Abstract
Adjuvant chemotherapy is associated with improvements in long-term cancer survival. However, reports of cognitive impairment following treatment emphasize the importance of understanding the long-term effects of chemotherapy on brain functioning. Cognitive deficits found in chemotherapy patients suggest a change in brain functioning that affects specific cognitive domains such as attentional processing and executive functioning. This study examined the processes potentially underlying these changes in cognition by examining brain functional connectivity pre- and post-chemotherapy in women with breast cancer. Functional connectivity examines the temporal correlation between spatially remote brain regions in an effort to understand how brain networks support specific cognitive functions. Nine women diagnosed with breast cancer completed a functional magnetic resonance imaging (fMRI) session before chemotherapy, one month after, and one year after the completion of chemotherapy. Seed-based functional connectivity analyses were completed using seeds in the intraparietal sulcus (IPS) to examine connectivity in the dorsal anterior attention network and in the posterior cingulate cortex (PCC) to examine connectivity in the default mode network. Results showed decreased functional connectivity one month after chemotherapy that partially returned to baseline at one year in the dorsal attention network. Decreased connectivity was seen in the default mode network at one month and one year following chemotherapy. In addition, increased subjective memory complaints were noted at one month and one year post-chemotherapy. These findings suggest a detrimental effect of chemotherapy on brain functional connectivity that is potentially related to subjective cognitive assessment.
Keywords: breast cancer, chemotherapy, functional connectivity, fMRI
As oncologic interventions improve and numbers of cancer survivors increase, quality of life issues are becoming important considerations for patients, physicians, and researchers. Patients have long reported adverse effects of chemotherapy on cognition and a number of studies have shown negative effects of chemotherapy on cognition (e.g. Vardy & Dhillon, 2010; Shilling et al., 2005; Kayl et al., 2006; Schagen et al., 2006). Much of the work focused on women with breast cancer and has found that women report subjective complaints of cognitive impairment (Hutchinson et al. 2012) and show objective impairment during neuropsychological testing (e.g. Weis et al., 2009). A study of breast cancer survivors 20 years past chemotherapy treatment performed worse on a battery of neuropsychological tests compared to matched healthy controls (Koppelmans et al. 2012). Thus, the effects of chemotherapy on the brain may be long lasting.
Recently neuroimaging methods have been used to examine the effects of chemotherapy for breast cancer on brain structure and function in an effort to understand underlying processes contributing to cognitive changes. Studies have identified structural changes in the brain after chemotherapy in gray and white matter (Deprez et al., 2012; de Ruiter et al., 2012; Inagaki et al., 2007; McDonald et al. 2012b). Thus, there is support for an anatomical basis to explain the functional impairments reported by survivors. Chemotherapy also affected task-related brain activation. In a study of breast cancer survivors who were 10 years past chemotherapy treatment, decreased brain activation was observed in regions of the parietal lobe that were involved in planning and episodic memory (de Ruiter et al. 2011). In a prospective longitudinal study, decreased working memory-related brain activity in the frontal lobes were seen one month after chemotherapy that partially recovered one year later (McDonald et al., 2012a; Collins et al., 2009). Thus, chemotherapy had negative effects on task-related brain activation and some of these effects recovered over time while other brain changes persisted into older age.
Studies have also begun to examine the effects of chemotherapy on whole brain functional connectivity (Bruno et al. 2012; Hosseini et al., 2012). Functional connectivity analyses can reveal how spatially separate brain regions fluctuate together implying network associations (Biswal et al. 2010). Chemotherapy’s impact on network connectivity through its disruption of gray matter integrity and/or white matter connectivity may contribute to the functional impairments or subjective complaints observed after chemotherapy. Breast cancer survivors five years past chemotherapy treatment appeared to have disrupted functional connectivity in frontal, temporal and striatal brain regions compared to healthy controls (Bruno et al., 2012). Survivors also reported more subjective complaints in executive functioning and memory difficulties compared to controls implying a relationship between network connectivity and subjective reports of cognition. Thus far, no study has examined functional connectivity in a prospective longitudinal design to examine the influence of chemotherapy immediately following and one year after treatment compared to pre-chemotherapy baseline.
