Abstract
The context pre-exposure facilitation effect (CPFE) is a modified form of standard contextual fear conditioning that dissociates learning about the context during a preexposure phase from learning the context-shock association during an immediate shock training phase conducted on separate days. Fear conditioning in the CPFE is an associative process in which only animals that are preexposed to the same context they are later given an immediate shock in demonstrate freezing when tested for conditioned fear memory. Previous research has shown that the hippocampus and amygdala are necessary for different phases of the CPFE, but whether other brain regions are also involved is unknown. The present study examined expression of the immediate-early gene early growth response gene 1 (Egr-1; also called Zif268, Ngfi-a, Krox-24) in the dorsal hippocampus, lateral nucleus of the amygdala, retrosplenial cortex, and several prefrontal cortex regions (infralimbic and prelimbic medial prefrontal cortex, anterior cingulate, and orbitofrontal cortex) following each phase of the CPFE in juvenile rats. Animals preexposed to the conditioning context displayed fear conditioned freezing during a retention test whereas rats preexposed to an alternate context did not. Following context preexposure, Egr-1 mRNA was elevated in context and alternate context exposed animals compared to homecaged control rats in almost all regions analyzed. Following the context-shock training phase, fear conditioned rats displayed significantly more Egr-1 mRNA expression in the infralimbic, prelimbic, and orbitofrontal cortices compared to the alternate context preexposed control rats. These differences in Egr-1 expression were not found in amygdala between the preexposed context and alternate context rats. No sex differences were observed following preexposure or training in any regions analyzed. The findings suggest that increased expression of Egr-1 within the prefrontal cortex is associated with contextual fear conditioning in the CPFE paradigm.
Keywords: hippocampus, amygdala, Zif268, immediate early genes, context fear conditioning, spatial learning
1. Introduction
Pavlovian fear conditioning has been extensively used to investigate the neurobiological bases of learning and memory (Fanselow and Gale, 2003; Lavond, Kim, and Thompson, 1993; Maren, 2001; Rosen, 2004). In a typical fear conditioning paradigm, a conditioned stimulus (CS), such as a tone, light, or context, is paired with an aversive unconditioned stimulus (US), such as a footshock. Animals form an association between the CS and US, and when subsequently tested with only presentation of the CS demonstrate a conditioned response (CR), commonly measured as the species-specific defensive response of freezing behavior (Blanchard and Blanchard, 1969; Fanselow, 1980; Maren, 2001; Stanton, 2000). For discrete-cue conditioning, such as a light or tone, CS information is transmitted via sensory pathways through the thalamus and neocortex to converge with footshock US activation in the lateral nucleus of the amygdala to form an association representing conditioned fear (Aggleton, 2000; Pare, Quirk, and Ledoux, 2004).
Unlike fear conditioning to a discrete cue, standard contextual fear conditioning (sCFC) typically recruits the hippocampus to acquire a conjunctive representation of the context, which is then associated with the aversive stimulus in the amygdala (Anagnostaras, Gale, and Fanselow, 2001; Maren, Aharonov, and Fanselow, 1997; O'Reilly and Rudy, 2001). However, sCFC is not always impaired by hippocampal lesions or inactivation at the time of training because context conditioning can be supported by “feature-based” associations that do not depend on hippocampus (cf. Rudy, 2009, for review). Moreover, when studying immediate-early gene (IEG) expression during sCFC, it is difficult to determine which aspect of the training experience---learning about the context vs. the context-shock association---is driving gene expression in the hippocampus or amygdala. A variant of sCFC, the context preexposure facilitation effect (CPFE), does not suffer from these problems. In the CPFE, learning about the context, associating the context memory with shock, and retrieval of the context-shock association occur during separate, successive phases of the procedure. The CPFE cannot be learned without hippocampus (Rudy, 2009) and IEG expression related to acquisition of the context representation versus the context-shock association can be measured during different experimental phases (Fanselow, 1990; Frankland, Cestari, Filipkowski, McDonald, and Silva, 1998; Rudy, 2009; Rudy, Barrientos, and O'Reilly, 2002; Rudy, Huff, and Matus-Amat, 2004).
In a typical CPFE experiment, animals are preexposed to Context A on Day 1 (PRE), given an immediate shock in Context A on Day 2, and demonstrate freezing to Context A during testing on Day 3. Animals preexposed to an alternate context on Day 1 (ALT-PRE) and given an immediate shock in Context A on Day 2 do not display elevated freezing when tested in Context A on Day 3 (Fanselow, 1990; Rudy et al., 2002; Rudy et al., 2004). The CPFE takes advantage of the immediate-shock deficit, in which animals that are not given enough time to form a representation of the context prior to the onset of a US demonstrate a lack of conditioned fear when later tested in that same context (Fanselow, 1990). However, by preexposing the animals to the training context for a few minutes on the day prior to training with an immediate shock, the CPFE enables animals to overcome this deficit (Fanselow, 1990). Preexposed rats learn fear because the previously acquired context representation is rapidly retrieved on the training day via “pattern completion” and associated with the immediate shock (e.g., Rudy, 2009).
Lesion and inactivation studies have shown that hippocampal function during all 3 phases of the procedure is necessary for the CPFE (Matus-Amat, Higgins, Barrientos, and Rudy, 2004; Matus-Amat, Higgins, Sprunger, Wright-Hardesty, and Rudy, 2007; Rudy et al., 2004; Schiffino, Murawski, Rosen, and Stanton, 2011). In adults, inactivation of hippocampus with local microinfusions of muscimol during any phase of training disrupts the CPFE (Matus-Amat et al., 2004). In contrast, studies blocking hippocampal NMDA receptors indicate that NMDA-dependent plasticity is necessary only during the preexposure phase (Matus-Amat et al., 2007; Schiffino et al., 2011; Stote and Fanselow, 2004), but not for acquisition of the context-shock association at training or expression of that association at testing (Matus-Amat et al., 2007). Further, antagonism of NMDA receptors in the basolateral complex of the amygdala during the context-shock association phase blocks contextual fear conditioning, but not during the context preexposure phase or prior to testing (Matus-Amat et al., 2007). These studies suggest a distinct role for the hippocampus and amygdala in the acquisition of context fear during different phases of the CPFE paradigm.
Recently, we have begun to apply the CPFE paradigm to the ontogeny of learning and memory and its disorders (Burman, Murawski, Schiffino, Rosen, and Stanton, 2009; Dokovna, Jablonski, and Stanton, 2013; Jablonski, Schiffino, and Stanton, 2012; Murawski, Klintsova, and Stanton, 2012; Murawski and Stanton, 2010; 2011; Schiffino et al., 2011). The CPFE emerges between postnatal day (PD) 17 and 24 (Schiffino et al., 2011) with PD19-21 representing a transitional period (Jablonski et al., 2012). The CPFE also depends on conjunctive (rather than feature-based) representations of the context on PD24 (Jablonski et al., 2012), as it does in older rats (Rudy and O'Reilly, 1999). Blocking hippocampal NMDA receptors during context preexposure disrupts the CPFE in PD24 rats (Schiffino et al., 2011) as it does in adult rats (Matus-Amat et al., 2007).
