Abstract
Parasitic dinoflagellates of the genus Amoebophrya infect free-living dinoflagellates, some of which can cause harmful algal blooms (HABs). High prevalence of Amoebophrya spp. has been linked to the decline of some HABs in marine systems. The objective of this study was to evaluate the impact of Amoebophrya spp. on the dynamics of dinoflagellate blooms in Salt Pond (MA, USA), particularly the harmful species Alexandrium fundyense. The abundance of Amoebophrya life stages was estimated 3–7 days per week through the full duration of an annual A. fundyense bloom using fluorescence in situ hybridization coupled with tyramide signal amplification (FISH- TSA). More than 20 potential hosts were recorded including Dinophysis spp., Protoperidinium spp. and Gonyaulax spp., but the only dinoflagellate cells infected by Amoebophrya spp. during the sampling period were A. fundyense. Maximum A. fundyense concentration co-occurred with an increase of infected hosts, followed by a massive release of Amoebophrya dinospores in the water column. On average, Amoebophrya spp. infected and killed ∼30% of the A. fundyense population per day in the end phase of the bloom. The decline of the host A. fundyense population coincided with a dramatic life-cycle transition from vegetative division to sexual fusion. This transition occurred after maximum infected host concentrations and before peak infection percentages were observed, suggesting that most A. fundyense escaped parasite infection through sexual fusion. The results of this work highlight the importance of high frequency sampling of both parasite and host populations to accurately assess the impact of parasites on natural plankton assemblages.
Introduction
Parasitism is a common interspecific interaction in aquatic habitats. Quantitative understanding of the mechanisms of parasitism has improved rapidly in the past decade and there is increasing evidence that parasites can exert significant influence on marine ecosystems through impacts to host population dynamics and interruption of interspecific interactions. In many systems, parasites are important drivers of biodiversity and exert significant influence on the flow of energy through microplanktonic and microbial food webs [1].
Amoebophrya spp. belong to Syndiales (Alveolata) and are a widespread group of marine parasites [2]. Amoebophrya spp. infect and kill a taxonomically broad variety of dinoflagellates, including harmful algal bloom (HAB) species within the genera Alexandrium, Dinophysis, Karlodinium and Akashiwo [3]. During their life-cycle, Amoebophrya alternates between a free-swimming infective stage (dinospore) and a multinuclear growth phase (trophont) within its host [4], [5]. An infection starts when a dinospore attaches to a host cell’s outer surface, then enters the host’s cytosol. Once inside, the parasitoid can either remain in the cytoplasm or invade the host’s nucleus where it transforms into a trophont. The trophont increases in size while undergoing a series of nuclear divisions and flagellar replications without completing cytokinesis. Late-infection trophonts are large, multinucleate and multiflagellate, occupying most of the host cell and have a characteristic “beehive” appearance [6]. Once fully matured, the trophont expands through the cell wall of the host and transforms into a strongly motile vermiform stage. The vermiform is short-lived, soon separating into many individual infective dinospores. Dinospores must find a new host soon since their survival time in the water column is short (∼10 days) [7].
Infection of dinoflagellates by Amoebophrya spp. has received particular interest in recent years, at least in part due to the potential of parasitism to exert top-down control of HABs [8]–[11]. Previous field studies have shown that Amoebophrya spp. infection success can be moderate to high (20%–80%), with the highest infection percentages occurring near the termination of host blooms [9], [11]. High host concentrations have been cited as a pre-condition for high infection rates [3], but Amoebophrya infections may also be a significant cause of host mortality at low host concentrations [12]–[14]. Under certain conditions, Amoebophrya infections may sometimes have a greater impact on the population dynamics of bloom-forming dinoflagellates than grazing by microzooplankton, and can potentially eliminate an entire host population within a few days [15], [16]. Still, there are significant gaps in our knowledge of the interactions between Amoebophrya spp. and their hosts, including the in situ host specificity of different Amoebophrya strains.
Recent advances in molecular techniques to study picoplankton communities have provided unprecedented tools to study the abundance and diversity of the Amoebophrya free-living dinospores and early-stage infections in their natural environment [11], [17]. The ability to detect and quantify free-living Amoebophrya spp. dinospores has increased our knowledge considerably and allows the understanding of Amoebophrya spp. infectivity and the characterization of parasite-host dynamics in natural assemblages. In this study we applied fluorescence in situ hybridization coupled with tyramide signal amplification (FISH- TSA) with an oligonucleotide probe for Amoebophrya. We use this method to examine the distribution and abundance of free-living Amoebophrya dinospores and estimate the abundance of infected dinoflagellate hosts in Salt Pond (Eastham, MA USA), a drowned kettle pond within the Nauset Marsh system that experiences annual shellfishing closures due Paralytic Shellfish Poisoning (PSP). These PSP events are caused by the HAB species Alexandrium tamarense Group I, which we refer to hereafter as A. fundyense, a renaming that has been proposed by U. John and others ([18]).
Salt Pond is an ideal study site because Amoebophrya spp. have long been known to infect A. fundyense there [19] and because host A. fundyense blooms are selectively retained within the pond due to an interaction between the pond’s circulation, bathymetry, and A. fundyense’s swimming behavior [20]. These aspects of the study site enabled us to plan our sampling using A. fundyense as a focal host species because changes in A. fundyense abundance could be directly attributed to growth, mortality and sexual processes without considering advection of the host population in and out of the system. This study also coincided with the first ever deployment of an Imaging FlowCytobot (IFCB) in Salt Pond. The IFCB is an in situ imaging-in-flow cytometer that records images of phytoplankton 10–100 µm in length, including A. fundyense cells [21]. The instrument provided near real-time estimates of A. fundyense abundance and also a detailed record of the A. fundyense population’s transition to the sexual phase of its life cycle [22].
The primary objective of this study was to assess the quantitative importance of Amoebophrya parasitism to A. fundyense bloom termination in Salt Pond.
Materials and Methods
2.1 Study Area
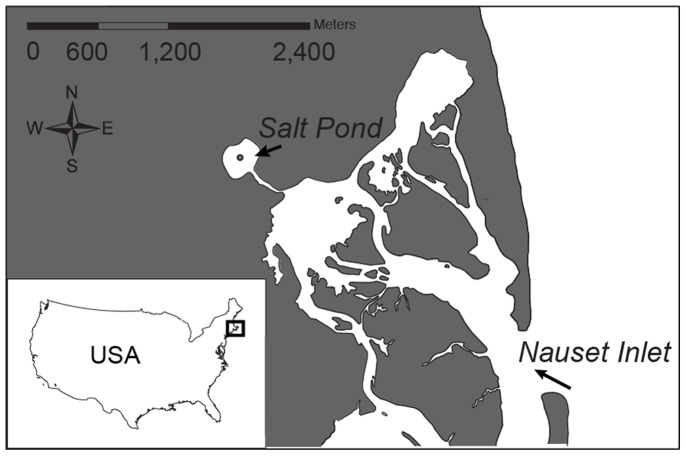
Salt Pond is a saline pond at the northwestern boundary of the Nauset Marsh system (NMS; Eastham, MA USA; Fig. 1). The NMS is a shallow estuary with extensive marshes protected from the Atlantic Ocean by a highly dynamic barrier beach with a single connection through Nauset Inlet [23]. Salt Pond is roughly circular, with a surface area of 82,200 m2, an average depth of 3.4 m, and a maximum depth of 9 m [20]. It is connected to the Nauset Marsh by a narrow inlet channel 0.5 km long, 30 m wide and 0.5 m deep at low tide. The pond receives freshwater input from groundwater but there are no direct stream or river inputs. Tidal currents are weaker and stratification due to salinity and temperature gradients are stronger than in the shallower central marsh.
