Abstract
Diabetic retinopathy (DR) is a sight-threatening complication of both type-1 and type-2 diabetes. The recent success of treatments inhibiting the function of vascular endothelial growth factor (VEGF) demonstrates that specific targeting of a growth factor responsible for vascular permeability and growth is an effective means of treating DR-associated vascular dysfunction, edema and angiogenesis. This has stimulated research of alternative therapeutic targets involved in the control of retinal vascular function. However, additional treatment options and preventative measures are still needed and these require a greater understanding of the pathological mechanisms leading to the disturbance of retinal tissue homeostasis in DR. Although severe DR can be treated as a vascular disease, abundant data suggests that inflammation is also occurring in the diabetic retina.Thus, anti-inflammatory therapies may also be useful for treatment and prevention of DR. Herein, the evidence for altered expression of angiogenic factors and cytokines in DR is reviewed and possible mechanisms by which the expression of VEGF and cytokines may be increased in the diabetic retina are examined. In addition, the potential role for microglial activation in diabetic retinal neuroinflammation is explored.
Keywords: Diabetic retinopathy, Vascular endothelial growth factor, Neuroinflammation, Leukocyte trafficking, Interleukin-6, Interleukin-8, Monocyte chemoattractant protein 1, Microglia
Introduction
Diabetic retinopathy (DR) is a major cause of blindness globally and a leading cause of legal blindness in the working age population of the developing world [1,2]. Diabetes affects the entire neurovascular unit of the retina, with gradual neurodegeneration, gliosis, neuroinflammation, compromise of the vascular blood-retinal barrier (BRB), edema, angiogenesis, and eventual fibrosis. Although neurodegeneration and inflammation have been implicated in the etiology of the disease [3,4], diagnosis and treatment of DR are focused on the vascular abnormalities that ultimately occur. This is both because the retinal vasculature can be visually examined and because vascular changes can cause perceptible aberrations in vision and lead to blindness. As DR severity increases, vascular abnormalities including plasma leakage, microaneurysms, hemorrhage, and growth of abnormal capillaries (angiogenesis), occur at increasing frequency. Diabetic macular edema (DME) is characterized by an increase in retinal thickness, as well as formation of light distorting fluid-filled cystoids within the retinal tissue, and serous or exudative deposits separating the neural retina from the retinal pigmented epithelium. Leakiness of the normally tight BRB is thought to be the primary cause of DME [5], although compromise of normal water removal mechanisms from the retina could also contribute. Retinal edema is noninvasively examined by optical coherence tomography (OCT) imaging of retinal thickness. OCT can also detect cystoids and exudative retinal detachments. In DR the retinal vasculature can exhibit dilations or microaneurysms appearing as small red dots under fundus examination. Microaneurysms are clearly visible as hyper-fluorescent dots during fluorescein angiography, which entails intravenous injection of fluorescein followed by fundus examination under fluorescent illumination. These microaneurysms are often associated with leakiness as evidenced by hazy fluorescein diffusion into the retina from their locations. Such focal vascular leakage during DR can lead to opaque deposits of plasma, known as hard exudates. Light can also be obstructed by what were previously called soft exudates, now referred to as cotton-wool spots. These are not exudates at all, but rather are accumulations of axoplasmic debris in the axons of retinal ganglion cells that make up the outermost nerve fiber layer of the retina [6]. Patient vision can also be suddenly impaired by retinal vascular hemorrhages, leading to obstruction of light by blood pooling in the vitreous fluid that fills the eye. Relatively large hemorrhages as well as small active hemorrhages are easily diagnosed by visible-light ophthalmoscopy. Hemorrhaging often occurs as DR progresses to proliferative DR (PDR), which is characterized by abnormal angiogenesis of the retinal vasculature. Feathery new vessels indicative of neovascularization of the optic disc (NVD) or elsewhere on the retina (NVE) are clearly highlighted in fluorescein angiograms. In addition to obscuring vision, these new blood vessels fail to form a tight barrier and thus contribute to edema. They are also prone to rupture. If left untreated severe PDR can lead to fibrovascular growth into the vitreous. The resulting epiretinal membranes attach to the vitreous and cause macular traction, which can lead to tractional retinal detachment and blindness.
In the not too distant past poor metabolic control of diabetes and a lack of treatments for DR meant that diabetic patients ran a very high risk of eventual blindness. In recent years, heightened screening and early diagnosis of diabetes, innovations in blood glucose level monitoring, and the advent of intensive glycemic control have counteracted the epidemic increase in diabetes to stabilize the prevalence of DR in the United States [2]. Tight control of blood sugar levels is the only proven preventative measure [7,8]; but with intensive insulin therapy comes increased risks of life-threatening hypoglycemic events. Novel treatments have also improved the outlook for patients with DR. For several decades, vascular dysfunction in DR has been treated by laser photocoagulation [9,10]. Focal photocoagulation targets specific edematous regions of the retina with a tight grouping of small laser burns, whereas grid photocoagulation distributes laser burns across the retina, avoiding the fovea. In pan-retinal photocoagulation (PRP) retinal tissue surrounding the macula is partially ablated by multiple relatively high intensity laser pulses. This procedure preserves central vision at the expense of loss of peripheral vision. PRP can prevent the progression of PDR as well as reverse DME. Intravitreal steroid injections have also been a DR treatment option for many years [10]. Steroids can rapidly reduce edema and significantly improve vision, demonstrating that a traditional anti-inflammatory treatment can diminish the pathology. Because ocular steroid injections often cause cataract formation, it is most beneficial to patients who have undergone cataract surgery or who have not responded to other DR treatments. Unfortunately, intravitreal steroids also increase intraocular pressure with the risk of developing glaucoma [11].
Recently, treatment of DME and PDR has greatly benefited from therapeutics designed to directly inhibit the function of a proangiogenic molecule, vascular endothelial growth factor (VEGF). As well as stimulating endothelial cell growth, VEGF also promotes the disassembly of junctions between endothelial cells and thus causes vascular permeability. In 1994, Aiello and co-workers demonstrated that VEGF protein levels are markedly upregulated in the vitreous fluid of DR patients [12]. This seminal report, and numerous subsequent studies, demonstrated that the mean vitreous VEGF concentrations in patients with DME and PDR are often increased to more than 10-times normal levels [12-21]. Furthermore, VEGF levels are significantly higher in the vitreous of patients with active PDR than in those with inactive or quiescent PDR, characterized by regressed or non-perfused neo-vessels and lack of active hemorrhage [19,20,22,23]. Targeting VEGF has proved highly effective in treating DR symptoms. Steroid treatment may, at least in part, decrease DME by inhibiting the expression of VEGF [24]. There are now several therapeutic agents available or undergoing clinical trials that are injected directly into the vitreous in order to bind and block the function of VEGF [25]. A humanized antibody against VEGF (bevacizumab, Avastin), which was developed and approved for cancer treatment, has been used to treat DME and PDR for several years. Although bevacizumab treatment for DME or PDR has never been evaluated in a large well-controlled clinical trial, several small trials have suggested efficacy for treatment of DME and improved vision in a fraction (on average 25%) of DR patients [26]. Recent clinical trials also demonstrated that repeated intravitreal injection of a VEGF-binding antibody fragment (ranibizumab, Lucentis) reduced the risk of DR progression over a two-year period by approximately 67%, compared to sham injection [27]. These anti-VEGF treatments thus represent a major advance in DR therapy, but are invasive, expensive, somewhat unpleasant and not without risk. In addition, the long-term effectiveness and safety of anti-VEGF treatments are not yet established.
The success of anti-VEGF treatments to treat DME and PDR has stimulated research of alternative therapeutic targets involved in the control of retinal vascular permeability and angiogenesis (reviewed in [28]). Targeting alternative permeability-inducing or pro-angiogenic factors will hopefully address the cases of DME and PDR for which anti-VEGF treatments are not effective. Although advanced DR can be treated as a vascular disease, there are no therapeutics to prevent the onset of DR. Abundant data suggests that inflammation is occurring in the diabetic retina and it is believed that anti-inflammatory therapies may be useful for treatment and prevention of DR (for recent reviews see [29,30]). This review focuses on the evidence for altered expression of angiogenic factors and cytokines in DR and examines possible mechanisms by which retinal expression of VEGF and cytokines may be increased. In addition, the relationship between microglial activation and neuroinflammation in the diabetic retina is explored.
The study of vitreous protein changes in diabetic retinopathy
Patient retinas cannot be ethically biopsied, so the contribution of angiogenic and inflammatory factors to DR progression in humans must be gleaned from studies examining the levels of these proteins in extracted vitreous samples, postmortem human retinas, and retinal tissue from experimental models. Vitrectomy (the surgical removal of vitreous fluid from within the eye) is a procedure that is called for when a persistent obstruction, such as blood, is hindering vision, or when a fibrovascular growth may cause retinal traction or detachment [10]. Thus, vitrectomy is usually only performed on a fraction of DR patients with unremitting DME or severe PDR, making large study population numbers difficult to acquire. In addition, vitreous is not removed from normal eyes, thus control samples must be obtained postmortem or, more often, from patients being treated for unrelated conditions that call for vitrectomy. These include macular pucker, macular hole, idiopathic vitreous hemorrhage or idiopathic epiretinal membrane formation. Thus, truly unperturbed, normal human vitreous is unobtainable. However, from a compilation of several relatively small studies a picture of the changes in the vitreous milieu that accompany DME and PDR is emerging. In addition, multiplex protein analysis has recently enabled simultaneous measurement of several angiogenic and inflammatory mediators in each vitreous sample, thus expanding the information gained from each invaluable set of samples.
An important aspect of interpreting increased level of proangiogenic factors or cytokines in the vitreous of DR patient is the origin of the proteins. Vitreous from DME and PDR patients exhibit markedly increased protein contents [31-33]. Because of vascular leakiness or hemorrhage, proteins from the blood contaminate these vitreal samples to various extents [31]. Therefore, it is not safe to assume that the concentration of a protein in the vitreous is an accurate reflection of its intraocular expression. Methods can be used to estimate and correct for the contribution of blood-born protein to vitreous levels [34]; however many studies simply report vitreous concentrations and do not test for the presence of plasma proteins or hemoglobin in vitreous samples. Studies often report the concentrations of factors in serum (or plasma) as well as vitreous, demonstrating that increased expression is unique to the eye or that vitreous levels do not correlate with serum levels. This is usually interpreted as indicating that the blood is not the source of increased ocular levels. However, for factors that are abundant in the blood, increased levels in the vitreous may still be due to leakage from the plasma. The only circumstance that effectively precludes the possibility that the majority of a protein measured in the vitreous did not originate in the blood is that in which the vitreous concentration is comparable to or higher than the concentration in blood. Thus, a vitreous/plasma concentration ratio can be reported, which should be ≥ 1 and should be increased for factors exhibiting elevated vitreous levels due to increased intraocular expression [35].
