Abstract
OBJECTIVE
Chlamydia trachomatis Infections are a significant cause of reproductive tract pathology. Protective and pathologic immune mediators must be differentiated in order to design a safe and effective vaccine.
METHODS
Wild-type mice and mice deficient in IL-22 and IL-23 were infected intravaginally with Chlamydia muridarum and their course of infection and oviduct pathology were compared. Local genital tract and draining lymph node immune responses were also examined in IL-23 deficient mice.
RESULTS
IL-22 and IL-23 deficient mice exhibited normal susceptibility to infection and oviduct pathology. IL-23 was required for development of a Chlamydia-specific Th17 response in the lymph nodes and for production of IL-22 and IL-17 in the genital tract. However, influx of Th1 and innate immune cells was not compromised in the absence of IL-23.
CONCLUSIONS
IL-22 and IL-23 play either redundant or minimal roles in the pathogenesis of Chlamydia infection in the mouse model. Induction of Th17-associated cytokines by a Chlamydia vaccine should be avoided since these responses are not central to resolution of infection and have pathologic potential.
Keywords: Th17, immunopathology, intracellular bacteria
INTRODUCTION
Chlamydia trachomatis infections of the female reproductive tract can lead to the development of pelvic inflammatory disease, chronic pelvic pain, infertility, and ectopic pregnancy. These sequelae result from the host inflammatory response, which is dually responsible for resolution of infection and the development of genital tract pathology. Differentiation of protective immune mediators from those that are primarily pathologic is critical for vaccine development. Studies in animal models of Chlamydia genital tract infection have repeatedly demonstrated a central role for IFNγ production by CD4+ T cells in controlling infection and preventing the development of oviduct disease.1–5 This protective Th1 response is counterbalanced by the anti-inflammatory cytokine IL-10, which inhibits Th1 activation and delays clearance of C. muridarum infection from the genital tract.6
IL-22 is member of the IL-10 family of cytokines that exhibits complex protective and pathologic effects depending on the disease model examined. Although IL-10 and IL-22 have limited homology, their heterodimeric receptor complexes share a common chain, IL-10 receptor beta, and predominately induce STAT3 activation.7–10 The unique subunit of the IL-22 receptor, IL-22 receptor alpha-1, is expressed exclusively by non-hematopoietic cells including epithelial cells, while the IL-10-specific receptor subunit, IL-10 receptor alpha, is widely expressed by both hematopoietic and non-hematopoietic cells.8, 11 The expression pattern of the IL-22 receptor explains the localization of IL-22-induced responses to environmental interfaces including the skin, lungs, and gastrointestinal tract.11–14 In addition, IL-22 receptor mRNA has been detected in the female reproductive tract including the ovaries, cervix, and placenta.11, 15, 16
IL-22 promotes mucosal immunity by enhancing epithelial barrier integrity, expression of anti-microbial molecules, and mucin production.12, 13, 17, 18 The importance of IL-22 in mucosal host defense was first documented in models of infection with extracellular bacteria including Klebsiella pneumoniae pulmonary infection and Citrobacter rodentium intestinal infection, where mice succumbed to infection when IL-22 was inhibited or absent.12, 13 In contrast, IL-22 induces immunopathology in the small intestine in response to peroral infection with the intracellular parasite Toxoplasma gondii.19,20 The reduced pathology observed upon neutralization of IL-22 in this model was associated with significant decreases in proinflammatory cytokine and chemokine production in the draining lymph nodes and ileum.19 Indeed, IL-22 has been demonstrated to induce production of several neutrophil chemokines (CXCL1, -2, -3, -5, -6, -8) in addition to up regulating expression of matrix metalloproteases (MMP1, -3, -10).13, 15, 21, 22 Enhanced neutrophil influx and MMP production are clearly associated with oviduct damage in response to Chlamydia infection in the mouse model.23–27. Thus, IL-22 induces responses in other models that are linked with disease development during chlamydial genital infection.
There are a limited number of studies examining the role of IL-22 in the female reproductive tract under both physiologic conditions and in the context of infection. IL-22-producing immature NK cells have been detected in the human uterus, where they have been proposed to play a role in tissue regeneration after cyclic shedding.28 In the context of infectious diseases, mouse models of vaginal infection with Candida albicans and Neisseria gonorrheoae failed to show a requirement for IL-22 in infection control.29, 30 We previously reported significantly increased levels of IL-22 in genital tract secretions from C. muridarum-infected IFNγ-deficient mice, which were associated with a heightened Th17 response, increased neutrophil infiltration, and the development of severe oviduct pathology.2 IL-22 and IL-17 production have also been observed by CD4+ T cells isolated from the cervical washes of women infected with C. trachomatis.31 These data indicate that IL-22 may be involved in the pathogenesis of Chlamydia genital tract infection.
IL-22 is produced by Th17 cells, Th22 cells, γδ T cells, lymphoid tissue inducer cells, and NK22 cells.14, 18, 32–36 Release of both IL-17 and IL-22 from the aforementioned cells is enhanced by IL-23.14,18, 32, 36–39 IL-22 and IL-17 can cooperatively induce the production of proinflammatory cytokines, neutrophil chemokines, and anti-microbial molecules.13, 18, 22 For example, IL-22 and IL-17 enhance production of S100A8 and S100A9, which form a heterodimeric complex known as calprotectin.18 Calprotectin induces neutrophil chemotaxis, and acts as an alarmin, potently amplifying inflammation.40, 41 The interplay between IL-17 and IL-22 has been shown to dictate whether IL-22 exhibits a tissue-protective or damaging role.42 Herein, we explored the possible cooperative effects of IL-17 and IL-22, by examining the course and outcome of chlamydial infection in mice deficient in IL-23.
The intracellular life cycle of Chlamydiae dictates that resolution of infection from the genital tract is dependent on the influx of CD4+ T cells. Even in the presence of a robust innate inflammatory cell influx, such as observed in MHC class II deficient mice, infection is sustained at high levels indefinitely.43 Thus, we hypothesized that IL-22-mediated induction of antimicrobial molecules was unlikely to be significantly beneficial in infection control in this model, which would be in accordance with findings in other models of intracellular bacterial infection including Mycobacterium tuberculosis and Listeria monocytogenes.19, 44, 45 However, given the importance of neutrophil activation and MMP production in development of chlamydial-induced oviduct damage,23–25, 27, 46 we hypothesized that IL-22-mediated induction of these processes would contribute to pathology, and this cytokine either independently, or in conjunction with IL-17, would play a detrimental rather than protective role during chlamydial genital tract infection. We tested this hypothesis by comparing the course and outcome of C. muridarum genital tract infection in mice genetically deficient for IL-22 with immunologically normal mice. In addition, we utilized mice genetically deficient for IL-23 to determine if reductions in both IL-22 and IL-17 would ameliorate oviduct pathology.