The current pilot study followed breast cancer survivors assessed at three times: before chemotherapy (baseline), one month after treatment (M1), and one year after treatment (Y1). Each study day included cognitive testing, fMRI scanning, and mood assessments. This study design allowed for the examination of the acute effects of chemotherapy on cognitive and brain functioning and the assessment of longer-term effects of chemotherapy on the brain one year later. A major focus of this pilot study was to examine the effects of chemotherapy on functional connectivity in a prospective longitudinal study design. Since prior studies suggest chemotherapy impacts executive functioning (de Ruiter et al. 2011; McDonald et al. 2012a) we evaluated the dorsal attention network (Kubler et al. 2003) that is involved in top-down directed attention and working memory (e.g Spreng et al. 2012). Brain regions associated with the dorsal attention network include the bilateral intraparietal sulci, superior frontal sulci, primary visual cortex, and anterior cingulate cortex. Additionally, changes in functional connectivity after chemotherapy may be observed in the default mode network which is involved in off-task, self-reflective processing and internally directed attention (e.g. Whitfield-Gabrieli & Ford 2012). Brain regions in the default mode network include the posterior cingulate cortex, precuneus, medial prefrontal cortex, and the lateral temporal cortex. Reuter-Lorenz and Cimprich (2013) proposed that self-reflective processes supported by the default mode network may become more prominent during and after chemotherapy and may be related to increased subjective reports of cognitive complaints. In addition, processes involving the default mode network may become dysregulated after chemotherapy. Thus, patterns of connectivity changes after chemotherapy may be important to examine in the “task positive” dorsal attention network and “task negative” default mode network.
Based on the pattern observed during task-based fMRI in a similarly designed study (McDonald et al. 2012a) we hypothesized that functional connectivity would be decreased in the dorsal attention network one month after chemotherapy and that partial recovery would be observed one year later. Based on the proposal of Reuter-Lorenz & Cimprich (2013) we hypothesized that the default mode network would show decreased connectivity one month and one year after chemotherapy in the presence of increased subjective memory complaints.
Method
Participants
Participants were nine cognitively normal women, ages 42–71 years, M(SD) = 57.10(8.6). See Table 1 for demographic characteristics. Women with a recent diagnosis of breast cancer were recruited through breast multidisciplinary clinics. Individuals who had received previous chemotherapy and with any known active neurologic disorder affecting the central nervous system were excluded. Exclusion criteria for MRI scanning included claustrophobia, cardiac pace makers, other implanted metal devices, injuries to the eye involving metal, tattoos on the head or neck, and other moveable metal implants in the body. If eligibility criteria were met individuals signed informed consent documents prior to testing.
Table 1.
Mean (standard deviation) demographic and screening data for breast cancer patients.
| Patients N=9 | |
|---|---|
| Age (y) | 57.1 (8.6) |
| Age range (y) | 42–71 |
| Education (y) | 15.3 (2.4) |
| MMSE | 28.7 (1.7) |
| IQ (WASI) | 119.0 (10.5) |
| DRS | 141.1 (4.4) |
| Mood (GDS) | 1.4 (0.5) |
Characteristics of the type of cancer and medications each for these participants were as follows. Four women were premenopausal and five were postmenopausal at baseline. Their cancer stages ranged from Ia to IIIa. The chemotherapy regimen for all nine women included cyclophosphamide, eight women received taxane, and four received anthracycline. Two women received monoclonal antibodies and one received carboplatin. Eight women received radiation. Five women received tamoxifen, two received aromotase inhibitors, and two received no anti-estrogen therapy after diagnosis. No participant received any cognitive rehabilitation therapy after breast cancer treatment.
Screening
Participants were screened for dementia and cognitively evaluated using the Mini Mental State Exam (MMSE; (Folstein et al., 1975), Brief Cognitive Rating Scale (Reisberg and Ferris, 1988), and the Mattis Dementia Rating Scale (DRS, (Jurica et al., 2001) to establish a Global Deterioration Scale score (GDS) which rated the degree of cognitive impairment (Reisberg and Ferris, 1988). To estimate IQ, participants completed the Wechsler Abbreviated Scale of Intelligence (WASI, Wechsler 1999). Participants were required to have an MMSE score greater than or equal to 27, a DRS score greater than or equal to 123, a GDS score of 1 or 2, and a WASI score greater than or equal to 80.
Behavioral screening consisted of a partial Structured Clinical Interview for DSM-IV-TR (SCID; (First et al., 2001) to establish the presence/absence of current major depression, mania or dysthymia. In addition, participants completed the Beck Depression Inventory-II (BDI; Beck et al. 1996). A cut off score of 15 was used for the BDI, and participants scoring over this criterion were discontinued from further participation. All participants met these criteria for the cognitive and behavioral screening.
Cognitive Testing
After meeting screening cognitive and behavioral criteria, participants were scheduled for the pre-chemotherapy baseline study day. Procedures described below were the same for each testing session. The one month assessment occurred within a six week window after the cessation of treatment and the one year follow up was one year ± one month after completing therapy.
Participants completed an extensive cognitive battery both in and out of the MRI scanner. The out of scanner battery consisted of tasks assessing attention, arousal, and verbal and spatial episodic memory. This battery was completed prior to the fMRI session and the data will be presented elsewhere when the main study is completed. Women were given a break between the testing sessions and no tasks overlapped between the in and out of scanner cognitive batteries.