Vitreous levels of angiogenesis factors in diabetic retinopathy
Changes in the vitreous level of several growth factors suggest that BRB breakdown and inappropriate retinal vessel growth in PDR is likely the result of an imbalance between pro-angiogenic and anti-angiogenic environmental influences (Figure 1). As mentioned previously, numerous studies have documented marked increases in vitreous VEGF levels in DME and PDR [12-21]. The rule of vitreous/plasma concentration ≥ 1 certainly applies to VEGF, where levels in DME and PDR vitreous can be many fold those found in plasma [17]. Several additional pro-angiogenic factors are also found to be upregulated in PDR, including: angiopoietin-2 (Ang-2) [19,20,36], osteopontin (OPN) [37,38], platelet-derived growth factor (PDGF) [39-42], erythropoietin (EPO) [18,20,43], stomal cell derived factor (SDF-1, CXCL12) [44,45] and cysteine-rich 61 (CYR61) [46,47]. Levels of several anti-angiogenic factors are decreased in the vitreous of PDR patients and/or increased following laser photocoagulation therapy. These include pigment epithelium derived growth factor (PEDGF) [18,36,37], endostatin (ES) [48], angiostatin (AS) [49] and tissue kallikrein (TK) [50]. In the most severe stage of PDR, when there is a transition from angiogenesis to fibrosis, vitreous levels of VEGF decrease while levels of the cytokine connective tissue growth factor (CTGF, CCN2) increase [51].
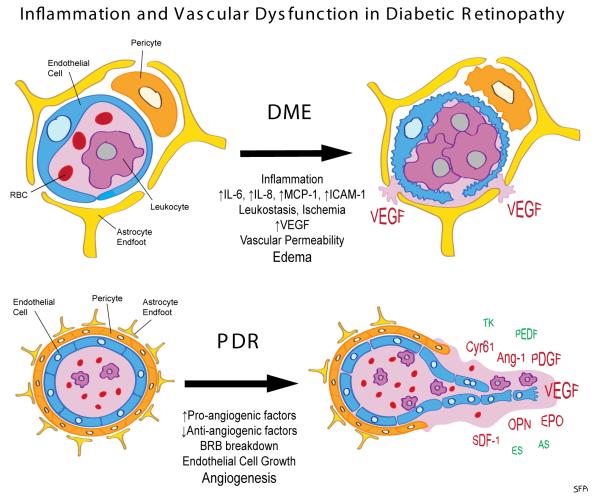
Figure 1. Inflammation and vascular dysfunctions in diabetic retinopathy (DR).
In diabetic macular edema (DME), neuroinflammation with upregulated expression of cytokines such as interleukin-6 (IL-6), IL-8 (CXCL8) and monocyte chemoattractant 1 (MCP-1, CCL2), as well as intercellular adhesion molecule 1 (ICAM-1) on the luminal surface on endothelial cells leads to increased adherence of leukocytes to the luminal endothelial surface (leukostasis). This can block blood flow and damage endothelial cells. Capillary obstruction due to leukostasis and capillary dropout due to death of vascular pericytes and endothelial cells leads to tissue ischemia. Resulting hypoxia may drive expression of vascular endothelial growth factor (VEGF). VEGF promotes vascular permeability resulting in leakage of plasma through the blood-retinal barrier (BRB) and tissue edema. In proliferative diabetic retinopathy (PDR) upregulated expression of pro-angiogenic factors and down regulation of anti-angiogenic factors leads to an imbalance that causes BRB disruption and unproductive angiogenesis. Pro-angiogenic factors include VEGF, angiopoietin-2 (Ang-2), osteopontin (OPN), platelet-derived. growth factor (PDGF), erythropoietin (EPO), stomal cell derived factor (SDF-1, CXCL12) and cysteine-rich 61 (CYR61). Anti-angiogenic factors include pigment epithelium derived growth factor (PEDGF), endostatin (ES), angiostatin (AS) and tissue kallikrein (TK). Red color indicates upregulation and green indicated downregulation.
The role of retinal ischemia in VEGF expression in DR
A prevailing hypothesis to explain increased VEGF levels in DR vitreous is that retinal capillary obstruction or capillary dropout leads to tissue ischemia and hypoxia causing increased retinal VEGF expression [52,53]. Capillary obstruction is caused by leukostasis, the adhesion of white blood cells to the retinal vasculature, and capillary dropout is caused by the death of retinal pericytes and endothelial cells. This hypothesis is compelling because VEGF expression is highly responsive to hypoxia through transcriptional regulation by hypoxia-inducible factor 1 alpha (HIF-1α) [54]. Under low oxygen conditions HIF-1α protein degradation is inhibited leading to its accumulation and subsequent transcriptional activation of target genes containing the hypoxia response element (HRE) to which it binds [55]. The overall angiogenesis program is responsive to a lack of oxygen, such that several pro-angiogenesis factors, including VEGF, EPO, Ang-2 and SDF-1, are hypoxia-responsive [52]. Thus, a shift in the balance of pro- and anti-angiogenesis factors in DR could be explained by retinal tissue ischemia resulting in a lack of oxygen delivery and stabilization of HIF-1α protein. Accordingly, Wang and co-workers measured VEGF and HIF-1α protein levels in vitreous sample from PDR patients and found that both were increased compared to levels in control subjects [56]. Furthermore, the vitreous levels of VEGF and HIF-1α were highly correlated in PDR patients. Han and colleagues demonstrated positive immunohistochemical staining for HIF-1α and VEGF proteins in epiretinal neovascular membranes obtained from PDR patients by vitrectomy [57]. Similarly, Abu El-Asrar et al. [58] demonstrated positive immunohistochemical staining for HIF-1α, VEGF and Ang-2 proteins in vessels of epiretinal membranes from PDR patients. However, whereas the fraction of HIF-1α positive vessels was invariably high in both active (proliferating) PDR and inactive (quiescent) PDR membranes, the fraction of VEGF-positive and Ang-2-positive vessels was significantly greater in active PDR membranes than in inactive PDR membranes. Thus, expression of HIF-1α and pro-angiogenic factors did not coincide. The study also demonstrated that HIF-1α, VEGF and Ang-2 proteins were co-localized with CD34-positive endothelial cells. Endothelia are usually thought of as the target of, rather than the producers of, pro-angiogenic factors. For example, Müller glial cells were largely responsible for increased VEGF expression in an experimental model of DR. This was demonstrated when conditional knockout of VEGF in Müller cells effectively blocked the increase in retinal VEGF expression observed in diabetic mice [59]. Furthermore, conditional HIF-1α knockout in mouse Müller cells abrogated the increase in retinal HIF-1α protein expression following STZ-induced diabetes, which in turn effectively blocked the diabetes-induced increases in retinal VEGF expression and vascular permeability [60].
Although expression of HIF-1α, VEGF and other pro-angiogenic factors are clearly involved in DR progression, the role of hypoxia is not fully defined. In an early experimental study, Linsenmeier and co-workers studied the oxygen levels in retinas of three cats with diabetes for over six years and concluded that partial pressure of oxygen (PO2) was decreased in regions of the inner retina that correlated with vascular abnormalities leading to tissue ischemia [61]. Ly and coworkers demonstrated increased HIF-1α protein expression in STZ-induced diabetic rats after six weeks of diabetes [62]. By using a chemical oxygen-dependent probe, pimonidazole, these authors also demonstrated the presence of hypoxia in the diabetic retinas. However, other studies in diabetic rodents have not confirmed retinal hypoxia. Using immunohistochemical staining, Wright and co-workers failed to detect any increase in HIF-1α or HIF-2α protein levels in retinas from diabetic mice or rats [63]. Using pimonidazole these authors found no increase in hypoxia in mice and a decrease in rats after 12 weeks of diabetes. A subsequent study by this group similarly suggested that oxygen levels were slightly increased in diabetic rat retinas after three weeks of diabetes [64]. Examination of retinal blood flow in Ins2Akita diabetic mice found it to be decreased and observed intermittent capillary stoppages [65]. However, this study also failed to find evidence of any resulting tissue hypoxia.
Although a majority of DR patients exhibit regions of capillary nonperfusion upon fluorescein angiogram examination [66], confirmation of hypoxia in human DR is also lacking. Lange and co-workers used an optical oxygen sensor to measure oxygen tension in the vitreous of PDR patients as vitrectomy was being performed [67]. Surprisingly, mean oxygen tension at the posterior pole (at the retinal surface) was increased in PDR patients and oxygen tension levels were positively correlated with vitreous VEGF levels. Thus, this study provides no evidence of hypoxia-induced VEGF expression in the diabetic retina. Several clinical studies have demonstrated an initial decrease in retinal blood flow in diabetic patients that is followed by an increase in retinal blood flow as DR pathology develops (for review see [68]). It has been speculated that diabetes-induced vasoconstriction causes early hypoperfusion resulting in hypoxia, and that hypoxia eventually reverses the vasoconstrictive effects of diabetes, leading to vascular dilation [68]. This scenario does not fit with the hypothesis hypoxia causes the marked increase in VEGF expression observed in the late stages of DR when DME and PDR are occurring. However, a small intervention study with nine DR patients found that supplemental inspired oxygen significantly diminished DME after three months, and that retinal edema returned in the majority of patients three month after discontinuing oxygen therapy [69]. This would suggest that oxygen delivery is lacking in DR. In contrast, a clinical study in which retinal vascular blood oxygen levels were obtained with an imaging oximeter found that retinal arterial blood oxygenation was not affected by DR, and was near saturation levels in control, nonproliferative DR and PDR patients [70]. Furthermore, venous oxygen saturation levels were significantly increased in diabetic retinas and progressively increased with DR severity. The decrease in arterio-venous oxygen saturation differences observed in DR would suggest that overall oxygen delivery to the tissue is ample, whereas oxygen extraction is decreasing with DR severity. It is important to note that the preponderance of oxygen delivery to the retina comes from the choroidal circulation and is consumed in the outer retina by the photoreceptor cells, making the inner retina relatively hypoxic under normal conditions [71]. This situation could render the inner retina especially susceptible to further reductions in oxygen levels due to capillary obstruction or dropout, but it may be quite difficult to detect decreases in inner retinal oxygen levels that result from focal ischemic events. Regardless, an unequivocal test of the hypothesis that ischemic hypoxia causes increased VEGF expression in DR is needed.