Figure 1. Nauset Marsh System map.
Map of the study area showing the location of Salt Pond inside the Nauset Marsh System. Station 21 in the center of the Pond is marked in grey.
2.2 Sampling Overview
Authors were issued a permit from the United States Department of the Interior, National Park Service, Cape Cod National Seashore to conduct survey work throughout the Nauset Marsh including Salt Pond in Eastham, MA for the years from 2010 to 2015. The title of the overarching project is “Toxic Alexandrium blooms in the Nauset Marsh System”, led by Dr. Donald Anderson (Permit ID: CACO-2010-SCI-0001).
Field collections were taken at 1–3 day intervals from a float near the center of Salt Pond, from early March to mid May 2012 (Fig. 1). Whole seawater samples of 200 mL were collected at 1, 3 and 5 m depth using a 2.5 L Niskin bottle. Additional samples were taken at 7 m depth on 27 and 29 Apr 2013. Samples were immediately fixed on board with formaldehyde (1% final concentration) and stored on ice. Once in the laboratory, samples were prescreened through a 100 µm pore size net to remove large zooplankton and divided into two size-fractions (<15 µm and 15–100 µm) with a 15 m sieve. Both size fractions were gently filtered (100 mm Hg) onto polycarbonate filters (Millipore, 25 mm diameter; 0.8 µm pore size for smaller fraction; 5 µm pore size for larger fraction) to collect Amoebophryidae dinospores (<15 µm) and the microplankton community (15–100 µm) separately. Filters were dehydrated in a series of ethanol washes (50, 80 and 100%, 3 min each), dried at room temperature and stored at −20°C until analysis (typically within 3 months of collection). On the day of analysis, filters were thawed at room temperature and then cut into pieces with a razor blade. To avoid cell loss, filter sections were dipped in low-gelling-point agarose gel (0.2%), dried face down on Parafilm, and then dehydrated with ethanol.
2.3 Enumeration of A. fundyense
A. fundyense abundances were quantified using whole cell fluorescence in situ hybridization (FISH) using the oligonucleotide probe NA1 (5′-AGT GCA ACA CTC CCA CCA-3′) conjugated to a Cy3® fluorochrome [24], [25]. Briefly, a 1/4 piece of the 5 µm filters was immersed in 1 mL of pre-hybridization buffer and incubated at RT for 5 minutes. Filters were removed from the pre-hybridization solution and immersed in 1 mL of hybridization buffer containing the probe. Hybridization was performed at 50°C for 1 hour in an aluminum foil wrapped microcentrifuge tube temperature block (Eppendorf NA). Filters were washed at 50°C for 5 min with 1 mL of freshly made washing buffer.
After hybridization, filters were mounted on a microscope slide and stored at 4°C in the dark until analysis. The entire filter section was counted using a Zeiss Axioskop epifluorescence microscope equipped with a 10X and 20X coupled with a Cy3® fluorescence filter set (Chroma #41032).
2.4 Amoebophrya spp. Prevalence and Dinospore Abundance
Fluorescence in situ hybridization coupled with tyramide signal amplification (FISH-TSA) was used to enumerate (1) infected dinoflagellate host cells, with special emphasis on A. fundyense and (2) free living Amoebophrya spp. dinospores. A horseradish peroxidase (HRP) conjugated, Amoebophrya genus-specific oligonucleotide probe (ALV01; 5′-GCC TGC CGT GAA CAC TCT-3′) was used because it has been tested to work well on different strains of Amoebophrya in both culture and field samples [11], [26] and targets most of the environmental sequences belonging to Amoebophyidae. (68% of 1970 sequences) [11].
The FISH-TSA procedure was adapted for the identification of infected dinoflagellate host cells and estimation of Amoebophrya spp. prevalence (% of infected dinoflagellate hosts) [13], [27]. One eighth sections of the 5 µm filter were immersed in 900 µL of 40% formamide hybridization buffer (40% deionized formamide, 10% dextran sulfate, 900 mM NaCl, 20 mM Tris-HCl pH 7.5, 0.01% sodium dodecylsulfate (SDS), 1% Blocking agent from Boehringer Mannheim) and 3 µL of oligonucleotide probe (50 ng µL−1 stock solution, final probe concentration 0.17 ng µL−1). Hybridization was performed at 35°C for 2–3 hours in a 1.5 mL microcentrifuge tube temperature block (Eppendorf NA). Filters were washed twice at 37°C for 5 min with 25 mL of fresh washing buffer. Each section was incubated in Tris-NaCl-Tween (TNT) buffer for 15 min at room temperature in the dark. Tyramide reactions were carried out using a TSA kit (NEL741001KT TSA Plus Fluorescein System, PerkinElmer); 30 µL of freshly made TSA mix was applied to cover each filter. Next, slides were incubated for 15 min at room temperature, then transferred to TNT buffer baths for 15 min.
Dinospore abundances were estimated following Siano et al., [13]. In brief, one eighth sections of the 0.8 µm filters were covered by 30 µL of hybridization buffer and 3 µl of oligonucleotide probe (50 ng µL stock solution) and incubated at 42°C for 3 h. Filters were then washed twice at 47°C for 30 min each. After the TSA reaction, sections were washed twice in 55°C pre-warmed TNT baths for 30 min. After hybridization, all filters were counterstained and mounted as described by Alves de Souza et al., [26]. Slides were stored at 4°C in the dark for a week before analysis. All hybridized and stained filters were observed with a Zeiss Axioskop epifluorescence microscope equipped with 40X and 63X objectives coupled with fluorescence filter sets for observing calcofluor, propidium iodide (PI) and fluorescein tyramide staining.
