Abstract
Introduction
The West and Central Africa (WCA) sub-region is the most populous region of sub-Saharan Africa (SSA), with an estimated population of 356 million living in 24 countries. The HIV epidemic in WCA appears to have distinct dynamics compared to the rest of SSA, being more concentrated among key populations such as female sex workers (FSWs), men who have sex with men (MSM), people who inject drugs (PWID) and clients of FSWs. To explore the epidemiology of HIV in the region, a systematic review of HIV literature among key populations in WCA was conducted since the onset of the HIV epidemic.
Methods
We searched the databases PubMed, CINAHL and others for peer-reviewed articles regarding FSWs, MSM and PWID in 24 countries with no date restriction. Inclusion criteria were sensitive and focused on inclusion of any HIV prevalence data among key populations. HIV prevalence was pooled, and in each country key themes were extracted from the literature.
Results
The search generated 885 titles, 214 abstracts and 122 full articles, of which 76 met inclusion and exclusion criteria providing HIV prevalence data. There were 60 articles characterizing the burden of disease among FSWs, eight for their clients, one for both, six for MSM and one for PWID. The pooled HIV prevalence among FSWs was 34.9% (n=14,388/41,270), among their clients was 7.3% (n=435/5986), among MSM was 17.7% (n=656/3714) and among PWID from one study in Nigeria was 3.8% (n=56/1459).
Conclusions
The disproportionate burden of HIV among FSWs appears to be consistent from the beginning of the HIV epidemic in WCA. While there are less data for other key populations such as clients of FSWs and MSM, the prevalence of HIV is higher among these men compared to other men in the region. There have been sporadic reports among PWID, but limited research on the burden of HIV among these men and women. These data affirm that the HIV epidemic in WCA appears to be far more concentrated among key populations than the epidemics in Southern and Eastern Africa. Evidence-based HIV prevention, treatment and care programmes in WCA should focus on engaging populations with the greatest burden of disease in the continuum of HIV care.
Keywords: men who have sex with men, sex work, people who inject drugs, HIV epidemiology, West Africa, Central Africa, prevalence, risk factors
Introduction
The sub-region of West and Central Africa (WCA) is the most populous of sub-Saharan Africa (SSA), with a combined population of roughly 356 million [1]. The region possesses a distinct cultural, economic and historical diversity. The majority of countries purport French as their national language, while English is the state language for four countries, and Spanish and Portuguese are both spoken within the region. Fifteen of the countries in WCA are classified by the World Bank Atlas method as low income (>US$1025), including Benin, Burkina Faso, Cape Verde, Central African Republic, Chad, the Democratic Republic of Congo (DRC), the Gambia, Guinea, Guinea-Bissau, Liberia, Mali, Mauritania, Niger, Sierra Leone and Togo [2]. Côte d'Ivoire, Cameroon, Ghana, Nigeria, the Republic of Congo, Senegal and São Tomé and Príncipe are categorized as low-middle income (US$1026 to US$4035) [2]. One country in the region is upper-middle income (Gabon), and one is ranked as a high-income country (Equatorial Guinea), mainly due to newly found oil reserves and a population under 1 million [2].
Historically and economically multifarious, the region has not been immune to the HIV epidemic. The first reported cases of HIV emerged in the mid-1980s, and national surveillance bodies such as National AIDS Committees (NACs) were established over the subsequent decade [3]. Early phylogenetic subtyping revealed unique regional dynamics, with both HIV-1 and HIV-2 circulating, and the majority of global cases of HIV-2 found in West Africa. Concurrently, the origins and greatest subtype diversity of HIV-1 were reported in Central Africa [4] (Figure 1).
Figure 1.
Map of West and Central Africa.
Nevertheless, regional epidemiological reporting has traditionally been immersed in the overall context of SSA. Trends in the HIV epidemic show that SSA possesses the highest burden of HIV, and 69% of the global population of people living with HIV reside within its borders [23.5 million (22.1–24.8 million)] [5, 6]. While these statistics show an important burden of disease on the continent, they mask disparities in HIV epidemics regionally [7]. Countries in East and South Africa report consistently generalized epidemics among reproductive-age adults (ages 15–49), which is defined through the Joint United Nations Programme on HIV/AIDS (UNAIDS) criteria as HIV prevalence consistently higher than 1% in antenatal clinics [8, 9]. Nine out of the 15 Southern African Development Community (SADC) members report national prevalence over 10% [5, 6, 10]. Reproductive-age adult estimates are as high as 25.9% in Swaziland and 24.8% in Botswana [11]. Comparatively, national prevalence in WCA has remained low or moderate since HIV surveillance reporting began, with current general-population estimates ranging from 0.02 to 4.5% [5, 6, 12]. Twelve countries in the sub-region report national prevalence under 2% [5]. Consequently, the majority of these countries’ HIV epidemics are classified as mixed, concentrated or borderline generalized [6, 12].
The international community has recently noted that classifications of the HIV epidemic based on prevalence data often limit understanding of the complexity of transmission and appropriate prevention strategies. However, concentrated epidemics have historically been defined as occurring in countries where HIV prevalence is consistently higher than 5% in at least one subgroup within the population, but less than 1% in antenatal clinics [7, 9]. These subgroups are generally considered to be female sex workers (FSWs), men who have sex with men (MSM) and people who inject drugs (PWID) [7, 13]. There is less clarity around mixed epidemics, although these are generally agreed to be low-level generalized epidemics ranging from 2 to 5% HIV prevalence in the general population, and high transmission rates in subgroups of the population [7]. Based on this, the HIV epidemics in countries in WCA are predominantly mixed or concentrated.
Researchers have suggested that the complexity of the regional dynamics in WCA has not been dissected adequately [12, 14–16]. Underlying drivers such as migration patterns, subtype diversity, significant regional variations of the disease and at-risk populations are understudied [11, 12, 16–19]. In an era where the global spread of HIV is on the decline, data are progressively emerging to show sustained or expanding transmission in populations at high-risk for HIV [15, 20–22]. However, national surveillance systems, particularly in low and middle-income countries, remain constructed on population-level studies such as the Demographic and Health Survey and antenatal care surveillance data [6, 13]. These methods provide a global overview of basic risk factors associated with transmission, but they do not capture data characterizing sex work and other transactional or compensated sex, same-sex practices and drug use outside of alcohol consumption, all of which are demonstrated high-risk factors and contributors to the acquisition and transmission of HIV [11, 21, 23].
Globally, surveillance shows that groups such as FSWs, their clients, MSM and PWID sustain a higher burden of disease in concentrated epidemics and substantially contribute to new infections annually [4, 7, 18, 22, 24]. In settings such as Southeast Asia and Latin America, general-population HIV prevalence remains similar to that of WCA, and a higher burden of disease is observed among key populations. For example, Pakistan and Indonesia report 25% and 35% prevalence among PWID, respectively [25]. Vietnam and Chile report an HIV prevalence rate of 15% and 20% among MSM, respectively [25, 26]. Myanmar (Burma) reports a prevalence of 10% among FSWs, and Brazil reports 4.9% [25, 26]. All of these reported levels are roughly five to thirty times higher than general-population prevalence in the specific countries listed [25, 26]. National-level responses on these continents have included programmes for key populations, and noteworthy advances in the reduction of new infections have been reported over time [27, 28]. In contrast, WCA reports partial or sporadic data for key populations and limited government-level policies defining key population treatment and prevention needs [5]. National surveillance and programming in WCA subsequently remain rooted in broad HIV prevention messaging and approaches similar to those seen across East and South Africa such as prevention of mother-to-child transmission (PMTCT) and non-targeted community-based behaviour change programmes [5, 7].
Lessons learned from other contexts such as Southeast Asia and Latin America, where limited prevalence of HIV among average-risk reproductive-age adults also exists, require us to examine the epidemiology of the HIV epidemic in WCA [11, 29]. This systematic review aims to complete a historic, situational and epidemiological analysis of the burden of disease among key populations in 24 countries located in WCA.
Methods
The US National Library of Medicine's MEDLINE database, one of the most comprehensive sources of healthcare information in the world, was searched using the PubMED interface to obtain biomedical markers for any of the three key populations: FSWs, MSM or PWID. The study objectives specified the need for epidemiologic studies that report biological endpoints (HIV prevalence) with defined methods; thus, it was decided a priori that MEDLINE would be sufficient. However, a sensitivity assessment was employed using the same search strategy to explore EBSCOhost CINAHL Plus, PsycINFO, Ovid, SocioFile and Popline, and no additional data points were obtained which met the defined inclusion and exclusion criteria. Google and Google Scholar were searched for contextual information and non-peer-reviewed literature. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRIMSA) guidelines were referenced for the development of the search protocol and study reporting structure [30, 31].
The medical subject headings (MeSH terms) for HIV and AIDS and key terms relating to “sex work,” “men who have sex with men” and “intravenous drug use” were cross-referenced with terms associated with 16 West African countries: the 15 countries of the Economic Community of West African States (ECOWAS: Benin, Burkina Faso, Cape Verde, Gambia, Ghana, Guinea (Conakry), Guinea-Bissau, Cote d'Ivoire, Liberia, Mali, Niger, Nigeria, Senegal, Sierra Leone and Togo) plus Mauritania. Eight Central African countries were included in the search: those in the Economic Community of Central African States (CEMAC: Cameroon, Chad, Equatorial Guinea, Central African Republic, Republic of Congo and Gabon), the Democratic Republic of Congo (DRC) and São Tomé and Príncipe. The search protocol was developed based on the objectives of this study and can be accessed as a Supplementary file with this manuscript.
The inclusion criteria for this study included reported HIV prevalence data for any of the three key populations, as well as clients of FSWs, in any of the 24 countries defined for this review. Publications were included if prevalence was listed in the article with sample size and sampling and HIV-testing methods described, regardless of the overall aim or topic of the study. Date of publication was not used as an inclusion criterion. Exclusion criteria included manuscripts not published in French, English or Spanish. Articles were downloaded and organized using Endnote (version X5), and data collection was finalized in April 2013.