Possible role of endoplasmic reticulum stress in VEGF expression in DR
An alternative mechanism whereby VEGF expression may be increased in DR involves endoplasmic reticulum (ER) stress and the activating transcription factor 4 (ATF4). ATF4 is one of several transcription factors that are activated as part of the unfolded protein response (UPR) to stress. Translation of ATF4 mRNA into ATF4 protein is induced in response to several stresses that result in inhibition of general protein synthesis via phosphorylation of the eukaryotic translation initiation factor 2 (eIF2) [72]. These stresses include nutrient deprivation, ER stress, oxidative stress, and hypoxia. ATF4 protein stability is also indexed to oxygen levels, with its degradation inhibited by anoxia [73,74]. In addition to hypoxia, starvation for nutrients, oxidative stress, conditions causing ER stress, and upregulation of the UPR increase VEGF expression [75-78]. Roybal and co-workers demonstrated that upregulation of VEGF expression in response to oxidative stress and ER stresses are dependent on ATF4 function and that the human VEGFA gene contains an ATF4 binding site [79,80]. Zong et al. demonstrated that ATF4 is necessary for VEGF expression by Müller cells exposed to high glucose levels and that blocking ATF4 activity inhibited expression of VEGF by Müller cells exposed to hypoxia [81]. Furthermore, two other transcription factors that contribute to the UPR, ATF6 and XBP-1, also stimulate VEGF expression [82]. Thus, ATF4 protein level control provides a complementary mechanism to increase VEGF expression in response to oxygen deprivation, and upregulation of VEGF expression in response the UPR provides a means to recruit additional blood supply in response to several stresses associated with ischemia or oxidative stress. Importantly, Zong et al. found that treatment of STZ-induced diabetic mice with a chemical protein chaperone to alleviate ER stress resulted in inhibition of both retinal VEGF expression and vascular permeability [81]. This group also demonstrated that retinal vascular permeability during STZ-induced diabetes was abrogated in ATF4 gene knockout mice [83]. However, at present there is no published evidence that ATF4 or the UPR is increased in clinical DR or that retinal VEGF expression in DR patients is affected by this mechanism.
Elevated vitreous levels of IL-6, IL-8 and MCP-1 in DR
In addition to factors that promote angiogenesis, three cytokines have consistently been reported as increased in the vitreous of DR patients; these are interleukin-6 (IL-6) [16,22,35,40,84-88], IL-8 (CXCL8) [39,40,85,86,88-91], and monocyte chemoattractant-1 (MCP-1, CCL2) [21,39,40,88-92]. Yoshimua et al. performed an analysis of 20 inflammatory mediators in vitreous from patients with a number of vitreoretinal diseases and found that only IL-6, IL-8, and MCP-1 were significantly elevated in all vitreoretinal diseases compared with the control group [88]. This study included patients with DME (n=92) and PDR (n=147), as well as branch retinal vein occlusion (BRVO, n= 30), central retinal vein occlusion (CRVO, n=13) and rhegmatogenous retinal detachment (RRD, n=63). As a control, vitreous samples from a total of 83 patients with either idiopathic macular hole or idiopathic epiretinal membrane were examined. Levels of IL-6, IL-8 and MCP-1 were highly correlated in all the pathologies examined, including DME and PDR. As would be expected, VEGF was significantly elevated in patients with PDR, but surprisingly not in DME patients. In PDR patients, the elevation of VEGF was significantly correlated with IL-6, IL-8, and MCP-1 levels. In a similar study of VEGF and IL-6 levels in the aqueous humor and vitreous of DME and PDR patients, the levels of both VEGF and IL-6 correlated with DR severity scores and were significantly greater in patients with active PDR than in those with quiescent PDR [22]. In this study, vitreous IL-6 levels were significantly correlated with those of VEGF. In a study of the effects of glucocorticoid treatment on edema and vitreous cytokine levels in DME patients, glucocorticoid injection reversed edema while significantly lowering vitreous levels of IL-6, MCP-1, and VEGF, but not IL-8 [93]. Thus, glucocorticoids may reverse edema by virtue of their anti-inflammatory effects as well as its ability to diminish VEGF levels and promote barrier tightening.
On the other hand, there is a relative lack of reports finding increased vitreous levels of inflammatory cytokines that are highly expressed during classical inflammation in non-neuronal tissues. For example, in a recent examination of IL-1β, TNF-α and IL-6 levels in vitreous of PDR patients, only IL-6 levels were increased. In this study, IL-1β was undetectable in vitreous and mean TNF-α concentration was slightly but significantly decreased in vitreous from PDR patients compared to normal patients without DR [35]. This is despite a significant increase in plasma TNF-α levels in the PDR subjects, suggesting the presence of low-grade systemic inflammation in these patients. Recently, when comparing the levels of 16 cytokines in vitreous samples from 13 PDR patients and 13 control patients, Shoenberger et al. found a significant increase in TNF-α levels with PDR [40]. However, the relative increase in TNF-α concentration (2.2 fold) was relatively small compared to increases in IL-6 (5.4 fold), IL-8 (14.2 fold), MCP-1 (3.4 fold), and VEGF (34 fold). Similarly, Ademiec-Mroczek and colleagues found that TNF-α concentrations were increased 2.2 fold in the vitreous of PDR patients compared to controls, while IL-6 concentrations in the vitreous were increased by 10.2 fold [87].
The functional role of IL-6 in the pathology of DR is uncertain. IL-6 was initially identified as an inducer of B-cell antibody production, but now it is known that this is an indirect effect due to induction of T-cell IL-21 production, which in turn stimulates B-cell differentiation and IgG production [94]. IL-6 exerts profound effects on immune cell responses by shifting T-helper cell populations, inhibiting the production of Th1 cells and promoting the differentiation of Th2 and Th17 cells (for review see [95]). IL-6 is well known as a muscle-produced myokine and inducer of fever and acute protein synthesis during infection. IL-6 is also classified as a neuropoietin, a group of cytokines including IL-6, IL-11, IL-27, IL-31, leukemia inhibitory factor, oncostatin M, cardiotrophin-1, neuropoietin and neurotrophin-1. IL-6 can exert numerous effects on the nervous system and is implicated in several neuroinflammatory and neurodegenerative diseases (for recent reviews see [96,97]). The role of IL-6 in nervous tissue is perplexing as it both increases acute damage by increasing neuroinflammation and provides neuroprotection by promoting neurotrophic factor expression [98,99]. IL-6 also supports neurogenesis, the production of new neurons and glial cells from neural stem cells. IL-6 is also a strong stimulator of reactive astrogliosis, which is characterized by increased content of intermediate filaments coinciding with increased expression of glial fibrillary acidic protein (GFAP), vimentin and nestin [100,101]. Müller cell astrogliosis is a prominent feature of experimental DR [102,103]. Astrogliosis during DR may provide an adaptive neuroprotective effect [104,105]. However, it may also impair the vascular support function of astrocytes and hinder the ability of Müller cells to uptake and process excess glutamate released by retinal neurons [106-108]. Glial cells, including Müller cells and astrocytes, may also be the initiators of neural inflammation in the diabetic retina. Reactive glial cells act as cytokine producers [109,110]. In addition, several studies have documented the inflammatory response of Müller cells to in vitro high glucose conditions, with the hypothesis that such a direct response may alter the balance between inflammatory and anti-inflammatory cytokine expression observed in DR [81,111,112]. Furthermore, in the CNS, astrocytes contribute greatly to the control of leukocyte infiltration due to both their role in the maintenance of the endothelial blood brain barrier, and by virtue of limiting leukocyte migration across the perivascular barrier formed by astrocyte end feet, the glia limitans [113]. This is evidenced by the fact that astrocyte depletion greatly amplified monocyte infiltration in experimental autoimmune encephalomyelitis and forebrain stab injury [114,115]. IL-6 can also play a direct role in leukocyte trafficking, particularly in the infiltration of monocytes and T-cells [116,117].
It is notable that DR is associated with elevated levels of IL-8 and MCP-1, which are chemokines targeting primarily neutrophils and monocytes, respectively [118]. Although the findings of high levels of IL-8 and MCP-1 in the vitreous of DR patients do not prove a role for neutrophil and monocyte recruitment in the pathology, this is a likely outcome of their presence. IL-8 induces the transmigration of neutrophils across the blood-brain barrier (BBB), followed by their degranulation with release of myeloperoxidase and a respiratory burst [119,120]. It is feasible that IL-8 elevation is a consequence of VEGF expression. Exposure of human brain microvascular endothelial cell monolayers to VEGF stimulated IL-8 expression, as well as neutrophil transmigration in an IL-8 dependent manner [121]. MCP-1 is thought to control monocyte homing to nervous tissue [122]. In addition, MCP-1 can directly promote BBB permeability [123]. MCP-1 induces transmigration of macrophages across the BBB [124]. This is dependent on expression of CCR2, the chemokine receptor to which MCP-1 binds, by both vascular endothelial cells and migrating macrophages [124]. Transgenic expression of MCP-1 in the CNS using an astrocyte-specific promoter (GFAP) caused encephalopathy characterized by BBB leakage and an increased number of tissue monocytes [125]. In experimental light-induced retinopathy (LIR), MCP-1 expression is increased in Müller cells, which coincides with the recruitment of monocytes into the retina [126]. Following light damage, invading monocytes cooperate with resident microglia to phagocytose dead photoreceptors and then leave in a debris-laden state [127].
Little is known about leukocyte trafficking in the retina during DR. However, leukostasis, the adherence of leukocytes to the diabetic retinal vasculature, is well documented in experimental models of DR (Figure 1). In 1991 Schröder and colleagues demonstrated increased leukostasis in retinas of rats made diabetic by alloxan treatment [128]. Using staining for esterase these authors concluded that the adherent cells were granulocytes (presumably neutrophils) and monocytes, with monocytes being most abundant. They also observed that the adherent cells were causing microvascular occlusion and capillary injury. Activated neutrophils produce reactive oxygen species that cause endothelial cell damage [129]. Adhered neutrophils also directly stimulate endothelial cell death through secretion of Fas-ligand (FasL) causing stimulation of the Fas death receptor on endothelial cells; and a FasL neutralizing antibody was able to prevent capillary loss and vascular permeability in diabetic rats [130]. In several studies published in the 1990’s Adamis and others quantitatively documented leukostasis in retinas of rats made diabetic with STZ and examined the mechanism of cell adhesion (for review see [131]). Endothelial activation and increased surface expression of intercellular adhesion molecules (i.e. ICAM-1) is thought to drive leukostasis in DR [132]. ICAM-1 blocking antibodies inhibited leukostasis in the STZ rat model by approximately 30% [133]. Heightened expression of ICAM-1 by endothelial cells was suggested to be a response to VEGF exposure or to activation of the receptor for advanced glycation end products (RAGE) [134,135]. Neutrophil expression of integrin β-2 (CD18) was also increased in STZ diabetic rats and a CD18 blocking antibody decreased retinal leukostasis by over 60% in these animals [136]. C18 combines with integrin α-L (CD11a) to form the leukocyte function-associated antigen 1 (LFA-1), with integrin α-M (CD11b) to form the macrophage adhesion receptor (MAC-1), and with integrin α-X to form the leukocyte adhesion receptor p150,95 [137]. Both LFA-1 and MAC-1 bind to ICAM-1 and contribute to leukocyte extravasation [138]. Knockout mice lacking CD18 or ICAM-1 each exhibited reduced retinal capillary damage and vascular permeability during STZ-induced diabetes [139]. Because CD18 is expressed by granulocytes, monocytes and lymphocytes, the effects of CD18 inhibition or deficiency could reflect inhibition of adhesion by any of these leukocytes. Thus, the adhesion of monocytes, neutrophils and other leukocytes in experimental DR is likely to be enhanced by the release of chemokines from retinal tissue, the expression of adhesion molecules on the retinal vasculature, and the expression of integrins by circulating leukocytes.
