Abstract
Plant-pollinator systems may be considered as biological markets in which pollinators choose between different flowers that advertise their nectar/pollen rewards. Although expected to play a major role in structuring plant-pollinator interactions, community-wide patterns of flower scent signals remain largely unexplored. Here we show for the first time that scent advertisement is higher in plant species that bloom early in the flowering period when pollinators are scarce relative to flowers than in species blooming later in the season when there is a surplus of pollinators relative to flowers. We also show that less abundant flowering species that may compete with dominant species for pollinator visitation early in the flowering period emit much higher proportions of the generalist attractant β-ocimene. Overall, we provide a first community-wide description of the key role of seasonal dynamics of plant-specific flower scent emissions, and reveal the coexistence of contrasting plant signaling strategies in a plant-pollinator market.
Many plants produce rewards in the form of nectar and pollen that attract pollinators, thus ensuring the transfer of pollen from flower to flower. Plant-pollinator communities may thus be considered as biological markets in which pollinators choose between different flowers that may compete for their visits1,2,3. Flowers rely on sensory signals to advertise their rewards, color and scent being the most important ones4. Historically, plant–pollinator relationships have mostly been considered as a visually-mediated process, and floral odors have received less consideration5. However, olfactory cues are often the basis upon which pollinators make flower choices, because scent cues are easily learned and remembered by pollinators6. Different studies have revealed that bees are able to detect pollen and nectar in flowers via odour cues (6,7,8,9,10, and references therein), that bees learn odours faster and remember them for longer than visual cues6,11, that specific pollen odour plays a key role in host recognition by oligolectic solitary bees7,8, and that floral odour differences are important for maintaining reproductive isolation between closely related plant species12. Other studies have revealed that plant and floral scents elicit a foraging response also in other insect pollinators13,14,15. In addition, floral scent has been found to improve plant fitness via increased pollinator attraction16. Nevertheless, in spite of the putative importance of flower odors in structuring plant-pollinator interactions, community-wide patterns of flower scent signals and their seasonal dynamics remain largely undescribed.
As in most markets, supply and demand in plant-pollinator systems fluctuate in time. Certain periods are characterized by a surplus of flowers relative to pollinators, which may result in competition between flowers and large investment in rewards and display1. Conversely, in periods exhibiting a surplus of pollinators relative to flowers, a reduction of investment in floral rewards and display is expected. In the Mediterranean region, the peak of flowering occurs in the early spring (March-April) and hot, dry summers present a physiological challenge to plants17,18,19. The early flowering peak results in a surplus of flowers relative to pollinators in spring, followed by a surplus of pollinators in relation to flower availability in summer1.
We studied a plant-pollinator community in a Mediterranean shrubland, in which flower and pollinator availability follow closely this model of a seasonal floral market20. To explore the existence of contrasting plant signaling strategies, we quantified floral scent compounds for each plant species as well as the seasonal variation of flower abundance, nectar and pollen availability, and flower visitation rates. Specifically, we examined two hypotheses associated with the emergence of differentiated plant-signaling strategies in plant-pollinator networks. Firstly, we hypothesized a greater investment in scent advertisement early rather than late in the flowering period associated with the lower pollinator availability (pollinator abundance hypothesis). Secondly, we examined whether less abundant flowering species, which, other factors being equal, might have difficulty attracting pollinators, produce a different scent from abundant species (plant abundance hypothesis). We tested these two hypotheses and provide a first integrative description of community-wide patterns of flower scent signals.
Results
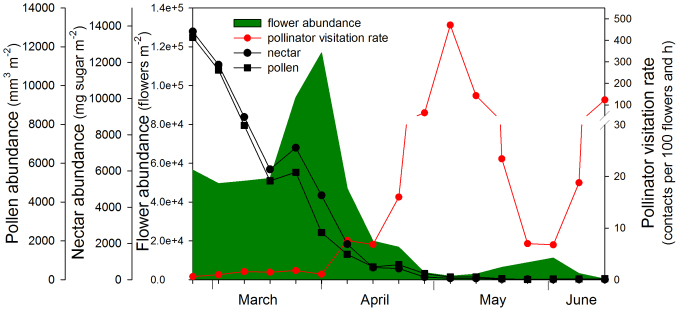
The overall flowering period extended from late February to June. The community presented a clear seasonal pattern with two contrasting scenarios. Early in the flowering period, from late February until early April, flower and floral reward availability (nectar and pollen) was high and visitation rates (pollinator visits per flower and unit time) low. On the other hand, from mid April until June, flower and floral reward availability were much lower, and pollinator visitation rates were much higher (Fig. 1). Plant species were therefore divided in two groups (early and late flowering species) using as a criterion the time when the drastic decline in flower availability coincided with a drastic increase in pollinator visitation (Fig. 1). The species with their peak of flowering early in the season (flowering from late February to early April) were Rosmarinus officinalis, Thymus vulgaris (hermaphrodite and female morphs), Muscari neglectum, Ranunculus gramineus, Euphorbia flavicoma and Iris lutescens. The species with their peak of flowering in the second half (flowering from early April to June) were Cistus salvifolius, Dorycnium hirsutum, Cistus albidus, Orobanche latisquama, Gladiolus illyricus, Galium aparine, Scorpiurus muricatus, Anagallis arvensis, Convolvulus althaeoides, Centaurea linifolia, Centaurea paniculata, Sideritis hirsuta, Phlomis lychnitis, Linum strictum, Leuzea conifera and Allium sphaerocephalon. One species, Biscutella laevigata, was in bloom during most of the flowering season (March to June) and was not included in the analyses. Bees were the main flower visitors until the end of may (see Supplementary Fig. S1 online).
