Abstract
Evening chronotypes not only differ from morning-types in their sleep and circadian timing, but they are prone to problematic outcomes involving reward function, including affective disturbance, sensation seeking, and substance involvement. We explored the neural mechanisms underlying these chronotype differences by comparing the neural response to reward in morning- and evening-types. Using a monetary reward fMRI paradigm, we compared the neural response to reward in 13 morning-types and 21 evening-types (all 20 y/o males). Region-of-interest (ROI) analyses focused on the medial prefrontal cortex (mPFC) and ventral striatum (VS), comparing the chronotype groups in these ROIs during anticipation and outcome conditions, and adjusting for time of scan. Chronotype groups were also compared on measures of sensation-seeking, substance involvement, and sleep quality. Evening-types reported significantly greater levels of alcohol dependence and worse sleep quality. Furthermore, evening-types showed an altered neural response to reward relative to morning-types, specifically, reduced mPFC reactivity during reward anticipation and increased VS reactivity during win outcome. In turn, less activation in the mPFC region in response to reward was associated with greater alcohol consumption, while increased activation in the VS in response to reward was associated with more symptoms of alcohol dependence. Increased reward-related problems among evening-types may be accompanied by altered neural responses to reward.
Keywords: Alcohol abuse, adolescence, brain imaging, circadian rhythms, chronotype, sleep
1. Introduction
Individual differences in chronotype, the preferred timing of sleep and activity, appear to moderate the risk for affective and behavioral dysregulation. Evening chronotypes, who prefer later sleep/wake schedules, consistently demonstrate higher levels of affective disturbance, sensation seeking, and substance use (e.g., (Gau et al., 2007; Hasler et al., 2010; Tonetti et al., 2010; Negriff et al., 2011), all problematic outcomes plausibly related to reward function. The associations between eveningness and indicators of dysregulated reward function hold cross-culturally, across adolescent and adult samples, and after accounting for numerous potential confounds including age, sex, and educational level. The developmental shift towards greater eveningness during adolescence and early adulthood (Roenneberg et al., 2004; Crowley et al., 2007) parallels marked changes in reward-seeking behavior and associated neural circuits ((Ernst and Fudge, 2009), suggesting that chronotype may be a particularly salient risk factor for reward-related impairments in these age groups. Further understanding of the neurobehavorial mechanisms linking eveningness to dysregulated reward function during late adolescence and early adulthood could inform the development of novel prevention and intervention approaches to affective and substance use disorders.
Although persuasive in the consistency of findings, the extant literature is almost entirely dependent on self-report measures related to reward function; few published studies have used objective measures such as neuroimaging. Several neuroimaging studies have demonstrated the effect of chronotype in other domains, including time-of-day variations in the activity of the motor cortex (Tamm et al., 2009; Peres et al., 2011), in hypothalamic and brainstem activation related to maintaining attention (Schmidt et al., 2009), and in brain reactivity to cognitive interference (Schmidt et al., 2012). Most germane to the current project, a recent study compared morning- and evening-type adults with primary insomnia using [18F]fluorodeoxyglucose-positron emission tomography (FDG-PET) during morning and evening wakefulness (Hasler et al., 2012b). The study focused on the medial prefrontal cortex (mPFC) and striatum, key elements of the reward circuit, in which the mPFC provides top-down modulation of the striatum (Ernst and Fudge, 2009). The evening-types displayed lower relative regional glucose metabolism in the mPFC and striatum during both morning and evening wakefulness. Furthermore, the evening-types displayed divergent diurnal patterns of brain activity within the mPFC and striatum that broadly paralleled the chronotype differences in diurnal patterns of positive affect. Specifically, the evening-types showed diurnal patterns that were not only later, but more blunted. Although preliminary, these findings suggest that alterations in reward-related brain function may contribute to the observed chronotype differences in behavioral indicators of reward function. Furthermore, these alterations may already be present during adolescence, based on a recent finding that later sleep timing was associated with reduced mPFC response to reward anticipation in healthy adolescents completing an fMRI monetary reward task (Forbes et al., 2012). Taken together, the PET and fMRI findings implicate chronotype in both top-down modulation of reward function (mPFC) and bottom-up reward motivation (striatum).
In addition to disturbances in reward-related behavior and brain function, evening-types display altered sleep and circadian rhythms. Evening-types show later timing of physiological markers of circadian timing in controlled laboratory conditions, indicating that chronotype is at least partially biologically-driven (Duffy et al., 2001; Mongrain et al., 2004). Under naturalistic conditions, evening-types tend to report later sleep-wake timing, longer latencies to sleep onset, lower subjective sleep quality, shorter total sleep time on weekdays, and larger swings between sleep-wake timing on work and free days (Taillard et al., 1999; Giannotti et al., 2002; Wittmann et al., 2006; Hasler et al., 2012b). This pattern led to the hypothesis that many evening-types are suffering from so-called “social jet lag”; that is, a mismatch between their internal circadian timing and conventionally early academic and/or work schedules (Wittmann et al., 2006), more broadly referred to as circadian misalignment. In this scenario, evening-types report longer sleep latencies because they are trying to initiate sleep at too early a biological time, they obtain less sleep because they are required to rise too early, and they show large differences in work-free day sleep-wake timing because they return to their preferred schedules on the weekend and sleep in to recover sleep lost during the work week. Thus, circadian misalignment and its accompanying sleep loss may partially explain the alterations in reward-related behavior and brain activity. This proposition is buttressed by a burgeoning literature demonstrating that the reward system is modulated by both circadian rhythms and sleep loss (Murray et al., 2009; Gujar et al., 2011; Venkatraman et al., 2011). Furthermore, we recently reported that larger differences in weekend-weekday sleep timing were associated with reduced mPFC reactivity to reward in a sample of healthy adolescents (Hasler et al., 2012a). The increased prevalence of eveningness during adolescence, with accompanying sleep and circadian disturbance, may partially explain the emergence of mood problems and substance use during this stage.