Leukostasis represents an appealing mechanism to explain both tissue ischemia and endothelial damage in DR. However, though well established in rodent models of DR, there is no direct clinical evidence that leukostasis is occurring in DR patients. Evidence for leukostasis leading to retinal capillary nonperfusion in the retinas of obese aging rhesis monkeys was obtained by Kim and co-workers [140]. Although the analysis was not blinded and included “selected regions of interest” rather than randomly-selected regions, these authors found increased numbers of esterase staining granulocytes in retinal capillaries of diabetic versus control monkeys. They also demonstrated decreased endothelial ADPase staining, indicative of nonperfusion, in obstructed capillary segments. There is also associative evidence that neutrophil adhesion may play a role in human DR. Song and co-workers compared the expression of CD18 on neutrophils from 38 DR patients and 10 controls and found it significantly elevated and increased with the severity of DR [141]. In a large retrospective study of almost 31,000 persons, Woo and coworkers found that blood neutrophil counts were increased by approximately 10% in diabetics and by 20% in patients with moderate nonproliferative DR or PDR [142]. The ratio of neutrophils to total white blood cell count (WBC) was also significantly increased in PDR. The neutrophil count was well correlated with DR severity score, and this parameter corresponded to a 2.7-fold odds ratio in DR patients within the highest quartile severity group.
Additional cytokines in DR
The fact that MCP-1 IL-8 and IL-6 are conspicuously elevated in vitreous of DR patients should not be interpreted to suggest that these are the only cytokines of importance in DR pathology. Additional cytokines and growth factors have been observed to be elevated in the vitreous of DME and PDR patients including: interleukin-1beta (IL-1β) [14], interferon-gamma-inducible protein 10 (IP-10, CXCL10) [21,40,91,143,144], monokine induced by gamma interferon (Mig, CXCL9) [21,91], growth-related oncogene (GRO, CXCL1) [40], stomal cell derived factor 1 (SDF-1, CXCL12) [44,45], transforming growth factor beta (TGF-β2) [145], granulocyte-colony stimulating factor (G-CSF) [15], macrophage-colony stimulating factor (M-CSF) [89], and platelet factor 4 (PF-4, CXCL4) [21]. However, it is important to consider the how plasma extravasation and hemorrhage may be influencing the inflammatory milieu of the vitreous, especially in PDR patients. Inflammatory factors may be expressed in response to leakage of plasma proteins and blood from the retinal vasculature. This possibility was recently supported when Goa and co-workers performed a proteomic analysis of vitreous from DR patients [146]. This analysis showed that vitreous of PDR patients contain abundant levels of proteins found in plasma and red blood cells, including those of the kallikrein-kinin, coagulation, and complement systems. These authors have also experimentally demonstrated that blood-born kallikrein, or the release of bradykinin in response to kallikrein, can promote retinal inflammation, leukostasis, and edema [147].
Other cytokines may play important roles in DR without accumulating markedly in the vitreous or even without increased expression in the retina. Huang and coworkers demonstrated that retinal cell death and vascular permeability in response to three months of diabetes was abrogated in TNF-α knockout mice, despite the fact that retinal TNF-α protein levels were not altered by diabetes in wild type mice with an intact TNF-α gene [148]. This raises the question: How might TNF-α deletion block DR pathologies when retinal TNF-α expression is not normally increased in wild type mice? The answer could be that this cytokine plays a permissive role in DR, with basal retinal levels being necessary and sufficient for DR progression. Alternatively, TNF-α knockout may block DR progression by removing TNF-α from the bloodstream. It is feasible that TNF-α represents an external factor that promotes or aggravates retinal inflammation and vascular dysfunction in DR. Several clinical studies have found that average serum levels of TNF-α are increased in patients with PDR and correlate with other systemic indicators of inflammation [85,87,149-153]. Relative to healthy controls, plasma levels of TNF-α progressively increase in diabetic patients with no retinopathy, with nonproliferative DR and with PDR [153]. A recent clinical study of eleven patients with severe DME that was refractory to conventional laser coagulation therapy found that systemic administration of an anti-TNF-α antibody (Infliximab, Remicade) for 16 weeks resulted in a significant 24% increase in visual acuity [154]. However, OCT measurements of retinal thicknesses and DR severity grading of fundus photographs showed no significant anatomical improvements with anti-TNF-α treatment. An earlier study of four patients treated with systemic Infliximab observed both functional and anatomical improvements in four of six eyes examined [155]. It should be noted that two recent studies examining intravitreal injections of anti-TNF-α therapeutics, including Infliximab and Adalimumab (Humira), in refractory DME patients found no benefits of treatment [156,157]. In fact, these studies found adverse effects, including vitritis and uveitis, in some eyes receiving Inflixamab. Thus, it seems that inhibition of circulating, but not intravitreal TNF-α, may be beneficial to DR patients.
Elevated plasma TNF-α could act by promoting endothelial activation or nervous tissue inflammation. TNF-α is a powerful regulator of endothelial activation and inducer of vascular dysfunction [158]. Peripheral inflammation or systemic delivery of TNF-α causes a sustained inflammatory response in the central nervous system in a TNF receptor-dependent fashion [159]. Thus, low-grade systemic inflammation associated with diabetes or with non-retinal complications of diabetes could increase the risk of DR progression. For example, the presence of diabetic foot ulcers is positively correlated with DR as well as with several signs of systemic inflammation, including markedly elevated serum TNF-α levels [160,161]. Recently, Nwanyanwu et al. retrospectively studied a group of 4,617 insurance beneficiaries initially diagnosed with nonproliferative DR and found that the presence of non-healing foot ulcers was the best single risk indicator for their progression to PDR [162]. These ulcers were associated with a 54% increase in the chance of progression. This compared to a 14% increase in hazard of progression for each 1-point rise in plasma HbA1c level. Thus, foot ulcers increased the risk by as much as hyperglycemia corresponding to a nearly 4-point increase in HbA1c. Tang and Kern have recently reasoned that inflammation in DR must originate in the retinal tissue because chronic systemic inflammation characterizes a number of disorders that do not cause retinopathy [29]. However, diabetes, or retinal changes due to diabetes, could alter the retinal response to systemic inflammation so that the combination of these factors results in an increased risk of disease progression.
Possible causes of retinal neuroinflammation in DR
An important question to consider is what leads to the neuroinflammation and cytokine expression in the diabetic retina. Numerous mechanisms have been hypothesized to explain the appearance of low-grade chronic inflammation in the diabetic retina (reviewed in [29,163]). These include: a direct effect of hyperglycemia, dyslipidemia, oxidative stress due to high glucose, advanced glycation end products, hypertension, and ER stress. As discussed above, one might add systemic inflammation to the list of possibilities. In addition, neuroinflammation may be initiated by activation of the retinal resident innate immune system, which is primarily composed of tissue-resident macrophage-like cells called microglia [164,165]. Because microglia are inherently hypersensitive to tissue damage and infection, it is generally presumed that microglia initiate neuroinflammation and that other glial cells respond to and amplify these responses [110,166]. It has been proposed that neuroinflammation should simply be described as “microglial activation,” or perhaps as neural “pseudo-inflammation” because astrocytes, as well as microglia, become activated and produce inflammatory mediators [167]. In fact, neuroinflammation is not synonymous with microglial activation, but microglial activation is the main mechanism by which neuroinflammation is instigated in response to nervous tissue perturbations.
Physically, microglia resembles dendritic cells more than macrophages, with a highly ramified morphology composed of a small cell body with several highly branched projections or processes. The main function of microglia is to monitor the environment and respond to abnormalities in an effort to maintain tissue homeostasis. An innate neural immune system response would presumably prevent a more extreme classical inflammatory response that may result in excessive collateral damage to irreplaceable neural tissue. Microglia are equipped with an array of molecular pattern recognition receptors and scavenger receptors that allow them to recognize and react to the presence of “nonself,” such as pathogens, and to “altered self,” such as damaged and apoptotic neurons [164]. The term microglial activation initially referred to an extreme response to pathogens resulting in a highly inflammatory phenotype with amoeboid morphology and increased motility [168,169]. This classically activated phenotype includes production of inflammatory cytokines, proteases, nitric oxide (NO•) and reactive oxygen species (ROS). Thus, fully activated microglia can actually cause neuronal death [167,170]. With the concept that macrophages can adopt a range of inflammatory (M1), anti-inflammatory and reparative (M2) phenotypes also came to the realization that microglial activation is not one-dimensional. Rather, it seems that microglia can convert from their surveilling phenotype into a spectrum of alternative activation states, depending on the type and extent of tissue dysfunction, damage, or infection [171].
In experimental models of DR, microglia are altered in ways suggesting their activation, but the precise nature of the activation has not been defined. A number of studies utilized immunohistochemistry to examine microglia, or to be more precise, cells expressing myeloid lineage or monocytic markers, within diabetic and control retinas. These studies reported increased numbers of microglia in retinas of rats made diabetic with STZ [103,172-175]. Many of these cells were less ramified than normal retinal microglia or were even amoeboid in shape [103,172,175]. Retinal microglia with short dendrites have also been reported in mice made diabetic by Alloxan injection [176], as well as Ins2Akita genetically diabetic mice [177,178]. Ibrahim et al. used immunohistochemistry with phospho-specific antibodies to demonstrate that phosphorylated forms of the mitogen-activated protein kinase (MAPK), proteins ERK1/2 and p38, co-localized with microglia in retinal sections from STZ-diabetic rats, thus suggesting activation of MEK1/2 and MKK3/6 in these cells [179]. Such activation of MAPK cascades is consistent with inflammatory activation of microglia [180]. However, it should be noted that in all these studies microglial cells were identified using antibodies to markers that are shared by microglia and other monocytes (i.e. Iba-1, ED1 antigen, Isolectin B4, CD11b, and CD68). By virtue of these markers alone it is virtually impossible to distinguish activated resident microglia from extravasated plasma monocytes (i.e. infiltrating macrophages), as well as the small number of retinal dendritic cells. The most reliable characteristics differentiating microglia from invading monocytes are a low CD45 expression and a highly ramified, dendritic morphology. However, during microglial activation CD45 expression can be increased and ramification can be lost to various extents [181,182]. In addition, circulating macrophages can infiltrate damaged nervous tissue, adopt a microglial phenotype and replace resident microglial populations [183,184]. Thus, it is unclear to what degree the increased numbers of monocytes resembling activated microglia are due to infiltration of the diabetic retina by circulating monocytic cells. Clearly, further studies are needed to differentiate the activation and proliferation of resident microglia from the infiltration and conversion of macrophages in DR.