Figure 1. Seasonal pattern of weekly flower abundance (number of flowers per m2), floral rewards (nectar and pollen, mg sugar and mm3 of pollen volume per m2), and pollinator visitation rate (number of pollinator contacts per 100 flowers and h) in the Garraf plant-pollinator community in 2008.
Note the break in the axis for the pollinator visitation rate. This seasonal pattern was consistent between years (authors' observations during the period 2006–2009).
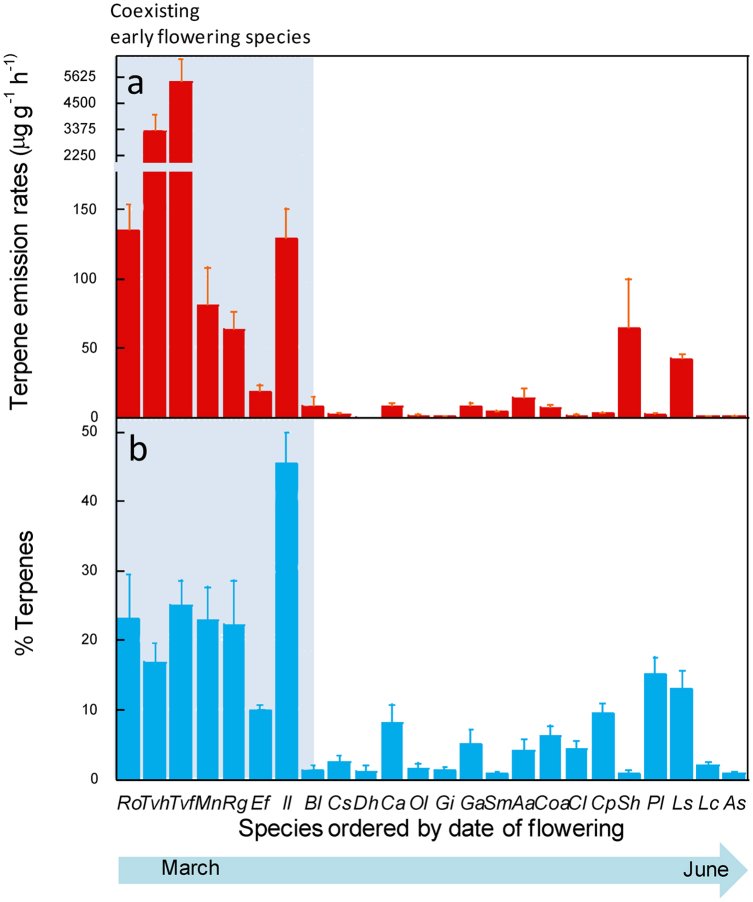
The floral scent of species flowering early in the season significantly differed from the floral scent of species flowering later (pseudo-F1,22 = 8.06, P < 0.001, PERMANOVA, see Supplementary Table S1 and Fig. S2 online). Species flowering early emitted higher amounts of terpenes per flower and per dry weight of flower (F1,21 = 12.8 P < 0.01, F1,21 = 6.03, P < 0.05, respectively, n = 23 species; Fig. 2a). After correcting emissions by field temperatures, species flowering early still emitted higher amounts of terpenes (per flower and per dry weight of flower) (F1,21 = 9.23 P < 0.01, F1,21 = 5.15 P < 0.05, respectively, n = 23 species). These species also emitted a higher proportion of terpenes relative to total volatiles (F1,21 = 34.2, p < 0.0001, ANOVA, Fig. 2b). There was not any significant phylogenetic signal in these differences for terpene emissions (p = 0.80) (see supplementary Fig. S3 online).