The present analyses explored the neural mechanisms underlying these chronotype differences by comparing reward-related brain function in morning- and evening-types from a sample of 20 year-old males. Studying our sample during late adolescence, on the cusp of adulthood, provides an opportunity to examine chronotype-reward interactions at a developmental stage when brain development is still ongoing but many reward-related problems, including substance use, are first emerging (Kandel and Logan, 1984; Ernst and Fudge, 2009). We build upon our previous findings of chronotype differences in the resting brain activity within the reward circuit by directly probing reward function in these regions using functional magnetic resonance imaging (fMRI) and a well-validated card-guessing paradigm designed to examine neural reactivity to anticipation and receipt of monetary reward. We focused on two regions—the mPFC and ventral striatum (VS)—that have been centrally implicated in reward processing (Haber and Knutson, 2010) and developmental models of reward-related behavior ((Ernst and Fudge, 2009). Our primary hypotheses rested on the presumption that the evening-types in our sample would be experiencing circadian misalignment and sleep loss, which we hypothesized would result in a combination of diminished top-down control and disinhibited reward responsiveness. Thus, consistent with diminished top-down modulation, we hypothesized that evening-types would display neural response to reward manifested by reduced mPFC reactivity, and greater basic reward motivation, manifested by increased VS reactivity. We did not have strong a priori hypotheses about differences across the anticipation and receipt phases of reward processing. In addition, consistent with reward function as a potential mechanism of the association between chronotype and substance use, we hypothesized that group differences in response to reward would occur in mPFC and VS subregions associated with substance use.
2. Methods
2.1 Participants and procedures
This study utilized data from the Pitt Mother & Child Project (PMCP), an ongoing longitudinal study examining vulnerability and resilience in low-income boys. The initial sample consisted of 310 boys and their families, recruited when boys were 6 to 17 months old through Women, Infants, and Children Nutritional Supplement (WIC) programs serving low-income families in the Pittsburgh metropolitan area (see (Shaw et al., 1999; Shaw et al., 2003). The current study utilizes data from a laboratory visit when participants were 20 years of age. This visit included a clinical interview, self-report measures, and an fMRI scan.
Participants were asked to refrain from using any illegal substances starting 48 hours prior to their fMRI scan and all participants were approved for the scan by a registered nurse. A combination of saliva drug screen, self report, and clinical judgment were used to determine which participants were approved to scan. Of those 254 young men completing age-20 visits, 184 participants completed a scan (25 were excluded due to past concussion, 17 excluded due to metal/bullet fragments/braces, 9 were unable to come in for the fMRI, 10 refused, 7 were too claustrophobic, 1 admitted to using illicit substances prior to the scan, and 1 was too large for the scanner). Of these, a total of 113 participants had both valid reward task fMRI data and had completed the Composite Scale of Morningness (CSM; described below). Dividing these 113 participants by chronotype resulted in 13 morning-types, 79 intermediate-types, and 21 evening-types. We focused our analyses on the well-defined morning- and evening-type groups in order to highlight differences attributable to circadian phase. Published evidence suggests that differences among individuals in the intermediate chronotype range are more subject to non-circadian influences (Mongrain and Dumont, 2007; Emens et al., 2009). Parallel analyses using the CSM as a continuous measure are reported in the Supplemental section (Table S1).
2.2 Measures of chronotype and sleep
We used the Composite Scale of Morningness (Smith et al., 1989)); 3=0.83) to assess chronotype. The score is obtained by the sum of 13 Likert-type items, and ranges from 13 (extreme eveningness) to 55 (extreme morningness). Sample items include “(1) Considering your own ‘feeling best’ rhythm, at what time would you get up if you were entirely free to plan your day?” (five options ranging from 5–6:30 AM to 11AM-noon) and “(9) One hears about ‘morning’ and ‘evening’ types of people. Which ONE of these types do you consider yourself to be?” (Definitely a morning type; more a morning than an evening type; more an evening than a morning type; definitely an evening type). Because the original CSM cut-offs were criticized for being unreliable across samples, we used more recent recommended cut-off scores (total score of 13–26 evening-type; 27–41 intermediate-type; 42–55 morning-type) (Natale and Alzani, 2001). We used the Pittsburgh Sleep Quality Index (PSQI; (Buysse et al., 1989)) to evaluate subjective sleep quality over the previous month. The 19 self-report items are combined into seven clinically-derived component scores, each weighted equally from 0 to 3, which are added to obtain a global score ranging from 0 to 21, with higher scores indicating worse sleep quality.
2.3 Clinical and substance use related variables
Psychiatric disorders were assessed using the Structured Clinical Interview for the DSM-IV (First et al., 2002). We evaluated impulsivity level via the Barratt Impulsiveness Scale–11 (BIS-11; (Patton et al., 1995); 3=0.80) and sensation-seeking via the impulsive sensation seeking subscale from the Zuckerman-Kuhlman Personality Questionnaire III ((Zuckerman et al., 1993); 3=0.79). Self-reported depressive symptoms were assessed using the Beck Depression Inventory (BDI; (Beck et al., 1961); α=0.86).
Interviewers administered the Alcohol and Drug Consumption Questionnaire (ADCQ; (Cahalan et al., 1969)) to participants to assess frequency of drug and alcohol use within the past year. Participants indicated their frequency of use of 12 different substances on an 8-point Likert scale (0=have never tried, 8= everyday use). Participants’ responses for alcohol, marijuana, and tobacco were used in these analyses. We used the Alcohol Dependence Scale (ADS; (Skinner and Allen, 1982); α=.83), a 25-item questionnaire to assess self-reported alcohol withdrawal symptoms, impaired control over drinking, awareness of a compulsion to drink, increased tolerance to alcohol, and salience of drink-seeking behavior in the past year. In the present study, only participants reporting current alcohol use completed the ADS. A score of 9 or more is highly indicative of current diagnosis of alcohol abuse or dependence.
2.4 Reward processing task
We employed a slow event-related fMRI card-guessing paradigm designed to examine neural reactivity to anticipation and receipt of monetary reward and loss. Each trial included an anticipation-period and outcome-period, where participants received win, loss, or no-change feedback for each trial. Although we describe the entire task here, the current analyses focused on possible-reward trials.