There is very little information regarding the role of microglia in clinical DR. In 1991 Weller and coworkers used immunohistochemistry to examine microglia in surgically removed preretinal traction membranes from patients with PDR, as well as patients with idiopathic and traumatic proliferative vitreo retinopathy (PVR) [185]. These authors concluded that microglia were prevalent in epiretinal membranes from patients with idiopathic PVR, significant in those from traumatic PVR patients, but inconsequential in those from patients with PDR. However, the lectin and antibodies used to identify microglia by immunohistochemistry do not differentiate between microglia and other monocytes. Green and Tso used immunohistochemistry to compare monocyte numbers and morphologies in 21 retinas from diabetic patients, with and without DR, and 10 retinas from control subjects [186]. They observed that retinas from DR patients contained monocytes near pathologic anomalies, including dilated veins, microaneurysms, hemorrhages, cotton-wool spots, as well as retinal and vitreal neovascularization. The prevalence of cells staining with monocytic markers located near the vasculature led these authors to coin the phrase “microvascular perivasculitis.” Thus, monocytes are coincident with the focal pathologies that occur in DR. These cells may originate from the retina (microglia), the vitreous (hyalocytes), or the circulation (macrophages). These cells may represent a causal agent in the pathology or, alternatively, are responding to the pathology. Monocytes located in the periphery of leaky vessels could represent microglia reacting to and disposing of extravasated plasma proteins. Phagocytic monocytes would seem necessary if the hemorrhage or exudates deposits are ever to be cleared away. Such phagocytic clearance is necessary for the resolution of neural inflammation, for when phagocytosis is blocked neuroinflammation is prolonged and amplified [187]. On the other hand, these cells could represent damaging inflammatory macrophages that have invaded by virtue of adherence and diapedesis through an activated endothelial cell layer.
Summary
Numerous studies have detected a shift toward expression of pro-angiogenic factors and cytokines in the vitreous of DR patients, particularly those with severe DME and PDR. The success of anti-VEGF biologic therapies demonstrates that targeting one of these factors can be of benefit in the latter stages of the disease. However, additional therapies are needed to treat DME and PDR patients not responding to anti-VEGF therapy. These may target other pro-angiogenic factors or bolster the anti-angiogenic side of the equation. Although periodic intravitreal injections seem less than ideal, these therapies have the benefit of delivery to a closed compartment of molecules that are relatively contained and long-lived in that compartment. Perhaps a similar approach aimed at cytokines would be of benefit. A better understanding of the roles of cytokines in DR onset and progression would help guide this strategy. Furthermore, a fuller understanding of the mechanisms leading to the development of DME and PDR may lead to preventative measures that block disease progression before vascular defects become apparent. Undetermined issues include: the cellular source and cause of the pro-angiogenic imbalance in DME and PDR, including the roles of focal ischemia, tissue hypoxia, and the UPR; the origin of increased cytokines in the vitreous; the effect of these cytokines on leukostasis and leukocyte trafficking in the diabetic retina; the potential consequences of altered leukocyte trafficking; the influence of systemic inflammation on DR progression; and the effects of microglial activation and/or macrophage infiltration on tissue homeostasis and neuroinflammation in the diabetic retina.
Acknowledgments
Funding This study was supported by grants from “Regulation of Retinal Cell Death in Diabetes” with grant number R01 EY020582 and “Bone marrow neuropathy drives diabetic retinopathy” with grant number R01EY007739.
References
- 1.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 2.Sivaprasad S, Gupta B, Crosby-Nwaobi R, Evans J. Prevalence of diabetic retinopathy in various ethnic groups: a worldwide perspective. Surv Ophthalmol. 2012;57:347–370. doi: 10.1016/j.survophthal.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Hernández C, Simó R. Neuroprotection in diabetic retinopathy. Curr Diab Rep. 2012;12:329–337. doi: 10.1007/s11892-012-0284-5. [DOI] [PubMed] [Google Scholar]
- 4.Gologorsky D, Thanos A, Vavvas D. Therapeutic interventions against inflammatory and angiogenic mediators in proliferative diabetic retinopathy. Mediators Inflamm. 2012;2012:629452. doi: 10.1155/2012/629452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang GE. Diabetic macular edema. Ophthalmologica. 2012;227:21–29. doi: 10.1159/000337156. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt D. The mystery of cotton-wool spots - a review of recent and historical descriptions. Eur J Med Res. 2008;13:231–266. [PubMed] [Google Scholar]
- 7.Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal T, et al. Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ. 2011;343:d6898. doi: 10.1136/bmj.d6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN, DCCT/EDIC Research Group Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial--revisited. Diabetes. 2008;57:995–1001. doi: 10.2337/db07-1618. [DOI] [PubMed] [Google Scholar]
- 9.Lock JH, Fong KC. An update on retinal laser therapy. Clin Exp Optom. 2011;94:43–51. doi: 10.1111/j.1444-0938.2010.00529.x. [DOI] [PubMed] [Google Scholar]
- 10.Giuliari GP. Diabetic retinopathy: current and new treatment options. Curr Diabetes Rev. 2012;8:32–41. doi: 10.2174/157339912798829188. [DOI] [PubMed] [Google Scholar]
- 11.Stewart MW. Corticosteroid use for diabetic macular edema: old fad or new trend? Curr Diab Rep. 2012;12:364–375. doi: 10.1007/s11892-012-0281-8. [DOI] [PubMed] [Google Scholar]
- 12.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 13.Abu El-Asrar AM, Nawaz MI, Kangave D, Siddiquei MM, Ola MS, et al. Angiogenesis regulatory factors in the vitreous from patients with proliferative diabetic retinopathy. Acta Diabetol. 2011 doi: 10.1007/s00592-011-0330-9. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Wang S, Xia X. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr Eye Res. 2012;37:416–420. doi: 10.3109/02713683.2012.661114. [DOI] [PubMed] [Google Scholar]
- 15.Abu El-Asrar AM, Nawaz MI, Kangave D, Abouammoh M, Mohammad G. High-mobility group box-1 and endothelial cell angiogenic markers in the vitreous from patients with proliferative diabetic retinopathy. Mediators Inflamm. 2012;2012:697489. doi: 10.1155/2012/697489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funatsu H, Yamashita H, Ikeda T, Mimura T, Eguchi S, et al. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2003;110:1690–1696. doi: 10.1016/S0161-6420(03)00568-2. [DOI] [PubMed] [Google Scholar]
- 17.Wakabayashi Y, Usui Y, Okunuki Y, Kezuka T, Takeuchi M, et al. Correlation of vascular endothelial growth factor with chemokines in the vitreous in diabetic retinopathy. Retina. 2010;30:339–344. doi: 10.1097/IAE.0b013e3181bd2f44. [DOI] [PubMed] [Google Scholar]
- 18.Mohan N, Monickaraj F, Balasubramanyam M, Rema M, Mohan V. Imbalanced levels of angiogenic and angiostatic factors in vitreous, plasma and postmortem retinal tissue of patients with proliferative diabetic retinopathy. J Diabetes Complications. 2012;26:435–441. doi: 10.1016/j.jdiacomp.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe D, Suzuma K, Suzuma I, Ohashi H, Ojima T, et al. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2005;139:476–481. doi: 10.1016/j.ajo.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Loukovaara S, Robciuc A, Holopainen JM, Lehti K, Pessi T, et al. Ang-2 upregulation correlates with increased levels of MMP-9, VEGF, EPO and TGFÎ21 in diabetic eyes undergoing vitrectomy. Acta Ophthalmol. 2012 doi: 10.1111/j.1755-3768.2012.02473.x. [DOI] [PubMed] [Google Scholar]
- 21.Nawaz MI, Van Raemdonck K, Mohammad G, Kangave D, Van Damme J, et al. Autocrine CCL2, CXCL4, CXCL9 and CXCL10 signal in retinal endothelial cells and are enhanced in diabetic retinopathy. Exp Eye Res. 2013;109C:67–76. doi: 10.1016/j.exer.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Funatsu H, Yamashita H, Noma H, Mimura T, Nakamura S, et al. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2005;243:3–8. doi: 10.1007/s00417-004-0950-7. [DOI] [PubMed] [Google Scholar]
- 23.Patel JI, Tombran-Tink J, Hykin PG, Gregor ZJ, Cree IA. Vitreous and aqueous concentrations of proangiogenic, antiangiogenic factors and other cytokines in diabetic retinopathy patients with macular edema: Implications for structural differences in macular profiles. Exp Eye Res. 2006;82:798–806. doi: 10.1016/j.exer.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Bao S, Lai D, Rapkins RW, Gillies MC. Intravitreal triamcinolone acetonide inhibits breakdown of the blood-retinal barrier through differential regulation of VEGF-A and its receptors in early diabetic rat retinas. Diabetes. 2008;57:1026–1033. doi: 10.2337/db07-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong V. Biological, preclinical and clinical characteristics of inhibitors of vascular endothelial growth factors. Ophthalmologica. 2012;227(Suppl 1):2–10. doi: 10.1159/000337152. [DOI] [PubMed] [Google Scholar]
- 26.Zechmeister-Koss I, Huic M. Vascular endothelial growth factor inhibitors (anti-VEGF) in the management of diabetic macular oedema: a systematic review. Br J Ophthalmol. 2012;96:167–178. doi: 10.1136/bjophthalmol-2011-300674. [DOI] [PubMed] [Google Scholar]
- 27.Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012;130:1145–1152. doi: 10.1001/archophthalmol.2012.1043. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Park JK, Duh EJ. Novel targets against retinal angiogenesis in diabetic retinopathy. Curr Diab Rep. 2012;12:355–363. doi: 10.1007/s11892-012-0289-0. [DOI] [PubMed] [Google Scholar]