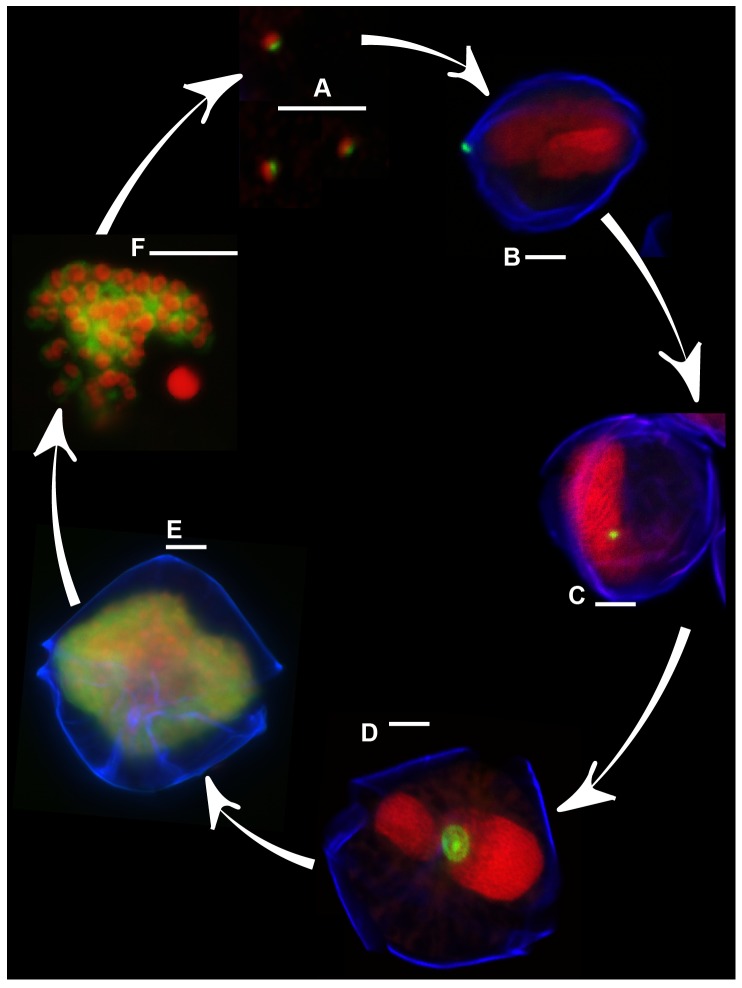
All infected A. fundyense on a filter section were counted. A. fundyense cells were considered infected when the nucleus of the cell and the ALV01 probe signal were both detected on the surface or inside the host. Infection prevalence was estimated when at least 400 A. fundyense host cells were observed (confidence limit at 95% significance level (+/−, 10%). Infected host cells were categorized into three groups according to the development of the Amoebophrya trophont (1) ‘early’ if the parasitoid was present as a small dot attached to the host theca or nucleus (Fig. 2B and Fig. 2C); (2) ‘intermediate’ if the parasitoid occupied a large portion of the host nucleus (Fig. 2D); and (3) ‘mature’ if a trophont was present (i.e. the “beehive” stage of infection; Fig. 2E).
Figure 2. Different stages of Amoebophrya sp. infecting A. fundyense in Salt Pond as revealed by FISH-TSA.
Green fluorescence shows the ALV01 probe targeting on the parasite. Red and blue fluorescence mark the host nucleus and theca respectively. (A) Dinospores, (B) initiation of the infection, dinospore attached to the host theca, (C) early, (D) intermediate and (E) beehive stages of infection (F) free-living vermiform. Scale bars = 10 µm.
Host mortality induced by Amoebophrya spp., the percentage of hosts killed per day, was estimated following a modification of an equation from Coats & Park [8] :
 |
where generation times for early, intermediate and mature stages were estimated to be 3.5, 2.5, and 1 day for early, intermediate and late-stage infections respectively. These maturation time estimates are based on a culture experiment (data not presented here).
Amoebophrya dinospores were counted with a 63X objective in either 20 randomly chosen fields or on the whole surface of the analyzed piece of filter depending on dinospore density. If less than 75 dinospores were found in 20 fields, filter sections were fully scanned. Amoebophrya spp. dinospore concentration was estimated when at least 400 free-living Amoebophrya cells were observed (confidence limit at 95% significance level (+/−, 10%).
2.5 Observations of A. fundyense Life-cycle Stages
A. fundyense life-cycle stages were enumerated on FISH-TSA stained filters. A minimum of 200 A. fundyense cells per sample was observed at 40X to calculate planozygote percentages and concentration (confidence limit at 95% significance level (+/−, 14%). Planozygotes were identified based on cell size (>45 µm diameter) and morphology as previously described by Anderson et al. [28] and Brosnahan et al. [29]. Examples of mating A. fundyense gamete pairs of A. fundyense cells were distinguished from dividing cells by their angle of attachment. Whereas dividing A. fundyense are always oriented relative to one another with their cingular grooves parallel [30], the cingular grooves of fusing gametes are often oriented obliquely, allowing them to be distinguished after fixation and staining.
Results
3.1 A. Fundyense Distribution in Salt Pond
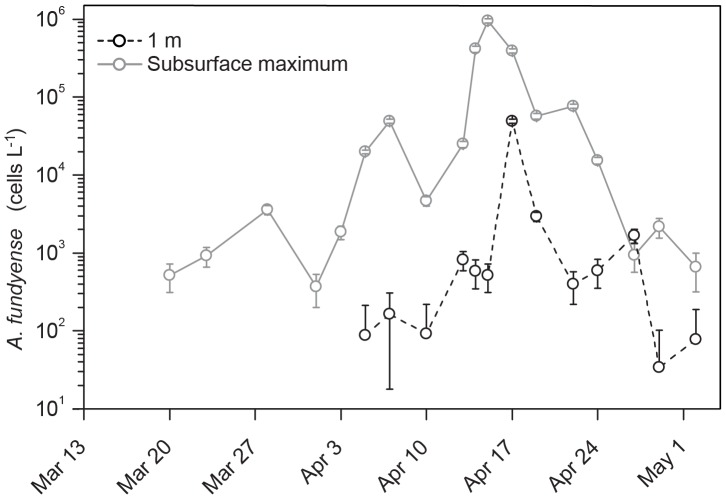
The maximum concentration of A. fundyense cells was always between 3 and 5 m depth throughout the 2012 bloom season, a pattern that reflected the diel vertical migration behavior of this organism. Because the sampling times were not synchronized with this diel migration cycle, the highest concentration obtained – either from 3 or 5 m depth - was chosen as the most representative sample of the A. fundyense population for each sampling day. At the start of the sampling period (20 March), the A. fundyense concentration was greater than 100 cells L−1 at the subsurface maximum in Salt Pond, with no cells observed in surface waters (Fig. 3). By April 5 and 7, A. fundyense concentrations had increased to greater than 100,000 cells L−1 and one week later on April 15, the population had increased to ∼1×106 cells L−1. Concentrations of A. fundyense remained high for 4 days (April 14–17), but then decreased more than 10-fold (from 390,231 to 10,180 cells L−1) over the next week (April 19–24). A. fundyense concentrations remained at less than 1,000 cells L−1 from April 24 until the end of the study (May 04). Throughout the development phase of the A. fundyense bloom, surface cell concentrations remained low, especially in comparison to the sub-surface where concentrations were typically 10–100 -fold higher. A single exception to this pattern was April 27 when the observed A. fundyense concentration at the surface was less than 2-fold higher than at 3 m, the subsurface maximum on that day.
Figure 3. Salt Pond A. fundyense vertical distribution from March to May 2012.
Subsurface maximum was obtained from the highest concentration – either from 3 or 5 m depth - as the most representative sample of the A. fundyense population for each sampling day.