Trials were presented in a pseudorandom order with predetermined outcomes. During each 20-second trial, participants had 4-seconds to guess, via button press, whether the value of a visually presented card with a possible value of 1–9 was higher or lower than 5. After a choice was made, the trial type was presented visually for a 6-second anticipation period, indicating whether the trial was a possible-reward (upward arrow) or possible-loss (presentation of a downward arrow) type. In reward trials participants would win $1 if their guess was correct and there would be no-change in earnings if their guess was incorrect. In loss trials participants would lose 50 cents if their guess was incorrect and there would be no-change in earnings if their guess was correct. The anticipation-period was immediately followed by the outcome-period, where participants were presented with the “actual” numerical value of the card (500ms) and received outcome feedback (additional 500ms): a green upward-facing arrow for win, a red downward-facing arrow for loss, or a yellow circle for no-change feedback. A crosshair was then presented for 9-seconds (inter-stimulus-interval, ISI). Twenty-four trials were presented in 1 run with 12 reward-anticipation and 12 loss-anticipation trials. Within reward-anticipation trials there were a balanced number of win-outcome and no-change outcome trials.
Contrasts generated from task data were reward anticipation > baseline and reward win > baseline, in which baseline was defined as the last 3 seconds of the ISI. We focused on reward trials rather than loss trials—and contrasted reward conditions with baseline—given evidence that chronotype is more strongly related to reward, appetitive motivation, and positive affect than to negative affect processes (Hasler et al., 2010). Outcome probabilities were fixed trial-wise in order to ensure an identical win/loss time series modeling and pattern of outcome experiences for every participant. Each participant was given $10 in earnings. Participants were unaware of the fixed outcome probabilities in the paradigm, and were led to believe their performance would determine net monetary gain.
2.5 fMRI data acquisition
Neuroimaging data were collected using a 3.0 Tesla Siemens Trio MRI scanner at the University of Pittsburgh. Structural 3D axial MPRAGE images were acquired in the same session (TE=3.29ms; TR=2200ms; Flip Angle=9°; Field of View=256×192mm; Slice-Thickness=1mm; Matrix: 256×256; 192 continuous slices). Mean blood-oxygenation-level-dependent (BOLD) images were then acquired with a gradient echo EPI sequence during 13-minutes covering 39 axial slices (3.1mm thick; TR/TE=2000/28msec; FOV=205×205mm; matrix 64×64; Flip Angle 90°).
2.6 fMRI data analysis
Data were preprocessed and analyzed with Statistical Parametric Mapping software, Version-8 (SPM8; http://www.fil.ion.ucl.ac.uk/spm). Data for each participant were realigned to the first volume in the time series to correct for head motion. Data sets were then selected for quality based on our standard small-motion correction (<2 mm). Participants were also removed from analysis if they missed 50% or more of the total trials or winning trials. Realigned images were then coregistered with the subject’s anatomical image, segmented to restrict analyses to gray matter, normalized to standard Montreal Neurological Institute (MNI) template, and spatially smoothed with a Gaussian kernel of 6mm full-width at half-maximum.
A first-level fixed-effect model was constructed for each participant and scan and predetermined condition effects at each voxel were calculated using a t-statistic, producing a statistical image for two contrasts: reward-anticipation-minus-baseline and win-outcome-after-reward-anticipation-minus-baseline.
First-level contrast images were then included in second-level whole-brain and region of interest (ROI) analyses. Given prior evidence of circadian rhythms in reward processes (Murray et al., 2009), we first conducted whole-brain voxel-wise regression analyses to examine whether there was a correlation between reward-related brain function and time of scan during reward anticipation or win outcome. We applied a height threshold of p<0.05, minimum extent of 10 contiguous voxels, and corrected cluster-level threshold of p<0.05 (based on Monte Carlo Simulations in AlphaSim) to these analyses. Time of scan ranged from 9 AM to 4 PM, although 76.5 % (30/34 participants) completed their scan between noon and 4 PM. Time of scan positively correlated with brain activation in large clusters during reward anticipation versus baseline (14,118 voxels, t=5.27, p<0.05, peak voxel[−16, −38,72]; 8,736 voxels, t=4.31, p<0.001, peak voxel [−46, −50, −2]) and win outcome versus baseline (3716 voxels, t=5.17, p<0.05, peak voxel[64, −30,12]; 9,721 voxels, t = 3.96, p<0.05, peak voxel[−58 18 12]). That is, multiple brain regions—for both chronotype groups, as well as the sample as a whole—exhibited increasing reward-related activation as the time of scan was later in the day, whereas no regions in our sample showed statistically significant decreasing reward-related activation with later scan times. In both contrasts, the clusters included portions of the mPFC and striatum (see further details in the Supplemental section); thus, we included time of scan as a covariate in subsequent analyses.
Next, we used two-sample t-tests in SPM to compare morning- and evening-type neural responses to reward within striatal and mPFC ROIs during reward anticipation and win outcome. The striatal region of interest (ROI) was anatomically-defined using the nucleus accumbens, caudate head, and putamen regions from WFU PickAtlas (v3.0.3). The mPFC ROI was constructed using the PickAtlas and defined as a 5,393-voxel sphere including medial Brodmann Area (BA) 10 and BA32, key projection targets of midbrain dopamine neurons, which demonstrate connectivity with the ventral striatum, and which have been implicated in evaluating the relative value of rewards and reward-directed behavior (Haber and Knutson, 2010). Time of scan was used as a covariate in these models. Next, regression analyses in SPM tested associations between reward-related brain function, time of scan, alcohol and marijuana use, and the Alcohol Dependence Scale. For all analyses, a threshold of p<0.05 and minimum extent of 10 contiguous voxels were applied. All results were corrected for multiple comparisons at the cluster-level via Monte Carlo simulations conducted in the AlphaSim program within AFNI (http://afni.nih.gov/afni/docpdf/AlphaSim.pdf). Regression models were also computed in SPSS (v 18.0.2) using extracted data to determine overall model fit.
3. Results
3.1 Chronotype differences in clinical and substance-related measures (Table 1)
Table 1.