Few studies have assessed the role of the prefrontal cortex (PFC) in the CPFE. The prelimbic and infralimbic medial PFC (PL and IL, respectively), as well as the anterior cingulate cortex (AC), orbitofrontal cortex (OFC), and retrospenial dysgranular cortex (RSD) play various roles in sCFC, such as fear acquisition, extinction, and expression of recent and remote memories (for reviews, see (Courtin, Bienvenu, Einarsson, and Herry, 2013; Euston, Gruber, and McNaughton, 2012; Maren, Phan, and Liberzon, 2013; Morrow, Elsworth, Inglis, and Roth, 1999; Schoenbaum, Roesch, Stalnaker, and Takahashi, 2009; Sotres-Bayon and Quirk, 2010). In the only study to date examining the role of the mPFC in the CPFE, infusions of the muscarinic receptor agonist oxotremorine into the AC facilitates acquisition of the context-shock association, but not learning of the context, in an inhibitory avoidance variant of the CPFE paradigm (Malin and McGaugh, 2006). The present report is the first to examine patterns of immediate early gene expression within the PFC of adolescent rats during the CPFE.
This study was designed to compare activation patterns in the prefrontal cortex, hippocampus, and amygdala following context preexposure and immediate shock training in the CPFE paradigm in juvenile rats. This age was chosen as a point of comparison with our previous developmental studies (Murawski et al., 2012; Murawski and Stanton, 2010; 2011; Schiffino et al., 2011) and as starting point for future studies involving younger rats. The IL, PL, OFC, AC, RSD, lateral nucleus of the amygdala (LA), and area CA1of dorsal hippocampus (CA1) were selected because of their importance in fear conditioning (Ji and Maren, 2008; Keene and Bucci, 2008; Matus-Amat et al., 2004; Morgan and LeDoux, 1995; Phillips and LeDoux, 1992; Rosen, 2004). The expression of the immediate-early gene early growth response gene-1 (Egr-1) mRNA was assessed because it has been shown to increase in the LA during acquisition of sCFC (Malkani and Rosen, 2001; Rosen, Fanselow, Young, Sitcoske, and Maren, 1998) and in CA1 during retrieval of sCFC (Hall, Thomas, and Everitt, 2001). Lee (2010) reported an increase in EGR-1 protein in the dorsal hippocampus during acquisition of the context-shock association. EGR-1 protein expression has also been used to investigate the activation of the AC and other PFC regions in remote contextual fear memory (Frankland, Bontempi, Talton, Kaczmarek, and Silva, 2004). Further, antisense knockdown of EGR-1 protein in the dorsal hippocampus disrupts acquisition of the context-shock association during the CPFE (Lee, 2010) and in the LA during sCFC (Malkani, Wallace, Donley, and Rosen, 2004). These studies suggest a specific role for EGR-1 in the dorsal hippocampus and LA in adult rats during the context-shock association phase of fear conditioning. This report expands the study of Egr-1 mRNA expression to include the LA, CA1, and PFC following the context preexposure and context-shock training phases of the CPFE paradigm in juvenile rats.
2. Materials and Methods
2.1 Subjects
Subjects and animal husbandry were as described in our previous reports (e.g., Schiffino et al., 2011). Subjects were 89 (42 males and 47 females) Long Evans rats derived from 18 time-bred dams in the University of Delaware breeding colony. Of this total, 34 were assigned to the preexposure assay (15 males, 19 females), 35 to the training assay (17 males 18 females), and 20 (10 males, 10 females) were assigned to behavioral testing (see design and procedure below). The date of birth was determined by checking for births during the light cycle on GD 21 and 22. On PD3, litters were culled to 8 pups (usually 4 males and 4 females) and paw-marked by subcutaneous injections of non-toxic black ink for identification purposes. Pups were kept with the dam in a clear polypropylene cage (45 × 24 × 21 cm) until PD 21, after which they were weaned and housed with same-sex littermates in 45 × 24 × 17 cm cages. Two days prior to the start of the experiment (PD 29±1), rats were individually housed in opaque white cages (24 × 18 × 13 cm), where they remained for the remainder of the study. Same-sex littermates were assigned to different behavioral conditions so that no more than one same-sex littermate was represented in a particular experimental condition. Animals had ad libidum access to food and water throughout the experiment. All subjects were treated in accordance with the Institutional Animal Care and Use Committee at the University of Delaware.
2.2 Apparatus and Stimuli
Contextual fear conditioning was based on previously reported methods from this lab (Murawski et al., 2012; Murawski and Stanton, 2011). Preexposure consisted of a 5-minute adaptation in one of two distinct contexts (Context A or Context B). Context A was a clear Plexiglas chamber measuring 16.5 × 21.1 × 21.6 cm3 with a floor consisting of 9 stainless steel bars floors (0.5 cm diameter placed 1.25 cm apart) connected to a shock scrambler that delivered a 2 sec 1.5 mA footshock (Med Associates, Georgia, VT ENV-414S). Four chambers (Context A) were placed on a Plexiglas stand (2 chambers per row and column) within a fume hood (which provided background light and ambient noise). The sides of each chamber were made opaque to prevent animals from viewing one another. Activity was recorded with a camera connected to a computer running FreezeFrame software (Actimetrics, Wilmette, IL). Freezing was defined as a bout of 0.75 seconds or longer without changes in pixel luminance. Context B consisted of modifications to Context A, including a wire mesh insert covering the floor and protruding into the chamber in order to alter the spatial configuration of the context. Opaque paper was also draped across three of the four outside walls of Context B such that only the wall facing the camera remained visible. Chambers were cleaned with a 5% ammonium hydroxide solution prior to use. Transport cages (11 × 11 × 18 cm) made of Lexan and surrounded with opaque paper on all four outside walls were used to move individual rats to and from their home cages in the colony room for experimental testing.
2.3 Design and Procedures
The general training procedure has been described previously (Murawski and Stanton, 2010). There were eight experimental conditions in this study with one littermate being assigned to each condition (Table 1). Contextual fear conditioning occurred over three days---Preexposure, Training, and Testing---starting on PD 31(±1). Three littermates were sacrificed on the Preexposure day, three on the Training Day, and 2 littermates were retained for behavioral testing on the final day, to confirm the CPFE observed in our previous studies (Murawski and Stanton, 2011; Schiffino et al., 2011).
Table 1.