3.2 A. fundyense Life Cycle Stages in the Water Column
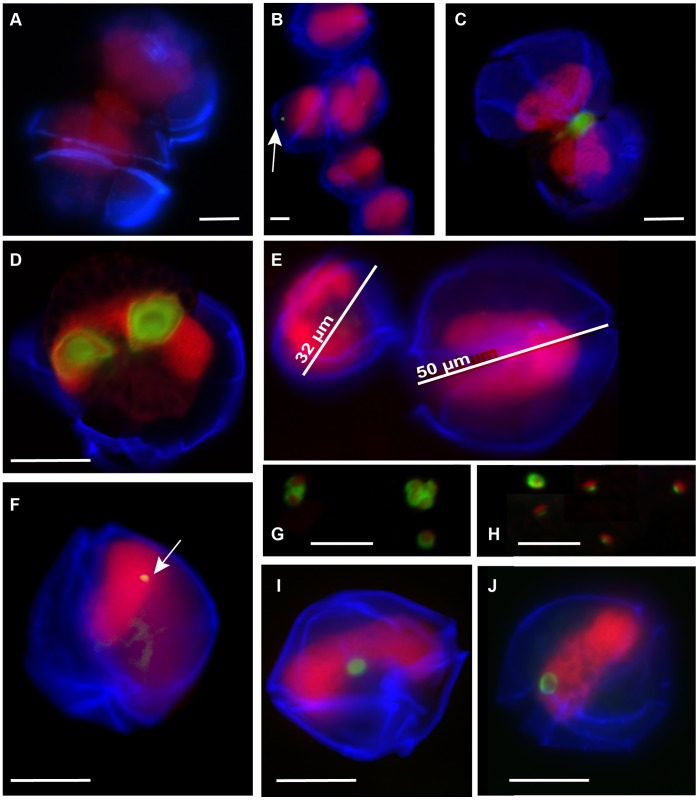
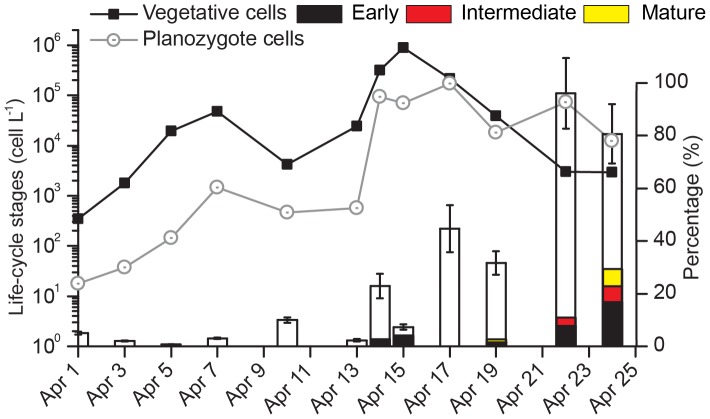
Planozygotes (defined here as large cells greater than 45 µm; Fig. 4E) represented between 2.3% and 96% of the total planktonic A. fundyense over the course of the bloom (Fig. 5). The lowest planozygote percentages were observed between April 3 and13. Following the peak (April 16), there was a significant increase in planozygote abundance with the maximum coinciding with the end of the bloom (April 22–24; Fig. 5). From April 15 to 19– just a few days prior to the bloom’s termination – a high proportion of fusing gametes (Fig. 4A) was observed in filtered samples (data not shown). These results are in agreement with a near continuous life-cycle analysis performed during the same time window with an Imaging FlowCytobot (IFCB) [21] that was deployed at 5 m depth below the float where water samples were taken for this study [22]. IFCB monitoring showed a rapid transformation to low volume cells (presumably gametes) on April 16 and a rapid decline in cell division (observed through a downward shift in the frequency of doublet vegetative cells; data not shown).
Figure 4. Micrographs of A. fundyense and Amoebophrya spp. life-cycle stages as detected by FISH-TSA.
Green fluorescence shows the ALV01 probe targeting on the parasite. Red and blue fluorescence mark the host nucleus and theca respectively. (A) Non infected fusing gametes; (B) A. fundyense chain showing one dinospore attached to a recently divided cell (white arrow); (C) infected A. fundyense gametes undergoing conjugation. (D) A. fundyense planozygote showing double infection with incipient mastigocoels. (E) Differences between a vegetative cell (32 µm diameter) and a planozygote (50 µm). (G) 2–4 clusters and (F) individual dinospores. Early infected planozygotes (F–J). Scale bars = 10 µm.
Figure 5. Time-series of A. fundyense life-cycle stages at the subsurface maximum from 1 to 24 April.
Vegetative cells (black squares), planozygotes (>45 µm diameter, grey open circles), and percentages of planozygotes (white bars) relative to the total A. fundyense population. Amoebophrya spp. infections and their maturation stage (%; black, red and yellow bars) are overlapped to the percentage of total planozygote abundance.
3.3 Infections by Amoebophrydae Parasitoids
The Amoebophrydae-specific probe ALV01 revealed that infections were restricted to just A. fundyense in Salt Pond in 2012. No infections were observed in any other potential dinoflagellate hosts present in the pond (Dinophysis spp., Gonyaulax spp., Neoceratium spp. among others). Multiple co-infecting dinospores (up to 5) were detected in some A. fundyense hosts (Fig. 4D), but only one vermiform was observed within mature parasitized hosts. FISH-TSA sensitivity allowed detection of all life-cycle stages of Amoebophrya spp., including very early infections (dinospores attached to their host surface; Fig. 2B and Fig. 4B) and free-living dinospores (∼5 µm equivalent spherical diameter, Fig. 2A and Fig. 4G–H). Asexually dividing cells were never found to contain parasites inside their nuclei. Conversely, very early Amoebophrya spp. infections - not yet associated with the cell nucleus - were observed in recently divided cells forming 2- and 4-cell chains (Fig. 4B). Infected gametes were observed to fuse with Amoebophrya spp. parasite inside at least one of their nuclei (Fig. 5C). Early infections were observed (up to 20%) in planozygotes after 19 Apr (Fig. 5 and Figs 4F–J).
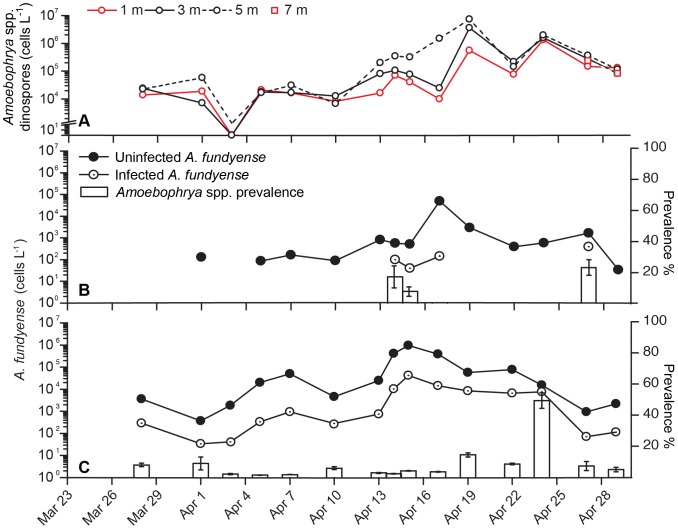
Infected A. fundyense cells were observed in the subsurface maximum throughout most of the study period (Fig. 6C). Beginning April 1, the number of infected A. fundyense cells increased steadily until April 15 when the overall A. fundyense concentration also reached its peak. Although the number of infected cells reached ∼50,000 cells L−1, the proportion of A. fundyense hosts infected by Amoebophrya spp. remained low (<5%). The proportion of infected hosts increased over the next week (April 16–23) as the A. fundyense host population declined. Highest proportions of hosts infected were observed on April 24 (50%), but the overall abundance of infected cells remained steady between 5,000 and 15,000 cells L−1. Infected cells were always scarce at the surface (1 m depth; Fig. 6B).