Clinical and substance-related measures
| Morning-types (n = 13) | Evening-types (n = 21) | t | p | d | |
|---|---|---|---|---|---|
| DSM-IV Diagnoses | |||||
| Major Depressive Disorder | 1 current | 3 past | |||
| Alcohol Abuse | 1 past | 1 current, 1 past | |||
| Alcohol Dependence | none | none | |||
| Substance Abuse | 1 current | 3 current, 1 past | |||
| Substance Dependence | none | 1 current, 2 past | |||
| Beck Depression Inventory | 4.85 ± 7.78 | 5.19 ± 5.54 | 0.15 | ns | 0.06 |
| Sensation Seeking Scale | 9.08 ± 4.41 | 9.29 ± 3.41 | 0.16 | ns | 0.06 |
| Barrett Impulsiveness Scale | 54.77 ± 12.31 | 60.19 ± 8.65 | 1.51 | ns | 0.55 |
| Alcohol Dependence Scalea | 2.30 ± 1.77 | 5.00 ± 3.50 | 2.60 | 0.016 | 1.09 |
| Alcohol and Drug Consumption Quest. | |||||
| Alcohol | 3.38 ± 1.12 | 3.86 ± 1.74 | 0.87 | ns | 0.32 |
| Marijuana | 2.69 ± 2.53 | 3.81 ± 2.86 | 1.16 | ns | 0.43 |
| Tobacco | 4.77 ± 3.83 | 4.86 ± 3.37 | 0.07 | ns | 0.03 |
The ADS was completed by 10 Morning-types and 16 Evening-types.
Groups are based on extreme cases from an original sample of 118 male adolescents.
d = Cohen’s d effect size.
Although evening-types reported greater degrees of dysregulation on all clinical measures and higher frequencies of substance use, the only statistically significant difference occurred on the Alcohol Dependence Scale (ADS). Based on two-tailed t-tests, evening-types reported more severe symptoms of alcohol dependence. Furthermore, small-to-medium effect sizes were observed for the Barrett Impulsiveness Scale and the frequency of alcohol and marijuana use on the Alcohol and Drug Consumption Questionnaire.
3.2 Chronotype differences in sleep quality (Table 2)
Table 2.
Chronotype differences on Pittsburgh Sleep Quality Index.
| PSQI Component Scale | Morning-types (n = 11) | Evening-types (n = 19) | t | p | d |
|---|---|---|---|---|---|
| Subjective sleep quality | 0.55 ± 0.52 | 1.42 ± 0.90 | 2.94 | 0.002 | 1.15 |
| Sleep latency | 0.55 ± 0.52 | 1.47 ± 1.26 | 2.81 | 0.009 | 1.10 |
| Sleep duration | 0.27 ± 0.90 | 0.68 ± 0.95 | 1.17 | ns | 0.46 |
| Sleep efficiency | 0.27 ± 0.47 | 0.79 ± 1.13 | 1.75 | 0.09 | 0.69 |
| Sleep disturbance | 0.64 ± 0.67 | 0.95 ± 0.71 | 1.18 | ns | 0.46 |
| Medications needed to sleep | 0.00 ± 0.00 | 0.16 ± 0.69 | 0.76 | ns | 0.30 |
| Daytime dysfunction | 0.27 ± 0.65 | 0.47 ± 0.77 | 0.73 | ns | 0.29 |
| TOTAL SCORE (0–21) | 2.55 ± 1.57 | 5.95 ± 4.80 | 2.84 | 0.009 | 1.11 |
NOTE: Subscale scores range from 0 (not at all during past month) to 3 (3+ times per week). Total scores greater than 5 are considered clinically significant. d = Cohen’s d effect size. PSQI data from 4 participants (2 from each group) was not included due to missing entries.
Due to missing data, analyses of the PSQI were based on 11 morning-types and 19 evening-types.. Based on two-tailed t-tests, evening-types reported statistically reliable worse overall sleep quality (PSQI Total Score) that was consistent with clinically-significant sleep disturbance (PSQI > 5). The difference in PSQI-Total reflected significantly worse scores on two PSQI subscales, including worse subjective sleep quality, and longer sleep latency (time to fall asleep). Given these findings, we also conducted the analyses below with PSQI score as a covariate. Results were the largely the same as those reported below.1
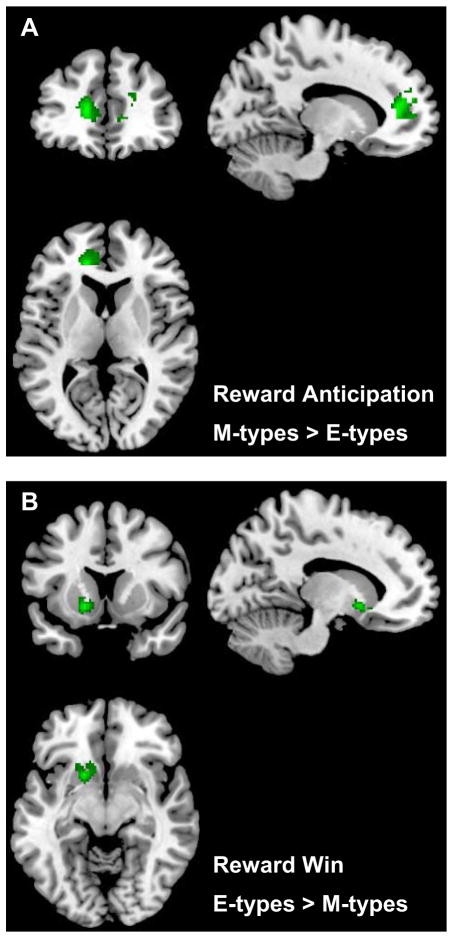
3.3 Chronotype differences in reward-related brain activity (Table 3)
Table 3.