Subject assignment and experimental design.
| --Exp'al Condition-- | ----------------Experimental Phase--------------- | ||||||
|---|---|---|---|---|---|---|---|
| Littermate | Sampling | Behavioral | Preexposure | Sac | Training | Sac | Testing |
| 1 | Preexp | Pre | Context A | x | |||
| 2 | Alt-Pre | Context B | x | ||||
| 3 | Baseline | Home cage | x | ||||
| 4 | Training | Pre | Context A | Context A | x | ||
| 5 | Alt-Pre | Context B | Context A | x | |||
| 6 | Baseline | Context A/B | Home cage | x | |||
| 7 | Behavior | Pre | Context A | Context A | Context A | ||
| 8 | Alt-Pre | Context B | Context A | Context A | |||
2.3.1 Preexposure
Rats were assigned to one of three groups: PRE (preexposed to Context A), ALT-PRE (preexposed to Context B), or HC (Home-caged controls). Animals were weighed and transported to a room adjacent to the conditioning room. Animals were loaded into individual contexts (A or B) and allowed to explore the context for a 5-min period, after which they were returned to their transport cages and brought back to their home cages in the colony room and then sacrificed 30 minutes (± 3) later. Home-cage controls were removed from their home cages and sacrificed while their Pre and Alt-Pre counterparts were being preexposed (see Brain Collection, below).
2.3.2 Training
Twenty-four hours after preexposure, animals in the Training condition (Table 1) were again weighed and transported four at a time to a room adjacent to the conditioning chambers (see Preexposure). Both PRE and ALT-PRE animals were then individually brought into the conditioning room and received a 2 sec 1.5 mA footshock immediately upon placement into Context A. Animals were immediately removed, returned to their transport cages, brought back to their home cages in the colony room, and sacrificed 30 minutes (± 3) later. Home-cage controls were sacrificed while their counterparts were being trained.
2.3.3 Retention Testing
Twenty-four hours after training, the remaining littermates in the Behavior condition (Table 1), one in the PRE and the other in the ALT-PRE group, were weighed and transported identically as described in the Preexposure and Training phases. Animals were loaded into the same chambers where they received training (Context A), and were monitored for freezing behavior over a 5-min testing period.
2.4 Brain Collection
All animals were sacrificed 30 (±3) minutes following chamber removal. HC (baseline control) animals remained undisturbed in their home cages and were sacrificed while their littermate counterparts were undergoing preexposure or training. Care was taken to ensure that HC controls had the same experimental history as their counterparts. For the training-day assay, the HC control group was comprised of rats that had received PRE and ALT-PRE exposures the previous day (Table 1). Rats were sacrificed by rapid decapitation, and brains were immediately removed and frozen in −45°C isopentane and stored at −80°C until sectioned. Sixteen micrometer coronal brain sections corresponding to the medial prefrontal cortex, orbitofrontal cortex, anterior cingulate, lateral nucleus of the amygdala, CA1 subfield of the dorsal hippocampus, and retrosplenial dysgranular cortex were sectioned on a cryostat (Leica Inc., Deerfield, IL) using the Paxinos and Watson stereotaxic brain atlas as a guide (Paxinos & Watson, 2007, 6th Ed.). Two brain sections were placed on each slide. Slides were stored at −80°C until processed for in situ hybridization.
2.5 In situ Hybridization
In situ hybridization was conducted identically to that described in (Asok, Ayers, Awoyemi, Schulkin, and Rosen, 2013). An antisense RNA probe (riboprobe) was transcribed from a plasmid containing a sense cDNA coding for a 230 bp sequence of Egr-1 (gift from J. Milbrandt, Washington University, St. Louis, MO). The transcribed riboprobe incorporated a radioactively labeled 35S UTP (approximately 1×106 dpm) using a T7 RNA polymerase Maxiscript kit according to the manufacturer's instructions (Life Technologies, Grand Island, NY). Following hybridization and washing, the dry slides were exposed to Kodak Biomax MR Film for 2 days.
2.6 In Situ Hybridization Image and Statistical Analysis
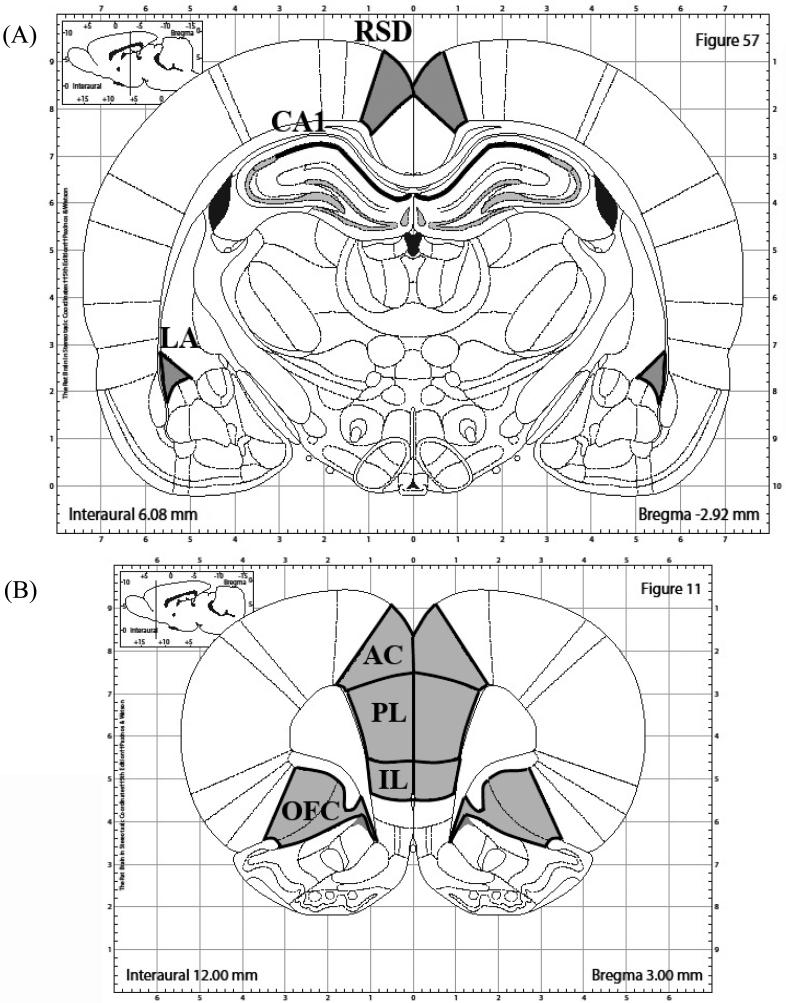
Autoradiograms were captured and digitized to 8-bit gray values via a Dage CCD video camera with ImageJ 1.45m program (Wayne Rasband, NIMH) on an Apple computer. ImageJ was used to subtract the background (2D-rolling ball radius of 50.0 pixels) and measure the mean density (mean gray value) within the regions of interest (see Figure 1 for illustration of areas analyzed). The mean density of all mRNA labeling was analyzed for the PL, IL, OFC (encompassing ventral and lateral parts) and AC (using Plate 11 of Paxinos and Watson as a guide), and LA, CA1, and RSD (using Plate 57 of Paxinos and Watson Guide).
Figure 1.
Illustrations of brain regions analyzed. (a) CA1, LA, and RSD regions included in Egr-1 analysis are outlined in black and shaded in grey. (b) PL, IL, AC, and OFC regions included in Egr-1 analysis are outlined in black and shaded in grey. Imaged are adapted from The Rat Brain in Stereotaxic Coordinates, 6th Ed (Paxinos and Watson, 2007).