Figure 2. Pollinator abundance hypothesis.
Seasonal pattern of (a) terpene emission rates and (b) percentage of terpenes emitted relative to the total emission of biogenic volatile compounds by the plant species of the Garraf shrubland community ordered by date of flowering peak. Note the break in the axis for the terpene emission rates. Early (from late February to early April): Ro- Rosmarinus officinalis, Tvh- Thymus vulgaris hermaphrodite, Tvf- Thymus vulgaris female, Mn- Muscari neglectum, Rg- Ranunculus gramineus, Ef- Euphorbia flavicoma, Il- Iris lutescens; late (from early April to June): Cs- Cistus salvifolius, Dh- Dorycnium hirsutum, Ca- Cistus albidus, Ol- Orobanche latisquama, Gi- Gladiolus illyricus, Ga- Galium aparine, Sm- Scorpiurus muricatus, Aa- Anagallis arvensis, Coa- Convolvulus althaeoides, Cl- Centaurea linifolia, Cp- Centaurea paniculata, Sh- Sideritis hirsuta, Pl- Phlomis lychnitis, Ls- Linum strictum, Lc- Leuzea conifera, As- Allium sphaerocephalon. Bl- Biscutella laevigata blooms during most of the flowering season (March to June). Error bars are SE (n = 5).
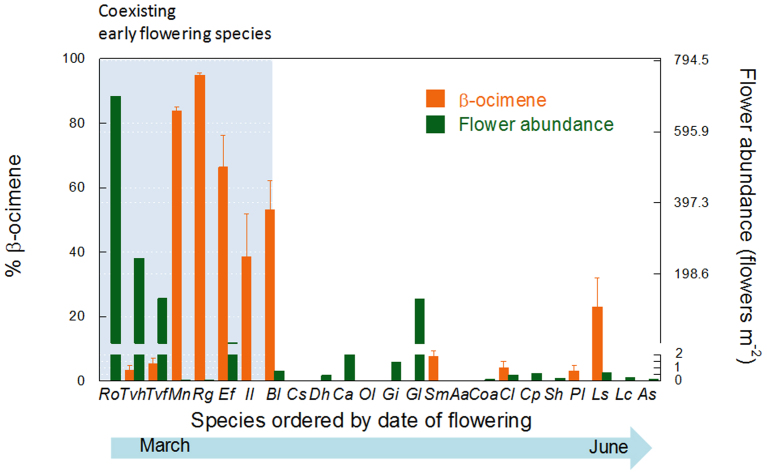
Among the species flowering early in the flowering period, Rosmarinus officinalis and Thymus vulgaris largely out-numbered the rest of species in number of individuals and number of flowers per individual (Fig. 3). These two species accounted by far for most of the nectar and pollen produced during this period20. Their scent was different from that of less abundant co-flowering species (pseudo-F1,6 = 12.27, P = 0.001). Notably, these less abundant species co-flowering with R. officinalis and T. vulgaris emitted a similar flower fragrance with a very high proportion of the monoterpene β-ocimene (Fig. 3). The percentage of β-ocimene emissions after controlling for phylogenetic relatedness was still higher in the less abundant species than in R. officinalis and T. vulgaris (p = 0.019, n = 5, PGLS, phylogenetic generalized least square regressions). Early flowering species shared pollinators (see Supplementary Fig. S1b online), most of which were generalists in their flower-visiting habitats (see Supplementary Fig. S1 online).
Figure 3. Plant abundance hypothesis.
Flower abundance (number of flowers per m2) and percentage of β-ocimene emitted relative to the total emission of terpenes by the plant species of the Garraf shrubland community ordered by date of flowering peak as described in Figure 2. Note the break in the axis for the flower abundance. Note that although peaking in the second half of the season, Bl- Biscutella laevigata overlaps with the early flowering species throughout March and April. Error bars are SE (n = 5).