Screening and data abstraction
A title and abstract search protocol was utilized based on previously validated methods for systematic reviews [32]. At each step in the search protocol, the titles, abstracts and available data were appraised by two independent reviewers (LA and EP), and compiled and synthesized using standardized forms. During the title and abstract reviews, if either of the two reviewers considered the article relevant, it was included. Articles classified as relevant at the title review stage were downloaded for abstract and full-text evaluation. Data were independently extracted by two reviewers (LA and EP), then compared and consolidated for analysis.
Data, including sampling methods, HIV-1, HIV-2 and dual HIV-1 and -2 (HIV-1/2) infections with sample size and number of participants living with HIV, were detailed and coded by the two independent reviewers (LA and EP). Information was categorized by key population studied, sampling techniques, country or countries, sample size, number of study participants living with HIV and notes. Discrepancies in abstracted data from the two reviewers were assessed by a third reviewer independently evaluating the article (SB), as was the final consolidated database (CH).
Results
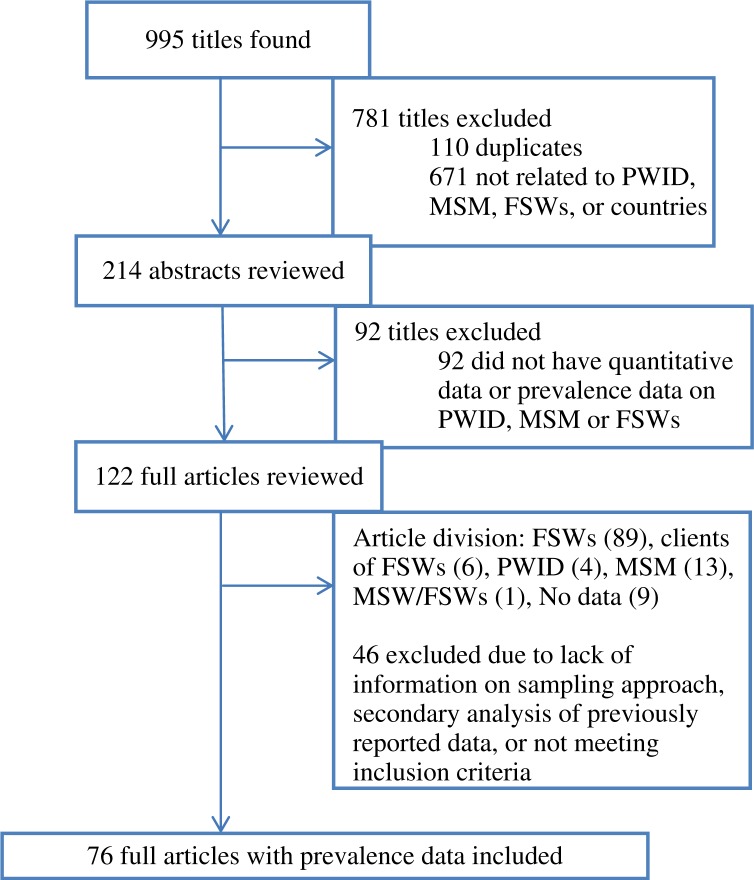
Our search generated 995 citations, including 885 unique titles with dates of publication from 1987 to 2013 (Figure 2). Based on the inclusion criteria, 122 full articles were reviewed for data extraction, and 76 of these contained relevant data for at least one of the key populations defined. HIV prevalence data for at least one key population existed in 13 of the 24 countries included in the search (54.2%). Eleven of these countries were located in West Africa, and two countries were in Central Africa (DRC and Cameroon).
Figure 2.
Flow chart of search findings and included studies.
The majority of publications were assessments regarding FSWs (78.9%, 60/76), and another 10.5% (8/76) provided HIV prevalence data for their clients. One publication provided prevalence data for FSWs and well as clients of FSWs in Togo [33]. Thus, 90.8% (69/76) of the publications included in this study were related to FSWs, representing 41,270 FSWs across 13 countries and 5,986 clients of FSWs across 6 countries.
Two countries (Senegal and Nigeria) had published HIV prevalence data among MSM, and one seroprevalence study was conducted among male sex workers (MSWs) in Côte d'Ivoire, which was included in the MSM pooled data for analysis [34]. A total of six publications combined for the three countries were found for MSM (7.9%, 6/76), and one publication was available with HIV prevalence data for PWID, totalling 3,714 MSM from three countries and 1,459 PWID represented in the region.
Results presented in Table 1 show a pooled HIV prevalence for the relevant key population(s) in each country, the 95% confidence interval (CI), and the date(s) of the publications retrieved per country. We include both HIV-1 and HIV-2 infections in the pooled prevalence data for the country, and, when possible, we display the division of HIV-1, HIV-2 and HIV-1/2 infections. The far-left data column in Table 1 displays the overall HIV prevalence among reproductive-age adults (15–49) per country as reported by UNAIDS’ most recent country-level surveillance data [6].
Table 1.
Pooled prevalence data for female sex workers (FSWs), clients of FSWs, MSM and PWID per country
| Country | Year of publication(s) | Key population | Pooled HIV prevalence % (95% confidence interval) | Pooled HIV prevalence (sample size N=) | HIV-1 prevalence % (sample size N=)* | HIV-2 prevalence % (sample size N=) | HIV 1 and 2 prevalence % (Sample size N=) | HIV prevalence % Among adults 15–49** | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Benin | 1992, 1997, 2001, 2002, 2007, 2009, 2012 | FSWs | 45.8 (44.2–47.4) | 3,885 | 41.8 (N=498) | 3.2 (N=498) | 11.2 (N=498) | 1.1 | [35–41] |
| 2000, 2007 | Clients | 6.7 (5.6–7.8) | 1,996 | [42,43] | |||||
| Burkina Faso | 1998, 2002 | FSWs | 45.8 (42.5–49.1) | 873 | 1.0 | [44,45] | |||
| Cameroon | 1991, 1995, 1998, 1998, 2001, 2009 | FSWs | 23.6 (22.4–24. 8) | 4,679 | 22.9 (N=2260) | 0.04 (N=2260) | 4.5 | [41,46–50] | |
| Cote d'Ivoire | 1987, 1988, 1992, 1995, 1995, 1997, 1998, 2000, 2002, 2012 | FSWs | 57.3 (56.1–58.5) | 7,014 | 40.0 (N=5204) | 2.7 (N=5204) | 21.1 (N=5204) | 3.2 | [17,29,66–73] |
| 2003 | Clients | 13.5 (10.2–16.8) | 423 | [74] | |||||
| 2012 | Male sex workers | 50.0 (40.0–60.0) | 96 | [34] | |||||
| DRC | 1988, 1988, 1991, 1998, 2007 | FSWs | 26.3 (24.6–28.0) | 2,518 | 1.1 | [51–55] | |||
| Gambia | 1991, 1991, 1993 | FSWs | 28.5 (25.0–32.0) | 627 | 1.3 (N=627) | 25.2 (N=627) | 2.1 (N=627) | 1.3 | [56–58] |
| 1992 | Clients | 6.1 (4.1–8.1) | 558 | [59] | |||||
| Ghana | 2000, 2001, 2012 | FSWs | 60.4 (58.3–62.6) | 1,982 | 46.7 (N=1348) | 2.2 (N=1348) | 6.7 (N=1348) | 1.4 | [40,60,61] |
| 2004 | Clients | 12.3 (9.4–15.2) | 497 | [62] | |||||
| Guinea | 2010, 2010, 2011 | FSWs | 36.9 (34.5–39.3) | 1,577 | 1.7 | [63–65] | |||
| Mali | 1988, 1998 | FSWs | 42.1 (37.3–46.9) | 406 | 35.8 (N=176) | 3.9 (N=176) | 6.2 (N=176) | 0.9 | [75, 76] |
| Niger | 1994, 1998, 2006, 2006 | FSWs | 31.2 (28.4–34.1) | 1,017 | 29.2 (N=767) | 0.9 (N=529) | 2.0 (N=767) | 0.5 | [77–80] |
| Nigeria | 1989, 1993, 1993, 1993, 1998, 2002, 2008, 2011, 2012, 2012, 2013 | FSWs | 24.3 (23.5–25.1) | 10,769 | 13.5 (N=2291) | 1.9 (N=2041) | 1.8 (N=610) | 3.2 | [81–91] |
| 2013 | PWID | 3.8 (2.8–4.8) | 1,459 | [92] | |||||
| 2011, 2012, 2013 | MSM | 15.1 (13.7–16.5) | 2,676 | [93–95] | |||||
| Senegal | 1992, 1996, 1997, 2003, 2007, 2009 | FSWs | 19.0 (17.9–20.1) | 4,612 | 7.6 (N=4008) | 10.1 (N=4008) | 1.1 (N=4008) | 0.5 | [96–101] |
| 1997, 2003 | Clients | 4.6 (3.6–5.7) | 1,515 | [102,103] | |||||
| 2005, 2009, 2010 | MSM | 21.7 (19.1–24.3) | 942 | 18.1 (N=442) | 0.5 (N=442) | 2.9 (N=442) | [104,105] | ||
| Togo | 2009 | FSWs | 36.2 (33.6–38.8) | 1,311 | 2.9 | [18] | |||
| 2009 | Clients | 7.9 (6.2–9.6) | 997 | [18] |
Where available, the distribution of HIV1, HIV2 and dual HIV1/2 infections in the available study or pooled per country is listed.
UNAIDS country prevalence data 2012 (6).
Female sex workers and their clients
Behavioural and seroprevalence studies in FSWs were conducted consistently over time; however, there was a significant lull in published data between 2002 and 2007. When pooled, the overall HIV prevalence for FSWs in WCA was 34.9% (95% CI 34.4–35.4) (Table 2). In the five countries with six or more publications, pooled HIV prevalence was high: 57.3% (N=7,014) in Côte d'Ivoire, 24.3% (N=10,769) in Nigeria, 45.8% (N=3,885) in Benin, 23.6% (N=4,679) in Cameroon and 19.0% (N =4,612) in Senegal. The pooled prevalence found among clients of FSWs was 7.3% (95% CI 6.6–8.0) (Table 2). Six countries had at minimum of one study reporting prevalence data for this demographic, with publications as early as 1992 and as late as 2009 (Table 1).
Table 2.
