Abstract
Opioid receptor agonists modulate both innate and adaptive immune responses. In this study, we examined the impact of long-term chronic morphine administration on the circulating T cell population dynamics in rhesus macaques. We found that the numbers of circulating Treg cells, and the functional activity of Th17 cells, were significantly increased with chronic morphine exposure. Our results also show that T cell populations with surface markers characteristic of gut-homing (CD161 and CCR6) and HIV-1 susceptibility (CCR5 and β7 integrin) were increased. These results represent the first detailed report of the impact of chronic morphine administration on circulating T cell dynamics.
Keywords: Treg cells, FoxP3, IL-17A, Th17 cells, CD161, β7 integrin, morphine
1. Introduction
It is well established that the activation of opioid receptors by the administration of morphine, or other selective or non-selective opioid agonists, alters both innate and adaptive immune competence. Acute administration studies conducted in vitro with cell culture, or in vivo analysis with rodents, have demonstrated modulation of antibody responses (Eisenstein et al., 1995; Guan et al., 1994; Taub et al., 1991), phagocytic cell function (Rojavin et al., 1993; Szabo et al., 1993), natural killer (NK) cell activity (Weber and Pert, 1989), and the development and function of T cells in the thymus (Linner et al., 1996; McCarthy et al., 2001; McCarthy Rogers, 2001) with opioid administration. Previous studies have shown that the production of IL-2 and IFNγ was inhibited following subcutaneous administration of morphine to rats (Lysle et al., 1993). Moreover, treatment in vitro of murine lymph node T cells with morphine resulted in a significant reduction in the expression of these cytokines in response to concanavalin A (ConA), and this effect was not observed in μ-opioid receptor (MOR) knockout mice (Wang et al., 2001). Similar results have been reported with morphine-treated human peripheral blood mononuclear cells (Peterson et al., 1987). Moreover, morphine treatment of mice in vivo induced a significant decrease in the levels of the proinflammatory cytokines TNFα, IL-1 and IL-6, and chemokines CXCL1 and CXCL2, in the bronchoalveolar lavage fluid (Wang et al., 2005). It appeared that the impaired cytokine and chemokine levels resulted in a reduction in the migration of protective neutrophils to the site of infection (Wang et al., 2005). Finally, the alteration of cytokine expression by morphine would be expected to change the characteristics of leukocytes in circulation, including the mobilization of cells from the bone marrow and lymph organs, and the activation and/or differentiation state of the cells.
In contrast to the results just described, studies carried out with human peripheral blood cells suggested that opioid administration did not induce a universal impairment in pro-inflammatory cytokine expression. For example, the activation of MOR with the μ-selective agonist, [D-Ala2,N-Me-Phe4,Gly-ol5]enkephalin (DAMGO)3 or morphine had the ability to increase the expression of several pro-inflammatory chemokines, including CCL4, CXCL12, CCL2, CCL5 and CXCL10, in astrocytes, as well as both non-activated and activated human PBMCs (Hu et al., 2000; Mahajan et al., 2005; Wetzel et al., 2000). Recent studies have also shown that activation of the MOR also induced a significant increase in CCL2 expression in primary human neurons (Rock et al., 2006). Moreover, activation of MOR induces significant up-regulation of the expression of the chemokine receptors CCR5 and CXCR4 on both monocytes and T cells (Steele et al., 2003). Results from our laboratory have implicated a role for TGF-β in MOR-mediated regulation of CCL5 (Happel et al., 2008), and while TGF-β is generally considered anti-inflammatory, this cytokine has been shown play a pivotal role in the function of the immune system, including both cell growth and differentiation (Li et al., 2006;Roberts, 1999).
Much of the work to determine the effects of morphine on the immune response has focused on analyses of acute effects of the drug, with an emphasis on rodent experiments carried out using cell culture. However, much less is known about the influence of chronic morphine administration on T cell population dynamics of cells in individual tissue compartments, including the peripheral circulation. Studies conducted with macaques have shown that chronic morphine treatment results in significantly reduced levels of circulating NK cells and NK cell activity (Carr France, 1993). These studies also showed that while circulating CD8 T cells were elevated, a decrease in circulating total CD4 T cells, and specifically, a decrease in the levels of CD4+CD45RA+ (naïve) T cells were observed. These results are consistent with similar studies carried out with chronic morphine-treated macaques which have shown an increase in circulating CD8 T cells (Carr France, 1993), and depressed levels of CD4+CD62L+ (resting naïve) T cells (Donahoe et al., 2001). Moreover, the levels of circulating CD4+CD29+ memory cells were found to increase in chronic morphine-treated macaques, suggesting that morphine administration may have opposing effects on the naïve and memory CD4 T cell populations (Carr France, 1993). However, little is known about the effects of chronic morphine administration in vivo on the T cell subpopulation dynamics in either human or non-human primates. In the present study we report results which show that chronic morphine administration to rhesus macaques leads to substantial changes in the circulating levels of regulatory T cell (Treg) subpopulation. We also find that circulating levels and functional activity of Th17 subsets are also altered in response to the chronic drug treatment. We believe this is the first report showing evidence that chronic morphine administration leads to a substantially modified circulating immune system.
2. Materials and methods
2.1. Animals
The Indian Rhesus macaques used in this study were housed at BIOQUAL, Inc. Rockville, MD, according to standards and guidelines as set forth in the Animal Welfare Act and The Guide for the Care and Use of Laboratory Animals, as well as according to animal care standards deemed acceptable by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC). All experiments were performed with the approvals of both the Temple University and Bioqual Institutional Animal Care and Use Committees (IACUC). Animals were housed on site for more than three months to allow for stabilization of the immune system prior to conducting the initial analysis of the immune system. In addition, the animals were tested for retroviral pathogens and herpes B virus and were negative. The 7 animals used in this study were composed of 3 males and 4 females and ranged in age from 2.5 to 4.5 years at study initiation.
2.2. Hematology
Hematology was performed by IDEXX (IDEXX Preclinical Research, West Sacramento, CA). For calculation of absolute cell numbers, whole blood was stained with anti-CD3-fluorescein isothiocyanate (FITC); anti-CD4-phycoerythrin (PE); anti-CD8-peridinin chlorophyll α protein (PerCP); anti-CD28-allophycocyanin (APC); anti-CD2-FITC; anti-CD20-PE, and red blood cells were lysed using lysing reagent (Beckman Coulter, Inc., Fullerton, Calif.). Samples were run on a FACSCalibur (BD Biosciences, San Jose, CA).
2.3. Morphine treatment
Morphine was administered by intramuscular injection to the animals at 5 mg/kg, 3 times daily beginning 24 hours after the baseline bleed was obtained. This dose of morphine was initially introduced stepwise from 3mg/kg to 5mg/kg over the initial 2 week period. Every 4 weeks following the beginning of the morphine treatment and for a total of 12 weeks, blood was drawn from the animals for analysis of the PBMCs.
2.4. Peripheral blood mononuclear cell (PBMC) isolation
Blood was collected into heparin vacutainers. Blood was shipped overnight at room temperature (RT). Blood (10 to 15ml) for each animal was pooled and layered onto 15ml of Ficoll-Paque (GE Healthcare) and centrifuged at 250g for 40 minutes at RT. PBMC layer was collected and diluted 1:1 with HBSS. Cells were pelleted at 250g for 10 min at 4C, and the cells were resuspended, and the erythrocytes were lysed with ACK solution (Invitrogen) for 10 minutes at 4C. Cells were washed and suspended in RPMI1640 containing 10% fetal calf serum (FCS) and counted using a Countess cell counter (Invitrogen).
2.5. Analysis of PBMCs by flow cytometry
For surface staining of markers, PBMCs (1 million) were blocked with 20ug of purified human IgG (Sigma) in FACS buffer (BD Bioscience) on ice for 30 minutes. The cells were centrifuged and the IgG was removed. PBMCs were next incubated with the appropriate antibody combinations (Table 1) in FACS buffer on ice for 30 minutes. PBMCs were washed twice with FACS buffer and fixed for 10 minutes on ice with 2% paraformaldehyde. The paraformaldehyde was removed, the cells transferred to staining buffer, and analyzed on BD LSRII cytometer.
