-control study of apparently healthy male physicians, plasma phospholipid cis-vaccenic acid concentration appeared to be inversely associated with HF with antecedent CHD. To the best of our knowledge, this is the first study to examine the relation of phospholipid cis-vaccenic acid with HF with antecedent HF. Furthermore, only limited data are available on the association of cis-vaccenic acid with HF or HF risk factors. Data from the Physicians' Health Study showed no overall association between cis-vaccenic acid and total HF24. However, a previous report revealed an inverse association between red blood cell cis-vaccenic acid with the risk of myocardial infarction19, suggesting that the observed inverse relation between cis-vaccenic acid and HF with antecedent CHD might be causal. Of note is that in our sample, we observed a Spearman correlation coefficient of 0.22 (p=0.0007) between red blood cell membrane and plasma phospholipid cis-vaccenic acid. In addition, data from the Cardiovascular Health Study reported a non-statistically significant lower risk of total CHD with plasma cis-vaccenic acid [RR=0.75 (95% CI:0.43-1.32)], WU et al.25. Given the paucity of data, additional investigation is required to elucidate the role of cis-vaccenic acid on the risk of HF with antecedent CHD and identify underlying biologic pathways. As integral components of cellular membranes, fatty acids could directly affect biologic processes relevant to the development of HF. For example, some fatty acids have been shown to enhance endoplasmic reticulum stress and inflammation, stimulate apoptosis, and impair endothelial function. Both inflammation and apoptosis play an important role in the development of HF. Additional studies are needed to confirm current results and help elucidate pathways by which cis-vaccenic acid could influence the risk of HF.
Fatty acids in the DNL may influence left ventricular hypertrophy and subsequent HF risk. Infusion of animals with a mixture of myristic, palmitic, and palmitoleic acids led to cardiac hypertrophy26. Furthermore, treatment of mice with a similar mixture of fatty acids led to increased left ventricular mass26. However, little is known about physiologic effects of cis- vacenic acid in humans. A precursor of cis-vaccenic acid, palmitoleic acid, may increase the risk of HF via hypertension.27 This suggests that conversion of palmitoleic acid to cis-vaccenic acid could mitigate blood pressure raising effect and lower the risk of HF. Additional studies are needed to elucidate biologic effects of cis-vaccenic acid in humans.
Study strengths and limitations
Our study has several limitations. First, we had a single measurement of cis-vaccenic acid and could not account for change of that fatty acid over time. Second, we did not collect information on dietary patterns to account for total protein or carbohydrate intake, factors that have been shown to influence DNL. Third, generalization of these findings to the general population and other ethnic groups is limited due to the fact that all subjects were male physicians and predominantly white. Fourth, we did not collect information on left ventricular ejection fraction or HF etiology for further subclassification of the outcome. Fifth, HF diagnosis was primarily self-reported by participants who were physicians and despite a 91% positive predictive value of self-reported HF against validation with medical record review in a subsample, it is possible that we may have missed some HF cases or erroneously classified healthy people as HF participants. However, if such misclassification were present, it is more likely to be non-differential with respect to plasma fatty acids assays and would likely have led to an underestimation of the true effect. Lastly, a lack of nutrients at baseline and during follow up prevented us from adjusting for other dietary determinants of cis-vaccenic acid, including carbohydrate and protein consumption. Nonetheless, the present study has numerous strengths including a large number of cases; use of valid and reproducible method to assess cis-vaccenic acid; availability of data on numerous covariates including oleic acid, omega-3 fatty acids, etc; use of risk set technique to match cases and controls on relevant and potential confounders; and high positive predictive value of self-reported HF against review of medical records in a subsample.
In conclusion, our data suggest that higher plasma levels of phospholipid cis-vaccenic acid are associated with a lower odds of HF with antecedent CHD. Confirmation of these findings in other cohorts that include women and other ethnic groups is warranted.
Acknowledgments
We are indebted to the participants in the PHS for their outstanding commitment and cooperation and to the entire PHS staff for their expert and unfailing assistance.
Funding: This study was funded by grants R01HL092946 and HL092946S1 (Djousse) from the National Heart, Lung, and Blood Institute and the Office of Dietary Supplement, Bethesda, MD. The Physicians' Health Study is supported by grants CA-34944, CA-40360, and CA-097193 from the National Cancer Institute and grants HL-26490 and HL-34595 from the National Heart, Lung, and Blood Institute, Bethesda, MD.
Footnotes
Statement of authorship: Study design (LD), Drafting the manuscript (LD), Measurement of fatty acids (MYT, NH, NW), Statistical analysis (LD, CM), Critical review of the paper and data interpretation (LD, CM, NH, NW, MYT, JMG), Securing funding for current project (LD)
Role of the sponsor: Funding agencies did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Financial disclosures: Dr Djousse is currently serving as PI on an investigator-initiated research funded by GlaxoSmithKline and California Walnut Commission. No other authors declare a conflict of interest.