The fMRI battery consisted of three tasks: the N-back test of working memory (Cohen et al. 1997), the modified Attention Network Task (mANT; Fan et al. 2002), and an emotion judgment task. We examined functional connectivity during the N-back task after statistically removing task-related activation (described below). The task-based fMRI data for all three tasks will also be presented elsewhere once the study has been completed.
To examine chemotherapy effects on cognitive processes supported by the dorsal attention network we examined working memory performance during the fMRI N-back task. During the visually presented verbal N-back sequential letter task, participants saw a string of consonants (except L, W, and Y), presented in upper case letters, one every 3 seconds. Four conditions were presented: 0-back, 1-back, 2-back, and 3-back. The 0-back control condition had a minimal working memory load; participants were asked to decide if the current letter matched a single target letter that was specified before the epoch began. During the working memory load conditions, participants were asked to decide if the current letter matched a letter that was presented either 1-, 2-, or 3-back in the sequence. The 0-, 1-, 2-, and 3-back conditions were repeated three times in a counterbalanced order such that the same condition was not repeated two times in a row. In this block design task, participants responded to nine items in each block that took 27 seconds. A rest break followed with a plus sign (+) fixation for 12 seconds. The total time of the task was eight minutes 12 seconds. Participants practiced the N-back task before each MRI session to ensure they understood the task instructions.
Participants responded to all items during the MRI scanning by button press through an MRI compatible fiber optic button response system (Eloquence System, Invivo Corp., Gainesville, FL) to indicate whether the item matched the target condition. Stimuli were delivered through an MR-safe computer monitor. Experimental tasks were programmed using the E-prime software package and presented by PC; the PC recorded subject responses and reaction times.
fMRI Scan Procedure
Participants were scanned on a Philips 3.0 Tesla Achieva scanner. All participants received the following MR sequences as part of the imaging protocol: (1) A sagittal T1- weighted spoiled gradient volumetric sequence oriented perpendicular to the anterior commissure (AC)-posterior commissure (PC) line using a repetition time (TR) of 9.9 ms, echo time (TE) of 4.6 ms, a flip angle of 8 degrees, number signal averages (NSA) 1.0, a field of view (FOV) of 256 mm, a 256 × 256 matrix, and 1.0 mm slice thickness with no gap for 140 contiguous slices. (2) A Fluid Attenuated Inversion Recovery (FLAIR) sequence was run using the AC-PC line for slice positioning. Three hundred twenty-seven slices of 1 mm thickness and no gap were acquired using TR 4822 ms, TE 380 ms, TI 1600 ms, NSA 2.0 and FOV of 256 mm. All images were reviewed by a board-certified neuroradiologist to exclude intracranial pathology. fMRI was performed using EpiBOLD (echoplanar blood oxygenation level dependent) imaging. For the fMRI sequences, a single-shot, gradient-echo, echoplanar pulse sequence was used (TR 2512 ms/TE 35 ms/flip angle 90 degrees/1 NSA for 197 volumes). Resolution was 2.5 mm × 2.8 mm × 5.0 mm. Thirty contiguous slices of 5mm thickness with no gap were obtained in the axial oblique plane, parallel to the AC-PC line using a FOV of 240 mm and a matrix size of 128 × 96. Field map correction for magnetic inhomogeneities was accomplished by acquiring images with offset TE at the end of the functional series.
Behavioral Measures
After the cognitive testing and MRI session, participants completed a number of questionnaires to assess mood and physical symptoms. On each study day participants completed the Memory Functioning Questionnaire (Gilewski et al. 1988) as a measure of subjective memory complainrs and the Beck Depression Inventory-II (Beck et al. 1996) to assess mood. We examined the effects of chemotherapy on subjective reports of memory functioning and mood because these constructs have been shown to be related to cognitive performance in some studies and are influenced by cancer and chemotherapy (Hutchinson et al. 2012).
Functional Connectivity Analyses
Functional connectivity analyses were completed using the 1000 Functional Connectomes Project Scripts available at www.nitrc.org/projects/fcon_1000/. Preprocessing was completed using both AFNI (Cox 1996) and FSL (www.fmrib.ox.ac.uk). The first five volumes of every scan were discarded. Motion correction was conducted by aligning each volume to the mean image volume. Data were spatially smoothed using a 6-mm FWHM Gaussian filter. Temporal filtering was completed with high pass of 0.01 Hz and low pass of 0.1 Hz filters followed by linear detrending to remove drift.