Figure 6. Vertical and temporal dynamics of Amoebophrya spp. dinospores and A. fundyense infections.
Top panel (A). Amoebophrya spp. dinospore concentrations observed at 1 (red solid line), 3 (black solid line), 5 (black dotted line) and 7 (square dots) meters depth. Bottom panels (B and C). Uninfected (black dots-black line) and infected (white dots-black line) A. fundyense cell concentrations (cell L−1) and Amoebophrya spp. prevalence (%; white bars) at 1 m (B) and the subsurface maximum (C) at Salt Pond, station 21, from 24 March to 5 May 2012.
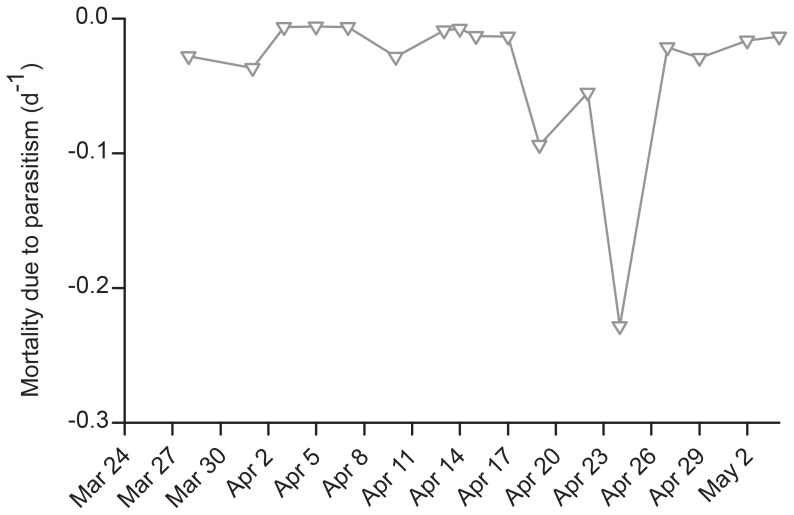
The estimated host mortalities due to Amoebophrya spp. infections at the subsurface maximum (3–5 m) during the peak on April 15 were −0.013 d−1 (Fig. 7). Assuming that all infected cells eventually died, infections killed less than 1% of the A. fundyense total population. The mortality rate due to parasitism subsequently increased from −0.013 d−1 April 17 to −0.09 April 19 and a maximum estimated rate of −0.23 d−1 April 24. These rates compare to a mean apparent population loss rates of −0.65 d−1 over the same time period. The difference in these estimates likely reflects losses due to other factors, including grazers and especially to sexual encystment. It is also important to note that each of these estimates likely reflects error associated with spatial patchiness in the distribution of cells and parasites in the pond. This is a potentially significant shortcoming of inferring rates from time-series of concentration in natural systems, even those like Salt Pond where advection by the mean flow is unlikely to have a significant impact. Despite the fact that spatial variability is a potentially significant source of error, the inferred rates are nevertheless valuable for assessing the impact of parasitism.
Figure 7. Estimates of mortality rates of the A. fundyense population due to parasitism by Amoebophrya.
Open triangles-grey line show the evolution of A. fundyense mortality due to Amoebophrya spp. parasitism (d−1) 30 March to 2 May at the subsurface maximum (3–5 m depth).
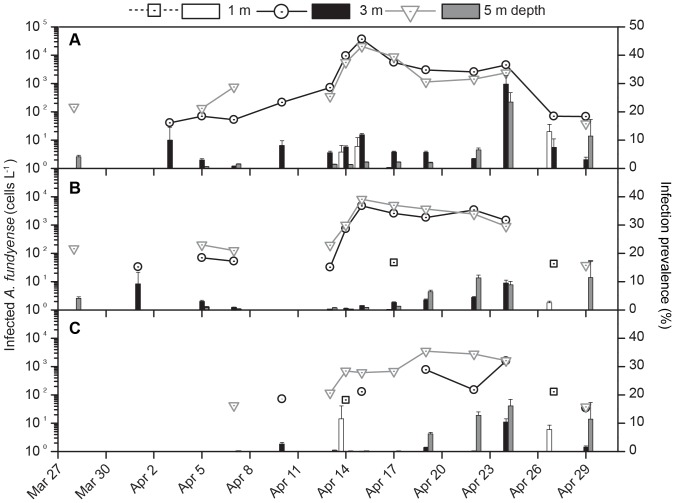
The maturation states of Amoebophrya spp. infections were considered when calculating general infection rates (Fig. 8). Early infections were more frequent at 3 m depth, and found throughout the entire time-series whereas intermediate and mature infections were usually found deeper (5 m) and - especially in the case of beehive stages - later in the season (from April 13 to 24).
Figure 8. Vertical distribution of Amoebophrya spp. maturation stages in infected A. fundyense cells.
Early (A), intermediate (B) and beehive (C) infected cells (scatter-lines; cell L−1) and prevalences (bars; %) of Amoebophrya spp. Infections at 1 m (open squares- dotted black lines and white bars), 3 m (open circles- solid black lines and black bars) and 5 m (open triangles-solid grey lines and grey bars) depth during the sampling season (27 March to 01 May).
Amoebophrya dinospores were present throughout the sampling period (March 28 to May 2; Fig. 6A) in concentrations that varied from 10,000 cells L−1 early in the sampling season (March 28) to <1,000 cells L−1 during surveys conducted through April 3 and up to 8×106 cells L−1 after the peak of the bloom on April 19th (Fig. 6A). This final, dramatic increase in dinospore abundance followed an increase in the number of infected A. fundyense hosts during the peak in A. fundyense abundance 3–4 days earlier (April 14–15; Fig. 6). A second spike in dinospore abundance was observed 5 days later on 24 Apr (∼ 1×106 cells L−1). Dinospore numbers then decreased steadily to level below the limit of detection (<100 cells L−1) after the April 28.
Dinospore appearance was different between the two main peaks in abundance. On April 19, vermiform structures (Fig. 2G) and dinospores clusters of 4 or 2 nuclei were abundant (Fig. 4G; data not shown). Individual dinospores were round, big and contained a large cytosol that surrounded a dense nucleus. In contrast, dinospores found on 24 Apr were smaller, less round and contained little cytoplasm (Fig. 4H). No vermiform or cluster structures were found at the end of the bloom.
Discussion
In situ observations [9], [11], [26] and modeling studies [15], [31] have suggested that Amoebophrya parasites can control and even prevent harmful algal blooms. However, host-parasite dynamics and their role in HAB termination in the field are not yet well understood. This study adds significantly to our knowledge of the Amoebophrya spp.-host dynamics in the field and contributes to our understanding of the quantitative importance of parasites to HAB termination that may be applicable to restricted embayments worldwide.