Chronotype differences in reward-related brain activity
| MNI Coordinates of Maximum Voxel in Cluster | |||||
|---|---|---|---|---|---|
|
| |||||
| x | y | z | Cluster Size | t | |
| M-type > E-type | |||||
| Reward Anticipation | |||||
| mPFC | −14 | 40 | 8 | 444 | 3.97 |
| E-type > M-type | |||||
| Win Outcome | |||||
| Ventral striatum | −16 | 12 | −8 | 181 | 4.32 |
NOTE: Both clusters survived correction for multiple comparisons in Monte Carlo simulations. df = 31
Morning- and evening-types differed in their patterns of brain reactivity to reward during the reward anticipation and win outcome conditions. Morning-types displayed greater left mPFC reactivity to reward anticipation (Figure 1A). No chronotype differences in striatal reactivity met the statistical threshold during reward anticipation. In contrast, during win outcome, evening-types displayed greater VS reactivity to reward (Figure 1B). No chronotype differences in mPFC reactivity met the statistical threshold during win outcome. These findings were broadly consistent with ROI analyses using CSM as a continuous measure (Table S2) and with findings for these regions within whole-brain analyses (Table S3).
Figure 1.
Chronotype differences in the neural response to the anticipation (1A) and outcome (1B) of monetary reward. Results are based on two-tailed t-tests run in SPM8, covarying for the time of scan, and masked for regions of interest in the medial prefrontal cortex (mPFC) and ventral striatum (VS). Evening-types displayed less mFPC activity during reward anticipation compared to morning-types (1A). Evening-types displayed more VS activity during win outcome compared to morning-types (1B).
3.4 Alcohol involvement and chronotype-relevant neural response to reward
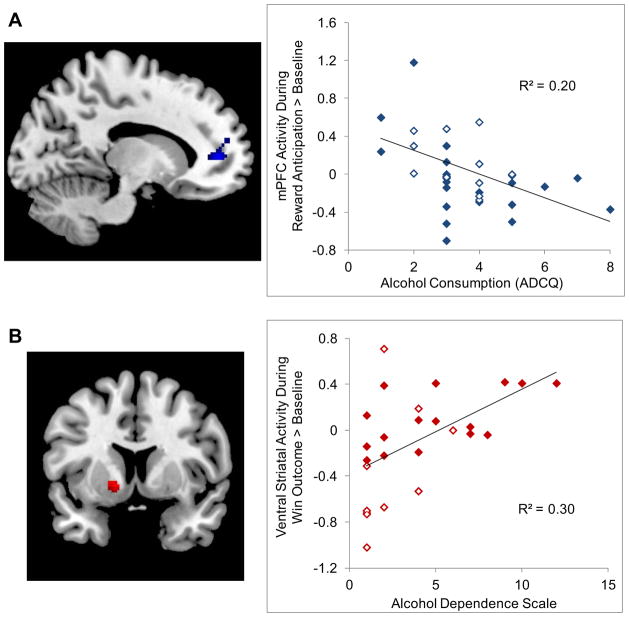
We tested whether the respective brain clusters showing chronotype difference in the neural response to reward also related to differences in alcohol or marijuana use, as based on the ADCQ scales. Alcohol and marijuana use showed larger effects sizes (Table 1) in the chronotype comparison, relative to tobacco use, and both were used by sizeable portions of the sample in the past year (82.4 and 41.2%, respectively). We constrained the analyses to the functional masks resulting from the chronotype comparisons; that is, analyses were constrained to mPFC cluster wherein morning-types displayed relatively greater reactivity during reward anticipation, and to the VS cluster wherein evening-types displayed relatively great reactivity during win outcome. A mPFC cluster showing negative correlation with alcohol use was found within mPFC regions showing decreased reactivity to reward anticipation in evening-types (151 voxels, t=4.63, pcorrected<0.05, peak voxel[−14,46,6]; Figure 2A). No associations were found with marijuana use.
Figure 2.
Medial prefrontal cortex (mPFC) activity during anticipation of monetary reward correlated with the alcohol consumption scale on the ADCQ (2A) and ventral striatal (VS) activity during the win outcome of monetary reward correlated with scores on the Alcohol Dependence Scale (2B). Results are based on regression analysis in SPM8, masked either for the mPFC cluster in which Evening-types displayed less mPFC activity during reward anticipation (see Figure 1A), or for the VS cluster in which Evening-types displayed greater VS activity during win outcome (see Figure 1B), respectively. Open diamonds = Morning-types; filled diamonds = Evening-types.
Given that the strongest chronotype difference among the clinical and substance-related measures was for alcohol dependence severity as assessed on the ADS, we speculated that the chronotype differences in the neural response to reward might relate to the observed differences in alcohol dependence. To explore this possibility, we used SPM regression analyses to examine the correlation between the ADS score and reward-related brain activity during reward anticipation and win outcome. As above, we constrained the analyses to the functional masks resulting from the chronotype comparisons. A VS cluster showing positive correlation with the ADS was found within striatal regions showing increased reactivity to win outcome in evening-types (96 voxels, t=3.55, pcorrected<0.05, peak voxel[−14,8, −4]; Figure 2B).
4. Discussion
This study is the first to investigate chronotype differences in the neural response to reward, thus exploring whether consistent reports of greater affective and behavioral dysregulation among evening-types may relate to chronotype differences within the reward circuit. Consistent with this hypothesis, we found that late adolescent evening-types displayed less mPFC reactivity during reward anticipation and greater VS reactivity during win outcome, a pattern that could indicate reduced regulatory control and elevated reward sensitivity. We controlled for time-of-day differences in these comparisons, thereby accounting for diurnal changes in reward-related brain function that could obscure the effects of chronotype. We also found that in this sample, at a vulnerable developmental period for alcohol-related problems (Kandel and Logan, 1984), evening-types reported more severe (albeit subclinical) symptoms of alcohol dependence and worse sleep quality, findings consistent with the greater affective and behavioral dysregulation observed in other evening-type samples. Furthermore, the mPFC and VS regions in which response differed by chronotype were associated with alcohol use and alcohol dependence, respectively. Taken together, our findings offer preliminary evidence that the greater dysregulation observed among late adolescent evening-types may have a basis in altered neural response to reward, and that this dysregulation is linked to substance use.