- 29.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30:343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W, Liu H, Rojas M, Caldwell RW, Caldwell RB. Anti-inflammatory therapy for diabetic retinopathy. Immunotherapy. 2011;3:609–628. doi: 10.2217/imt.11.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angi M, Kalirai H, Coupland SE, Damato BE, Semeraro F, et al. Proteomic analyses of the vitreous humour. Mediators Inflamm. 2012;2012:148039. doi: 10.1155/2012/148039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Feng L, Hu JW, Xie CL, Wang F. Characterisation of the vitreous proteome in proliferative diabetic retinopathy. Proteome Sci. 2012;10:15. doi: 10.1186/1477-5956-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feener EP. Proteomics in the viteous of diabetic retinopathy patients. In: Tombran-Tink J, Barnstable CJ, Gardner TW, editors. Visual Dysfunction in Diabetes. Humana Press, Springer; 2012. pp. 173–188. [Google Scholar]
- 34.Simó-Servat O, Hernández C, Simó R. Usefulness of the vitreous fluid analysis in the translational research of diabetic retinopathy. Mediators Inflamm. 2012;2012:872978. doi: 10.1155/2012/872978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gustavsson C, Agardh CD, Agardh E. Profile of intraocular tumour necrosis factor-α and interleukin-6 in diabetic subjects with different degrees of diabetic retinopathy. Acta Ophthalmol. 2012 doi: 10.1111/j.1755-3768.2012.02430.x. [DOI] [PubMed] [Google Scholar]
- 36.Huber M, Wachtlin J. Vitreous levels of proteins implicated in angiogenesis are modulated in patients with retinal or choroidal neovascularization. Ophthalmologica. 2012;228:188–193. doi: 10.1159/000339952. [DOI] [PubMed] [Google Scholar]
- 37.Abu El-Asrar AM, Imtiaz Nawaz M, Kangave D, Siddiquei MM, Geboes K. Osteopontin and other regulators of angiogenesis and fibrogenesis in the vitreous from patients with proliferative vitreoretinal disorders. Mediators Inflamm. 2012;2012:493043. doi: 10.1155/2012/493043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kase S, Yokoi M, Saito W, Furudate N, Ohgami K, et al. Increased osteopontin levels in the vitreous of patients with diabetic retinopathy. Ophthalmic Res. 2007;39:143–147. doi: 10.1159/000102936. [DOI] [PubMed] [Google Scholar]
- 39.Lee WJ, Kang MH, Seong M, Cho HY. Comparison of aqueous concentrations of angiogenic and inflammatory cytokines in diabetic macular oedema and macular oedema due to branch retinal vein occlusion. Br J Ophthalmol. 2012;96:1426–1430. doi: 10.1136/bjophthalmol-2012-301913. [DOI] [PubMed] [Google Scholar]
- 40.Schoenberger SD, Kim SJ, Sheng J, Rezaei KA, Lalezary M, et al. Increased prostaglandin E2 (PGE2) levels in proliferative diabetic retinopathy, and correlation with VEGF and inflammatory cytokines. Invest Ophthalmol Vis Sci. 2012;53:5906–5911. doi: 10.1167/iovs.12-10410. [DOI] [PubMed] [Google Scholar]
- 41.Praidou A, Papakonstantinou E, Androudi S, Georgiadis N, Karakiulakis G, et al. Vitreous and serum levels of vascular endothelial growth factor and platelet-derived growth factor and their correlation in patients with nonproliferative diabetic retinopathy and clinically significant macula oedema. Acta Ophthalmol. 2011;89:248–254. doi: 10.1111/j.1755-3768.2009.01661.x. [DOI] [PubMed] [Google Scholar]
- 42.Freyberger H, Bröcker M, Yakut H, Hammer J, Effert R, et al. Increased levels of platelet-derived growth factor in vitreous fluid of patients with proliferative diabetic retinopathy. Exp Clin Endocrinol Diabetes. 2000;108:106–109. doi: 10.1055/s-2000-5803. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe D, Suzuma K, Matsui S, Kurimoto M, Kiryu J, et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353:782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- 44.Butler JM, Guthrie SM, Koc M, Afzal A, Caballero S, et al. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest. 2005;115:86–93. doi: 10.1172/JCI22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brooks HL, Jr, Caballero S, Jr, Newell CK, Steinmetz RL, Watson D, et al. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol. 2004;122:1801–1807. doi: 10.1001/archopht.122.12.1801. [DOI] [PubMed] [Google Scholar]
- 46.You JJ, Yang CM, Chen MS, Yang CH. Elevation of angiogenic factor Cysteine-rich 61 levels in vitreous of patients with proliferative diabetic retinopathy. Retina. 2012;32:103–111. doi: 10.1097/IAE.0b013e318219e4ad. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, Yu W, Dong F. Cysteine-rich 61 (CYR61) is up-regulated in proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2012;250:661–668. doi: 10.1007/s00417-011-1882-7. [DOI] [PubMed] [Google Scholar]
- 48.Funatsu H, Yamashita H, Noma H, Mochizuki H, Mimura T, et al. Outcome of vitreous surgery and the balance between vascular endothelial growth factor and endostatin. Invest Ophthalmol Vis Sci. 2003;44:1042–1047. doi: 10.1167/iovs.02-0374. [DOI] [PubMed] [Google Scholar]
- 49.Spranger J, Hammes HP, Preissner KT, Schatz H, Pfeiffer AF. Release of the angiogenesis inhibitor angiostatin in patients with proliferative diabetic retinopathy: association with retinal photocoagulation. Diabetologia. 2000;43:1404–1407. doi: 10.1007/s001250051546. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura S, Morimoto N, Tsuruma K, Izuta H, Yasuda Y, et al. Tissue kallikrein inhibits retinal neovascularization via the cleavage of vascular endothelial growth factor-165. Arterioscler Thromb Vasc Biol. 2011;31:1041–1048. doi: 10.1161/ATVBAHA.111.223594. [DOI] [PubMed] [Google Scholar]
- 51.Kuiper EJ, Van Nieuwenhoven FA, de Smet MD, van Meurs JC, Tanck MW, et al. The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS One. 2008;3:e2675. doi: 10.1371/journal.pone.0002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campochiaro PA. Ocular neovascularization. J Mol Med. 2013;18:18. doi: 10.1007/s00109-013-0993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willard AL, Herman IM. Vascular complications and diabetes: current therapies and future challenges. J Ophthalmol. 2012;2012:209538. doi: 10.1155/2012/209538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arjamaa O, Nikinmaa M. Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Exp Eye Res. 2006;83:473–483. doi: 10.1016/j.exer.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 55.Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. EMBO J. 2012;31:2448–2460. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Wang G, Wang Y. Intravitreous vascular endothelial growth factor and hypoxia-inducible factor 1a in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2009;148:883–889. doi: 10.1016/j.ajo.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 57.Han XX, Guo CM, Li Y, Hui YN. Effects of bevacizumab on the neovascular membrane of proliferative diabetic retinopathy: reduction of endothelial cells and expressions of VEGF and HIF-1alpha. Mol Vis. 2012;18:1–9. [PMC free article] [PubMed] [Google Scholar]
- 58.Abu El-Asrar AM, Missotten L, Geboes K. Expression of hypoxia-inducible factor-1alpha and the protein products of its target genes in diabetic fibrovascular epiretinal membranes. Br J Ophthalmol. 2007;91:822–826. doi: 10.1136/bjo.2006.109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J, Xu X, Elliott MH, Zhu M, Le YZ. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59:2297–2305. doi: 10.2337/db09-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin M, Chen Y, Jin J, Hu Y, Zhou KK, et al. Ischaemia-induced retinal neovascularisation and diabetic retinopathy in mice with conditional knockout of hypoxia-inducible factor-1 in retinal Müller cells. Diabetologia. 2011;54:1554–1566. doi: 10.1007/s00125-011-2081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Linsenmeier RA, Braun RD, McRipley MA, Padnick LB, Ahmed J, et al. Retinal hypoxia in long-term diabetic cats. Invest Ophthalmol Vis Sci. 1998;39:1647–1657. [PubMed] [Google Scholar]
- 62.Ly A, Yee P, Vessey KA, Phipps JA, Jobling AI, et al. Early inner retinal astrocyte dysfunction during diabetes and development of hypoxia, retinal stress, and neuronal functional loss. Invest Ophthalmol Vis Sci. 2011;52:9316–9326. doi: 10.1167/iovs.11-7879. [DOI] [PubMed] [Google Scholar]
- 63.Wright WS, McElhatten RM, Messina JE, Harris NR. Hypoxia and the expression of HIF-1alpha and HIF-2alpha in the retina of streptozotocin-injected mice and rats. Exp Eye Res. 2010;90:405–412. doi: 10.1016/j.exer.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wright WS, McElhatten RM, Harris NR. Increase in retinal hypoxia-inducible factor-2α, but not hypoxia, early in the progression of diabetes in the rat. Exp Eye Res. 2011;93:437–441. doi: 10.1016/j.exer.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wright WS, Yadav AS, McElhatten RM, Harris NR. Retinal blood flow abnormalities following six months of hyperglycemia in the Ins2(Akita) mouse. Exp Eye Res. 2012;98:9–15. doi: 10.1016/j.exer.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kimble JA, Brandt BM, McGwin G., Jr Clinical examination accurately locates capillary nonperfusion in diabetic retinopathy. Am J Ophthalmol. 2005;139:555–557. doi: 10.1016/j.ajo.2004.08.073. [DOI] [PubMed] [Google Scholar]
- 67.Lange CA, Stavrakas P, Luhmann UF, de Silva DJ, Ali RR, et al. Intraocular oxygen distribution in advanced proliferative diabetic retinopathy. Am J Ophthalmol. 2011;152:406–412. doi: 10.1016/j.ajo.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 68.Curtis TM, Gardiner TA, Stitt AW. Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye (Lond) 2009;23:1496–1508. doi: 10.1038/eye.2009.108. [DOI] [PubMed] [Google Scholar]
- 69.Nguyen QD, Shah SM, Van Anden E, Sung JU, Vitale S, et al. Supplemental oxygen improves diabetic macular edema: a pilot study. Invest Ophthalmol Vis Sci. 2004;45:617–624. doi: 10.1167/iovs.03-0557. [DOI] [PubMed] [Google Scholar]