The A. fundyense-Amoebophrya dynamic in 2012 in Salt Pond shared some characteristics with observations of host-Amoebophrya spp. in other systems [11], [26], [32]. Peaks in A. fundyense cell concentrations were followed by increased infection levels and subsequently, by release of dinospores 4–5 days later. High concentrations of free-living dinospores were brief, as the swimming parasites must infect new hosts or they die within 3–4 days, based on the high mortality rate observed under culture conditions [7].
However, mortality due to Amoebophrya spp. infections was not responsible for the A. fundyense bloom decline, as a loss of 1% per day of the A. fundyense cells to parasitism could not have offset the increase of A. fundyense population during the early development of the bloom.
Dinospore: host ratios during the main two dinospores peak releases were as high as 200∶1 but infection prevalence never exceeded 55%. High dinospore inoculation (20∶1, 40∶1, 115∶1) in culture has been shown to result in significant high infections (up to 90%; [7]). However, on average, Amoebophrya spp. infected and killed ∼30% per day of the already declining A. fundyense population in the end phase of the bloom. Dinospore concentrations reported from natural systems have never been higher than 105–106 mL−1 nor the parasite-host ratio higher than 2∶1 [11], [13], [26]. Nevertheless, this is the first report of Amoebophrya spp. dinospore abundance as high as ∼8×106 cells L−1 (April 19, 5 m depth, Fig. 6A) in the field.
Many factors can affect infections in natural populations, such as host concentration; encounter rates, and genotypic variability within the host. Field and modeling studies have also shown that grazing by ciliates can limit the success of Amoebophrya spp. dinospores, with differential grazing pressure potentially influencing parasite prevalence [15], [33]. This process is called the ‘dilution effect’ and has been reported for many different parasite-host systems [34].
Sexual processes [35] and life-history shifts (e.g. the Cheshire cat strategy, [36]) have been pointed out as host refuge strategies to avoid parasite and viral infections. Dinoflagellates have been shown to stop dividing in culture once infected by Amoebophrya spp. [32] a result that is consistent with our observations in Salt Pond since no infections were found inside the nuclei of asexually diving A. fundyense pairs. However, we did observe the fusion of infected A. fundyense gametes (Fig. 4B and Fig. 4C) in the field suggesting that Amoebophrya spp. infections do not prevent sexual processes in their host populations. Based on comparison with culture experiments of trophont development, the size and location of trophonts found inside fusing cells (Fig. 4B) suggest that infection occurred at least 48 hours before fusion. However, it was not possible to document the fate of these fusing gametes, nor whether Amoebophrya spp. infections might have arrested gamete fusion/zygote development afterwards. Early trophonts were frequently observed in planozygotes (Fig. 5 and Fig. 4F–J) at the end of the bloom, suggesting that new dinospore recruitment, and not inherited gamete infection, was the main Amoebophrya spp. infection pathway for zygotic cells.
Although considerable scientific effort has been devoted to understand the effect of life-cycle strategies on the decline of HABs, questions regarding the role of biological factors causing the induction of sexuality remain unresolved. While most of the observed life-history shifts in dinoflagellate populations have been related to chemical factors [37], biological factors may also play an important role [35], [38]. Past studies at Salt Pond have reported the formation of A. fundyense planozygotes under favorable environmental conditions for cell growth with no obvious induction from physico-chemical factors [28]. Maximum values of planozygote percentages for A. fundyense observed by these authors were 35–40%. In 2012, planozygotes were present in low concentrations during the logarithmic growth phase of the bloom (14 Apr; Fig. 5). Yet, planozygote percentages reached very high proportions during the final stages of bloom termination. An apparent life-cycle shift occurred on April 15 in Salt Pond in 2012, and was already evident before the main release of dinospores on April 19. This fact suggests that the observed shifts (gamete formation, fusion, planozygote formation and encystment) during the A. fundyense bloom in Salt Pond is unlikely to be related to a high abundances of Amoebophrya spp. free-living dinospores (April 19), contrary to suggestions by several authors working in other systems [39], [40]. However, the increase in the number of parasitized A. fundyense cells at the time of the host peak (April 14) does suggest a possible trigger for sexual synchronization of A. fundyense populations in 2012. This hypothesis poses interesting questions. Can chemical cues such as water-borne signals or cell-to-cell interactions induce sexual changes in healthy A. fundyense cells? Similar findings have been recently reported for the coccolithophorid Emiliania huxleyi viral infections and could be compared with “quorum sensing” in bacterial biofilms [41]. Strongly supporting this hypothesis is the dramatic sexual response of A. fundyense cells. Further studies are needed to demonstrate if A. fundyense cells are capable of producing, sensing and responding to their own parasite-induced stress signals in both culture and field studies.
Amoebophrya spp. infected hosts have been observed to exhibit distinct swimming behavior and physiological patterns relative to healthy cells [32], [42]. Coats and Bockstahler [8] reported a sharp vertical separation of infected and uninfected hosts in the field, and detected a high percentage of mature infections several meters below the lightly infected surface population. Our results agree with these field [8] and culture [42] observations. In our study, maturation stages of Amoebophrya spp. infections also displayed vertical zonation (Fig. 8). Early infections were found in the upper 3 m while beehives were more common at 5 m depth. A. fundyense cells are known to follow a specific diel vertical migration in Salt Pond and are selectively retained due to general avoidance of surface layers [20]. However, infections by Amoebophrya spp. may interrupt internal mechanisms that control the vertical migration of their hosts.
To understand the quantitative effect of Amoebophrya spp. on host populations, it is important to recognize all parasite life stages. Newly formed Amoebophrya dinospores quickly lose their capacity to infect and most die after 2 to 10 days in cultures lacking hosts [7]. Furthermore, the mechanism that allows Amoebophrya spp. to survive during the dormancy period of their hosts remained an open question for many years. Recently, Chambouvet et al., [39] demonstrated the ability of some Amoebophrya strains to lie dormant inside the cyst of its host (Scrippsiella trochoidea). If this finding is generalizable, it would explain how Amoebophrya spp. can specifically infect the same host species year after year in natural environments like Salt Pond, even though the host has a meroplanktonic lifestyle and is present for most of the year in its cyst form, which is resistant to new infections [39]. A recent modeling study used a simplified representation of this shared-cyst survival strategy to understand parasite-host dynamic in close environments [43]. The authors concluded that when both parasites and hosts emerge from cysts through germination, hosts could reach high concentrations before becoming heavily infected. The intensity of the host bloom will depend on the number of parasitized cysts in the cyst beds. However, parasites would take over and the host population would be eliminated eventually unless the host population has a counter-balancing refuge strategy. Although A. fundyense blooms in Salt Pond are known to originate from germination from local cyst beds [23], there is still no evidence of a shared-cyst link between Amoebophrya and Alexandrium spp. Further work is needed to establish whether A. fundyense cysts provide a refuge for local Amoebophrya spp. populations.
Large numbers of dinospores were produced during the development and decline of the A. fundyense bloom in Salt Pond. Nevertheless, Amoebophrya spp. did not infect any other dinoflagellate species present. This strongly suggests that Amoebophrya spp. is host-specific for A. fundyense in our study area. Our results agree with the observations made by Chambouvet et al. [11] in the Penzé estuary (France). Those authors found highly host-specific interactions with a single Amoebophrya clade infecting a single dinoflagellate host throughout several years. However, isolated strains of Amoebophrya spp. from Salt Pond have been able to successfully infect different species of dinoflagellates (Alexandrium, Prorocentrum, Scrippsiella, and Heterocapsa) in culture [44], [45], suggesting that at least some Amoebophrya strain in Salt Pond are able to infect a wide range of hosts [3]. These conclusions, however, are based on culture observations alone and Amoebophrya species may have a narrower host range in the field.