The mean gray value of the left and right side of the brain was averaged within slices and then within slides. A 14C standard with known amounts of radioactivity was exposed and captured with the slides. The standard was used to generate a 3rd degree polynomial equation and convert the unknown mean grey values from the slides to known radioactivity (nCi/g). The nCi/g value was then normalized against the average nCi/g of all home-cage animals in that region to obtain a proportionate score. When nCi/g scores fell ± 1.96 standard deviations from the nCi/g group mean for a particular region, that score was defined as an outlier and was excluded from the calculation of proportionate scores and further analysis (typically 1 score/group/region was excluded). Three data points in the study were lost in particular brain regions because of tissue damage and/or poor labeling. To collapse across films, the proportionate scores were averaged together and multiplied by 100 so that the average of all homecaged animals would equal 100%.
PASW 20.0.0 (IBM, Chicago, Il) was used for all statistical analysis. Each region was analyzed separately by one-way ANOVA (HC, ALT-PRE, and PRE). First, Levene's Test for homogeneity of variance was conducted. Following acceptance of Levene's statistic, a one-way ANOVA was conducted to test for main effects at p<.05. Tukey's HSD post-hoc multiple means comparison was used at p<.05 to determine group differences following a main effect. Tukey-Kramer post-hoc analysis was used to control for unequal group size when necessary. If Levene's statistic was rejected, Welch's ANOVA, which controls for unequal variance, was used to test for significant main effects (Mendes and Akkartal, 2010). If Welch's test was rejected, p < .05, Games-Howell test, which controls for unequal variance and unequal group size, was used to test for group differences (Ruxton and Beauchamp, 2008; Stoline, 1981).
2.7 Behavioral Statistical Analysis
Freezing behavior was scored using FreezeFrame software by an observer blind to the experimental condition of the animals as described previously (Schiffino et al., 2011). Activity thresholds were adjusted on an individual basis to exclude small movements from being calculated as part of an animal's total freezing. An independent samples t-test was used to compare group differences of total freezing.
3. Results
3.1 CPFE Retention Testing
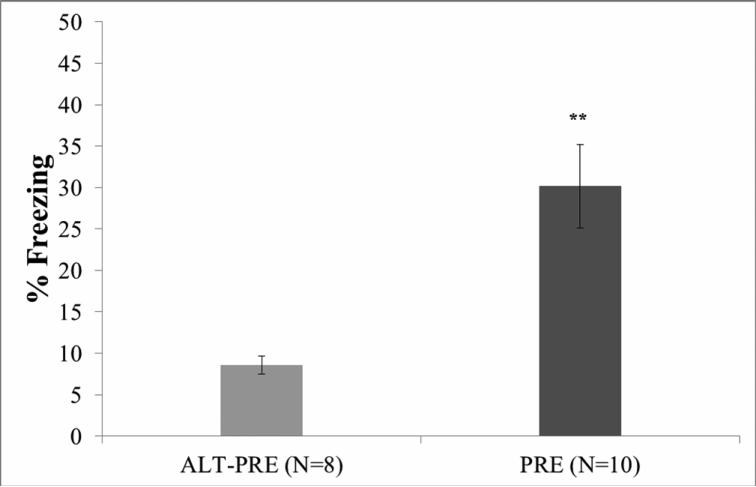
Of the original 20 animals, two (1 male ALT-PRE, 1 male PRE) were excluded from behavioral analysis because their mean percentage freezing scores were greater than 1.96 standard deviations from the mean. An independent samples t-test demonstrated that PRE animals froze significantly more during retention testing to the context alone than ALT-PRE animals, t(16) = −3.74, p < 0.002 (see Figure 2), indicating fear to the conditioning context only in those animals given prior exposure to that context. There were no significant behavioral differences between males and females in the PRE or ALT-PRE groups (p >.05).
Figure 2.
Mean percent freezing during the 5-min testing phase of the CPFE paradigm. Data are from littermates run through the full behavioral paradigm (groups 7 and 8 from Table 1). PRE animals froze significantly more than ALT-PRE animals. Error bars represent ± S.E.M. **p < .01.
3.2 Pre-exposure Egr-1 mRNA expression
Eleven animals in the HC and twelve animals in the PRE and ALT-PRE condition were assayed. One subject in the ALT-PRE group was excluded from OFC analysis because of tissue damage and labeling issues. After outlier exclusion (see methods), the final number of subjects in each group for the Egr-1 preexposure data analysis were: CA1 (nHC=10, nPRE=10, nALT-PRE=10), LA (nHC=9, nPRE=9, nALT-PRE=12), PL (nHC=9, nPRE=10, nALT-PRE=10), IL (nHC=9, nPRE=9, nALT-PRE=11), AC (nHC=10, nPRE=9, nALT-PRE=10), OFC (nHC=11, nPRE=9, nALT-PRE=10), and RSD (nHC=8, n PRE=10, nALT-PRE=11).
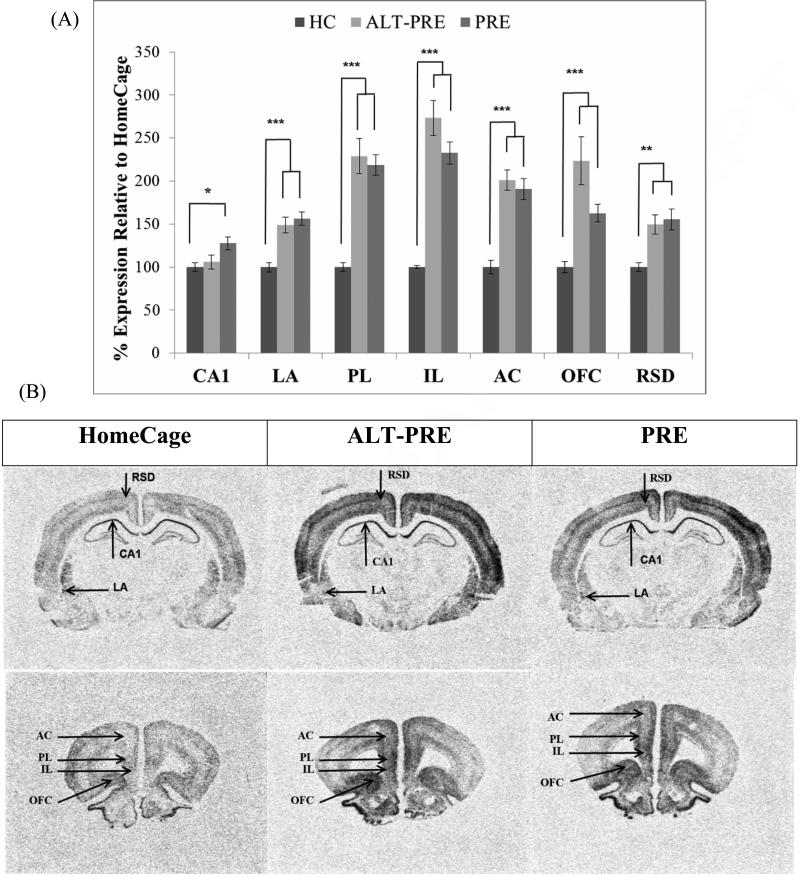
Following pre-exposure, PRE and ALT-PRE animals displayed significantly elevated Egr-1 in the LA, IL, PL, AC, OFC, and RSD when compared to HC controls, but did not differ from each other in any region (see Figure 3). In CA1 only PRE, but not ALT-PRE, animals displayed significantly elevated Egr-1 when compared to HC controls.