Pooled HIV prevalence data for female sex workers, clients of FSWs, MSM and PWID in West and Central Africa
| Key population | Pooled HIV prevalence (%) | 95% Confidence interval (%) | Pooled sample size, N= | n=Living with HIV |
|---|---|---|---|---|
| Female sex workers (FSWs) | 34.9 | 34.4–35.4 | 41,270 | 14,388 |
| Men who have sex with men (MSM) | 17.7 | 16.5–18.9 | 3,714 | 656 |
| People who inject drugs (PWID) | 3.8 | 2.8–4.8 | 1,459 | 56 |
| Clients of FSWs | 7.3 | 6.6–8.0 | 5,986 | 435 |
Men who have sex with men
While this review revealed a paucity of data for MSM, the pooled HIV prevalence in this review was 17.7% (95% CI 16.5–18.9) for MSM in WCA (Table 2). No studies included were published earlier than 2005, and all but one were published after 2010. Three relevant Nigerian studies showed a pooled prevalence of 15.1% compared to 3.2% in adults of reproductive age [6, 93–95, 106]. Senegal's pooled prevalence was 21.7% compared to 0.5% in the adults of reproductive age [6, 104, 105, 107]. The study conducted in Côte d'Ivoire among MSWs reported 50.0% prevalence among a sample of 96 men in Abidjan [34]. Snowball, convenience, purposive and respondent-driven sampling were the primary recruitment methods used to obtain these data.
People who inject drugs
One study included directly sampled PWID. The study found a slightly higher prevalence of HIV at 3.8% (95% CI 2.8–4.8), compared to 3.2% in the general population in Nigeria [6, 92]. The sample was recruited through respondent-driven sampling and mainly compromised of men (>90%) [6, 92].
Limitations
This study was conducted as a systematic review to understand the prevalence of key populations in WCA and compare historical HIV prevalence to general-population statistics. Data were obtained from peer-reviewed literature, and while this ensures some quality control, we acknowledge that some relevant data that exist in grey literature and other programmatic data may have been overlooked. Programmatic data were not included in this review as it was not possible to implement a standardized assessment of the quality of the methods used and to ascertain an overview of research sampling and testing methods. However, the grey literature obtained through this review played a key role in the contextual analysis and discussion section of this study. Certain limitations also include the use of only English, French and Spanish, as other publications in other languages may have relevant data not captured in these inclusion criteria. The study among MSWs from Côte d'Ivoire was included in the overall analysis; however, the sampling method directly recruited these individuals from an established sex worker clinic, and thus HIV prevalence may be overestimated in this subpopulation. Also, while the authors noted that the majority of MSWs in the Abidjan area were MSM, they did not collect data on types of partner [34]. The contextual description from the authors is supported by evidence from other contexts where partners of MSWs are male [108, 109]. Concurrently, systematic review methods were applied; however, sensitivity analysis and meta-analyses were not utilized. While odds ratios or aggregated comparison data were not generated, the overall analysis provides an overview of HIV prevalence among key populations and details of the epidemiology of key populations since the debut of HIV research in this region.
Discussion
Epidemiologic literature over the past 30 years has demonstrated a consistent and disproportionate burden of HIV among key populations in WCA. From the first published study in 1987 to the most recent in 2013, elevated levels of HIV among FSWs and their clients were consistently reported. In recent years, studies emerged to display an elevated burden of HIV among MSM within the region, although the number of studies in this subpopulation remains limited. Concurrently, there is nascent but growing evidence of the existence of PWID and, consequently, HIV infections in this subpopulation [92].
HIV prevalence
The elevated HIV prevalence among MSM, FSWs and clients of FSWs is important based on the determinants of the HIV epidemic in WCA and even more broadly across SSA. Surveillance has shown that women carry the highest burden of HIV on the continent, with national-level statistics constantly reporting that women have a higher HIV prevalence and incidence than men [13, 110]. While programmes are designed to address the various risks associated with female HIV acquisition, the results of this study demonstrate that HIV risks are significantly higher among FSWs than women who do not sell sex in WCA. These results are substantiated by a systematic review of FSWs in low and middle-income countries, which showed that FSWs in SSA have a pooled prevalence of 36.9% (95% CI 36.2–37.5) with a background prevalence on the continent of 7.42% in females [15]. Globally, FSWs were 13.5 (95% CI 10.0–18.1) times more likely to be living with HIV than women of reproductive age [15]. Thus, the results of this review and the epidemiology of HIV among FSWs worldwide suggest that inclusion of and significant focus on these women and their clients are of importance to address these populations’ high HIV acquisition and transmission risks in WCA [11, 72, 81, 84, 96].
On a continent where women are disproportionately burdened with HIV, prevalence of 17.7% (95% CI 16.5–18.9) among MSM demonstrates a potentially concentrated epidemic in this key population. A prevalence of 7.3% (95% CI 6.6–8.0) in clients of FSWs is also elevated compared to the general male population of the region and calls into question prevention programmes targeting this population. For clients of FSWs, male acquisition is linked to behavioural risks associated with multiple sexual partners, limited condom use and concomitant infection of an STI, amongst other determinants that are specific to men who engage in transactional sex [16, 74, 111]. For MSM, recent research has emerged that displays the increased transmission of HIV during anal sex, as well as sexual role versatility during same-sex practices that increases individual HIV risks and drives transmission within sexual networks of MSM [21]. Thus, the acknowledgement of a heightened burden of disease in these populations is important for the design and implementation of specialized HIV prevention, treatment and care programmes regionally [16, 26].
The heightened HIV prevalence in the MSM community found in these results is not unexpected, although the lack of data in WCA is noteworthy. The high prevalence reported in this review is comparable to other continents, with research indicating that MSM around the world are 19 times more likely to be infected with HIV than their adult male counterparts [18]. Interestingly, same-sex practices in WCA were reported as early as 1996 in a published population-based review [112]. The authors noted that the cumulative number of positive cases had exponentially increased from 1985 to 1995, and the primary modes of transmission were heterosexual practices (73.0%), homosexual practices (0.8%) and mother-to-child transmission (6.0%) [112]. More recent behavioural studies equally noted homosexual behaviour in different demographic studies. In Nigeria, 11.4% of sexually active secondary school students reported same-sex practices, and 12.4% reported anal sex [113]. In two Ghanaian studies in 2006 and 2008, prison inmates reported same-sex practices or identified as homosexual at 30.8% and 29.5%, respectively [114, 115]. While sporadic reports of same-sex practices and elevated HIV prevalence have been reported in the region, there is limited targeted programme activity for these men [5, 116, 117]. What does exist is limited in scale, based on community-driven initiatives, and functioning in highly stigmatized settings [33, 117, 118].
While HIV prevalence in PWID was found to be relatively low, the Nigerian study provides two important details for programming in WCA. Firstly, while it has generally been assumed that PWID constitute a minimal presence in WCA, the study's ability to generate a sample size of 1459 through respondent-driven sampling indicates that this population does exist. Secondly, while HIV prevalence appears low, we know from other contexts that once HIV is introduced into this specific subpopulation, the possibilities of rapid spread and sustained transmission are great [119, 120]. Contextually, policy makers are becoming aware of an increase of drug trafficking in the region, with large quantities of drugs confiscated in the past few years, and the recent conflict in Mali ascribed mainly to this trade [121]. Further supporting evidence of this regional trade was found in behavioural data in prisoners. In the same Ghanaian study in 2006, 41% of inmates reported imprisonment for narcotics; 7.3% had used cocaine, 5.2% heroin and 4.2% phencyclidine [114]. In the 2008 Ghanaian prison study, 35% of 1336 prisoners reported ever injecting drugs [115]. As was seen in Afghanistan as well as Thailand, Cambodia and other Southeast Asian countries, migration, trafficking, drug use and the HIV epidemic are intrinsically linked [119, 120, 122]. Thus, this is an important population to identify and appropriately engage in WCA in the coming decade of HIV prevention and control.
Historical perspective
This review also indicates that knowledge of HIV prevalence among key populations and the proportion of HIV infections attributable to key populations in WCA are not representative of new or changing dynamics of HIV transmission. In 1995, Djomand et al. noted that the male:female ratio of HIV infection in Côte d'Ivoire had declined over time and the gender ratio had shown females to be 4.8 times more likely to be infected than men in 1988, compared to 1.9 times more likely in 1991 [20]. The authors asserted that this decline displayed that the HIV epidemic was initially concentrated in a core group of FSWs and their male partners, and was potentially expanding in broader populations with less identifiable risk factors, similar to dynamics observed in other regions outside of SSA [20, 122–124]. In 2004, Côté et al. conducted a study of adult males (15–59) in Accra, Ghana, and attributed 84% of existing cases of HIV to sex work and other transactional sex [125]. A study in 2008 based on Demographic and Health Surveys across four countries in SSA, including Ghana, showed that men who ever paid for sex were more likely to have HIV than men who had not (odds ratio 1.89, 95% CI 1.57–2.28) [126].
In the capital city of Lomé, Togo, researchers estimated the attributable fraction of current HIV cases to sex work and other transactional sex was 32%, in contrast to only 2% of cases outside of Lomé [18]. Finally, recently in Nigeria, a modes of transmission study asserted that 23% of HIV infection was attributable to key populations, including 10% of new infections amongst MSM [93]. Despite high HIV prevalence among key populations and a high number of HIV in 2009, cases attributable to behaviours such as sex between men and sex work, systematic prevention and treatment programmes for key populations have not been implemented regionally [5]. While prevention programmes for FSWs and their clients have been noted in countries including Ghana, Côte d'Ivoire, Nigeria and Cameroon, the appropriate scale of these programmes and collected surveillance data are limited, and HIV prevention, treatment and care programming for key populations has failed to become a standard of best practices in the region [5].