After skull stripping using AFNI, each individual’s T1 was co-registered to the Montreal Neurological Institute’s 152 brain template (MNI152) using FSL’s linear affine transformation (FLIRT) and nonlinear transformation (FNIRT). Nuisance signals were removed with multiple regression to control for the effects of physiological processes such as heart rate and respiration. Each participant’s 4D signal was regressed on eight predictors: white matter, cerebral spinal fluid and the six motion parameters. An additional ninth predictor was included that represented the N-back task-related activity. A box car function adjusted for the canonical hemodynamic response function representing the task and rest periods of the block design of the N-back task was created in AFNI using the wavr function. This task regressor was added to the nuisance regressor step. A similar procedure was used in Fair et al. (2007) and Kelly et al. (2008). We did not include the global signal as a nuisance regressor because this procedure has been shown to artificially bias regional correlations in a way that is related to the underlying correlational structure (Saad et al. 2012).
We examined correlations between time courses of two seed regions separately with the time courses of the rest of the voxels in the brain. The seeds were 12 mm diameter spheres centered in previously published foci (Fox et al. 2005) in the intraparietal sulcus (IPS; −25, −57-46) and the posterior cingulate cortex (PCC; −5, −49, 40). The IPS seed should reveal the effects of chemotherapy on connectivity patterns in the dorsal attention network. The PCC seed should reveal the effects of chemotherapy on connectivity patterns on the default-mode network. Each participant’s residual 4D time series was spatially normalized to MNI152 standard space. The time series from each voxel in each seed was extracted and averaged. Correlations were then calculated for this average seed time series with all other voxels in the brain using 3dfim+ (AFNI) to produce correlation maps. These individual correlation maps were converted to Z maps using Fisher’s r-to-z transformation.
Second level analyses for the functional connectivity data were computed using Statistical Parametric Mapping, version 8 (SPM8; Wellcome Department of Cognitive Neurology, University College, London, United Kingdom). A one-way repeated measures ANOVA (time: baseline, one month post-chemotherapy (M1), one year post-chemotherapy (Y1)) was used to examine the effects of chemotherapy and time on functional connectivity in the dorsal attention and default mode networks. To test the hypothesis that connectivity was decreased at M1 and partially recovered at Y1 we used the following contrast vector, 1, −2, 1 in the ANOVA model. The inverse contrast was used to examine increased connectivity at M1 with −1, 2, −1. To test the hypothesis that chemotherapy caused decreased connectivity at one month and one year we used the contrast vector 1, 0, −1 and the inverse to examine increased connectivity at each assessment −1, 0, 1. Additionally, we applied the ICBM 152 gray matter mask to isolate connectivity effects in gray matter. Because this is a pilot study we used a p-value of .05 and k=1000 in each of these analyses.
The cognitive and behavioral measures were also analyzed with the one-way repeated measures ANOVA (time: baseline, M1, Y1) in SPSS 19 (IBM). Because the age range is large we examined correlations between age and changes in working memory performance, subjective memory complaints, and mood from baseline to M1 and from M1 to Y1. No change in these measures at M1 and Y1 showed any correlations with age thus the covariate models were not used.
Results
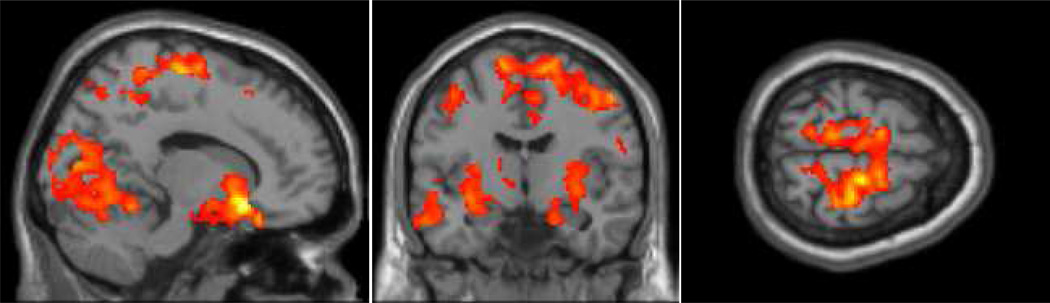
We examined the effects of chemotherapy on functional connectivity in breast cancer patients by comparing connectivity before treatment (baseline), one month (M1), and one year (Y1) after chemotherapy. At baseline the network generated by seeding the IPS included the bilateral intraparietal sulci (Brodmann’s areas (BA) 39 and 40), superior frontal sulci (BA 6 and 8), primary visual cortex (BA 17, 18, 19), and anterior cingulate cortex (BA 32). These areas are often associated with the dorsal attention network (Corbetta & Shulman 2002; Fox et al. 2005; Spreng et al 2013). We then examined connectivity patterns across the three time points. The primary analysis of interest revealed decreased connectivity at M1 that partially returned to baseline by Y1. The premotor cortex (BA 6), cuneus (BA 18), and the putamen had connectivity patterns reflecting a decrease at M1 and then partial return to baseline at Y1 (see Figure 1 and Table 2). There were no regions in the dorsal attention network that were increased at M1 and returned to baseline at Y1. We also examined the data for patterns of decreases at M1 and Y1 and found decreased connectivity over time the cerebellum (see Table 2).
