Abstract
Background
The present work aimed to find out the antibacterial activity of Nymphaea nouchali flower on human and plant pathogenic bacteria.
Methods
Antibacterial potency of methanol, acetone, ethyl acetate and petroleum spirit extracts of Nymphaea nouchali flower has been tested against four human pathogenic bacteria Bacillus subtilis (FO 3026) Escherichia coli (IFO 3007), Klebsiella pneumonia (ATTC 10031) and Sarcina lutea (IFO 3232) and one plant pathogenic bacterium Xanthomonas campestris (IAM 1671) by disc diffusion assay. Zone of inhibition produced by different extracts against the test bacteria was measured and compared with standard antibiotic disc.
Results
Methanol extract possessed better antibacterial activity against two pathogenic bacteria, B. subtilis (FO 3026) and S. lutea (IFO 3232) than commercial antibiotic nalidixic acid. Acetone extract showed moderate sensitivity whereas B. subtilis (FO 3026), S. lutea (IFO 3232) and X. campestris (IAM 1671) showed resistance to ethyl acetate and petroleum spirit extracts. The minimum inhibitory concentrations of various extracts were ranged between 128–2048 μgml-1.
Conclusions
Nymphaea nouchali flower could be a potential candidate for future development of novel broad spectrum antibacterial herbal formulation.
Keywords: Nymphaea nouchali flower, Antibacterial activity, Disc diffusion assay, Nalidixic acid
Introduction
The emergence of multiple antibiotic resistance pathogenic bacterial strains is a major medical problem worldwide and poses a big threat to human society [1,2]. Moreover almost all of the antibiotics have side effects including hypersensitivity, immune-suppression and allergic reactions, and they are expensive too [3]. These circumstances make it essential to search new and more potent antimicrobial compounds to combat these pathogens. Herbal treatment is one possible way to treat disease caused by multidrug resistant bacteria [1,4,5] which is the basis for this study.
Nymphaea nouchali (Burm. f) (Water lily in English and Shapla in Bangla) is the national flower of Bangladesh. It is an aquatic rooting herb belongs to the family Nympheaceae generally found in lakes and ponds throughout the country [6]. Various secondary metabolites like sterols (nymphayol, isolated from flower), alkaloids, saponins, tannins, and flavonoids has been isolated from this plant [7] and these metabolites may be responsible for antibacterial activities. It has been reported to use in treatment of diabetes, tumor, inflammation, liver and urinary disorders, menstruation problems, indigestion and also used as food by the local people [7-11]. There are many literatures reporting the ethno-medicinal values of N. nouchali, but there is little scientific proof for further using this plant commercially or in a more effective form. Therefore, an attempt was made to evaluate the antibacterial potential of N. nouchali flower extracts against human and plant pathogenic bacteria.
Materials and methods
Plant material
Healthy, disease free N. nouchali flower collected from different ponds of Kushtia and Jhenidah region, Bangladesh during the month of July, 2012. This plant was then botanically identified by Bushra Khan, Principal Scientific Officer, National Herbarium, Mirpur, Dhaka 1216, Bangladesh. A voucher (DACB 38572) has been deposited in National Herbarium, Mirpur, Dhaka 1216, Bangladesh.
Preparation of extracts
Fresh flowers were cleaned with deionized water and dried in shade for two to four weeks. After drying, the flowers were pulverized into fine powder by a grinding machine and stored in airtight container. This powder was used to prepare methanol, ethyl acetate, acetone and petroleum spirit extracts. These crude extracts were then filtered and concentrated by using a rotary evaporator. After that, the extracts were diluted to 4096, 2048, 1024, 512, 256, 128, 64, 32, 16, 8, 4, 2 and 1 μgml-1. All the extracts were stored in refrigerator at 4°C in sterile container for further use [12].
Test bacteria
Pure culture of Bacillus subtilis FO 3026, Sarcina lutea IFO 3232, Xanthomonas campestris IAM 1671, Escherichia coli IFO 3007, Klebsiella pneumonia ATTC 10031 obtained from the Microbiology Laboratory of Department of Biotechnology and Genetic Engineering, Islamic University, Kushtia, Bangladesh and were used in this study (106 CFUml-1).