Economic and regional migration
Underlying dynamics of the epidemic indicate external, economic and urban-centred disparities have contributed to the complexity of the HIV epidemic in WCA over time. Domestic and international migration patterns were repeatedly reported and significantly mirrored economic crises and fluctuations in specific countries. For example, a study in Côte d'Ivoire documenting the FSW population that accessed health clinics between 1991 and 1998 noted a major shift in country of origin over time, with Nigerian women surveyed increasing from 2 to 56% between 1992 and 1998, and Ghanaian women decreasing from 82 to 9% in the same time period [29]. Other studies reported the migration of Ghanaian FSWs to other countries in the 1990s and asserted that the significant economic and political crises in the country at that time contributed to this migration [3, 35]. The proportion of Liberian FSWs included in the same Ivorian study was shown to have increased from 0% in 1992 to 15% in 1995, and then to have declined to 2% in 1998 [94]. This evolution reflects the first internal conflict experienced in Liberia in the 1990s (1989–1996) [127, 128]. In a study reviewing the spread of HIV among FSWs in four cities across SSA, researchers noted that Cameroonian FSWs were more likely to have migrated internally to urban centres, while in Benin 86% of the FSWs sampled were from another country [41]. The only MSM study to discuss countries of origin was the MSW study in Côte d'Ivoire. Of the 96 MSWs sampled in Abidjan, 7.3% (7/96) reported a different country of origin [34].
The importance of these findings is revealed in the HIV prevalence among immigrants in the various studies. Nigerian and Ghanaian FSWs in the 2002 Côte d'Ivoire study were 1.03 (0.47–2.23) and 3.69 (2.28–5.97) times more likely to be infected than their counterparts from Côte d'Ivoire, Liberia and other West African countries [106]. In Lomé, two-thirds of FSWs were immigrants, and Ghanaian FSWs were 1.68 (1.06–2.66) times more likely to be living with HIV [126]. Addressing the needs of migrating populations at risk for or living with HIV is crucial, as these populations have less access to health services, are less likely to understand their human rights, and are more likely to contract a disease [129]. These populations are also more likely to be mobile; thus, successful prevention services for immigrant or mobile FSWs could potentially have an important impact in the overall reduction of HIV transmission and acquisition in the region [129].
Concurrently, disparity of HIV prevalence per locality was repeatedly reported in the various studies reviewed. In the same study that cited higher HIV levels among Ghanaian FSWs in Lomé, the prevalence among Lomé FSWs in 2005 was reported at 45.4% compared to 17.7% in the rest of Togo [18]. In two studies in Benin, there was significant spatial variation in the burden of HIV. For example, a study conducted in six cities in 2005 showed prevalence for HIV as high as 48.2% in Parakou, compared to 16.4% in Abomey/Bohicon [36]. A similar study found HIV prevalence in Cotonou, Benin, among FSWs to be 38.5%, compared to a pooled prevalence in three other large cities of the country of 58.9% [35]. Therefore, from an HIV prevention perspective, cross-border initiatives, effective community-based networking and standardized programmes across urban and regional landscapes for key populations are relevant for the WCA region.
Ways forward
Our review makes clear that there is a significant gap in the literature and subsequent HIV programmes for key populations in WCA. This may be ascribed to the application of the HIV response model of SSA to WCA epidemiological and prevention approaches. However, as reports of high HIV prevalence among key populations have existed in the literature since 1987, it also calls into question the structural barriers to healthcare for populations that engage in these defined sexual behaviours in this region. As in other contexts, sex work and other transactional sex, same-sex practice, and drug use are either criminalized or highly stigmatized in this region, and public policies have ignored or generally declined to address the specific health needs of key populations [5, 130, 131]. Research has shown that macro-level policies that impede or deter health service delivery for key populations ultimately increase vulnerability to disease acquisition [23, 130, 132].
Data presented here provide a useful framework for HIV programming in the region. The inclusion of relevant sexual history and behavioural questions in large-scale surveillance surveys, such as DHS, may also be of benefit in obtaining a better overview of the epidemiology of key populations, both in WCA and worldwide. While the delivery of sensitive questions such as engagement in sex work, transactional sex, same-sex practices and drug use must be carefully administered (ideally not within the household setting), standardized national data collection would go far to inform country and regional policy development in WCA.
Subsequently, emerging data have shown that addressing the epidemic in key populations requires combined behavioural, biomedical and structural approaches [23, 133]. Limited condom use with regular sexual partners, unawareness of HIV status and co-infections with genital ulcerative diseases are contributing factors to heightened prevalence [10, 21, 116]. High prevalence among key populations concurrently has implications for prioritized biomedical interventions [21, 134].
While the knowledge that these populations have a higher risk for transmission and acquisition of HIV and other STIs is acknowledged, the method in which prevention and treatment programmes address these risks has yet to be firmly cemented in HIV prevention programming [13]. Researchers in the United States and elsewhere have demonstrated the importance of engaging populations in the continuum of HIV care – from undiagnosed cases to testing and diagnosis, followed by linkage to ongoing care and treatment [135]. The continuum of HIV care significantly reduces the viral load among people living with HIV and ultimately reduces transmission [135, 136]. In two recent studies in the United States, researchers found that due to advances in antiretroviral regimes, with 70–80% adherence to antiretroviral therapy (ART) by participants, durable viral suppression occurred in most individuals, lowering the possibility for onward HIV transmission [136, 137]. The findings indicate that the key to community viral suppression is early diagnosis of the disease, well-developed referral systems to clinical services, and care and support programmes that encourage adherence and access to treatment [136]. This approach has been shown to be effective in contexts with both high and low prevalence, and recent research from South Africa affirms that adequate ART coverage at the community level reduces incidence over time [138]. Thus, prevention programmes are beginning to show that distribution of prevention commodities and messages should be in concert with interventions that address the virology and biomedical aspects of care and treatment [135]. This is even more relevant for key populations who carry a significant burden of disease and ultimately are people living with HIV.
Structural factors acting at the macro- and meso-levels should not be ignored in WCA and are essential when building combination biomedical programmes [23, 139]. Criminalization and public policy neglect substantially inhibit key populations’ ability to access appropriate, life-sustaining and prevention-oriented health services. Policy-level gaps and community-level stigma must be addressed if programmes are to adequately confront the needs of these populations [140, 141]. Studies from other countries on the continent indicate the stigma experienced within their communities and at health services, significantly deters the uptake at clinical services for key populations [130, 142]. Public policies that adequately address the intricate health needs, reduce stigma and discrimination, and facilitate community and provider level HIV care and treatment delivery will highly benefit the overall control and prevention of HIV among key populations in WCA [23].
Conclusions
This systematic review suggests that the concentrated HIV epidemic in WCA more closely resembles the epidemics in Southeast Asia and Latin America than those in the rest of SSA. This not only calls into question the response to the HIV epidemic in WCA but indicates that the region has an opportunity to adapt and develop region-specific prevention and treatment strategies. Targeted, cost-effective programmes that address not only behavioural but also biological and structural risk factors associated with HIV acquisition and transmission key populations should be engaged to reduce the onward spread of HIV. Prevention programmes should model strategies on appropriate programmes that reduce community viral loads, increase uptake of treatment among key populations and address the barriers to healthcare that exist in highly stigmatized settings. Ensuring that programmes rooted in community-based approaches address the continuum of HIV care, from diagnosis to viral suppression, will be a challenge but also a possible victory for HIV prevention and control in WCA.
Acknowledgements
The authors acknowledge all participants involved in the studies included in this systematic review. Their participation in research assists us in developing wide-range and appropriate evidence-based public health programmes. We are also grateful to the researchers of these studies for their dedication and efforts to ensure that their findings are in the public sphere.
Funding
The authors thank and acknowledge The USAID∣Project SEARCH Task Order No.2, funded by the US Agency for International Development under Contract No. GHH-I-00-07-00032-00, for continued staff support and encouragement in the development of this review. The Foundation for AIDS Research (amfAR) has equally been a strong supporter of the Key Population Program under the Center for Public Health and Human Rights at Johns Hopkins University, and we continue to be thankful for their collaboration.
To access the supplementary material to this article please see Supplementary Files under Article Tools online.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
EP and SB developed the conceptual framework and conducted the overall analysis of the study. SB provided guidance on research methods and sampling approach and EP led the execution of these methods. EP and LA reviewed and extracted data from all studies obtained. EP led the writing of the manuscript. CH, LA and EP consolidated the databases and tables and CH and EP reviewed the final databases versions. SB, AG, OK-Z, MT-N, NC, DD and FD all provided expert review and contextual analysis for the region of WCA and were instrumental in the interpretations of the results.
References
- 1.Bank TW. GPE Open Data Project 2012. Washington, DC: World Bank; 2012. Population estimates 2008–2012. [Google Scholar]
- 2.Bank TW. GPE Open Data Project. Washington, DC: World Bank; 2013. Economic assessment of lending groups, world atlas method. [Google Scholar]
- 3.Agyei-Mensah S. Twelve years of HIV/AIDS in Ghana: puzzles of interpretation. Can J Afr Stud. 2001;35(3):441–72. doi: 10.1080/00083968.2001.10751229. [DOI] [PubMed] [Google Scholar]
- 4.Gisselquist D. Emergence of the HIV type 1 epidemic in the twentieth century: comparing hypotheses to evidence. AIDS Res Hum Retroviruses. 2003;19(12):1071–8. doi: 10.1089/088922203771881158. [DOI] [PubMed] [Google Scholar]
- 5.UNAIDS. West and Central Africa: towards universal access to prevention, care and treatment; Dakar, Senegal: UNAIDS/0829E/JC1585E; 2008. [Google Scholar]
- 6.UNAIDS. Report on the global AIDS epidemic; Geneva: United Nations; 2013. [Google Scholar]
- 7.Wilson D, Halperin DT. “Know your epidemic, know your response”: a useful approach, if we get it right. Lancet. 2008;372(9637):423–6. doi: 10.1016/S0140-6736(08)60883-1. [DOI] [PubMed] [Google Scholar]
- 8.UNAIDS. Practical guidelines for intensifying HIV prevention: towards universal access [Internet] [cited 2013 May 1]. Available from: http://dataunaidsorg/pub/Manual/2007/20070306_2007.;Prevention_Guidelines_Towards_Universal_Access_en.pdf.