MATERIALS AND METHODS
Strains, cell lines, and culture conditions
Plaque-purified C. muridarum Nigg was used for all experiments and was isolated as previously described.47, 48 All chlamydial strains were propagated in L929 cells.49 Bacteria were titrated by plaque assay 48 or as inclusion forming units (IFU) using fluorescently tagged anti-chlamydial lipopolysaccharde monoclonal antibody (Bio-Rad, Hercules, CA).50
Animals
Female C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). The IL-22 knockout (IL-22 KO) mice were kindly provided by Dr. Wenjung Ouyang14 and the IL-23p19 knockout (IL-23p19 KO) mice by Dr. Nico Ghilardi,51 both at Genentech. IL-23p19 heterozygous mice used in these studies were the F1 progeny of a C57BL/6 and IL-23p19 KO mouse. Mice were at least 7 weeks of age at the time of infection. Mice were given food and water ad libitum in an environmentally controlled room with a cycle of 12 hours of light and 12 hours of darkness. All animal experiments were approved by the University Institutional Animal Care and Use Committee.
Immunohistochemical analysis of murine genital tract tissues for the IL-22 receptor
Analysis of IL-22 receptor alpha-1 (IL-22R1) expression was conducted as previously described.52 Genital tract tissues were harvested from C57BL/6 mice, and tissue sections were made as described below (See section Microscopic histopathological assessment). Sections were deparaffinized in xylene (3 × 10 minutes) and rehydrated through sequential washings with 100%, 95% and 75% ethanol (2 × 10 minutes). Antigen retrieval was performed by boiling for 10 minutes in 10mM citrate buffer followed by incubation for 30 minutes at room temperature. After peroxidase blocking (3% hydrogen peroxide for 10 minutes), slides were blocked following the Vectastain® ABC blocking protocol for Rat IgG (Vector Laboratories, Burlingame, CA). Receptor expression was visualized using Rat anti-mouse IL-22R1 (R&D Systems, Clone: 496514) at a dilution of 1:50. Additional tissues were incubated with Rat IgG2a (R&D Systems, Clone: 54447) as a control for nonspecific staining.
Murine infection and monitoring
Five to seven days prior to infection mice were subcutaneously injected with 2.5 mg of medroxyprogesterone (Depo-Provera®; Upjohn, Kalamazoo, MI) to induce a state of anestrous.53 Mice were intravaginally inoculated with 1×105 IFU of C. muridarum Nigg diluted in 30 μl of sucrose-sodium phosphate-glutamic acid (SPG) buffer unless otherwise indicated. Mice were monitored for cervicovaginal shedding via endocervical swabs,54 and IFU were calculated as previously described.50 Bacterial burden was measured in the oviducts by plaque assay.48 Lower genital tract (LGT) bacterial burden was enumerated for IL-22 KO and C57BL/6 mice in two independent experiments with 5–6 mice per strain per experiment. The course of infection in the LGT of IL-23p19 KO and C57BL/6 was compared in three independent experiments with 4–5 mice per strain. A group of five IL-23p19 heterozygous mice was added for one of these experiments. Bacteria were titrated from the oviducts of IL-23p19 KO and C57BL/6 mice in a single experiment with 3–4 mice per strain per day of analysis.
To determine the susceptibility of C57BL/6 and IL-23p19 KO mice to low doses of infection, 10-fold serial dilutions of C. muridarum Nigg ranging from 5×101 to 5×104 bacteria were resuspended in 30 μl of SPG buffer and intravaginally inoculated into groups of 6–7 mice per dose per strain. On day 6 post-infection, mice were euthanized, and their cervices were immediately processed for detection of infection via IFU.55
Processing of oviducts for flow cytometry
Oviducts and cervices were processed for flow cytometric analysis as previously described.2, 26 Briefly, tissues were harvested and minced with scissors. For measurement of cytokines and bacterial burden, an aliquot of the minced tissue was stored at −80°C until analysis. Cervices were digested with collagenase I (1mg/ml; Sigma-Aldrich, St. Louis, MO), and then cervices and oviducts were repeatedly passed through a 70 μm filter to yield a single cell suspension. Single cell suspensions were incubated with Fc Block (BD Pharmingen; Clone: 2.4G2), and cell surface proteins were subsequently stained with the indicated antibodies. Stimulation of cells for analysis of intracellular cytokines was conducted as below (See section Detection of Chlamydia-specific cytokine production by intracellular flow cytometry). Flow cytometry data were acquired using an LSR II Analyzer (BD Biosciences) and analyzed via FlowJo software (Tree Star, Ashland, OR).
Detection of Chlamydia-specific cytokine production by intracellular flow cytometry
For analysis of cytokine production, single cell suspensions generated from the cervix or oviducts of individual mice were incubated overnight in complete medium (DMEM containing 10% FBS, 2 mM glutamine, 100 μM non-essential amino acids, 50 μM β-mercaptoethanol, 100 μg/ml vancomycin and 50 μg/ml gentamicin) with 5 μg/ml of gradient purified UV-inactivated C. muridarum elementary bodies (UV-EBs).56 GolgiPlug™ (1:500 final dilution; BD Biosciences) was added for the last 4 hours of incubation. Cell surface proteins were stained with PerCP-Cy5.5 anti-mouse CD45 (Clone: 30-F11), V450 anti-mouse CD3 (Clone 500A2), and PE anti-mouse CD4 (Clone RM4-5) all from BD Biosciences. After surface staining, cells were fixed, permeabilized, and stained with APC anti-mouse IFNγ (BD 2Biosciences, clone XMG1.2) according to manufacturers instructions (Cytofix/Cytoperm™Kit, BD Biosciences). Cytokine production by WT and IL-23p19 KO cells was analyzed on days 7, 10, and 14 of infection with 3–4 mice per strain per day in two independent experiments.
Detection of cytokines in lower genital tract secretions and oviduct homogenates
LGT secretions were collected via washing the vaginal vault with 100 μl of phosphate-buffered saline with protease inhibitor (Complete EDTA-free protease inhibitor tablets, Roche Diagnostics) during the first ten days of infection as previously described.57 IL-17, TNFα, and IFNγ were quantified in these lavages and in the homogenized oviducts of C57BL/6 and IL-23p19 KO mice via multiparametric bead array (Millipore, Billerica, MA). IL-22 was measured by ELISA (R&D Systems, Minneapolis, MN). Cytokines were monitored in the LGT secretions of C57BL/6 and IL-23p19 KO mice in two independent experiments with 4–5 mice per group, and cytokines were measured in the oviducts of 3–5 mice per group per day.