Figure 3.
Mean percent expression of Egr-1 mRNA compared to HC following context preexposure in the CPFE paradigm (groups 1, 2 and 3 from Table 1). (a) PRE and ALT-PRE animals displayed significantly elevated Egr-1 mRNA expression in all areas except for CA1 when compared to HC. Only PRE had increased Egr-1 in CA1 compared to HC. (b) Digitized enhanced contrast images of animals in HC, ALT-PRE, and PRE conditions containing all brain regions analyzed. Error bars are ± S.E.M. *p < .05, **p < .01, ***p < .001.
These results were confirmed statistically in separate ANOVAs performed on each region. A significant main effect of condition (HC, ALT-PRE, PRE) was observed in CA1, F(2, 27)=4.13 (p<.05), LA F(2, 17.37=21.31 (p<.001), IL F(2, 12.21)=79.25 (p<.001), PL F(2, 27)=27.85, AC F(2, 26)=27.49 (p<.001), OFC F(2, 14.910)=19.48 (p<.001), and RSD F(2, 27)=7.62 (p<.005). In CA1, post-hoc analysis revealed that only PRE animals displayed significantly higher Egr-1 when compared to HC controls (p<.05), whereas ALT-PRE did not differ from PRE or HC controls. In the LA, IL, and PL, AC, and OFC, post-hoc analysis revealed that both PRE and ALT-PRE displayed significantly higher levels of Egr-1 than HC controls (p<.005), but did not differ from each other. There were no significant effects of sex in any of the regions analyzed (p>.05). Results indicate that no difference between ALT-PRE and PRE animals was observed following context preexposure in any region analyzed.
3.3 CPFE Post-training Egr-1 expression
Eleven animals in the HC group and twelve animals in the PRE and ALT-PRE condition were assayed. Two animals in the HC control group were excluded from PFC analysis because of tissue damage and labeling issues. After outlier exclusion (see methods), the final number of subjects in each group for the Egr-1 training data analysis were: CA1 (nHC=8, nPRE=11, nALT-PRE=10), LA (nHC=8, nPRE =10, nALT-PRE=10), PL (nHC=6, nPRE =9, nALT-PRE=10), IL (nHC=7, nPRE =9, n ALT-PRE=10), AC (nHC=6, nPRE =9, nALT-PRE=10), OFC (nHC=6, nPRE =9, nALT-PRE=10), and RSD (nHC=9, nPRE =9, nALT-PRE=10).
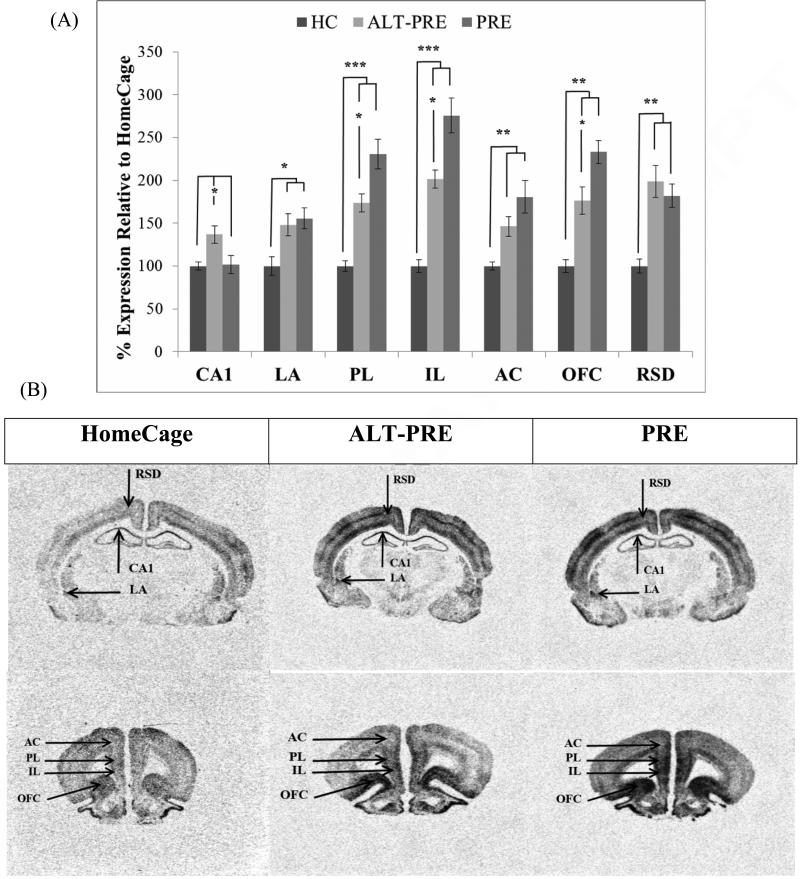
Following context-shock training, PRE and ALT-PRE animals displayed significantly elevated Egr-1 in the LA, PL, IL, AC, OFC, and RSD when compared to HC controls. Importantly, PRE animals also displayed elevated Egr-1 over ALT-PRE animals in the PL, IL, and OFC (see Figure 4). Only ALT-PRE, but not PRE, animals showed significantly higher Egr-1 in CA1 compared to HC controls.
Figure 4.
Mean percent expression of Egr-1 mRNA compared to HC following immediate shock training in the CPFE paradigm (groups 4, 5 and 6 from Table 1). (a) PRE animals displayed significantly higher Egr-1 mRNA than ALT-PRE animals in the PL, IL, and OFC. (b) Digitized enhanced contrast images of animals in HC, ALT-PRE, and PRE conditions containing all brain regions analyzed. Error bars are ± S.E.M. *p < .05, **p < .01, ***p < .001.
These results were confirmed statistically. A significant main effect was observed in CA1 F(2, 26)=4.88 (p<.05), LA F(2, 25)=5.66 (p<.01), IL F(2, 15.47)=50.38 (p<.001), PL F(2, 23)=23.12 (p<.001), AC F(2, 13.19)=13.31 (p<.001), OFC F(2, 22)=18.33 (p<.001), and RSD F(2, 15.38)=19.66 (p<.001). In CA1, post-hoc analysis revealed that the ALT-PRE group displayed significantly higher levels of Egr-1 when compared to HC controls (p<.05), and PRE animals (p<.05). HC and PRE animals did not statistically differ in CA1. In LA, AC, and RSD, post-hoc analysis revealed that PRE (p<.05) and ALT-PRE (p<.05) displayed significantly greater Egr-1 expression than HC animals, but PRE and ALT-PRE did not statistically differ from each other. In the IL, PL, and OFC post-hoc analysis revealed PRE (p<.005) and ALT-PRE (p<.005) displayed significantly higher levels of Egr-1 than HC controls, with PRE animals greater than ALT-PRE animals (p<.05). There were no significant effects of sex in any of the regions analyzed (p > .05). These results indicate that PRE animals display greater Egr-1 expression relative to ALT-PRE animals in the IL, PL, and OFC.