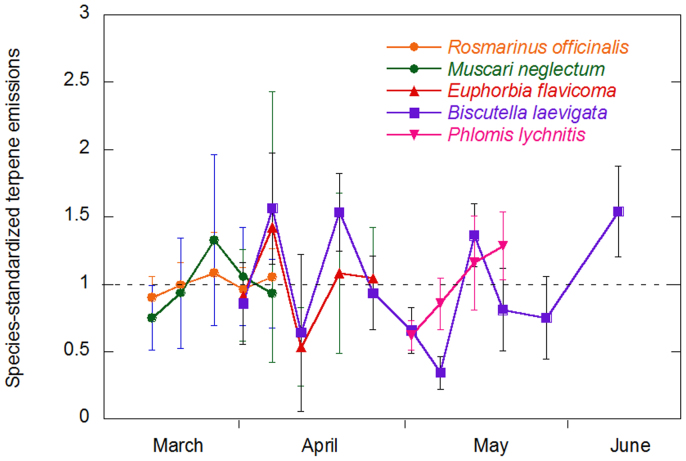
No significant seasonal trend was found in the emission rates of total terpenes, nor in the emission rates of β-ocimene in particular, in any of the five species studied throughout their entire blooming period (R. officinalis, E. flavicoma, M. neglectum- flowering mainly early in the season-, B. laevigata –flowering the whole season- and P. lychnitis-flowering late in the season) (Fig. 4).
Figure 4. Species-standardized terpene emission rates (μg g−1 h−1) (emission rates were divided by the mean emission rate of each species) of five representative species throughout their entire blooming period.
Error bars are SE (n = 5). Species standardized β-ocimene emission rates also followed no particular pattern (data not shown).
Discussion
In accordance with the pollinator abundance hypothesis, we found that species flowering early in the season presented a higher scent emission than plant species flowering later, with the former emitting a higher amount and proportion of terpenes. Floral scents dominated by terpenoids are common among plants pollinated by bees21, the main pollinator group during the early flowering period in this community (see Supplementary Fig. S1 online). Moreover, terpenoid emissions have been suggested to be major contributors to the effect of floral scent emissions on seed fitness16.
As expected based on the plant-abundance hypothesis, we found that Rosmarinus officinalis and Thymus vulgaris scent was different from that of less abundant co-flowering species, with the less abundant species emitting a similar fragrance dominated by β-ocimene. β-ocimene is known to be a general attractant, emitted by a wide range of plants pollinated by different groups of pollinators22, such as bees23,24, moths25,26, butterflies27, and beetles26,28 and has been found to be attractive to honey bees and bumblebees29,30,31. Early flowering species shared pollinators, which for the most part were generalists20. Non-dominant species would benefit from an increased capacity to attract pollinators and thus compensate for their low abundance. The existence of this shared long-range attraction odor does not prevent the existence of short-range differences among species that may lead to pollinator specialization. At least this seems to be the case in the genus Ranunculus, where it was found that β-ocimene presents an interesting spatial emission pattern within the flower with a marked increase in the emissions from the apical to basal part of the petals (nectariferous) paralleling optical nectar-guide patterns, and emission of protoanemonin associated exclusively with pollen and reproductive parts of the flowers32,33. While floral odours would operate at longer distances, the distinctiveness of the pollen's volatile profile suggests that it may serve a signaling role for pollinators specialized in collecting its pollen.
Variation in floral scent emission throughout the season could be the result of phenotypic plasticity34. The observed pattern could be attributed to a physiological flower response to pollinator abundance or to seasonal environmental changes (e.g., temperature, precipitation or air humidity). However, contrary to the expectations of a typical phenotypically plastic response, no significant seasonal trend was found in the studied species. Moreover, if anything, emissions would be expected to increase late in the season, when temperatures are higher and precipitation and air humidity lower35. The observed patterns could also be due to phylogenetic constraints, but there was no significant phylogenetic signal for the total terpene emissions and the phylogenetic signal for β-ocimene percentage was not sufficient to explain the differences between the less abundant species on the one hand and R. officinalis and T. vulgaris on the other. Alternatively, the observed inter-specific differences in scent signals may be the result of adaptive processes. Parachnowitsch et al.36 found floral scent to be under stronger natural selection than either flower size or color, which are much more frequently examined in studies of floral evolution. Successful pollinator attraction and ultimate sexual reproduction in a plant species depend not only on the efficiency of its own scent signal but also on the efficiency of the signals of co-flowering species, in combination with their relative abundances, distribution and spatial intermixing. Thus, scent emission is likely to be under strong selective pressure conditioned by seasonal pollinator availability, and plant community species composition. The seasonal pattern could result from selection for high flower attractiveness under low pollinator availability. The scent pattern found in species co-flowering with the dominant R. officinalis and T. vulgaris may result from selection of those species with a scent detectable for pollinators even in the presence of the abundant scent of the dominant species.
With few exceptions37, studies analyzing the factors underlying the structure of plant-pollinator networks have mostly focused on abundance (neutrality models) and complementary phenological and morphological traits (trait matching models), while the potential contribution of volatiles has been largely ignored5. For the first time we show a clear divergent seasonal pattern of scent emission in a plant-pollinator community, with different levels of investment in scent advertisement, and unveil contrasting plant-signaling strategies associated with pollinator seasonal abundance and local plant abundance. Overall, we provide a first community-wide description of the seasonal dynamics of flower scent emissions, and report patterns that suggest a key role of flower scent signals in structuring plant-pollinator networks.