Figure 1.
Functional connectivity in the dorsal attention network (p < .05; k = 1000) that decreased at one month and recovered one year after chemotherapy treatment.
Table 2.
Changes in functional connectivity at baseline, one month (M1) and one year after chemotherapy (Y1) including Talairach coordinates, cluster size, region descriptions (Brodmann’s areas, BA), peak t-values and uncorrected cluster-level p values in each region.
| Contrast | Talairach Coordinates |
Cluste Extent |
Region Description | t value | p value | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Dorsal Attention Network | ||||||||
| Decreased Connectivity at M1 recovered at Y1 | 18 | 18 | −12 | 11268 | Right superior frontal gyrus (BA 6) | 7.95 | p < .001 | |
| 24 | −86 | 16 | 6814 | Right cuneus (BA 18) | 4.89 | p < .001 | ||
| −24 | −4 | −4 | 2533 | Left putamen | 4.62 | p < .001 | ||
| Decreased Connectivity at M1 and Y1 | −30 | −50 | −16 | 1706 | Left cerebellum | 4.41 | p < .001 | |
| Default Mode Network | ||||||||
| Decreased Connectivity at M1 and Y1 | −10 | −54 | 16 | 1704 | Left precuneus (BA 31) | 4.36 | p = .001 | |
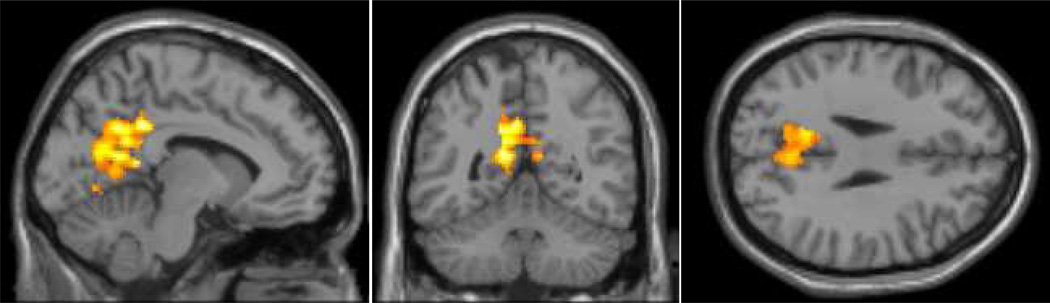
The seed in the PCC at baseline revealed the expected connectivity pattern of the default mode network in the posterior cingulate cortex/precuneus (BA 31), medial frontal cortex (BA 10), and the lateral temporal lobe (BA 22; Buckner et al. 2008). We found that connectivity decreased from baseline to M1 to Y1 in the precuneus (BA 31; see Figure 2 and Table 2). Unlike the partial return to baseline seen in the dorsal attention network at Y1, there was no similar pattern noted at Y1 in the default mode network.
Figure 2.
Functional connectivity in the default mode network (p < .05; k = 1000) that decreased one month and one year after chemotherapy.
As connectivity was assessed from a scan during which participants performed the N-back task, we examined the effects of chemotherapy on task performance. We used a 3 (time: baseline, M1, Y1) × 4 (Working memory load: 0- 1-, 2-, 3-back) repeated measures ANOVA to examine sensitivity and bias during the N-back task. Sensitivity (d’) is a measure of how similar two classes of items are (match and mismatch) and bias (C) is a measure of how likely a participant is to endorse an item as a match, both in standard deviation units. Both analyses showed no changes in working memory performance across the three assessments but there were the expected effects of working memory load on sensitivity and bias (d’: F(3,48) = 6.69, p = .002; C: F(3,48) = 7.40, p = .001; see Table 3). There were also no interactions of time and working memory load.
Table 3.
Performance measures (mean (standard deviation)) from the N-back, memory functioning questionnaire (MFQ), and Beck Depression Inventory (BDI-II) at baseline M1 and Y1.