- 70.Hammer M, Vilser W, Riemer T, Mandecka A, Schweitzer D, et al. Diabetic patients with retinopathy show increased retinal venous oxygen saturation. Graefes Arch Clin Exp Ophthalmol. 2009;247:1025–1030. doi: 10.1007/s00417-009-1078-6. [DOI] [PubMed] [Google Scholar]
- 71.Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003;121:547–557. doi: 10.1001/archopht.121.4.547. [DOI] [PubMed] [Google Scholar]
- 72.Baird TD, Wek RC. Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism. Adv Nutr. 2012;3:307–321. doi: 10.3945/an.112.002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ameri K, Lewis CE, Raida M, Sowter H, Hai T, et al. Anoxic induction of ATF-4 through HIF-1-independent pathways of protein stabilization in human cancer cells. Blood. 2004;103:1876–1882. doi: 10.1182/blood-2003-06-1859. [DOI] [PubMed] [Google Scholar]
- 74.Köditz J, Nesper J, Wottawa M, Stiehl DP, Camenisch G, et al. Oxygen-dependent ATF-4 stability is mediated by the PHD3 oxygen sensor. Blood. 2007;110:3610–3617. doi: 10.1182/blood-2007-06-094441. [DOI] [PubMed] [Google Scholar]
- 75.Abcouwer SF, Marjon PL, Loper RK, Vander Jagt DL. Response of VEGF expression to amino acid deprivation and inducers of endoplasmic reticulum stress. Invest Ophthalmol Vis Sci. 2002;43:2791–2798. [PubMed] [Google Scholar]
- 76.Marjon PL, Bobrovnikova-Marjon EV, Abcouwer SF. Expression of the pro-angiogenic factors vascular endothelial growth factor and interleukin-8/CXCL8 by human breast carcinomas is responsive to nutrient deprivation and endoplasmic reticulum stress. Mol Cancer. 2004;3:4. doi: 10.1186/1476-4598-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roybal CN, Marmorstein LY, Vander Jagt DL, Abcouwer SF. Aberrant accumulation of fibulin-3 in the endoplasmic reticulum leads to activation of the unfolded protein response and VEGF expression. Invest Ophthalmol Vis Sci. 2005;46:3973–3979. doi: 10.1167/iovs.05-0070. [DOI] [PubMed] [Google Scholar]
- 78.Pereira ER, Liao N, Neale GA, Hendershot LM. Transcriptional and post-transcriptional regulation of proangiogenic factors by the unfolded protein response. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roybal CN, Hunsaker LA, Barbash O, Vander Jagt DL, Abcouwer SF. The oxidative stressor arsenite activates vascular endothelial growth factor mRNA transcription by an ATF4-dependent mechanism. J Biol Chem. 2005;280:20331–20339. doi: 10.1074/jbc.M411275200. [DOI] [PubMed] [Google Scholar]
- 80.Roybal CN, Yang S, Sun CW, Hurtado D, Vander Jagt DL, et al. Homocysteine increases the expression of vascular endothelial growth factor by a mechanism involving endoplasmic reticulum stress and transcription factor ATF4. J Biol Chem. 2004;279:14844–14852. doi: 10.1074/jbc.M312948200. [DOI] [PubMed] [Google Scholar]
- 81.Zhong Y, Li J, Chen Y, Wang JJ, Ratan R, et al. Activation of endoplasmic reticulum stress by hyperglycemia is essential for Müller cell-derived inflammatory cytokine production in diabetes. Diabetes. 2012;61:492–504. doi: 10.2337/db11-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ghosh R, Lipson KL, Sargent KE, Mercurio AM, Hunt JS, et al. Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS One. 2010;5:e9575. doi: 10.1371/journal.pone.0009575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Y, Wang JJ, Li J, Hosoya KI, Ratan R, et al. Activating transcription factor 4 mediates hyperglycaemia-induced endothelial inflammation and retinal vascular leakage through activation of STAT3 in a mouse model of type 1 diabetes. Diabetologia. 2012;55:2533–2545. doi: 10.1007/s00125-012-2594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kauffmann DJ, van Meurs JC, Mertens DA, Peperkamp E, Master C, et al. Cytokines in vitreous humor: interleukin-6 is elevated in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1994;35:900–906. [PubMed] [Google Scholar]
- 85.Yuuki T, Kanda T, Kimura Y, Kotajima N, Tamura J, et al. Inflammatory cytokines in vitreous fluid and serum of patients with diabetic vitreoretinopathy. J Diabetes Complications. 2001;15:257–259. doi: 10.1016/s1056-8727(01)00155-6. [DOI] [PubMed] [Google Scholar]
- 86.Canataroglu H, Varinli I, Ozcan AA, Canataroglu A, Doran F, et al. Interleukin (IL)-6, interleukin (IL)-8 levels and cellular composition of the vitreous humor in proliferative diabetic retinopathy, proliferative vitreoretinopathy, and traumatic proliferative vitreoretinopathy. Ocul Immunol Inflamm. 2005;13:375–381. doi: 10.1080/09273940490518900. [DOI] [PubMed] [Google Scholar]
- 87.Adamiec-Mroczek J, Oficjalska-Mlynczak J. Assessment of selected adhesion molecule and proinflammatory cytokine levels in the vitreous body of patients with type 2 diabetes--role of the inflammatory-immune process in the pathogenesis of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2008;246:1665–1670. doi: 10.1007/s00417-008-0868-6. [DOI] [PubMed] [Google Scholar]
- 88.Yoshimura T, Sonoda KH, Sugahara M, Mochizuki Y, Enaida H, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009;4:e8158. doi: 10.1371/journal.pone.0008158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Elner SG, Elner VM, Jaffe GJ, Stuart A, Kunkel SL, et al. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res. 1995;14:1045–1053. doi: 10.3109/02713689508998529. [DOI] [PubMed] [Google Scholar]
- 90.Fonollosa A, Garcia-Arumi J, Santos E, Macia C, Fernandez P, et al. Vitreous levels of interleukine-8 and monocyte chemoattractant protein-1 in macular oedema with branch retinal vein occlusion. Eye (Lond) 2010;24:1284–1290. doi: 10.1038/eye.2009.340. [DOI] [PubMed] [Google Scholar]
- 91.Umazume K, Usui Y, Wakabayashi Y, Okunuki Y, Kezuka T, et al. Effects of soluble cd14 and cytokine levels on diabetic macular edema and visual acuity. Retina. 2012 doi: 10.1097/IAE.0b013e31826f0688. [DOI] [PubMed] [Google Scholar]
- 92.Mitamura Y, Takeuchi S, Yamamoto S, Yamamoto T, Tsukahara I, et al. Monocyte chemotactic protein-1 levels in the vitreous of patients with proliferative vitreoretinopathy. Jpn J Ophthalmol. 2002;46:218–221. doi: 10.1016/s0021-5155(01)00497-x. [DOI] [PubMed] [Google Scholar]
- 93.Sohn HJ, Han DH, Kim IT, Oh IK, Kim KH, et al. Changes in aqueous concentrations of various cytokines after intravitreal triamcinolone versus bevacizumab for diabetic macular edema. Am J Ophthalmol. 2011;152:686–694. doi: 10.1016/j.ajo.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 94.Dienz O, Eaton SM, Bond JP, Neveu W, Moquin D, et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J Exp Med. 2009;206:69–78. doi: 10.1084/jem.20081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rincon M. Interleukin-6: from an inflammatory marker to a target for inflammatory diseases. Trends Immunol. 2012;33:571–577. doi: 10.1016/j.it.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 96.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8:1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spooren A, Kolmus K, Laureys G, Clinckers R, De Keyser J, et al. Interleukin-6, a mental cytokine. Brain Res Rev. 2011;67:157–183. doi: 10.1016/j.brainresrev.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 98.Penkowa M, Giralt M, Lago N, Camats J, Carrasco J, et al. Astrocyte-targeted expression of IL-6 protects the CNS against a focal brain injury. Exp Neurol. 2003;181:130–148. doi: 10.1016/s0014-4886(02)00051-1. [DOI] [PubMed] [Google Scholar]
- 99.Suzuki S, Tanaka K, Suzuki N. Ambivalent aspects of interleukin-6 in cerebral ischemia: inflammatory versus neurotrophic aspects. J Cereb Blood Flow Metab. 2009;29:464–479. doi: 10.1038/jcbfm.2008.141. [DOI] [PubMed] [Google Scholar]
- 100.Okada S, Nakamura M, Mikami Y, Shimazaki T, Mihara M, et al. Blockade of interleukin-6 receptor suppresses reactive astrogliosis and ameliorates functional recovery in experimental spinal cord injury. J Neurosci Res. 2004;76:265–276. doi: 10.1002/jnr.20044. [DOI] [PubMed] [Google Scholar]
- 101.Tilgner J, Volk B, Kaltschmidt C. Continuous interleukin-6 application in vivo via macroencapsulation of interleukin-6-expressing COS-7 cells induces massive gliosis. Glia. 2001;35:234–245. doi: 10.1002/glia.1088. [DOI] [PubMed] [Google Scholar]
- 102.Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, et al. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes. 1998;47:815–820. doi: 10.2337/diabetes.47.5.815. [DOI] [PubMed] [Google Scholar]
- 103.Rungger-Brändle E, Dosso AA, Leuenberger PM. Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2000;41:1971–1980. [PubMed] [Google Scholar]
- 104.Liberto CM, Albrecht PJ, Herx LM, Yong VW, Levison SW. Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem. 2004;89:1092–1100. doi: 10.1111/j.1471-4159.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- 105.Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- 106.Li Q, Puro DG. Diabetes-induced dysfunction of the glutamate transporter in retinal Müller cells. Invest Ophthalmol Vis Sci. 2002;43:3109–3116. [PubMed] [Google Scholar]
- 107.Bringmann A, Pannicke T, Biedermann B, Francke M, Iandiev I, et al. Role of retinal glial cells in neurotransmitter uptake and metabolism. Neurochem Int. 2009;54:143–160. doi: 10.1016/j.neuint.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 108.Coorey NJ, Shen W, Chung SH, Zhu L, Gillies MC. The role of glia in retinal vascular disease. Clin Exp Optom. 2012;95:266–281. doi: 10.1111/j.1444-0938.2012.00741.x. [DOI] [PubMed] [Google Scholar]
- 109.Ambrosini E, Aloisi F. Chemokines and glial cells: a complex network in the central nervous system. Neurochem Res. 2004;29:1017–1038. doi: 10.1023/b:nere.0000021246.96864.89. [DOI] [PubMed] [Google Scholar]
- 110.Khandelwal PJ, Herman AM, Moussa CE. Inflammation in the early stages of neurodegenerative pathology. J Neuroimmunol. 2011;238:1–11. doi: 10.1016/j.jneuroim.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yego EC, Vincent JA, Sarthy V, Busik JV, Mohr S. Differential regulation of high glucose-induced glyceraldehyde-3-phosphate dehydrogenase nuclear accumulation in Müller cells by IL-1beta and IL-6. Invest Ophthalmol Vis Sci. 2009;50:1920–1928. doi: 10.1167/iovs.08-2082. [DOI] [PubMed] [Google Scholar]