4. Discussion
The CPFE paradigm provides a method for temporally dissociating the two major phases of context fear conditioning – the latent or incidental contextual learning, and the context-shock association (Fanselow, 2000). Our results indicate that Egr-1 mRNA expression 30 minutes following context-shock training results in a differential activation pattern within the PFC of animals preexposed to the training context.
4.1 Prefrontal Cortex
In all of the PFC regions assessed (i.e., PL, IL, AC and OFC), Egr-1 increased to similar levels in PRE and ALT-PRE animals following the preexposure phase. This suggests that the PFC is active following exposure to a novel context and may have a role in forming the contextual representation. Following training, all of these regions in PRE and ALT-PRE animals still displayed increased Egr-1 expression compared to the HC controls. It is not possible to determine the degree to which increased Egr-1 in the ALT-PRE group can be attributed to environmental novelty or footshock. The most interesting finding is the differential increase of prefrontal Egr-1 in PRE over ALT-PRE animals in the PL, IL, and OFC following training. Since only PRE rats display learning in the CPFE, this suggests that these prefrontal regions are involved in learning about a novel context and, to a greater degree, learning of the context-shock association. The learning and memory processes that contribute to Egr-1 in the PFC of PRE animals are not known yet, but may reflect retrieval of the same preexposed context, new context-shock associative learning, or updating/reconsolidation of the preexposed context with the context-shock association (Lee, 2010; Maddox, Monsey, and Schafe, 2011).
Different regions of the PFC are thought to be involved in distinct mechanisms of learning, memory, and expression of behavior. The IL is important for extinction of cued and contextually conditioned fear, whereas the PL is important for modulating the expression of freezing following fear conditioning (Sotres-Bayon and Quirk, 2010). The PL is also thought to be a site for storage of remote conditioned fear memory (Frankland and Bontempi, 2005; Frankland et al., 2004) and possibly involved in successful retrieval during weak fear conditioning (Rudy, Biedenkapp, and O'Reilly, 2005). Single unit recordings of neurons in the mPFC revealed that after auditory fear conditioning, exposure to the context alone evokes a significant increase in activity suggesting the cellular activity in the mPFC reflects re-exposure to an already acquired fear-evoking contextual representation (Baeg, Kim, Jang, Kim, Mook-Jung, and Jung, 2001). Hyman et al. (2012) showed that neurons in the mPFC selectively respond during repeated exposure to the same context (Hyman, Ma, Balaguer-Ballester, Durstewitz, and Seamans, 2012). This recent finding is of particular relevance to the present study, as it suggests that one of the proposed learning mechanisms underlying the CPFE (retrieval of the contextual representation) is correlated with activity in the PFC.
The OFC is involved in outcome expectancies, response inhibition, and rapid encoding of associative learning (Maren et al., 2013; Schoenbaum et al., 2009). Pattern completion and one-trial learning on the training day are features of the CPFE that may engage rapid encoding processes of the OFC. The AC appears to be involved in formation, consolidation, and reconsolidation of recent and remote contextual fear memory ((Einarsson and Nader, 2012; Frankland and Bontempi, 2005; Frankland et al., 2004; Malin, Ibrahim, Tu, and McGaugh, 2007). Further, expression of Egr-1 in the AC has been shown to increase during retrieval of contextual fear memory (Frankland et al., 2004; Thomas, Hall, and Everitt, 2002). Thus, it is possible that increased Egr-1 expression in OFC and AC following context-shock training reflects their role in rapid encoding, retrieval, or reconsolidation.
In summary, Egr-1 expression in different prefrontal regions corroborates other studies indicating a role for this IEG in learning and memory of context-shock associations. However, correlation is not causation and similarities in expression across prefrontal regions do not mean Egr-1 serves the same memory functions in these regions. Studies using infusion of antisense DNA or other methods of reducing expression of Egr-1 protein in specific PFC areas are needed to elucidate the functional role of prefrontal Egr-1 expression in contextual fear and the CPFE.
4.2 Hippocampus, Amygdala and Retrosplenial Cortex
In CA1, it was unexpected that Egr-1 would be elevated only in PRE animals during the preexposure phase of the CPFE and only in ALT-PRE animals during the training phase. If Egr-1 in the hippocampus increased simply in response to novelty, then both PRE and ALT-PRE animals should have displayed increased Egr-1 expression in CA1 following the preexposure phase to either Context A or B. However, this was not observed and features unique to each context might have produced these results (Desjardins, Mayo, Vallee, Hancock, Le Moal, Simon, and Abrous, 1997). Context B was modified from Context A in that it included a mesh insert and paper covering the walls of the chamber, which might have reduced the spatial cues associated with Context B. Speculatively, these spatial differences between Context A and Context B may have contributed to the pattern of Egr-1 expression observed in CA1. In both PRE and ALT-PRE animals, elevated Egr-1 was only apparent following the first exposure to Context A. Further, the PRE animals that were preexposed to and trained in Context A (i.e., briefly re-exposed to Context A) did not demonstrate an increase in Egr-1 expression following re-exposure. Thus, Context A itself (i.e., which includes access to more space and visual features than Context B) may drive Egr-1 expression in CA1. More research is needed to understand how configural differences between Contexts A and B may contribute to Egr-1 expression in CA1.
In the LA, PRE and ALT-PRE animals did not differ in Egr-1 expression following either context preexposure or training. These findings contrast with the sCFC findings of Rosen et al (1998) and Malkani and Rosen (2000), in which a delayed-shock group displayed significantly more Egr-1 mRNA in the LA than an immediate-shock group (Malkani and Rosen, 2000; Rosen et al., 1998). The behavioral paradigm in those studies did not involve a separate day of preexposure. Our data indicate that 5 minutes of preexposure to a context (either the prospective training context or an alternative context) increases Egr-1 expression within the LA, indicating that either context exposure alone or the presentation of an immediate shock may induce Egr-1 gene expression in the LA (Rosen and Donley, 2006). This suggests that Egr-1 in the LA might be activated by novelty (Alberini, 2009; Hall et al., 2001) or more precisely by uncertainty/unpredictability (Rosen and Donley, 2006), which is embedded in learning of fear, but not specific to fear conditioning. Blocking NMDA-dependent plasticity in the basolateral amygdala on the training day disrupts the CPFE (Matus-Amat et al., 2007). It is possible that the role of Egr-1 in plasticity during sCFC (Malkani and Rosen, 2000) is shifted to another molecular pathway or subregion within the amygdala during the CPFE. It is also possible that associative (fear) and nonassociative (novelty) processes are both driving Egr-1 on the training day (of the CPFE) in a way that obscures the role of Egr-1 in learning (Rosen and Donley, 2006). This could be tested via antisense microinfusion experiments (Malkani et al., 2004). All of these possibilities can be addressed by further research.