Methods
Study area field surveys
The study was conducted in a Mediterranean shrubland community in Garraf Natural Park (Barcelona, NE Spain), 340 m above sea level and 1700 m from the coastline. Field work was conducted in a ca. 1 ha plot, from late February to late June, encompassing the main flowering period in the area. No plants were in bloom during the dry summer season (July–August).
In 2008, we counted weekly the number of open flowers in six 50 × 1 m transects and conducted pollinator counts on 24 plant species, representing 99.96% of the total number of flowers in the study plot: Rosmarinus officinalis, Thymus vulgaris (hermaphrodite and female morphs), Muscari neglectum, Ranunculus gramineus, Euphorbia flavicoma, Iris lutescens, Biscutella laevigata Cistus salvifolius, Dorycnium hirsutum, Cistus albidus, Orobanche latisquama, Gladiolus illyricus, Galium aparine, Scorpiurus muricatus, Anagallis arvensis, Convolvulus althaeoides, Centaurea linifolia, Centaurea paniculata, Sideritis hirsuta, Phlomis lychnitis, Linum strictum, Leuzea conifera and Allium sphaerocephalon. Floral rewards were measured on 15–20 flowers of each species. To measure volume of pollen produced per flower, we estimated the number of pollen grains in undehisced anthers in a 70% ethanol-pollen suspending solution using an electronic particle counter (Coulter Multisizer), and measured pollen grain size under the microscope. To measure nectar production (mg of sugar produced per flower) we bagged flower buds and 24 h following anthesis, we used micropipettes to extract the accumulated nectar. Sugar concentration was measured with field refractometers.
Pollinator surveys were conducted twice a week throughout the blooming period. Flower patches were tagged, open flowers were counted and observed for 4 min periods throughout the day. During the observation time insects visiting the flowers were visually identified, and contacts were counted. Pollinators that could not be identified in the field were captured for later identification. From pollinator surveys, we obtained a measure of pollinator visitation rates (visits per flower and unit time).
Floral BVOC (biogenic volatile organic compounds) emission rates
We sampled the emission of flowers from 5 individuals of each plant species in its peak flowering week in 2009. Additionally, to test whether floral scent emission throughout the season could be the result of phenotypic plasticity, we sampled flowers from 5 individuals of 5 plant species (R. officinalis, E. flavicoma, M. neglectum- species flowering mainly early in the season-, B. laevigata –flowering the whole season- and P. lychnitis-flowering late in the season) throughout their entire blooming period in 2011. In both cases samples were taken in the field at midday. We carefully put our specimens in water vials and immediately transferred them to a portable 4°C cabinet prior to analyses with gas chromatography (GC-MS) and Proton Transfer Reaction Mass Spectrometry (PTR-MS). BVOC analyses, with special focus on isoprenoids, were performed through head space technique in the GC-MS (Agilent Technologies, GC: 7890A, MS: 5975C inert MSD with Triple-Axis Detector, Palo Alto, CA, USA). In the laboratory, flowers (inflorescences in the case of Centaurea spp. and Leuzea conifera) were separated from vegetative parts. This procedure was applied for each of the 10 individuals of each plant species. Flowers were introduced in 10 ml vials which were then placed in a Head Space incubator (CTC Analytics, MH 01-00B, Zwingen, Switzerland) and later processed with an automatic sample processor (Combi PAL, CTC Analytics, MXY 02-01B, Zwingen, Switzerland). Incubation time was 10 min. at 35°C. Two ml samples were injected into a 30 m × 0.25 mm × 0.25 μm film thickness capillary column (HP-5MS, Agilent Technologies). Helium flow was 0.5 ml min−1. Total run time was 30 min. and the solvent delay was 4 min. After the sample injection, the initial time was 1 min. and the initial temperature (40°C) was increased at 15°C.min−1 up to 150°C and kept for 5 min, and thereafter at 50°C.min−1 up to 250°C where the temperature was kept for 5 min., and thereafter at 30°C.min−1 up to 280°C, which was maintained for 5 min. The identification of monoterpenes was conducted by comparing retention times with liquid standards from Fluka (Buchs, Switzerland) volatilized in the vial, and the fractionation mass spectra with standards spectra and Nist05a and wiley7n mass spectra libraries. Terpene concentrations were determined using calibration curves for common monoterpenes, alpha-pinene, beta-pinene, 3-carene, linalool, and sesquiterpene alpha-humulene. The analyses of emission rates for all emitted volatiles were conducted with a PTR-MS. Flowers were enclosed in a leaf cuvette of a LCpro+ Photosynthesis System (ADC BioScientific Ltd., Hoddesdon, England) at 25°C, and the air exiting the leaf cuvette was monitored with flow meters and analyzed with a Proton-Transfer-Reaction Mass Spectrometer (PTR-MS-FTD hs) from Ionicon Analytik, Innsbruck, Austria. These VOC analyses were replicated three times for each sample. The quantification of VOCs was based on the use of replicated three times calibration standards (ethylene, methanol, isoprene, α-pinene, methyl salicylate and caryophylene, Sigma-Aldrich, Abelló- Linde). The PTR-MS drift tube was operated at 2.1 mbar and 40°C, with a drift field of 600 V cm−1. The parent ion signal was maintained at around 3 × 106 counts per second during the measurements. We conducted scans of all masses between 22 and 205 to determine which compounds were emitted by the different samples38. Previous to any measurement, we measured the background concentrations of VOCs in the empty cuvette, and considered these data to calculate the emission/uptake of every compound.