- 9.Unaids W. Guidelines for second generation HIV surveillance; Geneva: WHO; 2000. [Google Scholar]
- 10.Espirito Santo ME, Etheredge GD. How to reach clients of female sex workers: a survey by surprise in brothels in Dakar, Senegal. Bull World Health Organ. 2002;80(9):709–13. [PMC free article] [PubMed] [Google Scholar]
- 11.Unaids. Sub-Saharan Africa: AIDS epidemic update regional summary; Geneva: UNAIDS; 2008. [Google Scholar]
- 12.Lowndes CM, Alary M, Belleau M, Kofi Bosu W, Federic Kintin D, Asonye Nnorom, et al. West Africa HIV/AIDS epidemiology and response synthesis: characterisation of the HIV epidemic and reponse in West Africa: implications for prevention; Washington, DC: World Bank; 2008. [Google Scholar]
- 13.Baral S, Phaswana-Mafuya N. Rewriting the narrative of the epidemiology of HIV in sub-Saharan Africa. SAHARA J. 2012;9(3):127–30. doi: 10.1080/17290376.2012.743787. [DOI] [PubMed] [Google Scholar]
- 14.Drame FM, Peitzmeier S, Lopes M, Ndaw M, Sow A, Diouf D, et al. Gay men and other men who have sex with men in West Africa: evidence from the field. Cult Health Sex. 2013;15(Suppl):7–21. doi: 10.1080/13691058.2012.748935. [DOI] [PubMed] [Google Scholar]
- 15.Baral S, Beyrer C, Muessig K, Poteat T, Wirtz AL, Decker MR, et al. Burden of HIV among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(7):538–49. doi: 10.1016/S1473-3099(12)70066-X. [DOI] [PubMed] [Google Scholar]
- 16.Lowndes CM, Alary M, Meda H, Gnintoungbe CA, Mukenge-Tshibaka L, Adjovi C, et al. Role of core and bridging groups in the transmission dynamics of HIV and STIs in Cotonou, Benin, West Africa. Sex Transm Infect. 2002;78(Suppl 1):i69–77. doi: 10.1136/sti.78.suppl_1.i69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Djomand G, Greenberg AE, Sassan-Morokro M, Tossou O, Diallo MO, Ekpini E, et al. The epidemic of HIV/AIDS in Abidjan, Cote d'Ivoire: a review of data collected by Projet RETRO-CI from 1987 to 1993. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10(3):358–65. [PubMed] [Google Scholar]
- 18.Sobela F, Pepin J, Gbeleou S, Banla AK, Pitche VP, Adom W, et al. A tale of two countries: HIV among core groups in Togo. J Acquir Immune Defic Syndr. 2009;51(2):216–23. doi: 10.1097/QAI.0b013e31819c170f. [DOI] [PubMed] [Google Scholar]
- 19.Scott Kellerman M, Holtz S, Dutta A, Aliou S, Diallo I, Redding S, et al. The epidemiology of HIV epidemics in the 21-country West and Central Africa region: the impact of most at risk populations (MARPs); USAID AWARE II project; Washington, DC: World Bank; 2012. [Google Scholar]
- 20.Baral S, Sifakis F, Cleghorn F, Beyrer C. Elevated risk for HIV infection among men who have sex with men in low- and middle-income countries 2000–2006: a systematic review. PLoS Med. 2007;4(12):e339. doi: 10.1371/journal.pmed.0040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380(9839):367–77. doi: 10.1016/S0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathers BM, Degenhardt L, Ali H, Wiessing L, Hickman M, Mattick R. HIV prevention, treatment and care for people who inject drugs: a systematic review of global, regional and country level coverage. Lancet. 2010;385(9719):1014–28. doi: 10.1016/S0140-6736(10)60232-2. [DOI] [PubMed] [Google Scholar]
- 23.Baral S, Logie CH, Grosso A, Wirtz A, Beyrer C. Modified social ecological model: a tool to guide the assessment of the risks and risk contexts of HIV epidemics. BMC Public Health. 2013;13:482. doi: 10.1186/1471-2458-13-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson KE, Celentano DD, Eiumtrakol S, Hoover DR, Beyrer C, Suprasert S, et al. Changes in sexual behavior and a decline in HIV infection among young men in Thailand. N Engl J Med. 1996;335(5):297–303. doi: 10.1056/NEJM199608013350501. [DOI] [PubMed] [Google Scholar]
- 25.UNAIDS. Regional response fact sheet: Asia and the Pacific; Geneva: Joint United Nations Programme on HIV/AIDS; 2012. [Google Scholar]
- 26.UNAIDS. Regional fact sheet: Latin America and the Caribbean; Geneva: Joint United Nations Programme on HIV/AIDS; 2012. [Google Scholar]
- 27.UNAIDS. Report on the global AIDS epidemic 2010; Geneva: United Nations; 2010. [Google Scholar]
- 28.Hammett TM, Kling R, Johnston P, Liu W, Ngu D, Friedmann P, et al. Patterns of HIV prevalence and HIV risk behaviors among injection drug users prior to and 24 months following implementation of cross-border HIV prevention interventions in northern Vietnam and southern China. AIDS Educ Prev. 2006;18(2):97–115. doi: 10.1521/aeap.2006.18.2.97. [DOI] [PubMed] [Google Scholar]
- 29.Ghys PD, Diallo MO, Ettiegne-Traore V, Kale K, Tawil O, Carael M, et al. Increase in condom use and decline in HIV and sexually transmitted diseases among female sex workers in Abidjan, Cote d'Ivoire, 1991–1998. AIDS. 2002;16(2):251–8. doi: 10.1097/00002030-200201250-00015. [DOI] [PubMed] [Google Scholar]
- 30.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Lipsey MW, Wilson DB. Practical meta-analysis. Applied Social Research Methods Series, Volume 49; Thousand Oaks, California: Sages Publications; 2001. [Google Scholar]
- 32.Mateen FJ, Oh J, Tergas AI, Bhayani NH, Kamdar BB. Titles versus titles and abstracts for initial screening of articles for systematic reviews. Clin Epidemiol. 2013;6:89–95. doi: 10.2147/CLEP.S43118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beyrer C. Lesbian, gay, bisexual, and transgender populations in Africa: a social justice movement emerges in the era of HIV. SAHARA J. 2012;9(3):177–9. doi: 10.1080/17290376.2012.743813. [DOI] [PubMed] [Google Scholar]
- 34.Vuylsteke B, Semde G, Sika L, Crucitti T, Ettiegne Traore V, Buve A, et al. High prevalence of HIV and sexually transmitted infections among male sex workers in Abidjan, Cote d'Ivoire: need for services tailored to their needs. Sex Transm Infect. 2012;88(4):288–93. doi: 10.1136/sextrans-2011-050276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahoyo AB, Alary M, Meda H, Ndour M, Batona G, Bitera R, et al. Female sex workers in Benin, 2002. Behavioural survey and HIV and other STI screening. Sante. 2007;17(3):143–51. doi: 10.1684/san.2007.0080. [DOI] [PubMed] [Google Scholar]
- 36.Ahoyo AB, Alary M, Ndour M, Labbe AC, Ahoussinou C. HIV and sexually transmitted disease among female sex workers in Benin. Med Trop. 2009;69(5):457–62. [PubMed] [Google Scholar]
- 37.Alary M, Mukenge-Tshibaka L, Bernier F, Geraldo N, Lowndes CM, Meda H, et al. Decline in the prevalence of HIV and sexually transmitted diseases among female sex workers in Cotonou, Benin, 1993–1999. AIDS. 2002;16(3):463–70. doi: 10.1097/00002030-200202150-00019. [DOI] [PubMed] [Google Scholar]
- 38.Baganizi E, Alary M, Guedeme A, Padonou F, Davo N, Adjovi C, et al. HIV infection in female prostitutes from Benin: association with symptomatic but not asymptomatic gonococcal or chlamydial infections. AIDS. 1997;11(5):685–6. [PubMed] [Google Scholar]
- 39.Bigot A, Bodeus M, Burtonboy G, Ahouignan G, Zohoun I. Prevalence of HIV infection among prostitutes in Benin (West Africa) J Acquir Immune Defic Syndr. 1992;5(3):317–9. [PubMed] [Google Scholar]
- 40.Labbe AC, Pepin J, Khonde N, Dzokoto A, Meda H, Asamoah-Adu C, et al. Periodical antibiotic treatment for the control of gonococcal and chlamydial infections among sex workers in Benin and Ghana: a cluster-randomized placebo-controlled trial. Sex Transm Dis. 2012;39(4):253–9. doi: 10.1097/OLQ.0b013e318244aaa0. [DOI] [PubMed] [Google Scholar]
- 41.Morison L, Weiss HA, Buve A, Carael M, Abega SC, Kaona F, et al. Commercial sex and the spread of HIV in four cities in sub-Saharan Africa. AIDS. 2001;15(Suppl 4):S61–S9. doi: 10.1097/00002030-200108004-00007. [DOI] [PubMed] [Google Scholar]
- 42.Lowndes CM, Alary M, Gnintoungbe CA, Bedard E, Mukenge L, Geraldo N, et al. Management of sexually transmitted diseases and HIV prevention in men at high risk: targeting clients and non-paying sexual partners of female sex workers in Benin. AIDS. 2000;14(16):2523–34. doi: 10.1097/00002030-200011100-00015. [DOI] [PubMed] [Google Scholar]
- 43.Lowndes CM, Alary M, Labbe AC, Gnintoungbe C, Belleau M, Mukenge L, et al. Interventions among male clients of female sex workers in Benin, West Africa: an essential component of targeted HIV preventive interventions. Sex Transm Infect. 2007;83(7):577–81. doi: 10.1136/sti.2007.027441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lankoande S, Meda N, Sangare L, Compaore IP, Catraye J, Sanou PT, et al. Prevalence and risk of HIV infection among female sex workers in Burkina Faso. Int J STD AIDS. 1998;9(3):146–50. doi: 10.1258/0956462981921909. [DOI] [PubMed] [Google Scholar]