| Task | Baseline | M1 | Y1 | |
|---|---|---|---|---|
| N-back # | ||||
| 0-back | ||||
| d’ | 2.27 (0.9) | 2.64 (0.5) | 2.40 (0.8) | |
| C | 0.20 (0.3) | 0.38 (0.2) | 0.26 (0.3) | |
| 1-back | ||||
| d’ | 1.70 (1.2) | 2.13 (1.1) | 2.29 (1.0) | |
| C | 0.22 (0.5) | 0.16 (0.3) | 0.06 (0.2) | |
| 2-back | ||||
| d’ | 1.89 (0.8) | 1.88 (0.6) | 1.68 (0.7) | |
| C | 0.05 (0.4) | 0.23 (0.2) | 0.03 (.03) | |
| 3-back | ||||
| d’ | 1.32 (0.9) | 1.50 (0.8) | 1.60 (0.8) | |
| C | 0.61 (0.3) | 0.28 (0.4) | 0.32 (0.2) | |
| MFQ | ||||
| Total Memory Complaints* | 5.20 (0.8) | 4.99 (0.6) | 4.95 (0.7) | |
| Mood | ||||
| BDI-II | 3.33 (4.0) | 4.56 (3.1) | 3.44 (4.2) | |
p < .05 effect of time;
p < .0 effect of working memory load.
Next, we examined subjective reports of memory functioning (MFQ) and mood (BDI-II; see Table 3). On the MFQ the only measure to show any change across the three time points was the Seriousness of Memory Complaints (F(2,16) = 4.40, p = .03) that showed decreasing scores across the three time points indicating increased seriousness of memory complaints. Post hoc paired t-tests showed increased Seriousness of Memory Complaints from baseline to Y1 (t(8) = 2.63, p = .03).
Mood as measured by the BDI-II also did not change across the three assessments (F(2,14) = 1.31, p = .29). None of the means at any time point were near the clinical range (largest M(SD) = 4.56 (3.1) at M1).
Discussion
In this pilot study, functional connectivity changes after chemotherapy for breast cancer were observed in the dorsal attention and the default mode networks but the pattern of changes differed. Decreased connectivity was observed one month after chemotherapy in regions of the dorsal attention network that partially returned to baseline at the one-year assessment. The pattern of change was different in the default mode network where the results showed decreased connectivity at M1 and Y1. In addition, subjective cognitive complaints increased from baseline to M1 to Y1. The implications of these pilot findings for cognitive performance, brain functioning, and mood after chemotherapy are discussed below.
Changes in connectivity in the dorsal attention network have implications for understanding the impact of chemotherapy on cognitive performance. The dorsal attention network is involved in the top-down, voluntary control of attention and regions in this network have been shown to be involved in working memory and attention tasks using task-based fMRI. McDonald et al. (2012a) found decreased working memory-related activation in the frontal lobe one month after therapy that partially recovered at one year. We showed a similar pattern with the functional connectivity analyses in this pilot study in the premotor cortex, primary visual cortex and putamen. Middle-aged participants receiving chemotherapy may not be able to achieve full recovery of functional connectivity in task-related networks as a result of decreased brain plasticity resulting from chemotherapy, aging, or a combination of both. Following participants for longer periods of time after chemotherapy may result in further recovery. However, studies of longer-term cancer survivors 10 years after chemotherapy showed decreased network connectivity in the frontal, striatal and temporal regions compared to controls (Hosseini et al. 2012). Studies of longer-term cancer survivors that assessing the treatment aging interaction may amplify the negative effects of chemotherapy. In the current pilot study, while our age range was large, age was not related to the data patterns we observed. It may be that age interacts with time since chemotherapy to affect brain functioning in long term survivors. Studies that continue to follow women prospectively over time will be able to disentangle the contributions of cognitive aging versus chemotherapy on brain functioning.
Working memory tasks require engagement of brain regions that are part of the dorsal attention network and the disengagement of the default mode network. We found no differences in working memory performance after chemotherapy in this pilot study. It is possible that the sample size in this pilot study was too small to identify a change in working memory performance. In addition, the patients in this study had high IQ and studies have shown that women with high cognitive reserve before chemotherapy experienced less post-treatment cognitive decline (e.g. Ahles et al. 2010).
While the putamen is not classically part of the dorsal attention network we found that chemotherapy affected connectivity between the IPS and putamen. There are a number of studies in the literature that have shown that the putamen is involved in attentional processes (e.g. Galvin et al. 2011) and its connectivity to cortical networks supporting attentional processes is affected by disorders such as Parkinson’s (Hacker et al. 2012), Alzheimer’s (Galvin et al. 2011), and ADHD (Mills et al. 2012), as well as in normal aging (Ystad et al. 2011). The putamen has been shown to be involved in functional tasks requiring attention as well as connectivity with attention networks (Langner & Eickhoff 2012). Thus, while it was not anticipated that the putamen would be affected by chemotherapy after breast cancer, it is correlated with other regions in the dorsal attention network and was modulated by chemotherapy and time after treatment.