We estimated emission rates at the field temperature by using the equation
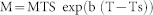 |
39where M is the emission rate at temperature T, MTS is emission rate at 303 K, b is an empirical coefficient and Ts = 303 K.
Data analysis
To test for differences between early and late-blooming plants in total BVOC emission, flower abundance, nectar and pollen content and pollinator abundance we conducted permutational multivariate ANOVAs (PERMANOVA)40 using the Bray Curtis index of similarity, with “season” (early flowering period, late flowering period) as a fixed factor. We also conducted a cluster analysis on the percentage of the different VOCs emitted by each species. All these analyses were conducted using the statistical packages PERMANOVA+ for PRIMER v.640 and Statistica 6.0 (Statsoft Inc., Tulsa, OK, USA). We also used the program PHYLOMATIC41 to build a phylogenetic tree of the plant species studied and test if total terpene emission showed a significant phylogenetic signal- i.e. the tendency of closely related species to resemble each other due to shared ancestry- as described in42. Briefly, PHYLOMATIC uses a backbone plant megatree based on a variety of sources involving primarily DNA studies to assemble a phylogenetic tree for the species of interest. Our phylogenetic hypothesis was based on the conservative megatree, where unresolved nodes were included as soft polytomies. We used the PDAP package43 to transform the phylogenetic tree into a matrix of phylogenetic distances, and tested if the studied traits showed significant phylogenetic signal with the randomization procedure in the PHYSIG module developed by45. This test compares the variance in phylogenetic independent contrasts observed in the real dataset against a null distribution obtained when the phenotypic data are randomized across the tips of the tree (breaking any pattern of phylogenetic resemblance between relatives). Phylogenetic signal was considered significant if the variance in contrasts of the real dataset was lower than the variance in 95% of the permuted datasets. These analyses were performed to determine if phylogenetic correction was necessary in subsequent regression analyses. When the dependent variable showed significant phylogenetic signal we used phylogenetic generalized least square regressions (PGLS). PGLS controls for phylogenetic relatedness by adjusting the expected variance/covariance of regression residuals using the matrix of phylogenetic distances (this approach is mathematically equivalent to analyzing the data with phylogenetically independent contrasts). These analyses were performed with the REGRESSIONV2 module in MATLAB 7.6.046. We used the stats package44 to draw the heatmap of volatile emissions in each species.
Author Contributions
I.F., A.R., J.B. and J.P. designed the research. I.F., C.P., A.M.G., G.F., A.R. and J.B. conducted the field work. I.F., J.L., R.S., G.F. and J.P. conducted the lab analyses. I.F. and J.P. drafted the paper. All authors contributed to the interpretation of the results and were deeply involved in the writing of the text.
Supplementary Material
Supplementary information
Table S1
Acknowledgments
This research was supported by funding from Spanish Government grants CGL2005-00491, CGL2009-12646, CGL2010-17172/BOS and Consolider-Ingenio Montes (CSD2008-0040) and from the Catalan Government grant CSGR2009/458. We thank J. Piñol for assistance with PERMANOVA analyses.