- 45.Nagot N, Ouangre A, Ouedraogo A, Cartoux M, Huygens P, Defer MC, et al. Spectrum of commercial sex activity in Burkina Faso: classification model and risk of exposure to HIV. J Acquir Immune Defic Syndr. 2002;29(5):517–21. doi: 10.1097/00126334-200204150-00013. [DOI] [PubMed] [Google Scholar]
- 46.Kaptue L, Zekeng L, Djoumessi S, Monny-Lobe M, Nichols D, Debuysscher R. HIV and chlamydia infections among prostitutes in Yaounde, Cameroon. Genitourin Med. 1991;67(2):143–5. doi: 10.1136/sti.67.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mauclere P, Mahieux R, Garcia-Calleja JM, Salla R, Tekaia F, Millan J, et al. A new HTLV type II subtype A isolate in an HIV type 1-infected prostitute from Cameroon, Central Africa. AIDS Res Hum Retroviruses. 1995;11(8):989–93. doi: 10.1089/aid.1995.11.989. [DOI] [PubMed] [Google Scholar]
- 48.Ryan KA, Roddy RE, Zekeng L, Weir SS, Tamoufe U. Characteristics associated with prevalent HIV infection among a cohort of sex workers in Cameroon. Sex Transm Infect. 1998;74(2):131–5. doi: 10.1136/sti.74.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bestetti G, Renon G, Mauclere P, Ruffie A, Mbopi Keou FX, Eme D, et al. High seroprevalence of human herpesvirus-8 in pregnant women and prostitutes from Cameroon. AIDS. 1998;12(5):541–3. [PubMed] [Google Scholar]
- 50.Mosoko JJ, Macauley IB, Zoungkanyi AC, Bella A, Koulla-Shiro S. Human immunodeficiency virus infection and associated factors among specific population subgroups in Cameroon. AIDS Behav. 2009;13(2):277–87. doi: 10.1007/s10461-007-9294-8. [DOI] [PubMed] [Google Scholar]
- 51.Mann JM, Nzilambi N, Piot P, Bosenge N, Kalala M, Francis H, et al. HIV infection and associated risk factors in female prostitutes in Kinshasa, Zaire. AIDS. 1988;2(4):249–54. doi: 10.1097/00002030-198808000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Mulanga-Kabeya C, Nzilambi N, Edidi B, Minlangu M, Tshimpaka T, Kambembo L, et al. Evidence of stable HIV seroprevalences in selected populations in the Democratic Republic of the Congo. AIDS. 1998;12(8):905–10. doi: 10.1097/00002030-199808000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Nzila N, Laga M, Thiam MA, Mayimona K, Edidi B, Van Dyck E, et al. HIV and other sexually transmitted diseases among female prostitutes in Kinshasa. AIDS. 1991;5(6):715–21. doi: 10.1097/00002030-199106000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Nzilambi N, De Cock KM, Forthal DN, Francis H, Ryder RW, Malebe I, et al. The prevalence of infection with human immunodeficiency virus over a 10-year period in rural Zaire. N Engl J Med. 1988;318(5):276–9. doi: 10.1056/NEJM198802043180503. [DOI] [PubMed] [Google Scholar]
- 55.Vandepitte JM, Malele F, Kivuvu DM, Edidi S, Muwonga J, Lepira F, et al. HIV and other sexually transmitted infections among female sex workers in Kinshasa, Democratic Republic of Congo, in 2002. Sex Transm Dis. 2007;34(4):203–8. doi: 10.1097/01.olq.0000233743.57334.6a. [DOI] [PubMed] [Google Scholar]
- 56.Pepin J, Morgan G, Dunn D, Gevao S, Mendy M, Gaye I, et al. HIV-2-induced immunosuppression among asymptomatic West African prostitutes: evidence that HIV-2 is pathogenic, but less so than HIV-1. AIDS. 1991;5(10):1165–72. [PubMed] [Google Scholar]
- 57.Pepin J, Dunn D, Gaye I, Alonso P, Egboga A, Tedder R, et al. HIV-2 infection among prostitutes working in The Gambia: association with serological evidence of genital ulcer diseases and with generalized lymphadenopathy. AIDS. 1991;5(1):69–75. [PubMed] [Google Scholar]
- 58.Pickering H, Quigley M, Pepin J, Todd J, Wilkins A. The effects of post-test counselling on condom use among prostitutes in The Gambia. AIDS. 1993;7(2):271–3. doi: 10.1097/00002030-199302000-00017. [DOI] [PubMed] [Google Scholar]
- 59.Pickering H, Todd J, Dunn D, Pepin J, Wilkins A. Prostitutes and their clients: a Gambian survey. Soc Sci Med. 1992;34(1):75–88. doi: 10.1016/0277-9536(92)90069-3. [DOI] [PubMed] [Google Scholar]
- 60.Asamoah-Adu C, Khonde N, Avorkliah M, Bekoe V, Alary M, Mondor M, et al. HIV infection among sex workers in Accra: need to target new recruits entering the trade. J Acquir Immune Defic Syndr. 2001;28(4):358–66. doi: 10.1097/00126334-200112010-00009. [DOI] [PubMed] [Google Scholar]
- 61.Deceuninck G, Asamoah-Adu C, Khonde N, Pepin J, Frost EH, Deslandes S, et al. Improvement of clinical algorithms for the diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis by the use of Gram-stained smears among female sex workers in Accra, Ghana. Sex Transm Dis. 2000;27(7):401–10. doi: 10.1097/00007435-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Côté AM, Sobela F, Dzokoto A, Nzambi K, Asamoah-Adu C, Labbe AC, et al. Transactional sex is the driving force in the dynamics of HIV in Accra, Ghana. AIDS. 2004;18(6):917–25. doi: 10.1097/00002030-200404090-00009. [DOI] [PubMed] [Google Scholar]
- 63.Aho J, Koushik A, Diakite SL, Loua KM, Nguyen VK, Rashed S. Biological validation of self-reported condom use among sex workers in Guinea. AIDS Behav. 2010;14(6):1287–93. doi: 10.1007/s10461-009-9602-6. [DOI] [PubMed] [Google Scholar]
- 64.Aho J, Nguyen VK, Diakite S, Sow A, Koushik A, Rashed S. High acceptability of HIV voluntary counselling and testing among female sex workers: impact of individual and social factors. HIV Med. 2012;13(3):156–65. doi: 10.1111/j.1468-1293.2011.00951.x. [DOI] [PubMed] [Google Scholar]
- 65.Diallo BL, Alary M, Rashed S, Barry A. HIV prevalence, associated risk factors and evolution among truck drivers from 2001 to 2007 in Guinea. Med Trop. 2011;71(2):142–6. [PubMed] [Google Scholar]
- 66.Denis F, Barin F, Gershy-Damet G, Rey JL, Lhuillier M, Mounier M, et al. Prevalence of human T-lymphotropic retroviruses type III (HIV) and type IV in Ivory Coast. Lancet. 1987;1(8530):408–11. doi: 10.1016/s0140-6736(87)90118-8. [DOI] [PubMed] [Google Scholar]
- 67.Ettiegne-Traore V, Ghys PD, Maurice C, Hoyi-Adonsou YM, Soroh D, Adom ML, et al. Evaluation of an HIV saliva test for the detection of HIV-1 and HIV-2 antibodies in high-risk populations in Abidjan, Cote d'Ivoire. Int J STD AIDS. 1998;9(3):173–4. doi: 10.1258/0956462981921819. [DOI] [PubMed] [Google Scholar]
- 68.Ghys PD, Diallo MO, Ettiegne-Traore V, Yeboue KM, Gnaore E, Lorougnon F, et al. Dual seroreactivity to HIV-1 and HIV-2 in female sex workers in Abidjan, Cote d'Ivoire. AIDS. 1995;9(8):955–8. doi: 10.1097/00002030-199508000-00019. [DOI] [PubMed] [Google Scholar]
- 69.Ghys PD, Fransen K, Diallo MO, Ettiegne-Traore V, Coulibaly IM, Yeboue KM, et al. The associations between cervicovaginal HIV shedding, sexually transmitted diseases and immunosuppression in female sex workers in Abidjan, Cote d'Ivoire. AIDS. 1997;11(12):F85–93. doi: 10.1097/00002030-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 70.Koffi K, Gershy-Damet GM, Peeters M, Soro B, Rey JL, Delaporte E. Rapid spread of HIV infections in Abidjan, Ivory Coast, 1987–1990. Eur J Clin Microbiol Infect Dis. 1992;11(3):271–3. doi: 10.1007/BF02098100. [DOI] [PubMed] [Google Scholar]
- 71.Nkengasong JN, Kestens L, Ghys PD, Koblavi-Deme S, Otten RA, Bile C, et al. Dual infection with human immunodeficiency virus type 1 and type 2: impact on HIV type 1 viral load and immune activation markers in HIV-seropositive female sex workers in Abidjan, Ivory Coast. AIDS Res Hum Retroviruses. 2000;16(14):1371–8. doi: 10.1089/08892220050140919. [DOI] [PubMed] [Google Scholar]
- 72.Vuylsteke B, Semde G, Sika L, Crucitti T, Ettiegne Traore V, Buve A, et al. HIV and STI prevalence among female sex workers in Cote d'Ivoire: why targeted prevention programs should be continued and strengthened. PLoS One. 2012;7(3):e32627. doi: 10.1371/journal.pone.0032627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ouattara SA, Diallo D, Meite M, Aron Y, Akran V, Gody M, et al. Epidemiology of infections caused by human immunodeficiency viruses HIV-1 and HIV-2 in the Ivory Coast. Med Trop. 1988;48(4):375–9. [PubMed] [Google Scholar]
- 74.Vuylsteke BL, Ghys PD, Traore M, Konan Y, Mah-Bi G, Maurice C, et al. HIV prevalence and risk behavior among clients of female sex workers in Abidjan, Cote d'Ivoire. AIDS. 2003;17(11):1691–4. doi: 10.1097/00002030-200307250-00014. [DOI] [PubMed] [Google Scholar]
- 75.Peeters M, Koumare B, Mulanga C, Brengues C, Mounirou B, Bougoudogo F, et al. Genetic subtypes of HIV type 1 and HIV type 2 strains in commercial sex workers from Bamako, Mali. AIDS Res Hum Retroviruses. 1998;14(1):51–8. doi: 10.1089/aid.1998.14.51. [DOI] [PubMed] [Google Scholar]