Table 1.
Flow cytometry panel description.
| Panel | FITC | PerCP- Cy5.5 |
PE-Cy7 | APC | Alexa700 | APC- Cy7 |
Pacific Blue |
Qdot- 605 |
Qdot- 655 |
|---|---|---|---|---|---|---|---|---|---|
| Treg | Fox P3 | CD25 | CD3 | CD4 | |||||
| Th/Tc | IFNγ | CD161 | IL-17A | CD3 | CCR6 | CD4 | CD8 | ||
| CCR5/CXCR4 | CCR5 | CXCR4 | β7 | CD4 | CD3 | CD8 | |||
| Integrin |
Abbreviations: FITC: fluorescein; PerCP: peridinin-chlorophyll; PE: phycoerythrin; APC: allophycocyanin; Qdot: quantum dot.
For intracellular staining of cytokines such as IL-17A and IFNγ, PBMCs were cultured in RPMI1640 containing 10% FCS for 8 hours at 37C and 5% CO2 in the presence of 50 ng/ml PMA (Sigma), 500ng/ml Ionomycin (Sigma), and 0.07% Golgi Stop (BD Bioscience). At the end of the culture period, the stimulus was removed and the cells were blocked and stained for 30 minutes on ice with a panel of antibodies specific for cell surface markers (Table 1) which included CD3-Alexa700 (BD Bioscience; clone SP34-2), CD4-Qdot605 (Invitrogen; clone S3.5), CD8-Qdot655 (Invitrogen; clone 3B5), CCR6-Biotin (BD Bioscience; clone 11A9; Streptavidin-V450 from BD Bioscience was used at a 1:400 dilution to label the CCR6 antibody), and CD161-PerCP-Cy5.5 (eBioscience; clone HP-3G10). The stained cells were washed, fixed, and permeabilized using BD Bioscience Cytofix/Perm kit. The permeabilized cells were blocked and stained with antibodies to intracellular cytokines including IFNγ -FITC (BD Bioscience; clone B27) and IL-17A-Alexa647 (eBioscience; clone eBio64CAP17). PBMCs were washed twice with FACS buffer and transferred to staining buffer, and analyzed using an LSRII cytometer.
Treg assessment was performed using an eBioscience FoxP2 staining buffer kit. Cells were blocked and stained first for surface markers (Table 1) using antibodies to CD3-PacBlue (BD Bioscience; clone SP34-2), CD4-Qdot605 (Invitrogen; clone S3.5), and CD25-APC-Cy7 (Biolegend; clone BC96) for 30 minutes on ice. The stained cells were washed twice with FACS buffer followed by fixation and permeabilizization. The cells were then washed, blocked, and stained with FoxP3-APC (eBioscience; clone PCH101) for 30 minutes on ice. The cells were next washed, transferred to staining buffer, and analyzed on a BD LSRII cytometer.
Analysis was conducted with a BD LSR II cytometer equipped with 4 lasers (355 nm, 405 nm, 488 nm, and 640 nm). BD Cytometer Setup & Tracking Beads were used to calibrate the cytometer and track performance over time. BD CompBeads were stained with each experimental antibody and used as single color control for compensation. Uncompensated data was acquired on LSR II cytometer in FCS 3.0 format using BD FACSDiva software version 6.1.3. Data analysis and compensation were performed on FlowJo software (version 7.6.4). PBMCs (typically 125,000 events) were discriminated based on size and granularity using forward light scatter (FSC) vs. side light scatter (SSC). Debris and dead cells were gated out from the FSC/SSC plot. CD3+ cells were first identified and further divided into CD4+ and CD8+ cell populations. Isotype-matched control antibodies were employed to determine the background of non-specific binding of antibodies. Blasting T cells were identified based on size and granularity as described previously (Ogata et al., 2002) using FSC/SSC analysis.
The gating strategy to define the Treg cells was similar except that the CD3+ cells were identified using anti-CD3 staining, and these cells were then analyzed for CD4 expression, and from this gate, the CD25 and FoxP3 expression was determined. For Th17 and Th1 cell populations the same strategy was used except that the CD3+ cells were separated based on CD4 expression and CD8 expression, and individual gates were drawn for CD4+ cells and CD8+ cells, and both populations were further separated based on IFNγ and IL-17A expression. CD4+IFNγ+ cells were defined as Th1 cells and CD4+IL-17A+ cells were defined as Th17 cells. CD8+IFNγ+ cells were defined as Tc1 cells and CD8+IL-17A+ cells were defined as Tc17 cells. The CD4+ and CD8+ populations were also separated based on co-expression of IL-17A and CD161, or CD161 and CCR6.
The gating strategy to characterize CXCR4 and CCR5 was to first draw a gate lymphocytes by FSC/SSC, and then gate a second population (blasting) which was brighter along the FCS axis from the first gate (Ogata et al., 2002). The CD3+ non-blasting and blasting cells were further separated based on CD4+ and CD8+ as described above. The CD3+CD4+ and CD3+CD8+ non-blasting and blasting gates were further separated based on expression of either CXCR4+ (CXCR4-PE-Cy7; BD Bioscience; clone 12G5) or CCR5+ (CCR5-PerCP-Cy5.5; BD Bioscience; clone 3A9). Alternatively, CCR5 expressing cells were analyzed for the expression of β7 Integrin+ (β7 Integrin-APC; BD Bioscience; clone FIB504), and the β7 Integrin+ cells were sub-divided into high and low expressing populations.
2.6. Statistical Analysis
The data from individual animals were reported in the figures as an average +/− the standard error of the mean (SEM). A Mann-Whitney test was used to determine statistical significance between the zero time point and the post-treatment time points.
3. Results
3.1. Chronic morphine administration elevates circulating levels of Treg cells
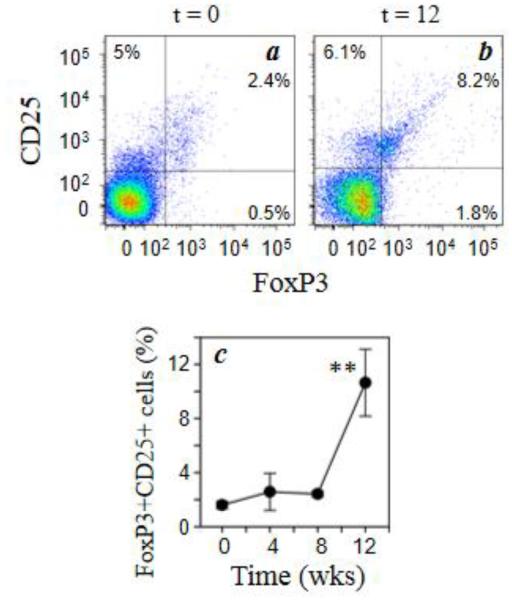
In order to understand the effects of chronic morphine exposure on circulating T cell dynamics, we evaluated the levels of Treg cells by multiparameter flow cytometry. PBMCs were obtained from each of the animals four weeks prior to, and one day prior to initiation of morphine exposure for baseline analysis. Immediately following the second baseline bleed, the morphine administration protocol was initiated, and the animals received morphine (5mg/kg) three times daily intravenously for the duration of the study. We initially determined the total numbers of circulating total CD3+, CD4+, and CD8+ cells. The data show that total CD3+, CD4+, CD8+ cells and the ratio of CD4 to CD8 cells were not changed with the administration of morphine (data not shown). We then wished to determine the levels of Tregs by measuring the frequency of FoxP3-positive cells within the CD3+CD4+CD25+ population. The results (Fig. 1) show that the levels of CD25+FoxP3+ dual positive cells increased by approximately 5-fold by the end of the 12 week treatment. On the other hand, the levels of CD25-positive, FoxP3-negative cells did not change at any point following morphine treatment (data not shown).
Fig. 1.