The evening-type group displayed a distinct pattern of neural response to monetary reward, including relatively less mPFC activation during anticipation and relatively greater VS activation during win outcome. This pattern could plausibly reflect reduced regulatory control and elevated reward sensitivity among the evening-types. This is consistent in turn with impulsivity acting as a risk factor among the evening-types, given links between the mPFC and individual differences in impulsivity (Sripada et al., 2011) and apparently greater impulsivity among the evening-types in our sample. Alternatively, the apparent mPFC decrement among evening-types could reflect impairments in the abstract calculation of intrinsic value, given that the cluster is proximal to the mesofrontal regions previously identified in reward appraisal paradigms comparing delayed versus immediate rewards (e.g., (Kable and Glimcher, 2007)) or healthy versus liked-but-unhealthy foods (Hare et al., 2009). Increased VS reactivity to win outcome in our sample is consistent with developmental models espousing hyper-reactivity to reward during adolescence (Spear, 2011), when the tendency towards eveningness is maximal (Roenneberg et al., 2004; Crowley et al., 2007). Finally, the chronotype differences during both the anticipation and outcome phases of our task suggest that chronotype may influence both anticipatory (“wanting”) and consummatory (“liking”) aspects of reward (Berridge and Robinson, 2003). Confirming this speculation will require more rigorous parsing of these two processes, given that fMRI tasks employing monetary rewards—rather than primary rewards such as food--are arguably unable to adequately tap consummatory processes.
The evening-types’ responses to reward in our study are consistent with the psychological and behavioral correlates of eveningness in other studies, which generally reflect disrupted reward responding. These include greater sensation-seeking and nicotine, alcohol, and marijuana use (e.g., (Adan, 1994; Gau et al., 2007; Tonetti et al., 2010; Broms et al., 2011). Furthermore, the reward-related reactivity in the mPFC and VS was related to reported alcohol use and alcohol dependence severity, respectively, grounding the apparent group-differences in brain function in clinically meaningful behaviors relevant to addiction. Reduced mPFC reactivity during reward anticipation was associated with increased alcohol consumption, while increased VS reactivity during win outcome was associated with greater subclinical alcohol dependence. Our mPFC finding is consistent with a recent finding that less mPFC activity during monetary reward-seeking is associated with elevated alcohol consumption (Bogg et al., 2012). Our VS finding appears less cohesive with models of alcoholism as a reward deficit disorder, where non-alcohol rewards such as money are less rewarding (Koob, in press). One possibility is that any blunted response to non-alcohol rewards emerges only after a longer period of alcohol involvement (i.e., from processes related to exposure to alcohol and/or development of addiction), which would be consistent with the duration and intensity of alcohol use among the young men in the current sample, who have had a lower degree of alcohol exposure than typical populations studied in human addiction research. Overall, the findings support a model in which neural changes linked to eveningness underlie the increased alcohol use reported in adolescent and adult evening-types (e.g., (Adan, 1994; Giannotti et al., 2002; Pieters et al., 2010; Negriff et al., 2011).
In contrast, other studies have observed reward-related dysregulation in the opposite direction among evening-types, including more severe symptoms of depression, reduced positive affect, and reduced self-reported reward responsiveness (e.g,, (Drennan et al., 1991; Hasler et al., 2010). Evening-types may also be less optimistic (Levy, 1985), which could influence their perception of expected value in the reward cues. Eveningness may be generally associated with disrupted reward function, which can lead individuals along different trajectories (e.g., depression and anhedonia versus substance use and reward-seeking) depending on other moderating influences. Adolescent evening-types may be at risk of landing on one of these problem trajectories.
Our findings complement a previous study by our group (Forbes et al., 2012) reporting that later midsleep times among 90 healthy adolescents (age 11–13) were associated with lower mPFC activation during the receipt of monetary reward. That analysis covaried for total sleep time, suggesting that greater sleep loss among the adolescents with later sleep-wake schedules (plausibly the result of greater eveningness) did not account for the effect. Furthermore, the Forbes et al study found that a polymorphism in the circadian gene PER2 (rs2304672 SNP) moderated the response to reward outcome in an overlapping region of the mPFC. This finding is coherent with animal studies indicating that circadian genes, including PER2, are active in reward-related brain regions and influence behavioral indices of reward function including alcohol consumption (e.g., (Spanagel et al., 2005; Amir and Stewart, 2009). Taken together with the present results, these findings implicate the circadian system in the modulation of the neural circuitry of reward, although a more definitive demonstration will require physiological measures of circadian timing (e.g., melatonin or core body temperature). Alternatively, the altered reward function could be driven by non-circadian sleep disturbances or sleep loss among the evening-types. Recent studies have reported that acute sleep deprivation induces increased reactivity to reward and pleasurable stimuli, reduced concern about losses, and diminished behavioral inhibition (Gujar et al., 2011; Venkatraman et al., 2011).
In this sample, chronotype differences in clinical and substance-related measures parallel those observed in the previous literature. The significantly greater ADS scores among evening-types is consistent with numerous papers reporting greater alcohol use (Adan, 1994; Giannotti et al., 2002; Pieters et al., 2010; Negriff et al., 2011). No other statistically significant chronotype differences occurred among the clinical and substance-related measures in our study. However, the differences were consistently in the direction of more dysregulation among the evening-types, and we observed small-to-medium effect sizes for the Barrett Impulsiveness Scale and the frequency of alcohol and marijuana use on the ADCQ. This suggests that these differences might be meaningful, albeit modest in magnitude, and require a larger sample to detect statistically reliable differences.
Consistent with other studies, the evening-types in our sample reported worse overall sleep quality (e.g., (Taillard et al., 1999; Giannotti et al., 2002; Hasler et al., 2012b). The worse sleep quality appears to be driven in part by longer sleep latencies (time to fall asleep), which may indicate that the evening-types were retiring at too early a biological time to fall asleep. This would be consistent with “social jet lag” among the evening-types, which has been hypothesized to account for their affective disturbance and increased substance involvement (Wittmann et al., 2006). We were limited by our use of a global, retrospective measure of sleep. More precise determination of the sleep patterns and disturbances among evening-types would be achieved through prospective assessment via diaries or wrist actigraphy. These methods would also allow determination of the difference in weekday-weekend sleep timing, which provides a measure of the extent of social jet lag (Wittmann et al., 2006).
The evening-types’ worse sleep quality could plausibly lead to reduced vigilance, which could provide an alternative mechanism for chronotype differences on the reward task, and might differentially impact the anticipation and outcome phases of the task.1 Although our study lacked a direct assessment of vigilance to address these questions, we would assert that our reward task is likely to be less subject to vigilance demands than the widely-used Monetary Incentive Delay task (Knutson et al., 2000), given that task’s reliance on reaction time.