Finally, we observed increased Egr-1 expression within the RSD of both groups following the preexposure and training phases. Lesions of the RSD disrupt contextual fear conditioning and plasticity within the RSD, indicating it is important for remote fear memory (Corcoran, Donnan, Tronson, Guzman, Gao, Jovasevic, Guedea, and Radulovic, 2011; Keene and Bucci, 2008; Vann, Aggleton, and Maguire, 2009). However, similar to the LA, it is not possible to determine whether the Egr-1 increases are a response to novelty or specific to contextual fear learning.
4.3 Development
The animals used in the present study were juvenile rats in the 5th week of postnatal life. This is an advanced stage of early development in which behavioral and neural mechanisms of learning are generally very similar to those of adult rats. The lack of sex differences might be due to training and testing at this juvenile stage of development. The CPFE is absent in PD17-19 rats, begins to emerge around on PD21, and produces adult levels of freezing by PD24-26, (Jablonski et al., 2012). It is currently unknown whether the emergence of the CPFE during this period of ontogeny depends on developmental changes in prefrontal Egr-1 activation observed in the present study. Furthermore, there is no published research on prefrontal Egr-1 expression during the CPFE in adult rats. Research examining the ontogenetic differences in basal expression of Egr-1 has shown that Egr-1 is expressed in the hippocampus and mPFC at the stage of development when the CPFE first emerges (Herms, Zurmohle, Schlingensiepen, Brysch, and Schlingensiepen, 1994). More studies are required to fully examine the role of Egr-1 expression patterns found in the present study during postnatal development and in adulthood of the CPFE.
4.4 Conclusion
The functional role of Egr-1 as correlate of novelty, associative learning, and memory reconsolidation has been debated (Alberini, 2009; 2011; Hall et al., 2001; Malkani and Rosen, 2000; Rosen and Donley, 2006; Yochiy, Britto, and Hunziker, 2012). Egr-1 is an inducible transcription factor, and synthesis of Egr-1 mRNA is a result of activation through three specific regulatory binding sites: SRE bound by ELK-1, CRE bound by CREB, and AP-1 bound by the Fos/Jun AP-1 complex, all of which have been implicated in associative learning (Alberini, 2009; Davis, Bozon, and Laroche, 2003). EGR-1 is also necessary for the maintenance of LTP and the persistence of long-term memory (Alberini, 2009; Katche, Goldin, Gonzalez, Bekinschtein, and Medina, 2012; Malkani and Rosen, 2000). Evidence from fear conditioning studies indicates the necessary role of EGR-1 in the LA during sCFC (Malkani et al., 2004) and the dHPC during the CPFE (Lee, 2010,Lee, 2010). The present study lends support to all of these views on the functional role of increased expression of Egr-1. Our findings following context preexposure showed that this gene is driven in multiple brain regions by exposure to a novel environment alone. During the training phase, associating a retrieved context representation with a footshock drives Egr-1 expression even further in prefrontal cortical regions. Because the CPFE provides a unique method to characterize the differential contributions of the spatial and aversive learning components inherent in context conditioning, future studies of the CPFE may help clarify the role of the PFC in contextual fear conditioning.
Highlights.
A CPFE paradigm was used to dissociate context learning from context-shock fear learning.
Preexposure to a context increases Egr-1 mRNA in the amygdala and PFC
Immediate shock in the same, but not alternate, preexposure context increases Egr-1 mRNA in PL, IL, and OFC
Egr-1 mRNA expression in the prefrontal cortex is associated with contextual fear conditioning
Acknowledgements
This research was supported by NIH grant 1-R21-HD070662-01 to Jeffrey B. Rosen and Mark E. Stanton. We would like to thank the UD Office of Laboratory Medicine staff for their care of animals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP. The amygdala : a functional analysis. 2nd ed. Oxford University Press; Oxford, OX ; New York: 2000. [Google Scholar]
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM. The role of reconsolidation and the dynamic process of long-term memory formation and storage. Front Behav Neurosci. 2011;5:12. doi: 10.3389/fnbeh.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Asok A, Ayers LW, Awoyemi B, Schulkin J, Rosen JB. Immediate early gene and neuropeptide expression following exposure to the predator odor 2,5-dihydro-2,4,5-trimethylthiazoline (TMT). Behav Brain Res. 2013;248:85–93. doi: 10.1016/j.bbr.2013.03.047. [DOI] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb Cortex. 2001;11:441–451. doi: 10.1093/cercor/11.5.441. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Burman MA, Murawski NJ, Schiffino FL, Rosen JB, Stanton ME. Factors governing single-trial contextual fear conditioning in the weanling rat. Behav Neurosci. 2009;123:1148–1152. doi: 10.1037/a0016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Donnan MD, Tronson NC, Guzman YF, Gao C, Jovasevic V, Guedea AL, Radulovic J. NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. J Neurosci. 2011;31:11655–11659. doi: 10.1523/JNEUROSCI.2107-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtin J, Bienvenu TC, Einarsson EO, Herry C. Medial prefrontal cortex neuronal circuits in fear behavior. Neuroscience. 2013;240:219–242. doi: 10.1016/j.neuroscience.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Davis S, Bozon B, Laroche S. How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behav Brain Res. 2003;142:17–30. doi: 10.1016/s0166-4328(02)00421-7. [DOI] [PubMed] [Google Scholar]
- Desjardins S, Mayo W, Vallee M, Hancock D, Le Moal M, Simon H, Abrous DN. Effect of aging on the basal expression of c-Fos, c-Jun, and Egr-1 proteins in the hippocampus. Neurobiol Aging. 1997;18:37–44. doi: 10.1016/s0197-4580(96)00206-0. [DOI] [PubMed] [Google Scholar]
- Dokovna LB, Jablonski SA, Stanton ME. Neonatal alcohol exposure impairs contextual fear conditioning in juvenile rats by disrupting cholinergic function. Behav Brain Res. 2013;248:114–120. doi: 10.1016/j.bbr.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarsson EO, Nader K. Involvement of the anterior cingulate cortex in formation, consolidation, and reconsolidation of recent and remote contextual fear memory. Learn Mem. 2012;19:449–452. doi: 10.1101/lm.027227.112. [DOI] [PubMed] [Google Scholar]