Assessment of Chlamydia-specific cytokine responses in the iliac nodes
Iliac nodes from IL-23p19 KO and C57BL/6 mice infected intravaginally with C. muridarum were harvested on days 0, 7, 14, 21, 28, 35, and 56 post infection. Lymph nodes were processed to a single cell suspension and placed in culture with media alone or UV-EBs (5 μg/well). Supernatants were collected after 96 hours in culture for quantification of cytokines as described above. Cytokine production by iliac lymph node mononuclear cells was evaluated using 5 mice per strain per day.
Microscopic histopathological assessment
Genital tracts were removed en bloc, fixed in 10% buffered formalin, and embedded in paraffin. Longitudinal 4-μm sections were cut and stained with hematoxylin and eosin. Oviduct epithelial cell erosion and oviduct dilatation were assessed for tissues harvested on day 42 using a four-tiered semi-quantitative scoring system by a pathologist blinded to the experimental design.50, 58 Oviduct pathology for IL-22 KO and C57BL/6 mice was compared on day 42 in two separate experiments with 5–6 mice per group per experiment, and the same comparison was conducted for IL-23p19 KO and C57BL/6 mice.
Statistics
Statistical comparison of flow cytometry data, cytokine levels, or the course of infection was conducted via two-way ANOVA with Bonferroni post-test analysis. A Mann-Whitney U test was used to determine significant differences in pathology scores. A Fisher’s exact test was used to determine differences in susceptibility to low dose infection. Comparisons of pathological data, cytokine responses over time, and course of infection over time requires 8–10 tissues or mice per group to yield a power of 0.74–0.90 to detect a 25–30% difference between groups since the variance may approach 20% because of biological variability of the infection and response to infection among individual animals. Prism software (GraphPad Software, LaJolla, CA) was utilized for all statistical analysis. Values of P < 0.05 were considered significant.
RESULTS
Murine genital tract epithelial cells express the IL-22 receptor
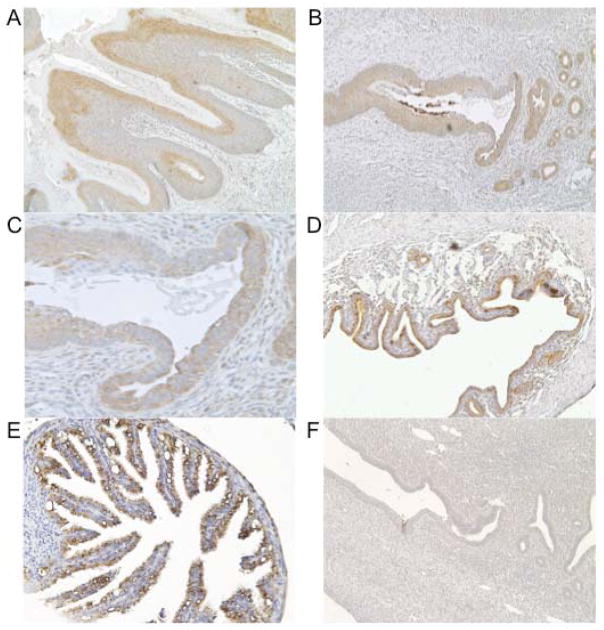
The IL-22 receptor is a dimeric complex of the IL-10 receptor beta chain (IL-10R2), which is ubiquitously expressed, and the IL-22 receptor alpha-1 chain (IL-22R1), which is only expressed by non-hematopoietic cells.8, 10, 11 Although a role for IL-22 receptor signaling has been reported at mucosal sites including the pulmonary and gastrointestinal tracts,12, 13 expression of this protein has not been previously described in the genital tract. Using immunohistochemistry, we detected IL-22R1 expression in the murine ectocervix (Fig. 1A), endocervix (Fig. 1B and 1C), uterine horns (Fig. 1D), and oviducts (Fig. 1E). Receptor expression was localized to the epithelium, and no staining was observed for stromal cells of the genital tract. No staining was observed when sections were incubated with the relevant immunoglobulin isotype (Fig. 1F).
FIG. 1. IL-22 receptor expression was detected in the murine genital tract.
Genital tracts from uninfected C57BL/6 mice were stained with anti-IL-22R1. Ectocervix (A; magnification, x100), Squamocolumnar junction (B; magnification, x100), Squamocolumnar junction (C; magnification, x200), Uterine horn (D; magnification, x100), Oviduct (E; magnification, x200), negative control (Rat IgG2a) uterine horn (F; magnification, x200).
IL-22 deficiency has no effect on bacterial burden or oviduct pathology
Since we detected expression of IL-22R1, and we previously documented IL-22 in murine genital tract secretions during active C. muridarum infection,2 we sought to determine if IL-22 was involved in resolution of infection from the genital tract. Comparison of the course of lower genital tract infection for C57BL/6 and IL-22 deficient mice revealed that infection resolved with normal kinetics in the absence of IL-22 (Fig. 2A). In addition, none of the mice exhibited clinical signs of bacterial dissemination as has been observed in models of infection with extracellular bacteria in the absence of IL-22.13, 39 We also examined the possibility that IL-22 could influence oviduct pathology in this model. Histological analysis revealed that erosion of the oviduct epithelium was comparable between strains (Fig. 2B). In addition, we detected similar degrees of oviduct dilatation in the presence and absence of IL-22, with 5 of 6 mice in both groups developing severe dilatation (Fig. 2C).
Fig. 2. Resolution of C. muridarum infection and the development of oviduct pathology are not influenced by the absence of IL-22.
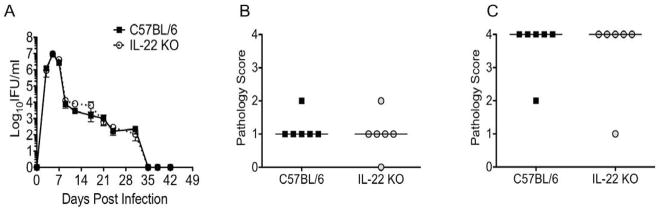
(A) The kinetics of lower genital tract infection for C57BL/6 (black squares) and IL-22 KO mice (open circles, dashed line) does not differ (P > 0.05 via two-way repeated measures ANOVA). Data points represent the mean ± SEM of IFU values from 6 mice per strain from a single representative experiment of two. (B,C) Histological analysis of oviduct epithelial cell erosion (B) and oviduct dilatation (C) in genital tracts harvested on day 42 post-infection revealed no difference between the strains. (P > 0.05 via Mann-Whitney U-test). Data points represent semi-quantitative scoring of oviduct pathology for individual mice with 6 mice per strain from a single experiment of two. C57BL/6 (black squares) and IL-22 KO mice (open circles). Median indicated by horizontal line.