Antibacterial bioassay
Disc diffusion method was used for in vitro antibacterial activity assesment [13]. Filter paper discs (6 mm diameter) of various extracts (methanol, ethyl acetate, acetone and petroleum spirit) were prepared by impregnating blank sterile paper discs to the respective extracts with different concentrations (4096, 2048, 1024, 512, 256, 128, 64, 32, 16, 8, 4, 2 and 1 μgml-1). These paper discs were placed on nutrient agar inoculated with the test bacteria and incubated at 37°C for 24 h. Nalidixic acid (30 μg/disc) (Invitrogen, USA) were used as positive control and blank discs (impregnated with solvents followed by evaporation) were used as negative control. After incubation the culture plates were examined and the zones of inhibition were measured in millimeter scale [14].
Statistical analysis
Each experiment was run in triplicate, and mean values were calculated with SD (standard deviation). SPSS version 11.0 was used for the data analysis.
Results
Antibacterial activities
The antibacterial activity of different extracts of N. nouchali flower is shown in Figure 1 in a comparative way with standard antibiotic disc- nalidixic acid (30 μg/disc). The present study revealed that, methanol extract possessed the highest zone of inhibition of 30 ± 0.18 mm against B. subtilis followed by 27 ± 0.28 mm and 20 ± 0.60 mm against S. lutea and E. coli, respectively. Acetone extract produced satisfactory sensitivity of 17 ± 0.23 mm and 16 ± 0.62 mm against B. subtilis and X. campestris, respectively. Ethyl acetate and petroleum spirit extracts showed relatively poor antibacterial activity. All the test extracts were somehow effective against both K. pneumoniae and E. coli whereas both B. subtilis and S. lutea were resistant by ethyl acetate extract. Petroleum sprit extract failed to inhibit the growth of X. campestris. Positive control produced significant zones of inhibition against all the test bacteria while no zone was formed by negative controls.
Figure 1.
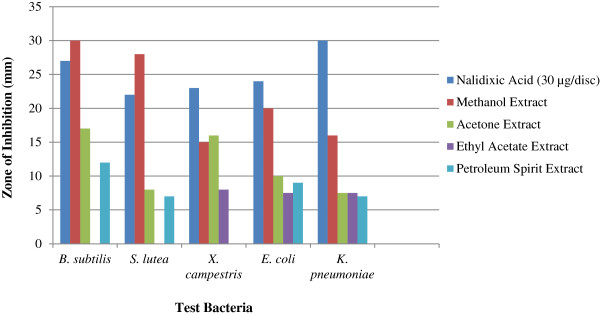
Antibacterial activity of different extracts of N. nouchali flower (4096 μgml-1).
Minimum inhibitory concentration (MIC)
The result of MIC assay is shown in Table 1. MIC values of various extracts were found between 128–2048 μgml-1. The best MIC value was 128 μgml-1 against B. subtilis by methanol extract producing 4.0 ± 0.00 mm zone of inhibition (Figure 2).
Table 1.
MIC values of various extracts of N. nouchali flower
|
Bacterial strains |
Methanol extract |
Acetone extract |
Ethyl acetate extract |
Petroleum spirit extract |
||||
|---|---|---|---|---|---|---|---|---|
| Concentration (μgml-1) | DIZ (mm) | Concentration (μgml-1) | DIZ (mm) | Concentration (μgml-1) | DIZ (mm) | Concentration (μgml-1) | DIZ (mm) | |
|
B. subtilis |
128 |
4.0 ± 0.00 |
256 |
3.5 ± 0.04 |
- |
- |
516 |
2.5 ± 0.01 |
|
S. lutea |
256 |
3.2 ± 0.00 |
1024 |
1.9 ± 0.03 |
- |
- |
1024 |
1.9 ± 0.03 |
|
X. campestris |
1024 |
2.0 ± 0.00 |
512 |
2.8 ± 0.05 |
1024 |
1.8 ± 0.13 |
- |
- |
|
E. coli |
512 |
3.5 ± 0.03 |
1024 |
2.3 ± 0.11 |
2048 |
2.0 ± 0.05 |
2048 |
1.2 ± 0.07 |
| K. pneumoniae | 1024 | 3.3 ± 0.00 | 2048 | 1.5 ± 0.07 | 1024 | 1.8 ± 0.00 | 2048 | 1.0 ± 0.00 |
DIZ = Diameter of Inhibition Zone.