References
- 1.Kannel WB. Incidence and epidemiology of heart failure. Heart Fail Rev. 2000;5:167–173. doi: 10.1023/A:1009884820941. [DOI] [PubMed] [Google Scholar]
- 2.Rich MW. Heart failure in the 21st century: a cardiogeriatric syndrome. J Gerontol A Biol Sci Med Sci. 2001;56:M88–M96. doi: 10.1093/gerona/56.2.m88. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg RJ, Spencer FA, Farmer C, Meyer TE, Pezzella S. Incidence and hospital death rates associated with heart failure: a community-wide perspective. Am J Med. 2005;118:728–734. doi: 10.1016/j.amjmed.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg RJ, Glatfelter K, Burbank-Schmidt E, Farmer C, Spencer FA, Meyer T. Trends in mortality attributed to heart failure in Worcester, Massachusetts, 1992 to 2001. Am J Cardiol. 2005;95:1324–1328. doi: 10.1016/j.amjcard.2005.01.076. [DOI] [PubMed] [Google Scholar]
- 5.Shahar E, Lee S, Kim J, Duval S, Barber C, Luepker RV. Hospitalized heart failure: rates and long-term mortality. J Card Fail. 2004;10:374–379. doi: 10.1016/j.cardfail.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, DeMets DL. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 7.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 8.Lopaschuk GD, Spafford MA, Marsh DR. Glycolysis is predominant source of myocardial ATP production immediately after birth. Am J Physiol. 1991;261:H1698–H1705. doi: 10.1152/ajpheart.1991.261.6.H1698. [DOI] [PubMed] [Google Scholar]
- 9.Collins JM, Neville MJ, Pinnick KE, Hodson L, Ruyter B, van Dijk TH, Reijngoud DJ, Fielding MD, Frayn KN. De novo lipogenesis in the differentiating human adipocyte can provide all fatty acids necessary for maturation. J Lipid Res. 2011;52:1683–1692. doi: 10.1194/jlr.M012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, Turk J, Semenkovich CF. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Chakravarthy MV, Lodhi IJ, Yin L, Malapaka RR, Xu HE, Turk J, Semenkovich CF. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell. 2009;138:476–488. doi: 10.1016/j.cell.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernal-Mizrachi C, Weng S, Feng C, Finck BN, Knutsen RH, Leone TC, Coleman T, Mecham RP, Kelly DP, Semenkovich CF. Dexamethasone induction of hypertension and diabetes is PPAR-alpha dependent in LDL receptor-null mice. Nat Med. 2003;9:1069–1075. doi: 10.1038/nm898. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Folsom AR, Zheng ZJ, Pankow JS, Eckfeldt JH. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2003;78:91–98. doi: 10.1093/ajcn/78.1.91. [DOI] [PubMed] [Google Scholar]
- 15.Hodge AM, English DR, O'Dea K, Sinclair AJ, Makrides M, Gibson RA, Giles GG. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr. 2007;86:189–197. doi: 10.1093/ajcn/86.1.189. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Tsai M, Manson JE, Djousse L, Gaziano JM, Buring JE, Sesso HD. Erythrocyte fatty acid composition is associated with the risk of hypertension in middle-aged and older women. J Nutr. 2011;141:1691–1697. doi: 10.3945/jn.111.138867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Folsom AR, Eckfeldt JH. Plasma fatty acid composition and incidence of coronary heart disease in middle aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Nutr Metab Cardiovasc Dis. 2003;13:256–266. doi: 10.1016/s0939-4753(03)80029-7. [DOI] [PubMed] [Google Scholar]
- 18.Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GS. Circulating palmitoleic acid and risk of metabolic abnormalities and new-onset diabetes. Am J Clin Nutr. 2010;92:1350–1358. doi: 10.3945/ajcn.110.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Djousse L, Matthan NR, Lichtenstein AH, Gaziano JM. Red blood cellmMembrane concentration of cis-palmitoleic and cis-vaccenic acids and risk of coronary heart disease. Am J Cardiol. 2012;110:539–544. doi: 10.1016/j.amjcard.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Final report on the aspirin component of the ongoing Physicians' Health Study. Steering Committee of the Physicians' Health Study Research Group. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 21.Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem. 2006;52:2265–2272. doi: 10.1373/clinchem.2006.072322. [DOI] [PubMed] [Google Scholar]
- 22.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 23.Djousse L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302:394–400. doi: 10.1001/jama.2009.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djousse L, Weir NL, Hanson NQ, Tsai MY, Gaziano JM. Plasma phospholipid concentration of cis palmitoleic acid and risk of heart failure. Circ Heart Fail. 2012 doi: 10.1161/CIRCHEARTFAILURE.112.967802. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu JH, Lemaitre RN, Imamura F, King IB, Song X, Spiegelman D, Siscovick DS, Mozaffarian D. Fatty acids in the de novo lipogenesis pathway and risk of coronary heart disease: the Cardiovascular Health Study. Am J Clin Nutr. 2011;94:431–438. doi: 10.3945/ajcn.111.012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riquelme CA, Magida JA, Harrison BC, Wall CE, Marr TG, Secor SM, Leinwand LA. Fatty acids identified in the Burmese python promote beneficial cardiac growth. Science. 2011;334:528–531. doi: 10.1126/science.1210558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng ZJ, Folsom AR, Ma J, Arnett DK, McGovern PG, Eckfeldt JH. Plasma fatty acid composition and 6-year incidence of hypertension in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 1999;150:492–500. doi: 10.1093/oxfordjournals.aje.a010038. [DOI] [PubMed] [Google Scholar]