Chronic morphine exposure increases the level of circulating Treg cells. Rhesus macaque PBMCs were stained for cell surface expression of CD3 (PacBlue), CD4 (Qdot605), and CD25 (APC-Cy7) followed by intracellular staining for FoxP3 (APC). Representative data for the co-expression of CD25 and FoxP3 (a, b) on CD3+CD4+ cells for animals at 0 week (a) and 12 weeks (b) of morphine administration are presented. The combined results for all of the macaque animals (5 animals) are presented as the percent of CD3+CD4+ which are CD25+FoxP3+ dual-positive cells (c). Data are presented as the mean (± sem) for the 5 animals. **p<0.01.
3.2. Circulating Th1, Tc1, total Th17 and total Tc17 levels are not altered by chronic morphine treatment
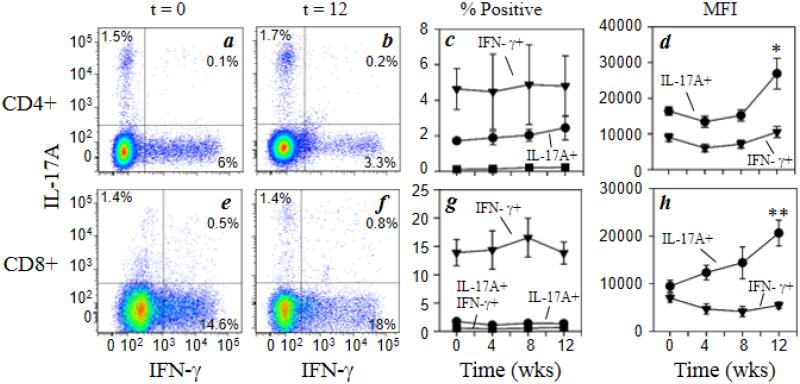
We conducted an analysis of the frequency of Th1 and Th17 T cell populations by analyzing the frequency of IFNγ and IL-17A within CD3+CD4+ cells and CD3+CD8+ cells. The results (Fig. 2a-c) show that the frequency of IFNγ+IL-17A− cells within the CD4+ population (Th1 cells) was not changed at any point following initiation of morphine administration. Analysis of IFNγ+IL-17A− cells within the CD8+ population (Tc1 cells) showed the same results (Fig. 2e-g). In addition, analysis of IFNγ-IL-17A+ cells within either the CD4 population (Th17 cells; Fig. 2a-c) or the CD8 population (Tc17 cells; Fig. 2e-g) also showed no statistically significant change following initiation of morphine treatment. Finally, an examination of the IFNγ+IL-17A+ dual-positive cells within either the CD4 or CD8 populations (Fig. 2a-c and e-g) also showed no change with morphine administration. These dual-positive cells are possibly cells in transition from Th17 to Th1, although this not currently clear (Annunziato et al., 2012).
Fig. 2.
Chronic morphine exposure increases the functional activity of Th17 and Tc17 cells. Rhesus macaque PBMCs were stained for cell surface expression of CD3 (Alexa700), CD4 (Qdot605), and CD8 (Qdot655) followed by intracellular staining for IFNγ (FITC) and IL-17A (Alexa647). Representative data for Th1 and Th17 CD4+ cells (a, b), and Tc1 and Tc17 CD8+ cells (e, f) for animals at 0 week (a, e) and 12 weeks (b, f) of morphine administration are presented. The pooled results for the full macaque panel (7 animals) are presented as the percent positive IFNγ + (▼), IL-17A + (●) and IFNγ +IL-17A + (■) for CD4+ cells (c) and CD8+ cells (g). The level of intracellular cytokine expression was also determined for the CD4+ (d) and CD8+ subpopulations (h) and is expressed as mean fluorescence intensity (MFI). Data are presented as the mean (± sem) for the 7 animals. *p<0.05; **p<0.01
3.3. Chronic morphine administration induces Th17 and Tc17 functional activity
We examined the level of expression of both IFNγ and IL-17A in the CD4 and CD8 populations using quantitative intracellular flow cytometry. These determinations are based on the mean fluorescence intensity of IFNγ in the IFNγ+IL-17A− cells (Th1 and Tc1) or IL-17A in the IFNγ-IL-17A+ cells (Th17 and Tc17). The results (Fig. 2d) show that the level of IFNγ expressed by CD4 cells was not altered by morphine treatment, but the level of expression of IL-17A was significantly increased at 12 weeks. Moreover, the level of expression of IFNγ expressed by CD8 cells (Fig. 2h) was not significantly altered by morphine treatment (except for a modest reduction at 8 weeks), while the level of expression of IL-17A was significantly induced with morphine administration at 12 weeks. These results show that while the numbers of total Th17 and total Tc17 cells were not altered with morphine treatment, the functional activity on a cell-by-cell basis was elevated with drug administration.
3.4. The CCR6+ and CD161+ Th17 and Tc17 sub-populations are elevated with chronic morphine administration
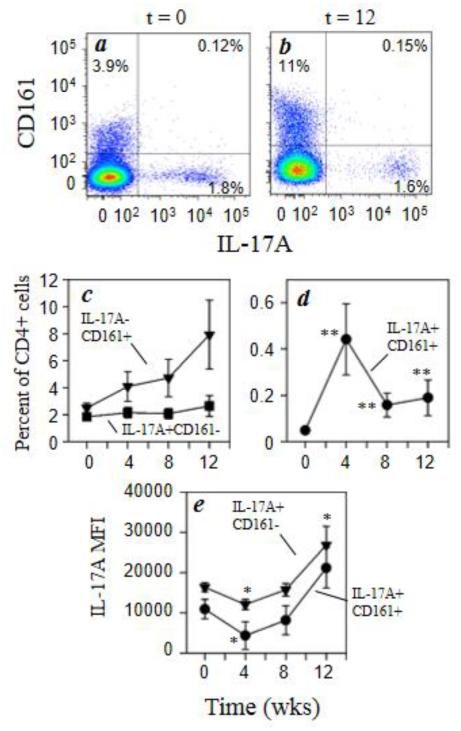
We examined the expression of the C-type lectin-like receptor, CD161, in the CD3+CD4+ and CD3+CD8+ populations since the expression of this marker in human T cells indicates a memory phenotype as well as a cell type with gut-homing properties. Figures 3a and 3b show the representative distribution of IL-17A and CD161 expression within CD3+CD4+ cells before and 12 weeks after morphine administration. The percentage of IL-17A−CD161+ CD4 cells were determined and the results show that this cell population increases nearly 3-fold over time (Fig. 3c) while IL-17A+CD161+ cells transiently increase by 4 week with morphine administration and returns to baseline levels by 12 weeks (Fig. 3d). However, the percentage of IL-17A+ cells that are negative for CD161 expression (Fig. 3c) does not change with morphine administration. The functional activity of the IL-17A+ (Th17 cells) was also measured by using mean fluorescence intensity for intracellular IL-17A expression. The data in figure 3e shows that functional activity in the Th17 cells was increased 2-fold by morphine administration independent of CD161 expression on these cells. Interestingly, there was a transient decrease in functional activity in the IL-17A+CD161+ cells by 4 weeks of morphine administration.
Fig. 3.