IL-23 induces IL-17 and IL-22 production in response to C. muridarum infection in the genital tract and iliac lymph nodes
IL-23 is composed of the shared IL-12p40 subunit and the unique IL-23p19 subunit.59 IL-23 enhances the release of IL-17 and IL-22 from both innate and adaptive immune cells.14,18, 32, 37–39 In order to determine the role of IL-23 in the cytokine response to C. muridarum genital tract infection, we intravaginally infected IL-23p19 deficient mice. Lower genital tract secretions were collected for the first 10 days of C. muridarum infection, and oviducts were harvested on day 10 post-infection. These time points represent peak days of cytokine production at both sites.26 Examination of cytokine levels in the absence of IL-23 revealed significantly reduced IL-17 (Fig. 3A and E) and IL-22 (Fig. 3B and F) but no difference in TNFα (Fig. 3C and G) or IFNγ (Fig. 3D and H) at either of these sites.
FIG. 3. IL-23 induces production of IL-17 and IL-22 but not TNFα or IFNγ in the genital tract during C. muridarum infection.
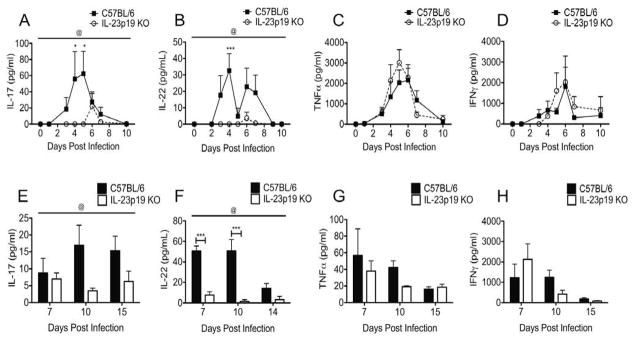
(A–D) Levels of IL-17 (A) and IL-22 (B) in the vaginal lavages of IL-23p19 KO mice (clear circles, dashed line) were significantly reduced over the course of infection compared to C57BL/6 mice (black squares), but no difference was detected for TNFα (C) or IFNγ (D). Data points represent the mean ± SEM for 4–5 mice per strain from one representative experiment of two. @, P < 0.05 for IL-17 and IL-22 (by two-way ANOVA over the interval measured). *, P < 0.05; ***, P < 0.001 on individual days (by two-way ANOVA with Bonferroni post-test analysis). (E–H) Measurement of cytokines in the homogenized oviducts of infected mice revealed significantly reduced levels of IL-17 (E) and IL-22 (F) over the course of infection in the absence of IL-23 but no difference in TNFα (G) and IFNγ (H). Data points represent the mean ± SEM for 3–5 mice strain per day. @, P < 0.05 for IL-17 and IL-22 (by two-way ANOVA over the interval measured). ***, P < 0.001 for IL-22 on individual days by (two-way ANOVA with Bonferroni post-test analysis).
IL-23 has been previously shown to promote the stability of the Th17 lineage.60 In order to determine the role of IL-23 in the adaptive immune response to Chlamydia, we harvested the iliac lymph nodes (ILN) from infected mice and stimulated them with C. muridarum elementary bodies. In the absence of IL-23, Chlamydia-specific release of both IL-17 and IL-22 was significantly reduced (Fig. 4A and B). Similar to our previous findings in IL-17 receptor deficient mice,2 IFNγ production was reduced on day 7 in the ILNs of IL-23p19 deficient mice, but levels were comparable to those detected for wild-type mice by day 14 (Fig. 4C). Despite early reductions of IFNγ in the ILN, flow cytometry revealed no difference in the frequency of Chlamydia-specific IFNγ-producing CD3+CD4+ T cells in either the cervix or oviducts on days 7, 10, or 14 post-infection (data not shown). These findings are in accordance with the detection of normal levels of IFNγ at both of these sites in the absence of IL-23 (Fig. 3D and H). These data indicate that IL-23p19 KO mice provide an appropriate model to examine the contributions of IL-17 and IL-22 to chlamydial pathogenesis without the confounding effects that would result from reductions in the protective cytokine IFNγ.2–4
Fig. 4. In the absence of IL-23, Chlamydia-specific cytokine production is reduced in the iliac lymph nodes.
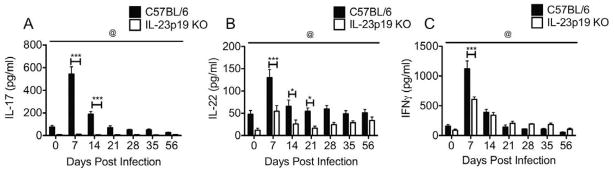
(A–C) Significantly reduced levels of IL-17 (A), IL-22 (B), and IFNγ (C) were measured in the supernatants of Iliac lymph node mononuclear cells from IL-23p19 KO (white bars) mice restimulated in vitro for 96 hours in the presence of UV-EBs relative to those from C57BL/6 mice (black bars). Bars represent the mean ± SEM for 5 mice/strain. @, P < 0.0001 for IL-17 and IL-22 and @, P < 0.05 for IFNγ (by two-way ANOVA over the interval measured). *, P < 0.05; **, P < 0.01; ***, P < 0.001 on individual days (by two-way ANOVA with Bonferroni post-test analysis).
Infection resolves with normal kinetics in the absence of IL-23
Given the role of IL-17 and IL-22 in enhancing mucosal immunity, we sought to determine if the reductions in IL-17 and IL-22 that we observed in the absence of IL-23 impacted the ability of mice to control infection in either the lower or upper genital tract. We followed the course of lower genital tract infection in C57BL/6, IL-23p19 deficient and IL-23p19 heterozygous mice and found no difference in the kinetics of infection between any of the strains (Fig. 5A). We also found no difference in the bacterial burden in the oviducts over the peak days of infection in the absence of IL-23 (Fig. 5B).
Fig. 5. C. muridarum infection resolves with normal kinetics in the absence of IL-23.