References
- Cohen D. & Shmida A. The evolution of flower display and reward. Evol. Biol. 27, 197–243 (1993). [Google Scholar]
- Chittka L. & Schürkens S. Successful invasion of a floral market. Nature 411, 653 (2001). [DOI] [PubMed] [Google Scholar]
- Noe R. & Hammerstein P. Biological markets. Trends Ecol. Evol. 10, 336–339 (1995). [DOI] [PubMed] [Google Scholar]
- Chittka L. & Raine N. E. Recognition of flowers by pollinators. Curr. Opin. Plant Biol. 9, 428–435 (2006). [DOI] [PubMed] [Google Scholar]
- Raguso R. A. Start making scents: the challenge of integrating chemistry into pollination ecology. Entomol. Exp. Appl. 128, 196–207 (2008). [Google Scholar]
- Wright G. & Schiestl F. P. The evolution of floral scent: the influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Funct. Ecol. 23, 841–851 (2009). [Google Scholar]
- Dobson H. E. M. Role of flower and pollen aromas in host plant recognition by solitary bees. Oecologia 72, 618–623 (1987). [DOI] [PubMed] [Google Scholar]
- Dobson H. E. M. & Bergström G. The ecology of pollen odor. Plant. Syst. Evol. 222, 63–87 (2000). [Google Scholar]
- Goulson D., Chapman J. W. & Hughes W. O. H. Discrimination of unrewarding flowers by bees; direct detection of rewards and use of repellent scent marks. J. Insect Behav. 14, 669–678 (2001). [Google Scholar]
- Howell A. D. & Alarcon R. Osmia bees (Hymenoptera: Megachilidae) can detect nectar-rewarding flowers using olfactory cues. Anim. Behav. 74, 199–205 (2007). [Google Scholar]
- Menzel R. in Neurobiology of learning and memory in honeybees. The Behaviour and Physiology of Bees (eds Goodman L. J., & Fisher R. C.) 323–354 (CAB International, Wallingford, 1991). [Google Scholar]
- Waelti M. O., Muhlemann J. K., Widmer A. & Schiestl F. P. Floral odour and reproductive isolation in two species of Silene. J. Evol. Biol. 21, 111–121 (2008). [DOI] [PubMed] [Google Scholar]
- Cook S. M., Bartlet E., Murray D. A. & Williams I. H. The role of pollen odour in the attraction of pollen beetles to oilseed rape flowers. Entomol. Exp. Appl. 104, 43–50 (2002). [Google Scholar]
- Anderson S. Foraging responses in the butterflies Inachis io, Aglais urticae (Nymphalidae), and Gonopteryx rhamni (Pieridae) to floral scents. Chemoecol. 13, 1–11 (2003). [Google Scholar]
- Primante C. & Dötterl S. A syrphid fly uses olfactory cues to find a non-yellow flower. J. Chem. Ecol. 36, 1207–1210 (2010). [DOI] [PubMed] [Google Scholar]
- Majetic C. J., Raguso R. A. & Ashman T. L. The sweet smell of success: floral scent affects pollinator attraction and seed fitness in Hesperis matronalis. Funct. Ecol. 23, 480–487 (2009). [Google Scholar]
- Kummerov J. Comparative phenology of Mediterranean type plant communities. In: Mediterranean plant ecosystem, (eds Kruger F. J., Mitchell D. L., & Jarvis J. U. M.) 300–317 (Springer Verlag, Berlin, 1983). [Google Scholar]
- Bosch J., Retana J. & Cerdá X. Flowering phenology, floral traits and pollinator composition in a herbaceous Mediterranean plant community. Oecologia 109, 583–591 (1997). [DOI] [PubMed] [Google Scholar]
- Petanidou T., Ellis W. N., Margaris N. S. & Vokou D. Constraints on flowering phenology in a phryganic (east Mediterranean shrub) community. Am. J. Bot. 82, 607–620 (1995). [Google Scholar]
- Bosch J., Martín González A. M., Rodrigo A. & Navarro D. Plant-pollinator networks: adding the pollinator's perspective. Ecol. Lett. 12, 409–419 (2009). [DOI] [PubMed] [Google Scholar]
- Dobson H. E. M. in Biology of Floral Scent (eds Dudareva N., & Pichersky E.) 147–198 (Taylor and Francis, Boca Raton, 2006). [Google Scholar]
- Knudsen J. T., Eriksson R., Gershenzon J. & Stahl B. Diversity and distribution of floral scent. Bot. Rev. 72, 1–120 (2006). [Google Scholar]
- Gerlach G. & Schill R. Composition of orchid scents attracting euglossine bees. Bot. Acta 104, 379–391 (1991). [Google Scholar]
- Borg-Karlson A., Valterova I. & Nilsson L. A. Volatile compounds from flowers of six species in the family Apiaceae: bouquets for different pollinators? Phytochem. 35, 111–119 (1994). [Google Scholar]