- 76.Pichard E, Guindo A, Grossetete G, Fofana Y, Maiga YI, Koumare B, et al. Human immunodeficiency virus (HIV) infection in Mali. Med Trop. 1988;48(4):345–9. [PubMed] [Google Scholar]
- 77.Gragnic G, Julvez J, Abari A, Alexandre Y. HIV-1 and HIV-2 seropositivity among female sex workers in the Tenere Desert, Niger. Trans R Soc Trop Med Hyg. 1998;92(1):29. doi: 10.1016/s0035-9203(98)90941-5. [DOI] [PubMed] [Google Scholar]
- 78.Mamadou S, Laouel Kader A, Rabiou S, Aboubacar A, Soumana O, Garba A, et al. Prevalence of the HIV infection and five other sexually-transmitted infections among sex workers in Niamey, Niger. Bull Soc Pathol Exot. 2006;99(1):19–22. doi: 10.3185/pathexo2623. [DOI] [PubMed] [Google Scholar]
- 79.Develoux M, Meynard D, Dupont A, Delaporte E. Hepatitis C virus antibodies in prostitutes in Niger. Trans R Soc Trop Med Hyg. 1994;88(5):536. doi: 10.1016/0035-9203(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 80.Tohon Z, Garba A, Amadou Hamidou A, Sidikou F, Ibrahim ML, Elhadj Mahamane A, et al. Behaviour and HIV seroprevalence investigation in sex workers of Dirkou, Niger, 2002. Bull Soc Pathol Exot. 2006;99(1):49–51. doi: 10.3185/pathexo2830. [DOI] [PubMed] [Google Scholar]
- 81.Ahmed S, Delaney K, Villalba-Diebold P, Aliyu G, Constantine N, Ememabelem M, et al. HIV counseling and testing and access-to-care needs of populations most-at-risk for HIV in Nigeria. AIDS Care. 2013;25(1):85–94. doi: 10.1080/09540121.2012.686597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dada AJ, Ajayi AO, Diamondstone L, Quinn TC, Blattner WA, Biggar RJ. A serosurvey of Haemophilus ducreyi, syphilis, and herpes simplex virus type 2 and their association with human immunodeficiency virus among female sex workers in Lagos, Nigeria. Sex Transm Dis. 1998;25(5):237–42. doi: 10.1097/00007435-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 83.Dada AJ, Oyewole F, Onofowokan R, Nasidi A, Harris B, Levin A, et al. Demographic characteristics of retroviral infections (HIV-1, HIV-2, and HTLV-I) among female professional sex workers in Lagos, Nigeria. J Acquir Immune Defic Syndr. 1993;6(12):1358–63. [PubMed] [Google Scholar]
- 84.Eluwa GI, Strathdee SA, Adebajo SB, Ahonsi B, Azeez A, Anyanti J. Sexual risk behaviors and HIV among female sex workers in Nigeria. J Acquir Immune Defic Syndr. 2012;61(4):507–14. doi: 10.1097/QAI.0b013e31826dfb41. [DOI] [PubMed] [Google Scholar]
- 85.Imade G, Sagay A, Egah D, Onwuliri V, Grigg M, Egbodo C, et al. Prevalence of HIV and other sexually transmissible infections in relation to lemon or lime juice douching among female sex workers in Jos, Nigeria. Sex Health. 2008;5(1):55–60. doi: 10.1071/sh07047. [DOI] [PubMed] [Google Scholar]
- 86.Lawan UM, Abubakar S, Ahmed A. Risk perceptions, prevention and treatment seeking for sexually transmitted infections and HIV/AIDS among female sex workers in Kano, Nigeria. Afr J Reprod Health. 2012;16(1):61–7. [PubMed] [Google Scholar]
- 87.Obi CL, Ogbonna BA, Igumbor EO, Ndip RN, Ajayi AO. HIV seropositivity among female prostitutes and nonprostitutes: obstetric and perinatal implications. Viral Immunol. 1993;6(3):171–4. doi: 10.1089/vim.1993.6.171. [DOI] [PubMed] [Google Scholar]
- 88.Olaleye OD, Bernstein L, Ekweozor CC, Sheng Z, Omilabu SA, Li XY, et al. Prevalence of human immunodeficiency virus types 1 and 2 infections in Nigeria. J Infect Dis. 1993;167(3):710–4. doi: 10.1093/infdis/167.3.710. [DOI] [PubMed] [Google Scholar]
- 89.Bakare RA, Oni AA, Umar US, Adewole IF, Shokunbi WA, Fayemiwo SA, et al. Pattern of sexually transmitted diseases among commercial sex workers (CSWs) in Ibadan, Nigeria. Afr J Med Sci. 2002;31(3):243–7. [PubMed] [Google Scholar]
- 90.Chikwem JO, Mohammed I, Ola T. Human immunodeficiency virus type 1 (HIV-1) infection among female prostitutes in Borno State of Nigeria: one year follow-up. East Afr Med J. 1989;66(11):752–6. [PubMed] [Google Scholar]
- 91.Fayemiwo SA, Odaibo GN, Oni AA, Ajayi AA, Bakare RA, Olaleye DO. Genital ulcer diseases among HIV-infected female commercial sex workers in Ibadan, Nigeria. Afr J Med Sci. 2011;40(1):39–46. [PubMed] [Google Scholar]
- 92.Eluwa GI, Strathdee SA, Adebayo SB, Ahonsi B, Adebajo SB. A profile on HIV prevalence and risk behaviors among injecting drug users in Nigeria: should we be alarmed? Drug Alcohol Depend. 2013;127(1–3):65–71. doi: 10.1016/j.drugalcdep.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 93.Adebajo SB, Eluwa GI, Allman D, Myers T, Ahonsi BA. Prevalence of internalized homophobia and HIV associated risks among men who have sex with men in Nigeria. Afr J Reprod Health. 2012;16(4):21–8. [PubMed] [Google Scholar]
- 94.Merrigan M, Azeez A, Afolabi B, Chabikuli ON, Onyekwena O, Eluwa G, et al. HIV prevalence and risk behaviours among men having sex with men in Nigeria. Sex Transm Infect. 2011;87(1):65–70. doi: 10.1136/sti.2008.034991. [DOI] [PubMed] [Google Scholar]
- 95.Vu L, Adebajo S, Tun W, Sheehy M, Karlyn A, Njab J, et al. High HIV prevalence among men who have sex with men in Nigeria: implications for combination prevention. J Acquir Immune Defic Syndr. 2013;63(2):221–7. doi: 10.1097/QAI.0b013e31828a3e60. [DOI] [PubMed] [Google Scholar]
- 96.Kane CT, Diawara S, Ndiaye HD, Diallo PA, Wade AS, Diallo AG, et al. Concentrated and linked epidemics of both HSV-2 and HIV-1/HIV-2 infections in Senegal: public health impacts of the spread of HIV. Int J STD AIDS. 2009;20(11):793–6. doi: 10.1258/ijsa.2008.008414. [DOI] [PubMed] [Google Scholar]
- 97.Kanki P, M'Boup S, Marlink R, Travers K, Hsieh CC, Gueye A, et al. Prevalence and risk determinants of human immunodeficiency virus type 2 (HIV-2) and human immunodeficiency virus type 1 (HIV-1) in west African female prostitutes. Am J Epidemiol. 1992;136(7):895–907. doi: 10.1093/aje/136.7.895. [DOI] [PubMed] [Google Scholar]
- 98.Langley CL, Benga-De E, Critchlow CW, Ndoye I, Mbengue-Ly MD, Kuypers J, et al. HIV-1, HIV-2, human papillomavirus infection and cervical neoplasia in high-risk African women. AIDS. 1996;10(4):413–17. doi: 10.1097/00002030-199604000-00010. [DOI] [PubMed] [Google Scholar]
- 99.Laurent C, Seck K, Coumba N, Kane T, Samb N, Wade A, et al. Prevalence of HIV and other sexually transmitted infections, and risk behaviours in unregistered sex workers in Dakar, Senegal. AIDS. 2003;17(12):1811–16. doi: 10.1097/00002030-200308150-00010. [DOI] [PubMed] [Google Scholar]
- 100.Ndiaye CF, Critchlow CW, Leggott PJ, Kiviat NB, Ndoye I, Robertson PB, et al. Periodontal status of HIV-1 and HIV-2 seropositive and HIV seronegative female commercial sex workers in Senegal. J Periodontol. 1997;68(9):827–31. doi: 10.1902/jop.1997.68.9.827. [DOI] [PubMed] [Google Scholar]
- 101.Wang C, Hawes SE, Gaye A, Sow PS, Ndoye I, Manhart LE, et al. HIV prevalence, previous HIV testing, and condom use with clients and regular partners among Senegalese commercial sex workers. Sex Transm Infect. 2007;83(7):534–40. doi: 10.1136/sti.2007.027151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thior I, Diouf G, Diaw IK, Sarr AD, Hsieh CC, Ndoye I, et al. Sexually transmitted diseases and risk of HIV infection in men attending a sexually transmitted diseases clinic in Dakar, Senegal. Afr J Reprod Health. 1997;1(2):26–35. [PubMed] [Google Scholar]
- 103.do Espirito Santo ME, Etheredge GD. HIV prevalence and sexual behaviour of male clients of brothels’ prostitutes in Dakar, Senegal. AIDS Care. 2003;15(1):53–62. doi: 10.1080/0954012021000039752. [DOI] [PubMed] [Google Scholar]
- 104.Wade AS, Larmarange J, Diop AK, Diop O, Gueye K, Marra A, et al. Reduction in risk-taking behaviors among MSM in Senegal between 2004 and 2007 and prevalence of HIV and other STIs. ELIHoS Project, ANRS 12139. AIDS Care. 2010;22(4):409–14. doi: 10.1080/09540120903253973. [DOI] [PubMed] [Google Scholar]
- 105.Wade AS, Kane CT, Diallo PA, Diop AK, Gueye K, Mboup S, et al. HIV infection and sexually transmitted infections among men who have sex with men in Senegal. AIDS. 2005;19(18):2133–40. doi: 10.1097/01.aids.0000194128.97640.07. [DOI] [PubMed] [Google Scholar]
- 106.Stromdahl S, Onigbanjo Williams A, Eziefule B, Emmanuel G, Iwuagwu S, Anene O, et al. Associations of consistent condom use among men who have sex with men in Abuja, Nigeria. AIDS Res Hum Retroviruses. 2012;28(12):1756–62. doi: 10.1089/AID.2012.0070. [DOI] [PubMed] [Google Scholar]
- 107.Ndiaye HD, Toure-Kane C, Vidal N, Niama FR, Niang-Diallo PA, Dieye T, et al. Surprisingly high prevalence of subtype C and specific HIV-1 subtype/CRF distribution in men having sex with men in Senegal. J Acquir Immune Defic Syndr. 2009;52(2):249–52. doi: 10.1097/QAI.0b013e3181af70a4. [DOI] [PubMed] [Google Scholar]