Morphine increases expression of CD161 by CD4+ T cells. Rhesus macaque PBMCs were stained for cell surface expression of CD3 (Alexa700), CD4 (Qdot605), and CD161 (PerCP-Cy5.5) followed by intracellular staining for IL-17A (Alexa647). Representative data for the co-expression of CD161 and IL-17A within the CD3+/CD4+ T cells for animals at 0 week (a) and 12 weeks (b) of morphine administration are presented. The pooled results for the full macaque panel (7 animals) are presented as the percent positive IL-17A−CD161+ (■) and IL-17A+CD161− (▼) and IL-17A+CD161+ (●) for CD4+ cells (c, d). The level of intracellular cytokine expression was also determined for the IL-17A+CD161− (●) and IL-17A+CD161+ (▼) and is expressed as mean fluorescence intensity (MFI) (e). Data are presented as the mean (± sem) for the 7 animals. *p<0.05; **p<0.01
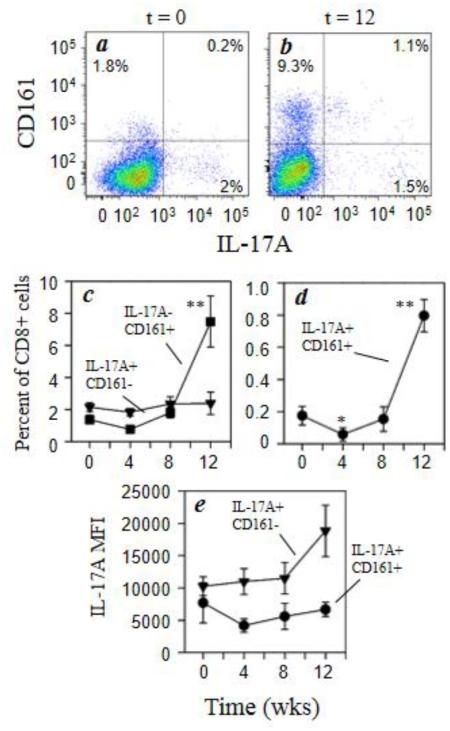
We also examined the distribution of IL-17A and CD161 expression within CD3+CD8+ cells with morphine administration (Fig. 4a and 4b). As with the Th17 cells, the results show that the CD3+CD8+ T cells expressing CD161 with or without IL-17A expression were increased 4- to 5-fold by morphine administration while the IL-17A+CD161− cells remained unchanged (Fig. 4c and 4d). Similarly, analysis of the functional activity of the Tc17 cells as measured by IL-17A expression was increased up to 2-fold, although not significantly, with morphine treatment, independent of CD161 expression on these cells (Fig. 4e).
Fig. 4.
Morphine increases expression of CD161 by CD8+ T cells. Rhesus macaque PBMCs were stained for cell surface expression of CD3 (Alexa700), CD8 (Qdot655), and CD161 (PerCP-Cy5.5) followed by intracellular staining for IL-17A (Alexa647). Representative data for the co-expression of CD161 and IL-17A within the CD3+CD8+ T cells (a, b) for animals at 0 week (a) and 12 weeks (b) of morphine administration are presented. The pooled results for the full macaque panel (7 animals) are presented as the percent positive IL-17A−CD161+ (■) and IL-17A+CD161− (▼) and IL-17A+CD161+ (●) for CD8+ cells (c, d). The level of intracellular cytokine expression was also determined for the IL-17A+CD161− (●) and IL-17A+CD161+ (▼) and is expressed as mean fluorescence intensity (MFI) (e). Data are presented as the mean (± sem) for the 7 animals. *p<0.05; **p<0.01
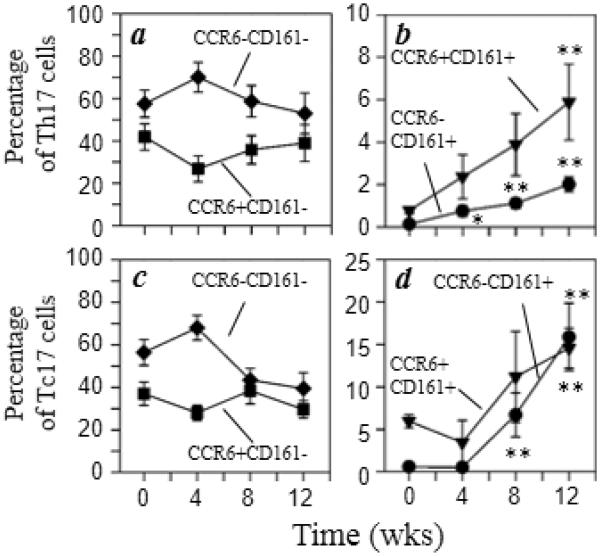
Since human Th17 cells exhibit preferential expression of mucosal-homing chemokine receptor CCR6, we examined the expression of CCR6 and CD161 by the Th17 and Tc17 cell populations (Fig. 5). The data show that both Th17 and Tc17 cells which express CD161 increased 2- to 8-fold with morphine administration independent of CCR6 expression (Fig. 5b and 5d). On the other hand, the frequency of CD161-negative Th17 cells were not changed significantly (Fig. 5a). However, Tc17 cells lacking both CCR6 and CD161 expression were reduced, although not significantly, following morphine administration (Fig. 5c). Taken together, these results show that chronic morphine administration increases the percentages of circulating Th17 and Tc17 cells with characteristics of mucosal homing.
Fig. 5.
Both CCR6+CD161+ and CCR6−CD161+ T cells are elevated following morphine administration. Rhesus macaque PBMCs stained for cell surface expression of CD3 (Alexa700), CD4 (Qdot605), CD8 (Qdot655), CD161 (PerCP-Cy5.5), and CCR6 (V450) followed by intracellular staining for IFNγ (FITC) and IL-17A (Alexa647). Multicolor flow cytometric analysis was performed and the expression of IFNγ and IL-17A was used to identify Th17 (a, b) and Tc17 (c, d) subpopulations. The results are presented as the percent of CCR6−CD161− (◆), CCR6+CD161− (■), CCR6+CD161+ (▼), and CCR6−CD161+ (●) cells. Data are presented as the mean (± sem) for the 7 animals. *p<0.05; **p<0.01
3.5. Chronic morphine administration alters CXCR4 and CCR5 expression by CD4+ and CD8+ T cells
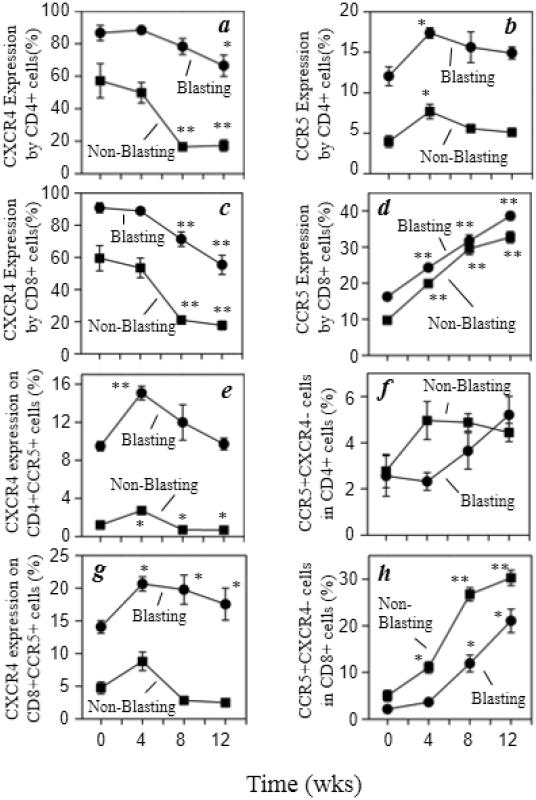
We analyzed CXCR4 and CCR5 expression by both blasting and non-blasting CD4+ and CD8+ T cells. The percentage of T cells expressing CXCR4 was reduced significantly regardless of CD4 or CD8 expression, or blasting versus non-blasting status, with chronic morphine administration (Fig. 6a and 6c). However, the percentage of T cells (blasting or non-blasting) expressing CCR5 was increased steadily over time in the CD8+ T cells but only transiently increased in the CD4+ T cells with morphine administration (Fig. 6b and 6d). A closer examination of CXCR4 expression within the CCR5+ T cell populations also shows a transient increase in the percentage of this cell population which returns to baseline levels in the blasting population or reduced levels in the non-blasting population with morphine administration (Fig. 6e). The frequency of a smaller fraction of the CCR5+CD4+ T cells which do not co-express CXCR4 exhibit a modest increase with morphine administration, although this was not statistically significant (Fig. 6f). Finally, the percentage of CD8+ T cells expressing CCR5 increased independent of CXCR4 co-expression (Fig. 6g and 6h). However, the increase was more dramatic in the CD8+CCR5+ T cells that did not co-express CXCR4 (Fig. 6h).
Fig. 6.