- Knudsen J. T. & Tollsten L. Trends in floral scent chemistry in pollination syndromes: floral scent composition in moth-pollinated taxa. Bot. J. Linn. Soc. 113, 263–284 (1993). [Google Scholar]
- Okamoto T., Kawakita A. & Kato M. Interspecific variation of floral scent composition in Glochidion and its association with host-specific pollinating seed parasite (Epicephala). J. Chem. Ecol. 33, 1065–1081 (2007). [DOI] [PubMed] [Google Scholar]
- Andersson S., Nilsson L. A., Groth I. & Bergström G. Floral scents in butterfly-pollinated plants: possible convergence in chemical composition. Bot. J. Linn. Soc. 140, 129–153 (2002). [Google Scholar]
- Dufaÿ M., Hossaert-McKey M. & Anstett M. C. When leaves act like flowers; how dwarf palms attract their pollinators. Ecol. Lett. 6, 28–34 (2003). [Google Scholar]
- Loper G. M., Waller G. D. & Berdel R. L. Olfactory screening of alfalfa clones for uniform honeybee selection. Crop Sci. 14, 120–122 (1974). [Google Scholar]
- Pecetti L., Tava A., Felicioli A., Pinzauti M. & Piano E. Effect of three volatile compounds from lucerne flowers on their attractiveness towards pollinators. B. Insectol. 55, 21–27 (2002). [Google Scholar]
- Granero A. M. et al. Chemical compounds of the foraging recruitment pheromone in bumblebees. Naturwissenschaften 92, 371–374 (2005). [DOI] [PubMed] [Google Scholar]
- Bergström G., Dobson H. E. M. & Groth I. Spatial fragrance patterns within the flowers of Ranunculus acris (Ranunculaceae). Plant Syst. Evol. 195, 221–242 (1995). [Google Scholar]
- Jürgens A. & Dötterl S. Chemical composition of anther volatiles in Ranunculaceae: Genera-specific profiles in Anemone, Aquilegia, Caltha, Pulsatilla, Ranunculus, and Trollius species. Am. J. Bot. 91, 1969–1980 (2004) [DOI] [PubMed] [Google Scholar]
- Majetic C. J., Raguso R. A. & Ashman T. L. Sources of floral scent variation. Can environment define floral scent phenotype? Plant. Signal Behav. 4, 129–131 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen H. B. & Olsen C. E. Influence of climatic factors on emission of flower volatiles in situ. Planta 192, 365–371 (1994). [Google Scholar]
- Parachnowitsch A. L., Raguso R. A. & Kessler A. Phenotypic selection to increase floral scent emission, but not flower size or colour in bee-pollinated Penstemon digitalis. New Phytol. 195, 667–675 (2012). [DOI] [PubMed] [Google Scholar]
- Junker R. R., Höcherl N. & Blüthgen N. Responses to olfactory signals reflect network structure of flower-visitor interactions. J. Anim. Ecol. 79, 818–23 (2010). [DOI] [PubMed] [Google Scholar]
- Peñuelas J., Filella I., Stefanescu C. & Llusià J. Caterpillars of Euphydryas aurinia (Lepidoptera: Nymphalidae) feeding on Succisa pratensis leaves induce large foliar emissions of methanol. New Phytol. 167, 851–857 (2005). [DOI] [PubMed] [Google Scholar]
- Guenther A., Zimmerman P., Harley P., Monson R. & Fall R. Isoprene and monoterpene emission rate variability: model evaluation and sensitivity analysis. J. Geophys. Res. 98, 12609–12617 (1993). [Google Scholar]
- Anderson M. J., Gorley R. N. & Clarke K. R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. Plymouth: PRIMER-E. 214 pp (2008). [Google Scholar]
- Webb C. O. & Donoghue M. J. Phylomatic: tree assembly for applied phylogenetics. Mol. Ecol. Notes 5, 181–183 (2005). [Google Scholar]
- Peñuelas J. et al. Faster returns on “leaf economics” and different biogeochemical niche in invasive compared with native plant species. Global Change Biol. 16, 2171–2185 (2010). [Google Scholar]
- Garland T. J., Harvey P. H. & Ives A. R. Procedures for the analysis of comparative data using phylogenetically independent contrast. Syst. Biol. 41, 18–32 (1993). [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. (2013).
- Blomberg S. P., Garland T. J. & Ives A. R. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745 (2003). [DOI] [PubMed] [Google Scholar]
- Lavin S. R., Karasov W. H., Ives A. R., Middleton K. M. & Garland T. J. Morphometrics of the avian small intestine compared with that of nonflying mammals: a phylogenetic approach. Physiol. Biochem. Zool. 81, 526–550 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Table S1