Patterns of connectivity after chemotherapy were different in the default mode network than in the dorsal attention network. We hypothesized that decreased connectivity would be observed at each time point as well as increased subjective memory complaints in the default mode network. The data supported both of these hypotheses. There was decreased connectivity in the precuneus at M1 and Y1. The precuneus is one of the main hubs of the default mode network and decreased connectivity has been observed in normal aging that was related to declines in memory performance in healthy older adults (Damoiseaux et al. 2008). Reuter-Lorenz and Cimprich (2013) hypothesized that changes in default mode connectivity may be related to increased self-reflection processes after chemotherapy. The current study found that participants reported increased subjective changes in memory. Interestingly, the connectivity pattern showed that this increased subjective memory complaints was observed during decreased default mode connectivity.
The current study was a pilot study and there are some limitations that should be considered. First, the small sample size may be responsible for the lack of significant findings in the cognitive performance measures. Second, the participants had high IQ and perhaps their high cognitive reserve was protective against adverse effects of chemotherapy on performance. There was also variability in cancer stage, anti-estrogen treatment, and menopause status that is likely to contribute to the effects of chemotherapy on brain functioning. Because of the decreased variability in performance across our small sample we did not run correlation analyses between connectivity, demographic, treatment, and performance measures. Future evaluation of this relationship may help clarify how functional connectivity impacts cognitive performance and mood.
Finally, we examined functional connectivity using an from a task-based fMRI run. In other studies functional connectivity was examined during a continuous resting state scan. However, Fair et al. (2007) and Kelly et al. (2008) showed that functional connectivity can be reliably examined during a task-based fMRI scan while statistically removing task-related activation. Although neither Fair et al. (2007) nor Kelly et al. (2008) used residuals derived from a block-designed task, this method reliably generated the expected dorsal attention and default mode networks the IPS and PCC seeds, respectively. In addition, our within-subjects prospective design allowed for a direct examination of the effects of chemotherapy and time on network connectivity. Future studies may include a continuous resting state scan to further examine chemotherapy effects on functional connectivity using a prospective longitudinal design.
In the context of the limitations described, the effects of chemotherapy on functional connectivity were similar to patterns observed in a similarly designed study that examined task-based fMRI (McDonald et al. 2012a). Changes in functional connectivity may be a more sensitive measure of chemotherapy’s impact on the central nervous system compared to performance on cognitive or neuropsychological tests. The relationship and clinical relevance between connectivity changes and subjective and objective measures of cognition remains to be determined. Larger studies with healthy older adults showed that disruptions to the dorsal attention and default mode networks were related to cognitive performance declines (Andrews-Hanna et al. 2007; Tomasi & Volkow 2012). This pilot study demonstrated that functional connectivity was affected by chemotherapy treatment for breast cancer. How these changes are related to cognitive performance, mood, cancer, and age remains to be examined in larger studies with longer longitudinal follow up.
Acknowledgements
This work was supported by the Breast Cancer Research Foundation, NIA K01 AG 030380, R01 AG021476, NCRR-00109, DoE SC 0001753. The authors wish to thank the research staff of the University of Vermont CRC and Vermont Cancer Center for their hard work and support of this study. The authors acknowledge the Vermont Advanced Computing Core supported by NASA (NNX 06AC88G) at the University of Vermont for providing High Performance Computing resources that have contributed to the results reported within this paper. The authors also thank Jay Gonyea, Scott Hipko, and Trevor Andrews, Ph.D. from the University of Vermont MRI Center for Biomedical Imaging. We specially thank our volunteers for their dedication to clinical research.
Footnotes
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno J, Hosseini SM, Kesler S. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol Dis. 2012;48:329–338. doi: 10.1016/j.nbd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Science. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psychooncology. 2009;18:134–143. doi: 10.1002/pon.1379. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance images. Computers in Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G, Lavini C, Linn SC, Boven E, van Dam FS, Schagen SB. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: Converging results from multimodal magnetic resonance imaging. Hum Brain Mapp. 2012;33:2971–2983. doi: 10.1002/hbm.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, van Dam FS, Nederveen AJ, Boven E, Schagen SB. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp. 2011;32:1206–1219. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprez S, Amant F, Smeets A, Peeters R, Leemans A, Van Hecke W, Verhoeven JS, Christiaens MR, Vandenberghe J, Vandenbulcke M, Sunaert S. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30:274–281. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- First MB, RL S, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition. Washington, D.C.: American Psychiatric Press Inc.; 2001. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin JE, Price JL, Yan Z, Morris JC, Sheline YI. Resting bold fMRI differentiates dementia with Lewy bodies vs Alzheimer disease. Neurology. 2011;76:1797–1803. doi: 10.1212/WNL.0b013e31821ccc83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilewski MJ, Zelinski EM, Schaie KW. The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychology of Aging. 1988;24:665–670. doi: 10.1037//0882-7974.5.4.482. [DOI] [PubMed] [Google Scholar]