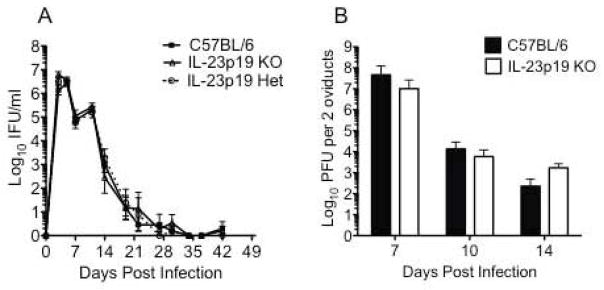
(A) The kinetics of lower genital tract infection for C57BL/6 (black squares), IL-23p19 KO (open triangles), and IL-23p19 heterozygous mice (open circles) does not differ. Data points represent the mean ± SEM of IFU values from 4–5 mice per strain from a single experiment of three. (B) Analysis of bacterial burden in the oviducts revealed no difference between C57BL/6 (black bars) and IL-23p19 KO mice (white bars). Bars represent the mean ± SEM of PFU for two pooled oviducts of individual mice with 3–4 mice per strain per day.
Although we did not detect a difference in the kinetics of infection when mice were infected with 100,000 bacteria, we recognized that with such a high dose of infection, innate defense mechanisms induced by IL-22 and IL-17 may be overwhelmed. To explore this possibility, we infected C57BL/6 and IL-23p19 deficient mice with doses of C. muridarum Nigg ranging from 50 to 50,000 microorganisms. All of the mice from both strains established an active infection upon inoculation with as few as 500 IFU (data not shown). When the innoculum was decreased to 50 IFU, 6 of 7 C57BL/6 mice and 2 of 6 IL-23p19 deficient mice developed an active infection, but these differences were not statistically significant (P > 0.05 Fisher’s exact test).
IL-23 is not required for influx of innate immune cells into the oviduct or the development of oviduct pathology
Innate immune responses are key for the development of chlamydia-induced immunopathology,24–27, 61, 62 and IL-17 and IL-22 have been shown to cooperate in inducing release of neutrophil chemokines and promoting innate inflammation13, 18, 22 Thus, we examined the influx of innate inflammatory cells into the oviducts of wild-type and IL-23p19 deficient mice on day 10 post-infection. Flow cytometry revealed no differences in the frequency of neutrophils, inflammatory monocytes, or macrophages between the strains at this time point (Fig. 6A). In accordance with these data, no improvement in the severity of oviduct dilatation was found in IL-23p19 deficient mice (Fig. 6B).
Fig. 6. IL-23 is not required for influx of acute inflammatory cells into the oviducts or the development of oviduct pathology.
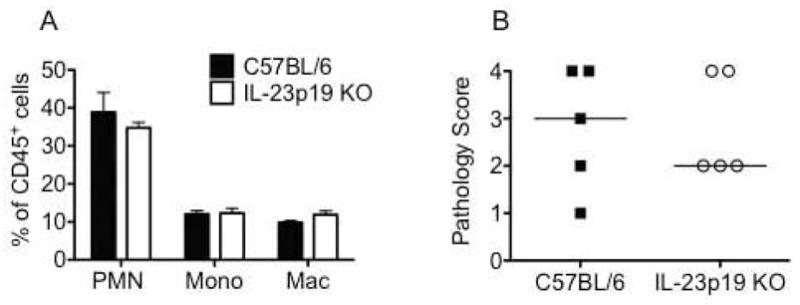
(A) On day 10 post-infection, flow cytometric analysis revealed no difference in the frequency of innate inflammatory cells in the oviducts of C57BL/6 (black bars) and IL-23p19 KO mice (white bars). Bars represent the mean ± SEM of the frequency of CD45+ cells in the oviducts of 4 mice per strain for one representative experiment of two. PMN: Ly6G/Chigh F4/80negCD11cneg; Mono (inflammatory monocytes): Ly6G med F4/80negCD11cneg; Mac (macrophages): F4/80pos (B) Histological analysis of oviduct dilatation in genital tracts harvested on day 42 post-infection revealed no difference between the strains (P > 0.05 via Mann-Whitney U-test). Data points represent semi-quantitative scoring of oviduct dilatation of individual mice for 5 mice per strain from one representative experiment of two. C57BL/6 (black squares) and IL-23p19 KO mice (open circles). Median indicated by horizontal line.
DISCUSSION
In the studies outlined in this manuscript, we explored the role of Th17 cells and the associated cytokines IL-22, IL-17, and IL-23 in the mouse model of Chlamydia genital tract infection. We show for the first time that the epithelial cells of the murine genital tract express the unique subunit of the IL-22 receptor, IL-22R1. We also demonstrate that IL-23 is required for IL-17 and IL-22 production in response to C. muridarum infection and is necessary for the maintenance of a Chlamydia-specific Th17 response in the draining lymph nodes. However, we were unable to detect a requirement for any of these cytokines in resolution of infection, susceptibility to low dose infection, or the development of oviduct pathology. The normal resolution of infection observed for IL-23p19 deficient mice could be predicted given these mice developed a Th1 response comparable to wild-type mice. Similarly, no compromise was seen in the ability of innate inflammatory cells to migrate into the oviducts of IL-23p19 deficient mice, and this influx was associated with oviduct pathology similar to that observed for C57BL/6 mice.