Chronic morphine administration alters CXCR4 and CCR5 expression by CD4 and CD8 T cells. Multicolor flow cytometric analysis was performed and the expression of CCR5 (PerCP-Cy5.5) and CXCR4 (PE-Cy7) expression on CD3+CD4+ (a, b) and CD3+CD8+ (c, d) cells that were either blasting (●) or non-blasting (■). The results are presented as the percent positive of total CXCR4+ (a, c), total CCR5+ (b, d), CXCR4+CCR5+ (e, g), and CXCR4−CCR5+ (f, h). Data are presented as the mean (± sem) for the 5 animals. *p<0.05; **p<0.01
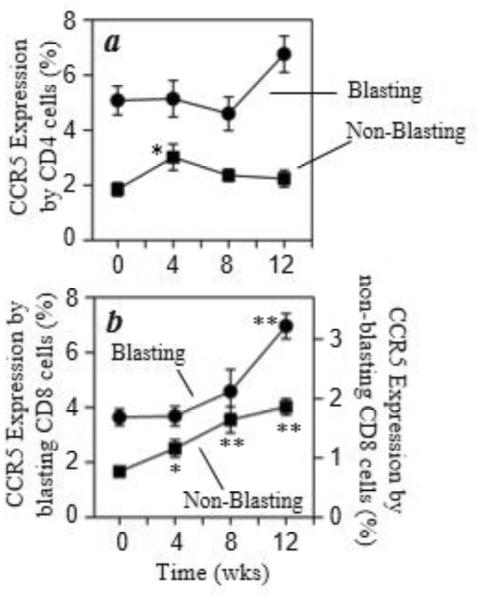
An analysis of CCR5 expression by T cells expressing high levels of β7 integrin was performed, and the results show that the percentage of CD4+ T cells co-expressing high β7 integrin and CCR5 was not significantly altered with morphine administration in blasting cells, but was transiently increased in the non-blasting population (Fig. 7a). This specific phenotype of high β7 integrin and CCR5 expression is reported to represent a highly HIV-1 susceptible T cell population (Cicala et al., 2009). In contrast, CD8+ T cells with high β7 integrin and CCR5 expression also increased significantly over time in both the blasting and non-blasting populations (Fig.7b). These results show that chronic morphine treatment can alter the expression of CCR5 and CXCR4 on a variety of T cell populations.
Fig. 7.
Chronic morphine administration increases CCR5 expression by β7 InterginHi CD8 T cells. Multicolor flow cytometric analysis was performed and the expression of CCR5 (PerCPCy5.5) on CD3+CD4+β7 IntegrinHi (a) or CD3+CD8+β7 IntegrinHi (b) cells was determined. The results are presented as the percent of CCR5+ cells on either blasting (●) or non-blasting (■) T cells within the β7 IntegrinHi (APC) populations. Data are presented as the mean (± sem) for the 5 animals. *p<0.05; **p<0.01.
4. Discussion
Immune competence is dependent on the interplay between the individual sub-populations of effector and regulatory T cells, and it is important to understand the influence of chronic exposure to μ-opioid drugs of abuse on the circulating levels of each of these T cell components. While the overall immunomodulatory effects of opioids are well described, the effects of opioids on T cell population dynamics are much less certain, particularly for humans or non-human primates. In general, studies on patients receiving acute opioid post-operative therapy have shown little effect on the total numbers of circulating T cells, or the CD4/CD8 ratio (Hashiguchi et al., 2005; Volk et al., 2004; Yokoyama et al., 2005). However, analysis of a cohort of patients receiving general anesthesia with fentanyl showed a rapid reduction in circulating T cells with an increase in the CD4/CD8 ratio, 20 min following anesthesia but prior to surgery (Brand et al., 2003). In contrast, analysis of subjects subjected to chronic opioid treatment has yielded much less consistent results. Analysis of peripheral blood from heroin addicts showed a reduction in circulating CD4 T cells, a reduced CD4/CD8 ratio, and an increase in CD8 cells (Donahoe et al., 1987; Govitrapong et al., 1998). However, disparate results have been reported which show that the circulating CD4/CD8 ratio is not significantly altered in heroin addicts (Novick et al., 1989). Finally, there is some evidence suggesting that chronic exposure of non-human primates to morphine results in reduced circulating levels of CD4 and CD8 T cells and a reduction in the CD4/CD8 ratio (Carr France, 1993; Donahoe et al., 2001). These latter studies were conducted with a different dosing regimen, and treatment duration, and this may account for the difference between their results and those presented here. Nevertheless, in the present study we failed to detect a significant change in the circulating levels of total CD4 or CD8 cells, or the CD4/CD8 ratio, following chronic morphine administration. Our results are consistent in this case with data reported for macaques receiving morphine 4 times daily for 3 months (Weed et al., 2006).
We wished to examine the effects of chronic morphine administration on the population dynamics of individual CD4 and CD8 T cell sub-populations. First we examined the circulating levels of Treg cells, and our results showed that exposure to morphine for 90 days results in a significant up-regulation of this sub-population. To our knowledge, this is the first report which characterizes the effect of chronic exposure to morphine on circulating Treg population dynamics. Treg cells are essential for the control of immune responsiveness, and the dysfunction of these cells results in potentially fatal autoimmune disease, chronic inflammatory disease, immunopathology and allergy (Sakaguchi et al., 2008; Sakaguchi et al., 2010). Treg cells can influence the function of the CD8 T cells, B cells, NK cells, dendritic cells and macrophages (Fields et al., 2005; Ghiringhelli et al., 2005; Green et al., 2003; Ito et al., 2008; Lim et al., 2005; Liu et al., 2011; Piccirillo et al., 2001; Tiemessen et al., 2007). It is clear that these cells also regulate the activity of CD4 effector T cell subpopulations as well, but there appears to be a hierarchy in the susceptibility of these cells to the influence of Treg cells. Recent studies suggest that Th1 cells are the most susceptible to Treg control, Th2 cells are less strongly regulated and Th17 cells are largely insensitive to Treg control (Annunziato et al., 2007; Huter et al., 2008; Stummvoll et al., 2008; Van et al., 2009). The greater sensitivity of Th1 cells to Treg control is interesting in light of reports which indicate that morphine and heroin administration induce a Th2-shift of the immune response (Azarang et al., 2007; Gao et al., 2012; Roy et al., 2001).
Our results showed that circulating Th1 and Th17 numbers were not significantly altered by chronic morphine administration. However, we did observe that the functional activity of Th17 cells, based on the production of IL-17A, was significantly increased. This population of effector T cells exerts pro-inflammatory effects, can contribute to autoimmune and other chronic inflammatory disease states, and can contribute significantly to host defense against infectious agents (Annunziato et al., 2012;Dong, 2009). Our results are somewhat surprising given previous reports showing that morphine administration to mice resulted in reduced dendritic cell IL-23 expression, and γ/δ T cell IL-17A production (Ma et al., 2010; Wang et al., 2011). The difference in results here may reflect the shorter duration of morphine treatment, and the difference in species. Nevertheless, our results also show a significant increase in the functional activity of Tc17 cells following chronic morphine administration, a population of cells which appears to arise under similar influences as those described for the Th17 population. For example, the development of Tc17 cells is STAT3-dependent, and develops from CD8-precusor cells in the periphery in response to IL-23 (Curtis et al., 2009; Yen et al., 2009). Tc17 cells have been reported to mediate protective immunity to both vaccinia and influenza virus infection, participate in anti-tumor immunity in hepatocellular carcinoma patients and a murine model of melanoma, promote autoimmunity in experimental autoimmune encephalitis, and regulate disease progression during pathogenic SIV infection (Garcia-Hernandez et al., 2010; Hamada et al., 2009; Huber et al., 2009; Kuang et al., 2010; Nigam et al., 2011; Yeh et al., 2010). It appears that Tc17 cells mediate weaker cytotoxic activity than classical Tc cells, but produce more pro-inflammatory mediators including TNFα, IL-21, IL-22, CCL5 and CXCL10 (Garcia-Hernandez et al., 2010; Kuang et al., 2010). However, a full understanding of the role of these cells in the immune response remains to be established.