Figure 2.
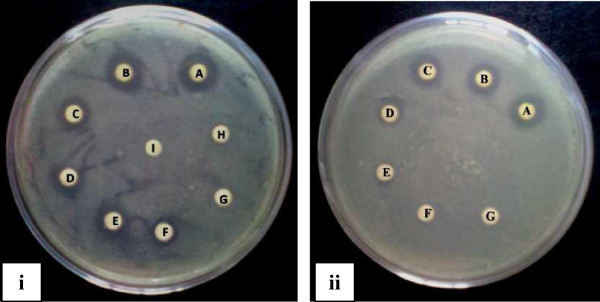
Methanol extract of N. nouchali flower produce zones of inhibition at different concentrations against (i) B. subtilis and (ii) S. lutea.
Concentration of disc A, B, C, D, E, F, G, H and I are 4096, 2048, 1024, 512, 256, 128, 64, 32 and 16 μgml-1 respectively.
Discussion
Infectious diseases caused by bacteria, fungi, viruses and parasites are a major threat to public health in developing countries due to unavailability and high cost of medicines [15,16]. This enabled us to evaluate N. nouchali flowers for its antimicrobial activity.
In this study methanol extract of N. nouchali flower showed better activity against both B. subtilis and S. lutea than nalidixic acid, a broad spectrum synthetic quinolone antibiotic, and it is mentionable that the antibacterial efficiency is 11% greater against B. subtilis as well as 27% greater against S. lutea than the antibiotic. The ability of plant extract to kill or inhibit pathogenic bacterial growth with great efficiency indicates the presence of some active compounds which have antibacterial activity. E. coli and K. pneumoniae, other two human pathogens also showed satisfactory sensitivity to methanol extract. B. subtilis was the most susceptible bacteria to all except the ethyl acetate extract. X. campestris, a pathogen for cabbage and cauliflower inhibited significantly by both methanol and acetone extracts; this suggests the effectiveness of this plant against plant pathogen. The MIC study also suggest that methanol and acetone extracts of N. nouchali flower could become an alternative to synthetic bactericides for using in pharmaceutical industry to control some pathogenic bacteria.
The antibacterial activity is believed to be due to the presence of secondary metabolites like alkaloids, tannins, steroids, phenol, saponins, flavonoids compounds, which are previously reported for their antimicrobial property [9-11]. It is therefore conceivable that these extract could be used against infections caused by these inhibited bacteria and the results showed a good correlation between the reported use of N. nouchali in traditional medicine against infectious diseases and in in vitro effectiveness. This study may not be adequate to suggest potential antibiotic agent considering the MIC value, and the zone of inhibition could be affected by the solubility and rate of diffusion in agar medium or its volatilization which could affect the results. However, this approach could be considered as preliminary step to find out promising candidates [1]. It is essential to do the quantitative analysis with the help of a chromatographer in gas phase and identify the metabolites responsible for antibacterial activity.
Conclusions
The outcome of present study suggests that some of the N. nouchali flower extracts possess compounds with high antibacterial properties that can be used as antibacterial agents in designing and developing new drugs. Further purification and characterization of the active compounds will provide a better understanding of the antibacterial mechanism.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BKD, MKS, KS and KH conducted laboratory analysis and drafted the manuscript. RI, NAB, SR and AHMJ participated in the design of the study and supervised the work. All authors were involved in the interpretation of the data and approved the final manuscript.
Contributor Information
Biplab Kumar Dash, Email: biplab.iu@gmail.com.
Monokesh Kumer Sen, Email: monokeshsen@yahoo.com.
Khasrul Alam, Email: manic.bge@gmail.com.
Kamal Hossain, Email: kamal.btge@gmail.com.
Rezuanul Islam, Email: rezwangbt@yahoo.com.
Nilufa Akhter Banu, Email: nab_bt_iu@yahoo.com.
Shahedur Rahman, Email: shahed.rajib@gmail.com.
Abu Hena Mostofa Jamal, Email: jamalbtg@gmail.com.