- Hacker CD, Perlmutter J, Criswell SR, Ances BM, Snyder AZ. Resting state functional connetivity of the striatum in Parkinson's disease. Brain. 2012;135(Pt 12):3699–3711. doi: 10.1093/brain/aws281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SM, Koovakkattu D, Kesler SR. Altered small-world properties of gray matter networks in breast cancer. BMC Neurol. 2012;12:28. doi: 10.1186/1471-2377-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, Wilson C. Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat Rev. 2012;38:926–934. doi: 10.1016/j.ctrv.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Inagaki M, Yoshikawa E, Matsuoka Y, Sugawara Y, Nakano T, Akechi T, Wada N, Imoto S, Murakami K, Uchitomi Y. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109:146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- Jurica PJ, Leitten CL, Mattis S. Dementia Rating Scale-2 Lutz. FL: Psychological Assessment Resources Inc.; 2001. [Google Scholar]
- Kayl AE, Wefel JS, Meyers CA. Chemotherapy and cognition: effects, potential mechanisms, and management. Am J Ther. 2006;13:362–369. doi: 10.1097/00045391-200607000-00013. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, Schagen SB. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;30:1080–1086. doi: 10.1200/JCO.2011.37.0189. [DOI] [PubMed] [Google Scholar]
- Kubler A, Murphy K, Kaufman J, Stein EA, Garavan H. Co-ordination within and between verbal and visuospatial working memory: network modulation and anterior frontal recruitment. Neuroimage. 2003;20:1298–1308. doi: 10.1016/S1053-8119(03)00400-2. [DOI] [PubMed] [Google Scholar]
- Langner R, Eickhoff SB. Sustaning attention to simple tasks: A meta-analytic review of the neurao mechanisms of vigilant attention. Psychological Bulletin epub ahead of print. 2012 doi: 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol. 2012a;30:2500–2508. doi: 10.1200/JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Smith DJ, West JD, Saykin AJ. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: A replication and extension study. Brain Behav Immun. 2012b doi: 10.1016/j.bbi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Bathula D, Dias TG, Iver SP, Fenesy MC, Musser ED, Stevens CA, Thurlow BL, Carpenter SD, Nagel BJ, Nigg JT, Fair DA. Altered cortico-striatal-thalamic connectivity in relation to spatial working memory capacity in children with ADHD. Frontiers in Psychiatry. 2012;3:1–17. doi: 10.3389/fpsyt.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg B, Ferris SH. Brief cognitive rating scale (BCRS) Psychopharmacol Bull. 1988;24:629–635. [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cimprich B. Cognitive function and breast cancer: promise and potential insights from functional brain imaging. Breast Cancer Res Treat. 2013;137:33–43. doi: 10.1007/s10549-012-2266-3. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. Trouble at rest: How correlation patterns and group differences become distorted after global signal regression. Brain Connectivity. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagen SB, Muller MJ, Boogerd W, Mellenbergh GJ, van Dam FS. Change in cognitive function after chemotherapy: a prospective longitudinal study in breast cancer patients. J Natl Cancer Inst. 2006;98:1742–1745. doi: 10.1093/jnci/djj470. [DOI] [PubMed] [Google Scholar]
- Shilling V, Jenkins V, Morris R, Deutsch G, Bloomfield D. The effects of adjuvant chemotherapy on cognition in women with breast cancer--preliminary results of an observational longitudinal study. The Breast. 2005;14:142–150. doi: 10.1016/j.breast.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic Architecture Underlying the Relations among the Default, Dorsal Attention, and Frontoparietal Control Networks of the Human Brain. J Cogn Neurosci. 2012;25:74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. Journal of Cognitive Neuroscience. 2013;25:74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Aging and functional brain networks. Mol Psychiatry. 2012;17:471, 549–458. doi: 10.1038/mp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy J, Dhillon H. The fog hasn't lifted on "chemobrain" yet: ongoing uncertainty regarding the effects of chemotherapy and breast cancer on cognition. Breast Cancer Res Treat. 2010;123:35–37. doi: 10.1007/s10549-009-0719-0. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence: The Psychological Corporation. 1999 [Google Scholar]
- Weis J, Poppelreuter M, Bartsch HH. Cognitive deficits as long-term side-effects of adjuvant therapy in breast cancer patients: 'subjective' complaints and 'objective' neuropsychological test results. Psychooncology. 2009;18:775–782. doi: 10.1002/pon.1472. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Ystad M, Hodneland E, Adolfsdottir S, Haasz J, Lundervold AJ, Eichele T, Lundervold A. Cortico-striatal connectivity and cognition in normal aging: a combined DTI and resting state fMRI study. Neuroimage. 2011;55:24–31. doi: 10.1016/j.neuroimage.2010.11.016. [DOI] [PubMed] [Google Scholar]