Subpopulations of Th17 and Tc17 cells have been identified, and we examined the levels of CD161-expressing subsets of these effector T cell populations. Our results showed that morphine treatment increased the circulating numbers of CD161+ Th17 and Tc17 cells, and increased the functional activity, particularly for the Th17 population, as well. CD161 is a C-type lectin-like receptor that is also expressed by subsets of NK cells (Lanier et al., 1994). The contribution of CD161 to the function of Th17 and Tc17 cells is not fully understood, but it has been suggested that CD161 is a marker for memory Th17 cells at sites of inflammation (Cosmi et al., 2008). However, CD161 appears to promote the transendothelial migration of Th17 and Tc17 cells at sites of inflammation by virtue of binding acidic oligosaccharides on the endothelial cell surface (Bezouska et al., 1994). CD161 appears to contribute to the tissue-homing properties of both Th17 and Tc17 cells, based on work showing gut-homing of CD161+ Th17 cells in patients with Crohn’s disease (Kleinschek et al., 2009), and liver- and joint-homing of CD161+ Tc17 cells in patients with hepatitis C virus infection and arthritis, respectively (Billerbeck et al., 2010). Finally, CD161+ Th17 cells exhibit profound depletion following HIV infection, and it has been suggested that this may impair mucosal immunity given the gut-homing properties of this T cell subpopulation (Guillot-Delost et al., 2012; Prendergast et al., 2010).
Our results also show that morphine substantially increases circulating levels of CD161+ Th17 and Tc17 cells that express CCR6. Like CD161, CCR6 defines a subset of Th17 cells that promote gut-homing, and CCR6+ Th17 cells appear to play a prominent role in HIV infection by contributing to the dissemination of HIV from the portal site of entry. In addition, CCR6+ Th17 cells are highly permissive to HIV infection, and circulating levels of CCR6+ Th17 cells are substantially diminished in chronically HIV-infected subjects despite viral-suppressive drug therapy (El et al., 2010; Gosselin et al., 2010). These results would suggest the possibility that morphine augments circulating levels of a cell population that is most likely to serve as a target for HIV infection, and allows the opioid to promote the traffic of virally-infected cells to the gut.
Recent reports suggest that CD4+ T cells that co-express the integrin α4β7 and CCR5 are highly susceptible to HIV infection (Cicala et al., 2009). Indeed, it is apparent that integrin α4β7 binds to HIV-1 gp120, and together with CD4 and CCR5, forms a complex with the viral envelope glycoprotein (Arthos et al., 2008; Cicala et al., 2009). Moreover, engagement of integrin α4β7 by gp120 initiates a signaling cascade which leads to the rapid activation of LFA-1, the central integrin involved in the establishment of viral synapses, and promotes the cell-cell spreading of HIV-1 (Arthos et al., 2008; Bromley et al., 2001; Piguet et al., 2004). The results reported here show that morphine does not alter the levels of circulating total CD4 or CD8 T cells which express integrin α4 β7. However, our studies show that chronic morphine administration results in a significant up-regulation of CCR5 expression on the integrin α4β7-positive CD8 T cells. The role of these cells in the immune response is not clear, although integrin α4β7 promotes gut-homing, and these cells would be expected to exert a pro-inflammatory influence in this tissue site.
Finally, our previous studies showed that acute administration in vitro to human PBMCs resulted in a significant induction of both CCR5 and CXCR4 by blasting T cells, and these cells were significantly more susceptible to infection with HIV-1 following treatment with either DAMGO or morphine (Steele et al., 2003). The results reported here extend these earlier studies and show that chronic morphine administration results in a modest induction of CCR5 by CD4 T cells, with a substantial up-regulation of CCR5 by CD8 T cells. However, in contrast to our earlier results with acute morphine treatment, in our present studies chronic morphine administration resulted in a substantial drop in CXCR4 expression by both CD4 and CD8 T cells. Both of these chemokine receptors possess pro-inflammatory activity for the immune system, and CXCR4 exerts critical homeostatic functional activity particularly for the brain, cardiovascular and immune systems. Our results suggest that chronic morphine exposure may exert opposing influences on the immune system by virtue of up-regulation of CCR5 and down-regulation of CXCR4. Taken together, the present studies show that chronic morphine administration exerts significant effects on the levels and functional activity of several T cell sub-populations, and the shift in circulating T cell dynamics would be expected to contribute to the immunomodulation observed following chronic opioid abuse.
Highlights.
• Chronic morphine administration induces an increase in circulating Treg cells
Morphine administration up-regulates Th17 functional activity
Morphine administration increases circulating T cells with gut-homing activity
Morphine increases the expression of CCR5, particularly for CD8 T cells
Morphine reduces the expression of CXCR4 by T cells
Acknowledgments
The authors wish to acknowledge the support from the National Institutes of Health for the following grant support: DA14230, DA25532, P30DA13429, PO1 DA23860, and S10 RR27910.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest to declare.
References
- Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Defining the human T helper 17 cell phenotype. Trends in Immunology. 2012;33:505–512. doi: 10.1016/j.it.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthos J, Cicala C, Martinelli E, Macleod K, Van RD, Wei D, Xiao Z, Veenstra TD, Conrad TP, Lempicki RA, McLaughlin S, Pascuccio M, Gopaul R, McNally J, Cruz CC, Censoplano N, Chung E, Reitano KN, Kottilil S, Goode DJ, Fauci AS. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nature Immunology. 2008;9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- Azarang A, Mahmoodi M, Rajabalian S, Shekari MA, Nosratabadi J, Rezaei N. T-helper 1 and 2 serum cytokine assay in chronic opioid addicts. European Cytokine Network. 2007;18:210–214. doi: 10.1684/ecn.2007.0107. [DOI] [PubMed] [Google Scholar]
- Bezouska K, Yuen CT, O'Brien J, Childs RA, Chai W, Lawson AM, Drbal K, Fiserova A, Pospisil M, Feizi T. Oligosaccharide ligands for NKR-P1 protein activate NK cells and cytotoxicity. Nature. 1994;372:150–157. doi: 10.1038/372150a0. [Erratum appears in Nature 1996 Apr 11;380(6574):559] [DOI] [PubMed] [Google Scholar]
- Billerbeck E, Kang YH, Walker L, Lockstone H, Grafmueller S, Fleming V, Flint J, Willberg CB, Bengsch B, Seigel B, Ramamurthy N, Zitzmann N, Barnes EJ, Thevanayagam J, Bhagwanani A, Leslie A, Oo YH, Kollnberger S, Bowness P, Drognitz O, Adams DH, Blum HE, Thimme R, Klenerman P. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc. Natl. Acad. Sci. (USA) 2010;107:3006–3011. doi: 10.1073/pnas.0914839107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand JM, Frohn C, Luhm J, Kirchner H, Schmucker P. Early alterations in the number of circulating lymphocyte subpopulations and enhanced proinflammatory immune response during opioid-based general anesthesia. Shock. 2003;20:213–217. doi: 10.1097/00024382-200309000-00003. [DOI] [PubMed] [Google Scholar]
- Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse. Ann. Rev. Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- Carr DJ, France CP. Immune alterations in morphine-treated rhesus monkeys. J. Pharmacol. Exp. Therapeut. 1993;267:9–15. [PubMed] [Google Scholar]