- Euston David R., Gruber Aaron J., McNaughton Bruce L. The Role of Medial Prefrontal Cortex in Memory and Decision Making. Neuron. 2012;76:1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Factors Governing One-Trial Contextual Conditioning. Animal Learning & Behavior. 1990;18:264–270. [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann N Y Acad Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci. 1998;112:863–874. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci. 2001;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herms J, Zurmohle U, Schlingensiepen R, Brysch W, Schlingensiepen KH. Developmental expression of the transcription factor zif268 in rat brain. Neurosci Lett. 1994;165:171–174. doi: 10.1016/0304-3940(94)90737-4. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Ma L, Balaguer-Ballester E, Durstewitz D, Seamans JK. Contextual encoding by ensembles of medial prefrontal cortex neurons. Proc Natl Acad Sci U S A. 2012;109:5086–5091. doi: 10.1073/pnas.1114415109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski SA, Schiffino FL, Stanton ME. Role of age, post-training consolidation, and conjunctive associations in the ontogeny of the context preexposure facilitation effect. Dev Psychobiol. 2012;54:714–722. doi: 10.1002/dev.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S. Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learn Mem. 2008;15:244–251. doi: 10.1101/lm.794808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katche C, Goldin A, Gonzalez C, Bekinschtein P, Medina JH. Maintenance of long-term memory storage is dependent on late posttraining Egr-1 expression. Neurobiol Learn Mem. 2012;98:220–227. doi: 10.1016/j.nlm.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behav Neurosci. 2008;122:89–97. doi: 10.1037/0735-7044.122.1.89. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Kim JJ, Thompson RF. Mammalian Brain Substrates of Aversive Classical-Conditioning. Annual Review of Psychology. 1993;44:317–342. doi: 10.1146/annurev.ps.44.020193.001533. [DOI] [PubMed] [Google Scholar]
- Lee JL. Memory reconsolidation mediates the updating of hippocampal memory content. Front Behav Neurosci. 2010;4:168. doi: 10.3389/fnbeh.2010.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox SA, Monsey MS, Schafe GE. Early growth response gene 1 (Egr-1) is required for new and reactivated fear memories in the lateral amygdala. Learn Mem. 2011;18:24–38. doi: 10.1101/lm.1980211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin EL, Ibrahim DY, Tu JW, McGaugh JL. Involvement of the rostral anterior cingulate cortex in consolidation of inhibitory avoidance memory: interaction with the basolateral amygdala. Neurobiol Learn Mem. 2007;87:295–302. doi: 10.1016/j.nlm.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin EL, McGaugh JL. Differential involvement of the hippocampus, anterior cingulate cortex, and basolateral amygdala in memory for context and footshock. Proc Natl Acad Sci U S A. 2006;103:1959–1963. doi: 10.1073/pnas.0510890103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkani S, Rosen JB. Specific induction of early growth response gene 1 in the lateral nucleus of the amygdala following contextual fear conditioning in rats. Neuroscience. 2000;97:693–702. doi: 10.1016/s0306-4522(00)00058-0. [DOI] [PubMed] [Google Scholar]
- Malkani S, Rosen JB. N-Methyl-D-aspartate receptor antagonism blocks contextual fear conditioning and differentially regulates early growth response-1 messenger RNA expression in the amygdala: implications for a functional amygdaloid circuit of fear. Neuroscience. 2001;102:853–861. doi: 10.1016/s0306-4522(00)00531-5. [DOI] [PubMed] [Google Scholar]
- Malkani S, Wallace KJ, Donley MP, Rosen JB. An egr-1 (zif268) antisense oligodeoxynucleotide infused into the amygdala disrupts fear conditioning. Learn Mem. 2004;11:617–624. doi: 10.1101/lm.73104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy JW. The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behav Neurosci. 2007;121:721–731. doi: 10.1037/0735-7044.121.4.721. [DOI] [PubMed] [Google Scholar]
- Mendes M, Akkartal E. Comparison of ANOVA F and WELCH Tests with Their Respective Permutation Versions in Terms of Type I Error Rates and Test Power. Kafkas Universitesi Veteriner Fakultesi Dergisi. 2010;16:711–716. [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Inglis FM, Roth RH. An antisense oligonucleotide reverses the footshock-induced expression of fos in the rat medial prefrontal cortex and the subsequent expression of conditioned fear-induced immobility. J Neurosci. 1999;19:5666–5673. doi: 10.1523/JNEUROSCI.19-13-05666.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski NJ, Klintsova AY, Stanton ME. Neonatal alcohol exposure and the hippocampus in developing male rats: effects on behaviorally induced CA1 c-Fos expression, CA1 pyramidal cell number, and contextual fear conditioning. Neuroscience. 2012;206:89–99. doi: 10.1016/j.neuroscience.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski NJ, Stanton ME. Variants of contextual fear conditioning are differentially impaired in the juvenile rat by binge ethanol exposure on postnatal days 4-9. Behav Brain Res. 2010;212:133–142. doi: 10.1016/j.bbr.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski NJ, Stanton ME. Effects of dose and period of neonatal alcohol exposure on the context preexposure facilitation effect. Alcohol Clin Exp Res. 2011;35:1160–1170. doi: 10.1111/j.1530-0277.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly RC, Rudy JW. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychological Review. 2001;108:311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Rosen JB. The neurobiology of conditioned and unconditioned fear: a neurobehavioral system analysis of the amygdala. Behav Cogn Neurosci Rev. 2004;3:23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Donley MP. Animal studies of amygdala function in fear and uncertainty: relevance to human research. Biol Psychol. 2006;73:49–60. doi: 10.1016/j.biopsycho.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Fanselow MS, Young SL, Sitcoske M, Maren S. Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning. Brain Res. 1998;796:132–142. doi: 10.1016/s0006-8993(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Context representations, context functions, and the parahippocampalhippocampal system. Learn Mem. 2009;16:573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O'Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116:530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Biedenkapp JC, O'Reilly RC. Prefrontal cortex and the organization of recent and remote memories: an alternative view. Learn Mem. 2005;12:445–446. doi: 10.1101/lm.97905. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O'Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav Neurosci. 1999;113:867–880. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- Ruxton GD, Beauchamp G. Time for some a priori thinking about post hoc testing. Behavioral Ecology. 2008;19:690–693. [Google Scholar]
- Schiffino FL, Murawski NJ, Rosen JB, Stanton ME. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiol Learn Mem. 2011;95:190–198. doi: 10.1016/j.nlm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat Rev Neurosci. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ME. Multiple memory systems, development and conditioning. Behav Brain Res. 2000;110:25–37. doi: 10.1016/s0166-4328(99)00182-5. [DOI] [PubMed] [Google Scholar]
- Stoline MR. The Status of Multiple Comparisons - Simultaneous Estimation of All Pairwise Comparisons in One-Way Anova Designs. American Statistician. 1981;35:134–141. [Google Scholar]
- Stote DL, Fanselow MS. NMDA receptor modulation of incidental learning in Pavlovian context conditioning. Behav Neurosci. 2004;118:253–257. doi: 10.1037/0735-7044.118.1.253. [DOI] [PubMed] [Google Scholar]
- Thomas KL, Hall J, Everitt BJ. Cellular imaging with zif268 expression in the rat nucleus accumbens and frontal cortex further dissociates the neural pathways activated following the retrieval of contextual and cued fear memory. Eur J Neurosci. 2002;16:1789–1796. doi: 10.1046/j.1460-9568.2002.02247.x. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Yochiy A, Britto LR, Hunziker MH. Novelty, but not operant aversive learning, enhances Fos and Egr-1 expression in the medial prefrontal cortex and hippocampal areas of rats. Behav Neurosci. 2012;126:826–834. doi: 10.1037/a0030721. [DOI] [PubMed] [Google Scholar]