- 108.Baral S, Kizub D, Masenior NF, Peryskina A, Stachowiak J, Stibich M, et al. Male sex workers in Moscow, Russia: a pilot study of demographics, substance use patterns, and prevalence of HIV-1 and sexually transmitted infections. AIDS Care. 2010;22(1):112–18. doi: 10.1080/09540120903012551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Infante C, Sosa-Rubi SG, Cuadra SM. Sex work in Mexico: vulnerability of male, travesti, transgender and transsexual sex workers. Cult Health Sex. 2009;11(2):125–37. doi: 10.1080/13691050802431314. [DOI] [PubMed] [Google Scholar]
- 110.Ngugi E. Female sex workers in Africa: epidemiology overview, data gaps, ways forward. SAHARA J. 2012;9(3):148–53. doi: 10.1080/17290376.2012.743825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lawoyin TO. Condom use with sex workers and abstinence behaviour among men in Nigeria. J R Soc Promot Health. 2004;124(5):230–3. doi: 10.1177/146642400412400521. [DOI] [PubMed] [Google Scholar]
- 112.Fourn L, Ducic S. Epidemiological portrait of acquired immunodeficiency syndrome and its implications in Benin. Sante (Montrouge France) 1996;6(6):371–6. [PubMed] [Google Scholar]
- 113.Bamidele JO, Abodunrin OL, Adebimpe WO. Sexual behavior and risk of HIV/AIDS among adolescents in public secondary schools in Osogbo, Osun State, Nigeria. Int J Adolesc Med Health. 2009;21(3):387–94. doi: 10.1515/ijamh.2009.21.3.387. [DOI] [PubMed] [Google Scholar]
- 114.Adjei AA, Armah HB, Gbagbo F, Ampofo WK, Quaye IK, Hesse IF, et al. Prevalence of human immunodeficiency virus, hepatitis B virus, hepatitis C virus and syphilis among prison inmates and officers at Nsawam and Accra, Ghana. J Med Microbiol. 2006;55(Pt 5):593–7. doi: 10.1099/jmm.0.46414-0. [DOI] [PubMed] [Google Scholar]
- 115.Adjei AA, Armah HB, Gbagbo F, Ampofo WK, Boamah I, Adu-Gyamfi C, et al. Correlates of HIV, HBV, HCV and syphilis infections among prison inmates and officers in Ghana: a national multicenter study. BMC Infect Dis. 2008;8:33. doi: 10.1186/1471-2334-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alary M, Lowndes CM, Mukenge-Tshibaka L, Gnintoungbe CA, Bedard E, Geraldo N, et al. Sexually transmitted infections in male clients of female sex workers in Benin: risk factors and reassessment of the leucocyte esterase dipstick for screening of urethral infections. Sex Transm Infect. 2003;79(5):388–92. doi: 10.1136/sti.79.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Henry E, Marcellin F, Yomb Y, Fugon L, Nemande S, Gueboguo C, et al. Factors associated with unprotected anal intercourse among men who have sex with men in Douala, Cameroon. Sex Transm Infect. 2010;86(2):136–40. doi: 10.1136/sti.2009.036939. [DOI] [PubMed] [Google Scholar]
- 118.Kalamar M, Maharaj P, Gresh A. HIV-prevention interventions targeting men having sex with men in Africa: field experiences from Cameroon. Cult Health Sex. 2011;13(10):1135–49. doi: 10.1080/13691058.2011.607244. [DOI] [PubMed] [Google Scholar]
- 119.Beyrer C, Jittiwutikarn J, Teokul W, Razak MH, Suriyanon V, Srirak N, et al. Drug use, increasing incarceration rates, and prison-associated HIV risks in Thailand. AIDS Behav. 2003;7(2):153–61. doi: 10.1023/a:1023946324822. [DOI] [PubMed] [Google Scholar]
- 120.Beyrer C, Mehta SH, Baral SD. The international drug epidemic. In: Pizer H, Mayer K, editors. Social ecology of infectious disease. London: Elsevier; 2006. pp. 77–112. [Google Scholar]
- 121.Perry A. The cocaine crisis: how the drug trade is ruining West Africa; Time Magazine; 2012. Oct 22, [Google Scholar]
- 122.Beyrer C. War in the blood; Sex, politics and HIV/AIDS in SouthEast Asia; London, UK: Zed Books; 1998. [Google Scholar]
- 123.Beyrer C. Fearful symmetry: heroin trafficking and HIV spread. Bull Narc. 2002;LIV(1):103. [Google Scholar]
- 124.Mathers BM, Degenhardt L, Adam P, Toskin I, Nashkhoev M, Lyerla R, et al. Estimating the level of HIV prevention coverage, knowledge and protective behavior among injecting drug users: what does the 2008 UNGASS reporting round tell us? J Acquir Immune Defic Syndr. 2009;52(Suppl 2):S132–S42. doi: 10.1097/QAI.0b013e3181baf0c5. [DOI] [PubMed] [Google Scholar]
- 125.Cote AM, Sobela F, Dzokoto A, Nzambi K, samoah-Adu C, Labbe AC, et al. Transactional sex is the driving force in the dynamics of HIV in Accra, Ghana. AIDS. 2004;18(6):917–25. doi: 10.1097/00002030-200404090-00009. [DOI] [PubMed] [Google Scholar]
- 126.Leclerc PM, Garenne M. Commercial sex and HIV transmission in mature epidemics: a study of five African countries. Int J STD AIDS. 2008;19(10):660–4. doi: 10.1258/ijsa.2008.008099. [DOI] [PubMed] [Google Scholar]
- 127.Decosas J, Kane F, Anarfi JK, Sodji KD, Wagner HU. Migration and AIDS. Lancet. 1995;346(8978):826–8. doi: 10.1016/s0140-6736(95)91631-8. [DOI] [PubMed] [Google Scholar]
- 128.Meredith M. The state of Africa: a history of fifty years of independence; London: The Free Press; 2006. [Google Scholar]
- 129.Beyrer C, Baral S, Zenilman J. STDs, HIV/AIDS and migrant populations. Sexually transmitted diseases. In: Holmes KK, Sparling PF, Mardh PA, Lemon SM, Stamm WE, Piot P, et al., editors. Seattle: McGraw-Hill Professional; 2006. pp. 257–68. [Google Scholar]
- 130.Poteat TD, Diouf D, Baral S, Ndaw M, Drame F, Traore C, Wirtz A, et al., editors. The impact of criminalization of same sex practices on HIV risk among men who have sex with men (MSM) in Senegal: results of a qualitative rapid assessment (TUPE0709). International AIDS Conference; July 18–23, 2010; Vienna: IAS; [Google Scholar]
- 131.Munoz J, Adedimeji A, Alawode O. ‘They bring AIDS to us and say we give it to them’: socio-structural context of female sex workers’ vulnerability to HIV infection in Ibadan, Nigeria. SAHARA J. 2010;7(2):52–61. doi: 10.1080/17290376.2010.9724957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Millett GA, Flores SA, Peterson JL, Bakeman R. Explaining disparities in HIV infection among black and white men who have sex with men: a meta-analysis of HIV risk behaviors. AIDS. 2007;21(15):2083–91. doi: 10.1097/QAD.0b013e3282e9a64b. [DOI] [PubMed] [Google Scholar]
- 133.Baral SD, Wirtz A, Sifakis F, Johns B, Walker D, Beyrer C. The highest attainable standard of evidence (HASTE) for HIV/AIDS interventions: toward a public health approach to defining evidence. Public Health Rep. 2012;127(6):572–84. doi: 10.1177/003335491212700607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dreazen Y. Mali: Welcome to Cocainebougou Foreign Policy Magazine, FP Group; Washington, DC. Published online March 27, 2013. [Google Scholar]
- 135.Beyrer C, Baral S, Kerrigan D, El-Bassel N, Bekker LG, Celentano DD. Expanding the space: inclusion of most-at-risk populations in HIV prevention, treatment, and care services. J Acquir Immune Defic Syndr. 2011;57(Suppl 2):S96–9. doi: 10.1097/QAI.0b013e31821db944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gross R, Yip B, Lo Re V, 3rd, Wood E, Alexander CS, Harrigan PR, et al. A simple, dynamic measure of antiretroviral therapy adherence predicts failure to maintain HIV-1 suppression. J Infect Dis. 2006;194(8):1108–14. doi: 10.1086/507680. [DOI] [PubMed] [Google Scholar]
- 138.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339(6122):966–71. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Auerbach JD, Parkhurst JO, Cáceres CF. Addressing social drivers of HIV/AIDS for the long-term response: conceptual and methodological considerations. Glob Public Health. 2011;6(Suppl 3):S293–S309. doi: 10.1080/17441692.2011.594451. [DOI] [PubMed] [Google Scholar]
- 140.Logie C. The case for the World Health Organization's Commission on the Social Determinants of Health to address sexual orientation. Am J Public Health. 2012;102(7):1243–6. doi: 10.2105/AJPH.2011.300599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chakrapani V, Newman PA, Shunmugam M, Kurian AK, Dubrow R. Barriers to free antiretroviral treatment access for female sex workers in Chennai, India. AIDS Patient Care and STDs. 2009;23(11):973–80. doi: 10.1089/apc.2009.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Baral S, Semugoma P, Diouf D, Trapence G, Poteat T, Ndaw M, Drame F, et al., editors. Criminalization of same sex practices as a structural driver of HIV risk among men who have sex with men (MSM): the cases of Senegal, Malawi, and Uganda (MOPE0951). International AIDS Conference; July 18–23, 2010; Vienna: IAS; [Google Scholar]