- Cicala C, Martinelli E, McNally JP, Goode DJ, Gopaul R, Hiatt J, Jelicic K, Kottilil S, Macleod K, O'shea A, Patel N, Van RD, Wei D, Pascuccio M, Yi L, McKinnon L, Izulla P, Kimani J, Kaul R, Fauci AS, Arthos J. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc. Natl. Acad. Sci. (USA) 2009;106:20877–20882. doi: 10.1073/pnas.0911796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmi L, De PR, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, Berrino L, Fambrini M, Caproni M, Tonelli F, Lazzeri E, Parronchi P, Liotta F, Maggi E, Romagnani S, Annunziato F. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J. Exp. Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MM, Way SS, Wilson CB. IL-23 promotes the production of IL-17 by antigen-specific CD8 T cells in the absence of IL-12 and type-I interferons. J. Immunol. 2009;183:381–387. doi: 10.4049/jimmunol.0900939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahoe RM, Bueso-Ramos C, Donahoe F, Madden JJ, Falek, Nicholson JK, Bokos P. Mechanistic implications of the findings that opiates and other drugs of abuse moderate T-cell surface receptors and antigenic markers. Annals of the New York Academy of Sciences. 1987;496:711–721. doi: 10.1111/j.1749-6632.1987.tb35834.x. [DOI] [PubMed] [Google Scholar]
- Donahoe RM, Byrd LD, McClure HM, Brantley M, Wenzel D, Ansari AA, Marsteller F. Effects of morphine on T-cell recirculation in rhesus monkeys. Adv. Exp. Med. Biol. 2001;493:89–101. doi: 10.1007/0-306-47611-8_11. [DOI] [PubMed] [Google Scholar]
- Dong C. Differentiation and function of pro-inflammatory Th17 cells. Microbes. Infect. 2009;11:584–588. doi: 10.1016/j.micinf.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein TK, Meissler JJ, Jr., Rogers TJ, Geller EB, Adler MW. Mouse strain differences in immunosuppression by opioids in vitro. J. Pharmacol. Exp. Therapeut. 1995;275:1484–1489. [PubMed] [Google Scholar]
- El HA, Khaitan A, Kozhaya L, Manel N, Daskalakis D, Borkowsky W, Valentine F, Littman DR, Unutmaz D. Susceptibility of human Th17 cells to human immunodeficiency virus and their perturbation during infection. J. Infect. Dis. 2010;201:843–854. doi: 10.1086/651021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields ML, Hondowicz BD, Metzgar MH, Nish SA, Wharton GN, Picca CC, Caton AJ, Erikson J. CD4+ CD25+ regulatory T cells inhibit the maturation but not the initiation of an autoantibody response. J. Immunol. 2005;175:4255–4264. doi: 10.4049/jimmunol.175.7.4255. [DOI] [PubMed] [Google Scholar]
- Gao M, Sun J, Jin W, Qian Y. Morphine, but not ketamine, decreases the ratio of Th1/Th2 in CD4-positive cells through T-bet and GATA3. Inflammation. 2012;35:1069–1077. doi: 10.1007/s10753-011-9413-6. [DOI] [PubMed] [Google Scholar]
- Garcia-Hernandez ML, Hamada H, Reome JB, Misra SK, Tighe MP, Dutton RW. Adoptive transfer of tumor-specific Tc17 effector T cells controls the growth of B16 melanoma in mice. J. Immunol. 2010;184:4215–4227. doi: 10.4049/jimmunol.0902995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, Lecesne A, Robert C, Blay JY, Bernard J, Caillat-Zucman S, Freitas A, Tursz T, Wagner-Ballon O, Capron C, Vainchencker W, Martin F, Zitvogel L. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J. Exp. Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin A, Monteiro P, Chomont N, Diaz-Griffero F, Said EA, Fonseca S, Wacleche V, El-Far M, Boulassel MR, Routy JP, Sekaly RP, Ancuta P. Peripheral blood CCR4+CCR6+ and CXCR3+CCR6+CD4+ T cells are highly permissive to HIV-1 infection. J. Immunol. 2010;184:1604–1616. doi: 10.4049/jimmunol.0903058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govitrapong P, Suttitum T, Kotchabhakdi N, Uneklabh T. Alterations of immune functions in heroin addicts and heroin withdrawal subjects. J. Pharmacol. Exp. Therapeut. 1998;286:883–889. [PubMed] [Google Scholar]
- Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc. Natl. Acad. Sci. (USA) 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan L, Townsend R, Eisenstein TK, Adler MW, Rogers TJ. Both T cells and macrophages are targets of kappa-opioid-induced immunosuppression. Brain Behavior Immun. 1994;8:229–240. doi: 10.1006/brbi.1994.1021. [DOI] [PubMed] [Google Scholar]
- Guillot-Delost M, Le GS, Mesel-Lemoine M, Cherai M, Baillou C, Simon A, Levy Y, Weiss L, Louafi S, Chaput N, Berrehar F, Kerbrat S, Klatzmann D, Lemoine FM. Human CD90 identifies Th17/Tc17 T cell subsets that are depleted in HIV-infected patients. J. Immunol. 2012;188:981–991. doi: 10.4049/jimmunol.1101592. [DOI] [PubMed] [Google Scholar]
- Hamada H, Garcia-Hernandez ML, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J. Immunol. 2009;182:3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel C, Steele AD, Finley MJ, Kutzler MA, Rogers TJ. DAMGO-induced expression of chemokines and chemokine receptors: the role of TGF-{beta}1. J. Leukoc. Biol. 2008;83:956–963. doi: 10.1189/jlb.1007685. [DOI] [PubMed] [Google Scholar]
- Hashiguchi S, Morisaki H, Kotake Y, Takeda J. Effects of morphine and its metabolites on immune function in advanced cancer patients. Journal of Clinical Anesthesia. 2005;17:575–580. doi: 10.1016/j.jclinane.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Hu S, Chao CC, Hegg CC, Thayer S, Peterson PK. Morphine inhibits human microglial cell production of, and migration towards, RANTES. Journal of Psychopharmacology. 2000;14:238–243. doi: 10.1177/026988110001400307. [DOI] [PubMed] [Google Scholar]
- Huber M, Heink S, Grothe H, Guralnik A, Reinhard K, Elflein K, Hunig T, Mittrucker HW, Brustle A, Kamradt T, Lohoff M. A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur. J. Immunol. 2009;39:1716–1725. doi: 10.1002/eji.200939412. [DOI] [PubMed] [Google Scholar]
- Huter EN, Stummvoll GH, DiPaolo RJ, Glass DD, Shevach EM. Cutting edge: antigen-specific TGF beta-induced regulatory T cells suppress Th17-mediated autoimmune disease. J. Immunol. 2008;181:8209–8213. doi: 10.4049/jimmunol.181.12.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, Qin FX, Gilliet M, Liu YJ. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28:870–880. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, Raskin L, Desai B, Faubion WA, de Waal MR, Pierce RH, McClanahan T, Kastelein RA. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J. Exp. Med. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang DM, Peng C, Zhao Q, Wu Y, Zhu LY, Wang J, Yin XY, Li L, Zheng L. Tumor-activated monocytes promote expansion of IL-17-producing CD8+ T cells in hepatocellular carcinoma patients. J. Immunol. 2010;185:1544–1549. doi: 10.4049/jimmunol.0904094. [DOI] [PubMed] [Google Scholar]
- Lanier LL, Chang C, Phillips JH. Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J. Immunol. 1994;153:2417–2428. [PubMed] [Google Scholar]
- Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Ann. Rev. Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J. Immunol. 2005;175:4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- Linner KM, Quist HE, Sharp BM. Expression and function of proenkephalin A messenger ribonucleic acid in murine fetal thymocytes. Endocrinol. 1996;137:857–863. doi: 10.1210/endo.137.3.8603595. [DOI] [PubMed] [Google Scholar]
- Liu G, Ma H, Qiu L, Li L, Cao Y, Ma J, Zhao Y. Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice. Immunology & Cell Biology. 2011;89:130–142. doi: 10.1038/icb.2010.70. [DOI] [PubMed] [Google Scholar]
- Lysle DT, Coussons ME, Watts VJ, Bennett EH, Dykstra LA. Morphine-induced modulation of immune status: evidence for opioid receptor mediation and compartment specificity. Adv. Exp. Med. Biol. 1993;335:53–59. doi: 10.1007/978-1-4615-2980-4_8. [DOI] [PubMed] [Google Scholar]