Acknowledgement
The authors are grateful to the Department of Biotechnology and Genetic Engineering, Faculty of Applied Science and Technology, Islamic University, Kushtia-7003, Bangladesh for funding this research work (grant number IUBT2012) and providing laboratory facilities.
References
- Rahman S, Parvez AK, Islam R, Khan MH. Antibacterial activity of natural spices on multiple drug resistant Escherichia coli isolated from drinking water, Bangladesh. Ann Clin Microbiol Antimicrob. 2011;10(1):10. doi: 10.1186/1476-0711-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abubacker M, Ramanathan R. Antibacterial Activities of Argemone mexicana L. (Papavaraceae) Leaf Extract on Pathogenic Bacterial Strains. Drug Invention Today. 2012;4(6):385–387. [Google Scholar]
- Londonkar RL, Madire Kattegouga U, Shivsharanappa K, Hanchinalmath JV. Phytochemical screening and in vitro antimicrobial activity of Typha angustifolia Linn leaves extract against pathogenic gram negative micro organisms. J Pharm Res. 2013;6(2):280–283. doi: 10.1016/j.jopr.2013.02.010. [DOI] [Google Scholar]
- Aliero A, Afolayan A. Antimicrobial activity of Solanum tomentosum. African J Biotechnol. 2006;5(4):369–372. [Google Scholar]
- Alam M, Karim M, Khan SN. Antibacterial activity of different organic extracts of Achyranthes aspera and Cassia alata. J Sci Res. 2009;1(2):393–398. [Google Scholar]
- Sikder M, Jisha H, Kuddus M, Rumi F, Kaisar MA, Rashid MA. Evaluation of Bioactivities of Nymphaea nouchali (Burm. f)-the National Flower of Bangladesh. Bang Pharm J. 2012;15:1–5. [Google Scholar]
- Raja MMM, Sethiya NK, Mishra S. A comprehensive review on Nymphaea stellata: A traditionally used bitter. J Adv Pharm Technol Res. 2010;1(3):311. doi: 10.4103/0110-5558.72424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandarkar MR, Khan A. Antihepatotoxic effect of Nymphaea stellata willd., against carbon tetrachloride-induced hepatic damage in albino rats. J Ethnopharmacol. 2004;91(1):61–64. doi: 10.1016/j.jep.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Murakami A, Ali AM, Mat-Salleh K, Koshimizu K, Ohigashi H. Screening for the in vitro anti-tumor-promoting activities of edible plants from Malaysia. Biosci Biotechnol Biochem. 2000;64(1):9–16. doi: 10.1271/bbb.64.9. [DOI] [PubMed] [Google Scholar]
- Rajagopal K, Sasikala K, Ragavan B. Hypoglycemic and antihyperglycemic activity of Nymphaea stellata flowers in normal and alloxan diabetic rats. Pharm Biol. 2008;46(9):654–659. doi: 10.1080/13880200802182554. [DOI] [Google Scholar]
- Sarma H, Sarma A, Sarma C. Traditional knowledge of weeds: a study of herbal medicines and vegetables used by the Assamese people [India] Herba Pol. 2008;54(2):80–88. [Google Scholar]
- Mamun-or-Rashid A, Islam MR, Dash BK. In vitro Antibacterial Effect of Bushy Matgrass (Lippia alba Mill.) Extracts. Res J Med Plant. 2012;6:334–340. doi: 10.3923/rjmp.2012.334.340. [DOI] [Google Scholar]
- Bauer A, Kirby W, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4):493. [PubMed] [Google Scholar]
- Jamal M, Rahman S, Islam A, Karim R, Alam S, Rahman Z. Minimum Inhibitory Concentration Analysis of Nerium oleander against Bacterial Pathogens. Asian Pacific J Tropical Biomed. 2012;2(3):S1664–S1666. doi: 10.1016/S2221-1691(12)60473-0. [DOI] [Google Scholar]
- Radfar M, Sudarshana M, Kavitha H, Satish S, Niranjan M. Evaluation of antibacterial and antifungal activity of root and root callus extracts of Trianthema decandra L. Afr J Biotechnol. 2012;11(2):510–515. [Google Scholar]
- Caldera A, Zarnic Z. Affordability of Pharmaceutical Drugs in Developing Countries. Working paper.