The IL-22 receptor is a heterodimer of IL-22R1 and IL-10R2, with expression of IL-22R1 limited to non-hematopoietic cells.11 IL-22R1 expression was previously detected in the human cervix and ovary by microarray,15 but protein expression in vivo has not been previously demonstrated. We detected IL-22R1 expression localized to the epithelium of the ectocervix, endocervix, uterine horns, and oviducts of uninfected C57BL/6 mice. Inflammatory stimuli including LPS, IFNγ, TNFα, and IL-1β may induce higher levels of IL-22R1 expression during acute chlamydial infection.11, 63 Detection of this receptor on the epithelium of the genital tract is in accordance with reports of receptor expression by epithelial cells at environmental interfaces including the skin, lung, and gastrointestinal tract.11–14 Columnar epithelial cells of the genital tract are the site of chlamydial replication and are highly susceptible to infection-induced damage. Thus, the IL-22 receptor is appropriately located to play a role during chlamydial infection. Despite detection of this receptor, we determined that C. muridarum infection resolved normally in IL-22 deficient mice. Resolution of infection was also normal for IL-23p19 deficient mice despite nearly undetectable levels of both IL-17 and IL-22 in the lower and upper genital tract. These findings are not unique to C. muridarum, as pulmonary infection with the intracellular bacterium Mycobacterium tuberculosis resolved normally in the absence of IL-17 and IL-22.19, 64
There are several possible explanations for why we observed normal control of chlamydial infection in IL-22 and IL-23p19 deficient mice. It is likely that the intracellular replicative niche of chlamydiae hinders the potential protective capacity of anti-microbial proteins induced by these cytokines, which include S100A proteins, β-defensins, Reg proteins, and lipocalins.13, 15, 18 Chlamydiae are susceptible to anti-microbial peptides in vitro,65, 66 but innate defense mechanisms have a limited ability to resolve C. muridarum infection in vivo independently of the adaptive immune response.43 It is also possible that the ability of chlamydiae to directly stimulate pattern recognition receptors 67 on epithelial cells and tissue resident immune cells obviates the requirement for epithelial-targeting cytokines peripheral to the Th1 response.62, 67, 68 Although IL-17 and IL-22 can induce the production of Th1 chemokines including CXCL9,13 we observed no deficit in IFNγ production or Th1 migration to the genital tract of IL-23p19 deficient mice. PRR stimulation by Chlamydia induces the production of many proinflammatory cytokines that can activate the same pathways as IL-22 and IL-17 62, 67. These cytokines, in combination with pathways induced directly by PRR stimulation, likely augment production of chemokines necessary for innate and adaptive inflammatory cell influx into the genital tract, thus obviating the requirement for IL-22 and IL-17.
It was previously observed that IL-17 played an important role in inducing the Th1 response to C. muridarum infection in the lung and was required for normal resolution of pulmonary infection.69 This contrasts with our findings of only slight reductions in IFNγ and no compromise in resolution of genital tract infection in the absence of IL-17 receptor signaling,2 or IL-23-dependent induction of the Th17-related cytokines, IL-22 and IL-17 (current work). The genital tract is a mucosal site that must maintain a tolerogenic environment for proper reproductive fitness. Thus, there appears to be a site-specific role for IL-17 in defense against chlamydial infections, and this may hold true for IL-22 as well. Such tissue-specificity is described for Candida albicans infection where Th17 cytokines were required for control of oropharyngeal candidiasis 70, but in vulvovaginal candidasis, infection resolved normally in the absence of IL-22, IL-17 and IL-23.29
We also determined that Chlamydia-induced genital tract pathology was not altered in the absence of either IL-22 or IL-23. It was difficult to predict whether IL-22 would prevent or induce tissue damage in this model given its complex and dual roles in other models. We hypothesized that since IL-17 and IL-22 enhance neutrophil chemokine production and promote MMP release,13, 15, 21, 22 these cytokines would promote damage. We expected to observe decreased epithelial erosion and oviduct hydrosalpinx in IL-22 and IL-23p19 deficient mice. However, we determined that similar degrees of oviduct pathology developed in both the presence and absence of IL-17,2 IL-22, and IL-23, which indicates that if these cytokines promote pathologic responses, their role is redundant with other cytokines. On the other hand, IL-22 has been demonstrated to enhance epithelial regeneration after an inflammatory insult.13, 17, 42, 45, 52, 71 Despite known regenerative effects of this cytokine, we did not observe any difference in the degree of epithelial erosion in the oviducts of IL-22-deficient and wild-type mice. When fully virulent C. muridarum are used for infection, enhanced early control of infection and prevention of ascension of Chlamydia to the oviduct may be required to prevent oviduct damage.57, 72 This does not preclude the potential for IL-22 to play a protective and regenerative role in the human genital tract, where Chlamydia trachomatis infection is frequently more indolent and chronic in nature.73
We previously demonstrated that during C. muridarum genital infection, IFNγ-deficient mice exhibited significantly increased bacterial burden, enhanced production of Th17-differentiating cytokines, predominant Th17 and neutrophilic responses, an increased IL-22 response, and enhanced genital tract tissue damage.2 Chlamydia trachomatis has been shown to induce IL-23 by a combination of toll-like receptor stimulation and endoplasmic reticulum stress signals,74 both of which would be augmented in the presence of a suboptimal Th1 response and increased bacterial burden. CD4+ T cells isolated from the cervix of women actively infected with C. trachomatis have been observed to produce IL-17 and IL-22.31 These cells may play dual roles due to the complex interactions of these cytokines in vivo. 13, 42 However, data in the mouse model indicate a primary role for Th1 cells in host defense and resolution of infection, and our data in mice deficient for the IL-17 receptor,2 or for Th17 cells and their downstream cytokines reveal that this pathway is dispensable for inducing a robust Th1 response and for resolution of genital tract infection and does not contribute substantially to protection from tissue damage. Given the fragile nature of the female oviduct, and the documented complex and often tissue injurious roles for IL-17 and IL-22, we propose that chlamydial vaccine strategies should avoid induction of these cytokines and focus on selective enhancement of the IFNγ response to chlamydial antigens.
Acknowledgments
This work was supported by the NIH-NIAID via grants R01 AI054624 and U19 AI084024 (NIAID) to T.D and by a grant from Children’s Hospital of Pittsburgh of the UPMC Health System to L.F. We are grateful to Alison Logar for assistance with flow cytometry.