Our study was most notably limited by the small sample size and reliance on self-report measures of chronotype. The apparent effects of chronotype on the neural response to reward must be interpreted with caution until replicated with a larger sample. Follow-up studies should also include female participants, emerging adults from higher SES backgrounds, and participants living in both rural and suburban (versus urban) communities. Our measure of chronotype is widely-used and practical, but not strictly synonymous with endogenous circadian timing, which is best measured via physiological measures (e.g., melatonin) under controlled conditions. Finally, our study was limited by its cross-sectional and nonexperimental design, which precludes determination of causality. An important eventual step would be to conduct longitudinal studies assessing whether there are any parallels in the trajectories of chronotype, reward-related brain function, and reward-related behavior during the course of adolescence.
These preliminary data indicate that oft-noted increases in reward-related problems among late adolescent evening-types are accompanied by altered neural responses to reward that are consistent with reduced regulatory control and elevated reward reactivity. The findings may have clinical relevance. These chronotype-reward function associations could have meaning for the development of problems at this age, with individuals showing this combination of characteristics more likely progress to full-blown addiction, and thus chronotype may be important to assess in the context of drug and alcohol use evaluations. On the other hand, elevated reward sensitivity among the evening-types may also indicate appropriate interventions; for example, evening-types may be more amenable to contingency management for alcohol or drug abstinence. These findings underscore the importance of further investigations aimed at elucidating the possible mechanisms (e.g., “social jet lag”) linking chronotype to reward-related brain function and behavior. Demonstrating a role for “social jet lag” in the observed reward-related problems among evening-types would provide yet another argument in the ongoing efforts to move school start times later in secondary education. Greater understanding of these mechanisms may inform sleep- and circadian-based preventive measures and interventions for alcohol use disorders during a pivotal time of development.
Supplementary Material
Acknowledgments
The authors would like to thank the participants and their parents who participated in this study. The authors would also like to thank the staff of the University of Pittsburgh Magnetic Resonance Research Center for their invaluable technical assistance. This work was supported by grants from the National Institutes of Health, including DA026222 (Forbes, Shaw), T32HL082610 (Buysse) and K01DA032557 (Hasler).
Footnotes
We also explored sleep quality’s influence on our findings by including it as a covariate along with time of scan in all of our primary models. The results were in the same direction, with the mPFC effect for reward anticipation a bit stronger (680 voxels; t=5.10, p<0.001) and the VS effect for reward outcome somewhat weaker (72 voxels, t=3.52,p<0.001) and no longer meeting Alphasim threshold (141 voxels). These results suggest that sleep quality is not a strong mediator of the chronotype effect overall, and is at best a partial mediator of the chronotype effect on VS reactivity. Furthermore, the sleep quality measured by the PSQI is distinct from sleep loss, which we hypothesize may partially mediate the association between chronotype and neural response to reward.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adan A. Chronotype and personality factors in the daily consumption of alcohol and psychostimulants. Addiction. 1994;89:455–462. doi: 10.1111/j.1360-0443.1994.tb00926.x. [DOI] [PubMed] [Google Scholar]
- Amir S, Stewart J. Motivational modulation of rhythms of the expression of the clock protein PER2 in the limbic forebrain. Biological Psychiatry. 2009;65:829–834. doi: 10.1016/j.biopsych.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neurosciences. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bogg T, Fukunaga R, Finn PR, Brown JW. Cognitive control links alcohol use, trait disinhibition, and reduced cognitive capacity: Evidence for medial prefrontal cortex dysregulation during reward-seeking behavior. Drug and Alcohol Dependence. 2012;122:112–118. doi: 10.1016/j.drugalcdep.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broms U, Kaprio J, Hublin C, Partinen M, Madden PA, Koskenvuo M. Evening types are more often current smokers and nicotine-dependent-a study of Finnish adult twins. Addiction. 2011;106:170–177. doi: 10.1111/j.1360-0443.2010.03112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cisin I, Crossley H. American drinking practices. Center for Alcohol Studies, Rutgers University; New Brunswick, N.J: 1969. [Google Scholar]
- Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep medicine. 2007;8:602–612. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Drennan MD, Klauber MR, Kripke DF, Goyette LM. The effects of depression and age on the Horne-Ostberg morningness-eveningness score. Journal of affective disorders. 1991;23:93–98. doi: 10.1016/0165-0327(91)90096-b. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behavioral Neuroscience. 2001;115:895–599. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- Emens JS, Yuhas K, Rough J, Kochar N, Peters D, Lewy AJ. Phase angle of entrainment in morning- and evening-types under naturalistic conditions. Chronobiology International. 2009;26:474–493. doi: 10.1080/07420520902821077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience & Biobehavioral Reviews. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RC, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Edition. Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Forbes EE, Dahl RE, Almeida JR, Ferrell RE, Nimgaonkar VL, Mansour H, Sciarrillo SR, Holm SM, Rodriguez EE, Phillips ML. PER2 rs2304672 polymorphism moderates circadian-relevant reward circuitry activity in adolescents. Biological Psychiatry. 2012;71:451–457. doi: 10.1016/j.biopsych.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gau SS, Shang CY, Merikangas KR, Chiu YN, Soong WT, Cheng AT. Association between morningness-eveningness and behavioral/emotional problems among adolescents. Journal of Biological Rhythms. 2007;22:268–274. doi: 10.1177/0748730406298447. [DOI] [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. Journal of Sleep Research. 2002;11:191–199. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- Gujar N, Yoo SS, Hu P, Walker MP. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. Journal of Neuroscience. 