- Ma J, Wang J, Wan J, Charboneau R, Chang Y, Barke RA, Roy S. Morphine Disrupts Interleukin-23 (IL-23)/IL-17-Mediated Pulmonary Mucosal Host Defense against Streptococcus pneumoniae Infection. Infect. Immun. 2010;78:830–837. doi: 10.1128/IAI.00914-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan SD, Schwartz SA, Aalinkeel R, Chawda RP, Sykes DE, Nair MP. Morphine modulates chemokine gene regulation in normal human astrocytes. Clin. Immunol. 2005;115:323–332. doi: 10.1016/j.clim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- McCarthy L, Szabo I, Nitsche JF, Pintar JE, Rogers TJ. Expression of functional mu-opioid receptors during T cell development. J. Neuroimmunol. 2001;114:173–180. doi: 10.1016/s0165-5728(01)00248-x. [DOI] [PubMed] [Google Scholar]
- McCarthy LE, Rogers TJ. Alteration of early T cell development by opioid and superantigen stimulation. Adv. Exp. Med. Biol. 2001;493:163–167. doi: 10.1007/0-306-47611-8_19. [DOI] [PubMed] [Google Scholar]
- Nigam P, Kwa S, Velu V, Amara RR. Loss of IL-17-producing CD8 T cells during late chronic stage of pathogenic simian immunodeficiency virus infection. J. Immunol. 2011;186:745–753. doi: 10.4049/jimmunol.1002807. [DOI] [PubMed] [Google Scholar]
- Novick DM, Ochshorn M, Ghali V, Croxson TS, Mercer WD, Chiorazzi N, Kreek MJ. Natural killer cell activity and lymphocyte subsets in parenteral heroin abusers and long-term methadone maintenance patients. J. Pharmacol. Exp. Therapeut. 1989;250:606–610. [PubMed] [Google Scholar]
- Ogata K, Nakamura K, Yokose N, Tamura H, Tachibana M, Taniguchi O, Iwakiri R, Hayashi T, Sakamaki H, Murai Y, Tohyama K, Tomoyasu S, Nonaka Y, Mori M, Dan K, Yoshida Y. Clinical significance of phenotypic features of blasts in patients with myelodysplastic syndrome. Blood. 2002;100:3887–3896. doi: 10.1182/blood-2002-01-0222. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Sharp B, Gekker G, Brummitt, Keane WF. Opioid-mediated suppression of interferon-gamma production by cultured peripheral blood mononuclear cells. J. Clin. Invest. 1987;80:824–831. doi: 10.1172/JCI113140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo CA, Shevach EM, Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J. Immunol. 2001;167:1137–1140. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- Piguet V, Sattentau Q, Piguet V, Sattentau Q. Dangerous liaisons at the virological synapse. J. Clin. Invest. 2004;114:605–610. doi: 10.1172/JCI22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast A, Prado JG, Kang YH, Chen F, Riddell LA, Luzzi G, Goulder P, Klenerman P. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. Aids. 2010;24:491–502. doi: 10.1097/QAD.0b013e3283344895. [DOI] [PubMed] [Google Scholar]
- Roberts AB. TGF-beta signaling from receptors to the nucleus. Microbes & Infection. 1999;1:1265–1273. doi: 10.1016/s1286-4579(99)00258-0. [DOI] [PubMed] [Google Scholar]
- Rock RB, Hu S, Sheng WS, Peterson PK. Morphine stimulates CCL2 production by human neurons. J. Neuroinflammation. 2006;3:32. doi: 10.1186/1742-2094-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojavin M, Szabo I, Bussiere JL, Rogers TJ, Adler MW, Eisenstein TK. Morphine treatment in vitro or in vivo decreases phagocytic functions of murine macrophages. Life Sci. 1993;53:997–1006. doi: 10.1016/0024-3205(93)90122-j. [DOI] [PubMed] [Google Scholar]
- Roy S, Balasubramanian S, Sumandeep S, Charboneau R, Wang J, Melnyk D, Beilman GJ, Vatassery R, Barke RA. Morphine directs T cells toward T(H2) differentiation. Surgery. 2001;130:304–309. doi: 10.1067/msy.2001.116033. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nature Reviews, Immunology. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309:99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- Stummvoll GH, DiPaolo RJ, Huter EN, Davidson TS, Glass D, Ward JM, Shevach EM. Th1, Th2, and Th17 effector T cell-induced autoimmune gastritis differs in pathological pattern and in susceptibility to suppression by regulatory T cells. J. Immunol. 2008;181:1908–1916. doi: 10.4049/jimmunol.181.3.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Rojavin M, Bussiere JL, Eisenstein TK, Adler MW, Rogers TJ. Suppression of peritoneal macrophage phagocytosis of Candida albicans by opioids. J. Pharmacol. Exp. Therapeut. 1993;267:703–706. [PubMed] [Google Scholar]
- Taub DD, Eisenstein TK, Geller EB, Adler MW, Rogers TJ. Immunomodulatory activity of mu- and kappa-selective opioid agonists. Proc. Natl. Acad. Sci. (USA) 1991;88:360–364. doi: 10.1073/pnas.88.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc. Natl. Acad. Sci. (USA) 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van YH, Lee WH, Ortiz S, Lee MH, Qin HJ, Liu CP. All-trans retinoic acid inhibits type 1 diabetes by T regulatory (Treg)-dependent suppression of interferon-gamma-producing T-cells without affecting Th17 cells. Diabetes. 2009;58:146–155. doi: 10.2337/db08-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk T, Schenk M, Voigt K, Tohtz S, Putzier M, Kox WJ. Postoperative epidural anesthesia preserves lymphocyte, but not monocyte, immune function after major spine surgery. Anesthesia & Analgesia. 2004;98:1086–1092. doi: 10.1213/01.ANE.0000104586.12700.3A. [DOI] [PubMed] [Google Scholar]
- Wang J, Barke RA, Charboneau R, Roy S. Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J. Immunol. 2005;174:426–434. doi: 10.4049/jimmunol.174.1.426. [DOI] [PubMed] [Google Scholar]
- Wang J, Charboneau R, Balasubramanian S, Barke RA, Loh HH, Roy S. Morphine modulates lymph node-derived T lymphocyte function: role of caspase-3, -8, and nitric oxide. J. Leuk. Biol. 2001;70:527–536. [PubMed] [Google Scholar]
- Wang J, Ma J, Charboneau R, Barke R, Roy S. Morphine inhibits murine dendritic cell IL-23 production by modulating Toll-like receptor 2 and Nod2 signaling. J. Biol. Chem. 2011;286:10225–10232. doi: 10.1074/jbc.M110.188680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber RJ, Pert A. The periaqueductal gray matter mediates opiate-induced immunosuppression. Science. 1989;245:188–190. doi: 10.1126/science.2749256. [DOI] [PubMed] [Google Scholar]
- Weed MR, Carruth LM, Adams RJ, Ator NA, Hienz RD. Morphine withdrawal dramatically reduces lymphocytes in morphine-dependent macaques. Journal Of Neuroimmune Pharmacology: The Official Journal Of The Society On NeuroImmune Pharmacology. 2006;1:250–259. doi: 10.1007/s11481-006-9029-z. [DOI] [PubMed] [Google Scholar]
- Wetzel MA, Steele AD, Eisenstein TK, Adler MW, Henderson EE, Rogers TJ. Muopioid induction of monocyte chemoattractant protein-1, RANTES, and IFN-gamma-inducible protein-10 expression in human peripheral blood mononuclear cells. J. Immunol. 2000;165:6519–6524. doi: 10.4049/jimmunol.165.11.6519. [DOI] [PubMed] [Google Scholar]
- Yeh N, Glosson NL, Wang N, Guindon L, McKinley C, Hamada H, Li Q, Dutton RW, Shrikant P, Zhou B, Brutkiewicz RR, Blum JS, Kaplan MH. Tc17 cells are capable of mediating immunity to vaccinia virus by acquisition of a cytotoxic phenotype. J. Immunol. 2010;185:2089–2098. doi: 10.4049/jimmunol.1000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen HR, Harris TJ, Wada S, Grosso JF, Getnet D, Goldberg MV, Liang KL, Bruno TC, Pyle KJ, Chan SL, Anders RA, Trimble CL, Adler AJ, Lin TY, Pardoll DM, Huang CT, Drake CG. Tc17 CD8 T cells: functional plasticity and subset diversity. J. Immunol. 2009;183:7161–7168. doi: 10.4049/jimmunol.0900368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama M, Itano Y, Katayama H, Morimatsu H, Takeda Y, Takahashi T, Nagano O, Morita K. The effects of continuous epidural anesthesia and analgesia on stress response and immune function in patients undergoing radical esophagectomy. Anesthesia & Analgesia. 2005;101:1521–1527. doi: 10.1213/01.ANE.0000184287.15086.1E. [DOI] [PubMed] [Google Scholar]