Understanding host-Amoebophrya interactions is a high priority research topic with implications for HAB control and prevention. Host-parasite interactions are characterized by an ‘arms race’ that results in the fast co-evolution between both populations (the Red Queen hypothesis, [46]). The Red Queen hypothesis [47] explains how pathogens may maintain sexual reproduction in hosts and suggests that parasites have a great impact on driving host biodiversity. A. fundyense blooms in Salt Pond have been characterized by extensive genetic diversity and rapid temporal genetic differentiation [48]. Amoebophrya infections were suggested by these authors as one of the underlying reasons for these genetic differences. Results from our study provide direct, empirical evidence that Amoebophrya specifically infect A. fundyense in Salt Pond, suggesting that parasitism may control the genetic structure of the pond’s A. fundyense population.
Acknowledgments
The authors are grateful to the staff and students from the Anderson Laboratory at WHOI who assisted with the collection of samples, especially to B. Keafer, K. Norton and D. Kulis. We also thank Linda Amaral-Zettler, Leslie Murphy, Julie Reveillaud who helped with the surveys and Catharina Alves de Souza and Laure Guillou for their invaluable assistance and encouragement with the TSA-FISH technique.
Funding Statement
L. Velo-Suárez was supported by a Marie Curie International Outgoing Fellowship (IOF; grant agreement: MOHAB PIOF-GA-252260). This work was supported in part by NSF grants OCE-0430724 and OCE-0911031 and National Institute of Environmental Health Sciences grants 1P50-ES01274201 and 1P01ES021923-01 to D.M. Anderson and D.J. McGillicuddy through the Woods Hole Center for Oceans and Human Health, National Park Service Cooperative Agreement H238015504 to D.M. Anderson. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hudson PJ, Dobson AP, Lafferty KD (2006) Is a healthy ecosystem one that is rich in parasites? Trends Ecol Evol 21: 381–385. [DOI] [PubMed] [Google Scholar]
- 2. Guillou L, Viprey M, Chambouvet A, Welsh RM, Kirkham AR, et al. (2008) Widespread occurrence and genetic diversity of marine parasitoids belonging to Syndiniales (Alveolata). Environ Microbiol 10: 3349–3365. [DOI] [PubMed] [Google Scholar]
- 3. Park MG, Yih W, Coats DW (2004) Parasites and phytoplankton, with special emphasis on dinoflagellate infections. J Eukaryot Microbiol 51: 145–155. [DOI] [PubMed] [Google Scholar]
- 4. Cachon J (1969) Contribution à l’étude des péridiniens parasites: cytologie, cycles évolutifs. Ann Sci Nat Zool 6: 1–158. [Google Scholar]
- 5. Miller JJ, Delwiche CF, Coats DW (2012) Ultrastructure of Amoebophrya sp. and its changes during the course of infection. Protist 163: 720–745. [DOI] [PubMed] [Google Scholar]
- 6. Fritz L, Nass M (1992) Developemnt of the endoparasitic dinoflagellate Amoebophrya ceratii within host dinoflagellate species J Phycol. 28: 312–320. [Google Scholar]
- 7. Coats DW, Park MG (2002) Parasitism of photosynthetic dinoflagellates by three strains of Amoebophrya (Dinophyta): Parasite survival, infectivity, generation time, and host specificity. J Phycol 38: 520–528. [Google Scholar]
- 8. Coats DW, Bockstahler KR (1994) Occurrence of the parasitic dinoflagellate Amoebophrya ceratii in the Chesapeake Bay populations of Gymnodinium sanguineum . J Eukaryot Microbiol 41: 586–593. [Google Scholar]
- 9. Coats DW, Adam EJ, Gallegos CL, Hedrick S (1996) Parasitism of photosynthetic dinoflagellates in a shallow subestuary of Chesapeake Bay, USA. Aquat Microb Ecol 11: 1–9. [Google Scholar]
- 10. Kim S, Park MG, Yih W, Coats DW (2004) Infection of the bloom-forming thecate dinoflagellates Alexandrium affine and Gonyaulax spinifera by two strains of Amoebophrya (Dinophyta). J Phycol 40: 815–822. [Google Scholar]
- 11. Chambouvet A, Morin P, Marie D, Guillou L (2008) Control of toxic marine dinoflagellate blooms by serial parasitic killers. Science 322: 1254–1257. [DOI] [PubMed] [Google Scholar]
- 12. Salomon PS, Graneli E, Neves MHCB, Rodriguez EG (2009) Infection by Amoebophrya spp. parasitoids of dinoflagellates in a tropical marine coastal area. Aquat Microb Ecol 55: 143–153. [Google Scholar]
- 13. Siano R, Alves-de-Souza C, Foulon E, Bendif EM, Simon N, et al. (2011) Distribution and host diversity of Amoebophryidae parasites across oligotrophic waters of the Mediterranean Sea. Biogeosciences 8: 267–278. [Google Scholar]
- 14.Velo-Suárez L, González-Gil S, Pazos Y, Reguera B (2013) The growth season of Dinophysis acuminata in an upwelling system embayment: A conceptual model based on in situ measurements. Deep Sea Res (II Top Stud Oceanogr) DOI: 10.1016/j.dsr2.2013.03.033
- 15. Montagnes DJS, Chambouvet A, Guillou L, Fenton A (2008) Responsibility of microzooplankton and parasite pressure for the demise of toxic dinoflagellate blooms. Aquat Microb Ecol 53: 211–225. [Google Scholar]
- 16. Salomon PS, Stolte W (2010) Predicting the population dynamics in Amoebophrya parasitoids and their dinoflagellate hosts using a mathematical model. Mar Ecol Prog Ser 419: 1–10. [Google Scholar]
- 17. Not F, Simon N, Biegala IC, Vaulot D (2002) Application of fluorescent in situ hybridization coupled with tyramide signal amplification (FISH-TSA) to assess eukaryotic picoplankton composition. Aquat Microb Ecol 28: 157–166. [Google Scholar]
- 18. Lilly EL, Halanych KM, Anderson DM (2007) Species boundaries and global biogeography of the Alexandrium tamarense complex (Dinophyceae). J Phycol 43: 1329–1338. [Google Scholar]
- 19.Jacobson DM (1987) The ecology and feeding biology of thecate heterotrophic dinoflagellates: WHOI-MIT Joint Program in Oceanography and Oceanographic Engineering.
- 20. Anderson DM, Stolzenbach KD (1985) Selective retention of 2 dinoflagellates in a well-mixed estuarine embayment- Importance of diel vertical migration and surface avoidance. Mar Ecol Prog Ser 25: 39–50. [Google Scholar]
- 21. Olson RJ, Sosik HM (2007) A submersible imaging-in-flow instrument to analyze nano-and microplankton: Imaging FlowCytobot. Limnol Oceanogr Methods 5: 195–203. [Google Scholar]
- 22.Brosnahan ML, Anderson DM, Sosik HM, Olson RJ (2012) New insights into the development and termination of Alexandrium tamarense blooms through imaging flow cytometry. 15th International Conferance on Harmful Algae. CECO, Gyeongnam, Korea.