2011;31:4466–4474. doi: 10.1523/JNEUROSCI.3220-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Allen JJB, Sbarra DA, Bootzin RR, Bernert RA. Morningness-eveningness and depression: Preliminary evidence for the role of BAS and positive affect. Psychiatry Research. 2010;176:166–173. doi: 10.1016/j.psychres.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Dahl RE, Holm SM, Jakubcak JL, Ryan ND, Silk JS, Phillips ML, Forbes EE. Weekend-weekday advances in sleep timing are associated with altered reward-related brain function in healthy adolescents. Biological psychology. 2012a;91:334–341. doi: 10.1016/j.biopsycho.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Germain A, Nofzinger EA, Kupfer DJ, Krafty RT, Rothenberger SD, James JA, Bi W, Buysse DJ. Chronotype and diurnal patterns of positive affect and affective neural circuitry in primary insomnia. Journal of Sleep Research. 2012b doi: 10.1111/j.1365-2869.2012.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Logan JA. Patterns of drug use from adolescence to young adulthood: I. Periods of risk for initiation, continued use, and discontinuation. American Journal of Public Health. 1984;74:660–666. doi: 10.2105/ajph.74.7.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Koob GF. Theoretical Frameworks and Mechanistic Aspects of Alcohol Addiction: Alcohol Addiction as a Reward Deficit Disorder. Current topics in behavioral neurosciences. doi: 10.1007/7854_2011_129. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DA. Optimism and pessimism: Relationships to circadian rhythms. Psychological Reports. 1985;57:1123–1126. [Google Scholar]
- Mongrain V, Dumont M. Increased homeostatic response to behavioral sleep fragmentation in morning types compared to evening types. Sleep. 2007;30:773–780. doi: 10.1093/sleep/30.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongrain V, Lavoie S, Selmaoui B, Paquet J, Dumont M. Phase relationships between sleep-wake cycle and underlying circadian rhythms in morningness-eveningness. Journal of Biological Rhythms. 2004;19:248–257. doi: 10.1177/0748730404264365. [DOI] [PubMed] [Google Scholar]
- Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB, Trinder J. Nature’s clocks and human mood: the circadian system modulates reward motivation. Emotion. 2009;9:705–716. doi: 10.1037/a0017080. [DOI] [PubMed] [Google Scholar]
- Natale V, Alzani A. Additional validity evidence for the composite scale of morningness. Personality and Individual Differences. 2001;30:293–301. [Google Scholar]
- Negriff S, Dorn LD, Pabst SR, Susman EJ. Morningness/eveningness, pubertal timing, and substance use in adolescent girls. Psychiatry Research. 2011;185:408–413. doi: 10.1016/j.psychres.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Peres I, Vetter C, Blautzik J, Reiser M, Poppel E, Meindl T, Roenneberg T, Gutyrchik E. Chronotype predicts activity patterns in the neural underpinnings of the motor system during the day. Chronobiology International. 2011;28:883–889. doi: 10.3109/07420528.2011.619084. [DOI] [PubMed] [Google Scholar]
- Pieters S, Van Der Vorst H, Burk WJ, Wiers RW, Engels RC. Puberty-dependent sleep regulation and alcohol use in early adolescents. Alcoholism: Clinical and Experimental Research. 2010;34:1512–1518. doi: 10.1111/j.1530-0277.2010.01235.x. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. A marker for the end of adolescence. Current Biology. 2004;14:R1038–1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Collette F, Leclercq Y, Sterpenich V, Vandewalle G, Berthomier P, Berthomier C, Phillips C, Tinguely G, Darsaud A, Gais S, Schabus M, Desseilles M, Dang-Vu TT, Salmon E, Balteau E, Degueldre C, Luxen A, Maquet P, Cajochen C, Peigneux P. Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area. Science. 2009;324:516–519. doi: 10.1126/science.1167337. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Peigneux P, Leclercq Y, Sterpenich V, Vandewalle G, Phillips C, Berthomier P, Berthomier C, Tinguely G, Gais S, Schabus M, Desseilles M, Dang-Vu T, Salmon E, Degueldre C, Balteau E, Luxen A, Cajochen C, Maquet P, Collette F. Circadian preference modulates the neural substrate of conflict processing across the day. PloS one. 2012;7:e29658. doi: 10.1371/journal.pone.0029658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DS, Gilliom M, Ingoldsby EM, Nagin DS. Trajectories leading to school-age conduct problems. Developmental psychology. 2003;39:189–200. doi: 10.1037//0012-1649.39.2.189. [DOI] [PubMed] [Google Scholar]
- Shaw DS, Winslow EB, Flanagan C. A prospective study of the effects of marital status and family relations on young children’s adjustment among African American and European American families. Child Development. 1999;70:742–755. doi: 10.1111/1467-8624.00053. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. Journal of Abnormal Psychology. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. Journal of Applied Psychology. 1989;74:728–738. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nature Medicine. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: Emerging convergences across laboratory animal and human data. Developmental cognitive neuroscience. 2011;1:392–400. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada CS, Gonzalez R, Phan KL, Liberzon I. The neural correlates of intertemporal decision-making: contributions of subjective value, stimulus type, and trait impulsivity. Human Brain Mapping. 2011;32:1637–1648. doi: 10.1002/hbm.21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taillard J, Philip P, Bioulac B. Morningness/eveningness and the need for sleep. Journal of Sleep Research. 1999;8:291–295. doi: 10.1046/j.1365-2869.1999.00176.x. [DOI] [PubMed] [Google Scholar]
- Tamm AS, Lagerquist O, Ley AL, Collins DF. Chronotype influences diurnal variations in the excitability of the human motor cortex and the ability to generate torque during a maximum voluntary contraction. Journal of Biological Rhythms. 2009;24:211–224. doi: 10.1177/0748730409334135. [DOI] [PubMed] [Google Scholar]
- Tonetti L, Adan A, Caci H, De Pascalis V, Fabbri M, Natale V. Morningness-eveningness preference and sensation seeking. European Psychiatry. 2010;25:111–115. doi: 10.1016/j.eurpsy.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Venkatraman V, Huettel SA, Chuah LY, Payne JW, Chee MW. Sleep deprivation biases the neural mechanisms underlying economic preferences. Journal of Neuroscience. 2011;31:3712–3718. doi: 10.1523/JNEUROSCI.4407-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: Misalignment of biological and social time. Chronobiology International. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman DM, Joireman J, Teta P, Kraft M. A comparison of three structural models for personality: The Big Three, the Big Five, and the Alternative Five. Journal of Personality and Social Psychology. 1993;64:757–768. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.