References
- 1.Rank RG, Ramsey KH, Pack EA, Williams DM. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect Immun. 1992;60:4427–4429. doi: 10.1128/iai.60.10.4427-4429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scurlock AM, Frazer LC, Andrews CW, Jr, O’Connell CM, Foote IP, Bailey SL, Chandra-Kuntal K, Kolls JK, Darville T. IL-17 contributes to generation of Th1 immunity and neutrophil recruitment during Chlamydia muridarum genital tract infection but is not required for macrophage influx or normal resolution of infection. Infect Immun. 2011;79:1349–1362. doi: 10.1128/IAI.00984-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 4.Cotter TW, Ramsey KH, Miranpuri GS, Poulsen CE, Byrne GI. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infection and Immunity. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su H, Caldwell HD. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infection and Immunity. 1995;63:3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Igietseme JU, Ananaba GA, Bolier J, Bowers S, Moore T, Belay T, Eko FO, Lyn D, Black CM. Suppression of endogenous IL-10 gene expression in dendritic cells enhances antigen presentation for specific Th1 induction: potential for cellular vaccine development. JImmunol. 2000;164:4212–4219. doi: 10.4049/jimmunol.164.8.4212. [DOI] [PubMed] [Google Scholar]
- 7.Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164:1814–1819. doi: 10.4049/jimmunol.164.4.1814. [DOI] [PubMed] [Google Scholar]
- 8.Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem. 2001;276:2725–2732. doi: 10.1074/jbc.M007837200. [DOI] [PubMed] [Google Scholar]
- 9.Wehinger J, Gouilleux F, Groner B, Finke J, Mertelsmann R, Weber-Nordt RM. IL-10 induces DNA binding activity of three STAT proteins (Stat1, Stat3, and Stat5) and their distinct combinatorial assembly in the promoters of selected genes. FEBS Lett. 1996;394:365–370. doi: 10.1016/0014-5793(96)00990-8. [DOI] [PubMed] [Google Scholar]
- 10.Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, Gurney AL. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem. 2000;275:31335–31339. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 11.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 13.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 15.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 16.Gruenberg BH, Schoenemeyer A, Weiss B, Toschi L, Kunz S, Wolk K, Asadullah K, Sabat R. A novel, soluble homologue of the human IL-10 receptor with preferential expression in placenta. Genes Immun. 2001;2:329–334. doi: 10.1038/sj.gene.6363786. [DOI] [PubMed] [Google Scholar]
- 17.Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson MS, Feng CG, Barber DL, Yarovinsky F, Cheever AW, Sher A, Grigg M, Collins M, Fouser L, Wynn TA. Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J Immunol. 2010;184:4378–4390. doi: 10.4049/jimmunol.0903416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, Plickert R, Bereswill S, Fischer A, Dunay IR, Wolk K, Loddenkemper C, Krell HW, Libert C, Lund LR, Frey O, Holscher C, Iwakura Y, Ghilardi N, Ouyang W, Kamradt T, Sabat R, Liesenfeld O. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrix metalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med. 2009;206:3047–3059. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sestito R, Madonna S, Scarponi C, Cianfarani F, Failla CM, Cavani A, Girolomoni G, Albanesi C. STAT3-dependent effects of IL-22 in human keratinocytes are counterregulated by sirtuin 1 through a direct inhibition of STAT3 acetylation. FASEB J. 2011;25:916–927. doi: 10.1096/fj.10-172288. [DOI] [PubMed] [Google Scholar]
- 22.Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A, Shimizu N, Fujiyama Y. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969–984. doi: 10.1053/j.gastro.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 23.Imtiaz MT, Schripsema JH, Sigar IM, Kasimos JN, Ramsey KH. Inhibition of matrix metalloproteinases protects mice from ascending infection and chronic disease manifestations resulting from urogenital Chlamydia muridarum infection. Infect Immun. 2006;74:5513–5521. doi: 10.1128/IAI.00730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HY, Schripsema JH, Sigar IM, Lacy SR, Kasimos JN, Murray CM, Ramsey KH. A role for CXC chemokine receptor-2 in the pathogenesis of urogenital Chlamydia muridarum infection in mice. FEMS Immunology & Medical Microbiology. 2010;60:49–56. doi: 10.1111/j.1574-695X.2010.00715.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee HY, Schripsema JH, Sigar IM, Murray CM, Lacy SR, Ramsey KH. A link between neutrophils and chronic disease manifestations of Chlamydia muridarum urogenital infection of mice. FEMS Immunol Med Microbiol. 2010;59:108–116. doi: 10.1111/j.1574-695X.2010.00668.x. [DOI] [PubMed] [Google Scholar]
- 26.Frazer LC, O’Connell CM, Andrews CW, Jr, Zurenski MA, Darville T. Enhanced neutrophil longevity and recruitment contribute to the severity of oviduct pathology during Chlamydia muridarum infection. Infect Immun. 2011;79:4029–4041. doi: 10.1128/IAI.05535-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacy HM, Bowlin AK, Hennings L, Scurlock AM, Nagarajan UM, Rank RG. Essential role for neutrophils in pathogenesis and adaptive immunity in Chlamydia caviae ocular infections. Infect Immun. 2011;79:1889–1897. doi: 10.1128/IAI.01257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Male V, Hughes T, McClory S, Colucci F, Caligiuri MA, Moffett A. Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J Immunol. 2010;185:3913–3918. doi: 10.4049/jimmunol.1001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yano J, Kolls JK, Happel KI, Wormley F, Wozniak KL, Fidel PL., Jr The acute neutrophil response mediated by S100 alarmins during vaginal Candida infections is independent of the Th17-pathway. PLoS One. 2012;7:e46311. doi: 10.1371/journal.pone.0046311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feinen B, Russell MW. Contrasting Roles of IL-22 and IL-17 in Murine Genital Tract Infection by Neisseria gonorrhoeae. Front Immunol. 2012;3:11. doi: 10.3389/fimmu.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jha R, Srivastava P, Salhan S, Finckh A, Gabay C, Mittal A, Bas S. Spontaneous secretion of interleukin-17 and -22 by human cervical cells in Chlamydia trachomatis infection. Microbes Infect. 2011;13:167–178. doi: 10.1016/j.micinf.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujita H, Nograles KE, Kikuchi T, Gonzalez J, Carucci JA, Krueger JG. Human Langerhans cells induce distinct IL-22-producing CD4+ T cells lacking IL-17 production. Proc Natl Acad Sci U S A. 2009;106:21795–21800. doi: 10.1073/pnas.0911472106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov, Littman DR, O’Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 37.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Buonocore S, Ahern PP, Uhlig HH, Ivanov, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, Tardif MR, Sathaliyawala T, Kubota M, Farber DL, Collman RG, Shaked A, Fouser LA, Weiner DB, Tessier PA, Friedman JR, Kiyono H, Bushman FD, Chang KM, Artis D. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol. 2003;170:3233–3242. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- 41.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 42.Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med. 2010;207:1293–1305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infection and Immunity. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graham AC, Carr KD, Sieve AN, Indramohan M, Break TJ, Berg RE. IL-22 production is regulated by IL-23 during Listeria monocytogenes infection but is not required for bacterial clearance or tissue protection. PLoS One. 2011;6:e17171. doi: 10.1371/journal.pone.0017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imtiaz MT, Distelhorst JT, Schripsema JH, Sigar IM, Kasimos JN, Lacy SR, Ramsey KH. A role for matrix metalloproteinase-9 in pathogenesis of urogenital Chlamydia muridarum infection in mice. Microbes Infect. 2007 doi: 10.1016/j.micinf.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Connell CM, Ingalls RR, Andrews CW, Jr, Skurlock AM, Darville T. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. Journal of Immunology. 2007;179:4027–4034. doi: 10.4049/jimmunol.179.6.4027. [DOI] [PubMed] [Google Scholar]