- 23. Crespo BG, Keafer BA, Ralston DK, Lind H, Farber D, et al. (2011) Dynamics of Alexandrium fundyense blooms and shellfish toxicity in the Nauset Marsh System of Cape Cod (Massachusetts, USA). Harmful Algae 12: 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scholin CA, Herzog M, Sogin M, Anderson DM (1994) Identification of the group-specific and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence-analysis of a fragment of the LSU ribosomal RNA gene. J Phycol 30: 999–1011. [Google Scholar]
- 25. Anderson DM, Kulis DM, Keafer BA, Gribble KE, Marin R, et al. (2005) Identification and enumeration of Alexandrium spp. from the Gulf of Maine using molecular probes. Deep Sea Res (II Top Stud Oceanogr) 52: 2467–2490. [Google Scholar]
- 26. Alves-de-Souza C, Varela D, Luis Iriarte J, Gonzalez HE, Guillou L (2012) Infection dynamics of Amoebophryidae parasitoids on harmful dinoflagellates in a southern Chilean fjord dominated by diatoms. Aquat Microb Ecol 66: 183–197. [Google Scholar]
- 27.Pernthaler A, Pernthaler J, Amann R (2004) Sensitive multi-color fluorescence in situ hybridization for the identification of environmental microorganisms. In: Kowalchuk G, De Bruin F, Head I, Akkermans A, Van Elsas J, editors. Molecular microbial ecology manual. 2nd ed. Dordrecht, The Netherlands.: Kluwer Academic Publishers. 711–726.
- 28. Anderson DM, Chisholm SW, Watras CJ (1983) Importance of the life-cycle events in the population dynamics of Gonyaulax tamarensis . Mar Biol 76: 179–189. [Google Scholar]
- 29.Brosnahan ML, Farzan S, Keafer BA, Sosik HM, Olson RJ, et al. (2013) Complexities of bloom dynamics in the toxic dinoflagellate Alexandrium fundyense revealed through DNA measurements by imaging flow cytometry coupled with species-specific rRNA probes. Deep Sea Res (II Top Stud Oceanogr) DOI: 10.1016/j.dsr2.2013.05.034 [DOI] [PMC free article] [PubMed]
- 30. Tomas RN (1974) Cell-division in Gonyaulax catenella, marine catenate dinoflagellate. J Protozool 21: 316–321. [DOI] [PubMed] [Google Scholar]
- 31.Alves-de-Souza C (2011) Interactions parasitoid-host between Amoebophryidae (MALV II) and planktonic dinoflagellates in marine systems with different trophic status: Universidad Austral de Chile.
- 32. Park MG, Cooney SK, Yih W, Coats DW (2002) Effects of two strains of the parasitic dinoflagellate Amoebophrya on growth, photosynthesis, light absorption, and quantum yield of bloom-forming dinoflagellates. Mar Ecol Prog Ser 227: 281–292. [Google Scholar]
- 33. Johansson M, Coats DW (2002) Ciliate grazing on the parasite Amoebophrya sp decreases infection of the red-tide dinoflagellate Akashiwo sanguinea . Aquat Microb Ecol 28: 69–78. [Google Scholar]
- 34.Goedknegt A, Welsh J, Thieltges DW (2012) Parasites as Prey. eLS: John Wiley & Sons, Ltd.
- 35. Figueroa RI, Garces E, Camp J (2010) Reproductive plasticity and local adaptation in the host-parasite system formed by the toxic Alexandrium minutum and the dinoflagellate parasite Parvilucifera sinerae . Harmful Algae 10: 56–63. [Google Scholar]
- 36. Frada M, Probert I, Allen MJ, Wilson WH, de Vargas C (2008) The “Cheshire Cat” escape strategy of the coccolithophore Emiliania huxleyi in response to viral infection. Proc Natl Acad Sci USA 105: 15944–15949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kremp A, Rengefors K, Montresor M (2009) Species-specific encystment patterns in three Baltic cold-water dinoflagellates: The role of multiple cues in resting cyst formation. Limnol Oceanogr 54: 1125–1138. [Google Scholar]
- 38. Rengefors K, Karlsson I, Hansson LA (1998) Algal cyst dormancy: a temporal escape from herbivory. Proc R Soc Lond, Ser B: Biol Sci 265: 1353–1358. [Google Scholar]
- 39. Chambouvet A, Alves-de-Souza C, Cueff V, Marie D, Karpov S, et al. (2011) Interplay between the parasite Amoebophrya sp (Alveolata) and the cyst formation of the red tide dinoflagellate Scrippsiella trochoidea . Protist 162: 637–649. [DOI] [PubMed] [Google Scholar]
- 40. Toth GB, Noren F, Selander E, Pavia H (2004) Marine dinoflagellates show induced life-history shifts to escape parasite infection in response to water-borne signals. Proc R Soc Lond, Ser B: Biol Sci 271: 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shapiro JA (1998) Thinking about bacterial populations as multicellular organisms. Annu Rev Microbiol 52: 81–104. [DOI] [PubMed] [Google Scholar]
- 42. Park MG, Cooney SK, Kim JS, Coats DW (2002) Effects of parasitism on diel vertical migration, phototaxis/geotaxis, and swimming speed of the bloom-forming dinoflagellate Akashiwo sanguinea . Aquat Microb Ecol 29: 11–18. [Google Scholar]
- 43.Arancio M, Sourisseau M, Souissi S (Accepted) Oscillation sources in homogeneous host-parasitoid system with a shared survival stage. Infection dynamics of marine dinoflagellate-dinoflagellate couple. Ecol Model.
- 44. Chambouvet A, Laabir M, Sengco M, Vaquer A, Guillou L (2011) Genetic diversity of Amoebophryidae (Syndiniales) during Alexandrium catenella/tamarense (Dinophyceae) blooms in the Thau lagoon (Mediterranean Sea, France). Res Microbiol 162: 959–968. [DOI] [PubMed] [Google Scholar]
- 45.Sengco MR, Coats DW, Popendorf KJ, Erdner DL, Gribble KE, et al. (2003) Biological and phylogenetic characterization of Amoebophrya sp. ex Alexandrium tamarense http://www.whoi.edu/cms/files/Abstracts_-_2nd_Symposium_24224.pdf: Accessed 2013 Oct 26.
- 46. Van Valen L (1973) A new evolutionary law. Evol Theory 1: 1–30. [Google Scholar]
- 47. Howard RS, Lively CM (1994) Parasitism, mutation accumulation and the maintenance of sex Nature. 368: 358–358. [DOI] [PubMed] [Google Scholar]
- 48. Richlen ML, Erdner DL, McCauley LAR, Libera K, Anderson DM (2012) Extensive genetic diversity and rapid population differentiation during blooms of Alexandrium fundyense (Dinophyceae) in an isolated salt pond on Cape Cod, MA, USA. Ecol Evol 2: 2588–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]