- 48.O’Connell CM, Nicks KM. A plasmid-cured Chlamydia muridarum strain displays altered plaque morphology and reduced infectivity in cell culture. Microbiology. 2006;152:1601–1607. doi: 10.1099/mic.0.28658-0. [DOI] [PubMed] [Google Scholar]
- 49.O’Connell CM, Abdelrahman YM, Green E, Darville HK, Saira K, Smith B, Darville T, Scurlock AM, Meyer CR, Belland RJ. TLR2 activation by Chlamydia trachomatis is plasmid-dependent and plasmid-responsive chromosomal loci are coordinately regulated in response to glucose limitation by C. trachomatis but not by C. muridarum. Infect Immun. 2011 doi: 10.1128/IAI.01118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Darville T, Andrews CW, Jr, Laffoon KK, Shymasani W, Kishen LR, Rank RG. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect Immun. 1997;65:3065–3073. doi: 10.1128/iai.65.8.3065-3073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghilardi N, Kljavin N, Chen Q, Lucas S, Gurney AL, De Sauvage FJ. Compromised humoral and delayed-type hypersensitivity responses in IL-23-deficient mice. J Immunol. 2004;172:2827–2833. doi: 10.4049/jimmunol.172.5.2827. [DOI] [PubMed] [Google Scholar]
- 52.Pociask DA, Scheller EV, Mandalapu S, McHugh KJ, Enelow RI, Fattman CL, Kolls JK, Alcorn JF. IL-22 Is Essential for Lung Epithelial Repair following Influenza Infection. Am J Pathol. 2013;182:1286–1296. doi: 10.1016/j.ajpath.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tuffrey M, Taylor-Robinson D. Progesterone as a key factor in the development of a mouse model for genital-tract infection with Chlamydia trachomatis. FEMS Microbiol Let. 1981;12:111–115. [Google Scholar]
- 54.Kelly KA, Robinson EA, Rank RG. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infect Immun. 1996;64:4976–4983. doi: 10.1128/iai.64.12.4976-4983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagarajan UM, Sikes J, Prantner D, Andrews CW, Jr, Frazer L, Goodwin A, Snowden JN, Darville T. MyD88 deficiency leads to decreased NK cell gamma interferon production and T cell recruitment during Chlamydia muridarum genital tract infection, but a predominant Th1 response and enhanced monocytic inflammation are associated with infection resolution. Infect Immun. 2011;79:486–498. doi: 10.1128/IAI.00843-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramsey KH, Soderberg LS, Rank RG. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect Immun. 1988;56:1320–1325. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riley MM, Zurenski MA, Frazer LC, O’Connell CM, Andrews CW, Jr, Mintus M, Darville T. The recall response induced by genital challenge with Chlamydia muridarum protects the oviduct from pathology but not from reinfection. Infect Immun. 2012;80:2194–2203. doi: 10.1128/IAI.00169-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Darville T, Andrews CW, Jr, Sikes JD, Fraley PL, Braswell L, Rank RG. Mouse strain-dependent chemokine regulation of the genital tract T helper cell type 1 immune response. Infect Immun. 2001;69:7419–7424. doi: 10.1128/IAI.69.12.7419-7424.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F, To W, Wagner J, O’Farrell AM, McClanahan T, Zurawski S, Hannum C, Gorman D, Rennick DM, Kastelein RA, de Waal Malefyt R, Moore KW. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 60.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prantner D, Darville T, Sikes JD, Andrews CW, Jr, Brade H, Rank RG, Nagarajan UM. Critical role for interleukin-1beta (IL-1beta) during Chlamydia muridarum genital infection and bacterial replication-independent secretion of IL-1beta in mouse macrophages. Infection and Immunity. 2009;77:5334–5346. doi: 10.1128/IAI.00883-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Darville T, O’Neill JM, Andrews CW, Jr, Nagarajan UM, Stahl L, Ojcius DM. Toll-like receptor-2, but not toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J Immunol. 2003;171:6187–6197. doi: 10.4049/jimmunol.171.11.6187. [DOI] [PubMed] [Google Scholar]
- 63.Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A, Leclair S, Herrmann K, Seiderer J, Ochsenkuhn T, Goke B, Auernhammer CJ, Dambacher J. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827–838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- 64.Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, Ghilardi N, deSauvage F, Cooper AM. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 65.Yasin B, Harwig SS, Lehrer RI, Wagar EA. Susceptibility of Chlamydia trachomatis to protegrins and defensins. Infection and Immunity. 1996;64:709–713. doi: 10.1128/iai.64.3.709-713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yasin B, Lehrer RI, Harwig SS, Wagar EA. Protegrins: structural requirements for inactivating elementary bodies of Chlamydia trachomatis. Infection and Immunity. 1996;64:4863–4866. doi: 10.1128/iai.64.11.4863-4866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson RM. Murine oviduct epithelial cell cytokine responses to Chlamydia muridarum infection include interleukin-12-p70 secretion. Infection and Immunity. 2004;72:3951–3960. doi: 10.1128/IAI.72.7.3951-3960.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Derbigny WA, Kerr MS, Johnson RM. Pattern recognition molecules activated by Chlamydia muridarum infection of cloned murine oviduct epithelial cell lines. The Journal of Immunology. 2005;175:6065–6075. doi: 10.4049/jimmunol.175.9.6065. [DOI] [PubMed] [Google Scholar]
- 69.Bai H, Cheng J, Gao X, Joyee AG, Fan Y, Wang S, Jiao L, Yao Z, Yang X. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J Immunol. 2009;183:5886–5895. doi: 10.4049/jimmunol.0901584. [DOI] [PubMed] [Google Scholar]
- 70.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, Ouyang W, Neurath MF, Becker C. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Su H, Morrison R, Messer R, Whitmire W, Hughes S, Caldwell HD. The effect of doxycycline treatment on the development of protective immunity in a murine model of chlamydial genital infection. J Infect Dis. 1999;180:1252–1258. doi: 10.1086/315046. [DOI] [PubMed] [Google Scholar]
- 73.Molano M, Meijer CJ, Weiderpass E, Arslan A, Posso H, Franceschi S, Ronderos M, Munoz N, van den Brule AJ. The natural course of Chlamydia trachomatis infection in asymptomatic Colombian women: a 5-year follow-up study. J InfectDis. 2005;191:907–916. doi: 10.1086/428287. [DOI] [PubMed] [Google Scholar]
- 74.Goodall JC, Wu C, Zhang Y, McNeill L, Ellis L, Saudek V, Gaston JS. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc Natl Acad Sci U S A. 2010;107:17698–17703. doi: 10.1073/pnas.1011736107. [DOI] [PMC free article] [PubMed] [Google Scholar]