- 112.Shelton MD, Kern TS, Mieyal JJ. Glutaredoxin regulates nuclear factor kappa-B and intercellular adhesion molecule in Müller cells: model of diabetic retinopathy. J Biol Chem. 2007;282:12467–12474. doi: 10.1074/jbc.M610863200. [DOI] [PubMed] [Google Scholar]
- 113.Owens T, Bechmann I, Engelhardt B. Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol. 2008;67:1113–1121. doi: 10.1097/NEN.0b013e31818f9ca8. [DOI] [PubMed] [Google Scholar]
- 114.Toft-Hansen H, Fuchtbauer L, Owens T. Inhibition of reactive astrocytosis in established experimental autoimmune encephalomyelitis favors infiltration by myeloid cells over T cells and enhances severity of disease. Glia. 2011;59:166–176. doi: 10.1002/glia.21088. [DOI] [PubMed] [Google Scholar]
- 115.Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- 116.Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, et al. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol. 2008;181:2189–2195. doi: 10.4049/jimmunol.181.3.2189. [DOI] [PubMed] [Google Scholar]
- 117.McLoughlin RM, Jenkins BJ, Grail D, Williams AS, Fielding CA, et al. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc Natl Acad Sci U S A. 2005;102:9589–9594. doi: 10.1073/pnas.0501794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Semple BD, Kossmann T, Morganti-Kossmann MC. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J Cereb Blood Flow Metab. 2010;30:459–473. doi: 10.1038/jcbfm.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bell MD, Taub DD, Perry VH. Overriding the brain’s intrinsic resistance to leukocyte recruitment with intraparenchymal injections of recombinant chemokines. Neuroscience. 1996;74:283–292. doi: 10.1016/0306-4522(96)00083-8. [DOI] [PubMed] [Google Scholar]
- 121.Lee TH, Avraham H, Lee SH, Avraham S. Vascular endothelial growth factor modulates neutrophil transendothelial migration via up-regulation of interleukin-8 in human brain microvascular endothelial cells. J Biol Chem. 2002;277:10445–10451. doi: 10.1074/jbc.M107348200. [DOI] [PubMed] [Google Scholar]
- 122.Prinz M, Priller J. Tickets to the brain: role of CCR2 and CX3CR1 in myeloid cell entry in the CNS. J Neuroimmunol. 2010;224:80–84. doi: 10.1016/j.jneuroim.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 123.Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, et al. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- 124.Dzenko KA, Song L, Ge S, Kuziel WA, Pachter JS. CCR2 expression by brain microvascular endothelial cells is critical for macrophage transendothelial migration in response to CCL2. Microvasc Res. 2005;70:53–64. doi: 10.1016/j.mvr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 125.Huang D, Wujek J, Kidd G, He TT, Cardona A, et al. Chronic expression of monocyte chemoattractant protein-1 in the central nervous system causes delayed encephalopathy and impaired microglial function in mice. FASEB J. 2005;19:761–772. doi: 10.1096/fj.04-3104com. [DOI] [PubMed] [Google Scholar]
- 126.Rutar M, Natoli R, Valter K, Provis JM. Early focal expression of the chemokine Ccl2 by Müller cells during exposure to damage-inducing bright continuous light. Invest Ophthalmol Vis Sci. 2011;52:2379–2388. doi: 10.1167/iovs.10-6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Joly S, Francke M, Ulbricht E, Beck S, Seeliger M, et al. Cooperative phagocytes: resident microglia and bone marrow immigrants remove dead photoreceptors in retinal lesions. Am J Pathol. 2009;174:2310–2323. doi: 10.2353/ajpath.2009.090023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schröder S, Palinski W, Schmid-Schönbein GW. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol. 1991;139:81–100. [PMC free article] [PubMed] [Google Scholar]
- 129.He P. Leucocyte/endothelium interactions and microvessel permeability: coupled or uncoupled? Cardiovasc Res. 2010;87:281–290. doi: 10.1093/cvr/cvq140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Joussen AM, Poulaki V, Mitsiades N, Cai WY, Suzuma I, et al. Suppression of Fas-FasL-induced endothelial cell apoptosis prevents diabetic blood-retinal barrier breakdown in a model of streptozotocin-induced diabetes. Faseb J. 2003;17:76–78. doi: 10.1096/fj.02-0157fje. [DOI] [PubMed] [Google Scholar]
- 131.Adamis AP. Is diabetic retinopathy an inflammatory disease? Br J Ophthalmol. 2002;86:363–365. doi: 10.1136/bjo.86.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Patel N. Targeting leukostasis for the treatment of early diabetic retinopathy. Cardiovasc Hematol Disord Drug Targets. 2009;9:222–229. doi: 10.2174/187152909789007052. [DOI] [PubMed] [Google Scholar]
- 133.Miyamoto K, Khosrof S, Bursell SE, Rohan R, Murata T, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 1999;96:10836–10841. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kaji Y, Usui T, Ishida S, Yamashiro K, Moore TC, et al. Inhibition of diabetic leukostasis and blood-retinal barrier breakdown with a soluble form of a receptor for advanced glycation end products. Invest Ophthalmol Vis Sci. 2007;48:858–865. doi: 10.1167/iovs.06-0495. [DOI] [PubMed] [Google Scholar]
- 135.Joussen AM, Poulaki V, Qin W, Kirchhof B, Mitsiades N, et al. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol. 2002;160:501–509. doi: 10.1016/S0002-9440(10)64869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Barouch FC, Miyamoto K, Allport JR, Fujita K, Bursell SE, et al. Integrin-mediated neutrophil adhesion and retinal leukostasis in diabetes. Invest Ophthalmol Vis Sci. 2000;41:1153–1158. [PubMed] [Google Scholar]
- 137.Wautier JL, Setiadi H, Vilette D, Weill D, Wautier MP. Leukocyte adhesion to endothelial cells. Biorheology. 1990;27:425–432. doi: 10.3233/bir-1990-273-419. [DOI] [PubMed] [Google Scholar]
- 138.Smith CW. Endothelial adhesion molecules and their role in inflammation. Can J Physiol Pharmacol. 1993;71:76–87. doi: 10.1139/y93-012. [DOI] [PubMed] [Google Scholar]
- 139.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 140.Kim SY, Johnson MA, McLeod DS, Alexander T, Hansen BC, et al. Neutrophils is associated with capillary closure in spontaneously diabetic monkey retinas. Diabetes. 2005;54:1534–1542. doi: 10.2337/diabetes.54.5.1534. [DOI] [PubMed] [Google Scholar]
- 141.Song H, Wang L, Hui Y. Expression of CD18 on the neutrophils of patients with diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2007;245:24–31. doi: 10.1007/s00417-006-0379-2. [DOI] [PubMed] [Google Scholar]
- 142.Woo SJ, Ahn SJ, Ahn J, Park KH, Lee K. Elevated systemic neutrophil count in diabetic retinopathy and diabetes: a hospital-based cross-sectional study of 30,793 Korean subjects. Invest Ophthalmol Vis Sci. 2011;52:7697–7703. doi: 10.1167/iovs.11-7784. [DOI] [PubMed] [Google Scholar]
- 143.Abu El, Asrar AM, Struyf S, Kangave D, Geboes K, Van Damme J. Chemokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Eur Cytokine Netw. 2006;17:155–165. [PubMed] [Google Scholar]
- 144.Elner SG, Strieter R, Bian ZM, Kunkel S, Mokhtarzaden L, et al. Interferon-induced protein 10 and interleukin 8. C-X-C chemokines present in proliferative diabetic retinopathy. Arch Ophthalmol. 1998;116:1597–1601. doi: 10.1001/archopht.116.12.1597. [DOI] [PubMed] [Google Scholar]
- 145.Van Geest RJ, Klaassen I, Lesnik-Oberstein SY, Tan HS, Mura M, et al. Vitreous TIMP-1 levels associate with neovascularization and TGF-beta2 levels but not with fibrosis in the clinical course of proliferative diabetic retinopathy. J Cell Commun Signal. 2013;7:1–9. doi: 10.1007/s12079-012-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gao BB, Chen X, Timothy N, Aiello LP, Feener EP. Characterization of the vitreous proteome in diabetes without diabetic retinopathy and diabetes with proliferative diabetic retinopathy. J Proteome Res. 2008;7:2516–2525. doi: 10.1021/pr800112g. [DOI] [PubMed] [Google Scholar]
- 147.Liu J, Clermont AC, Gao BB, Feener EP. Intraocular Hemorrhage Causes Retinal Vascular Dysfunction via Plasma Kallikrein. Invest Ophthalmol Vis Sci. 2013;54:1086–1094. doi: 10.1167/iovs.12-10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Huang H, Gandhi JK, Zhong X, Wei Y, Gong J, et al. TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest Ophthalmol Vis Sci. 2011;52:1336–1344. doi: 10.1167/iovs.10-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Koleva-Georgieva DN, Sivkova NP, Terzieva D. Serum inflammatory cytokines IL-1beta, IL-6, TNF-alpha and VEGF have influence on the development of diabetic retinopathy. Folia Med (Plovdiv) 2011;53:44–50. doi: 10.2478/v10153-010-0036-8. [DOI] [PubMed] [Google Scholar]
- 150.Myśliwiec M, Balcerska A, Zorena K, Myśliwska J, Lipowski P, et al. The role of vascular endothelial growth factor, tumor necrosis factor alpha and interleukin-6 in pathogenesis of diabetic retinopathy. Diabetes Res Clin Pract. 2008;79:141–146. doi: 10.1016/j.diabres.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 151.Doganay S, Evereklioglu C, Er H, Türköz Y, Sevinç A, et al. Comparison of serum NO, TNF-alpha, IL-1beta, sIL-2R, IL-6 and IL-8 levels with grades of retinopathy in patients with diabetes mellitus. Eye (Lond) 2002;16:163–170. doi: 10.1038/sj.eye.6700095. [DOI] [PubMed] [Google Scholar]
- 152.Gustavsson C, Agardh E, Bengtsson B, Agardh CD. TNF-alpha is an independent serum marker for proliferative retinopathy in type 1 diabetic patients. J Diabetes Complications. 2008;22:309–316. doi: 10.1016/j.jdiacomp.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 153.Ben-Mahmud BM, Chan WH, Abdulahad RM, Datti A, Orlacchio A, et al. Clinical validation of a link between TNF-alpha and the glycosylation enzyme core 2 GlcNAc-T and the relationship of this link to diabetic retinopathy. Diabetologia. 2006;49:2185–2191. doi: 10.1007/s00125-006-0332-2. [DOI] [PubMed] [Google Scholar]
- 154.Sfikakis PP, Grigoropoulos V, Emfietzoglou I, Theodossiadis G, Tentolouris N, et al. Infliximab for diabetic macular edema refractory to laser photocoagulation: a randomized, double-blind, placebo-controlled, crossover, 32-week study. Diabetes Care. 2010;33:1523–1528. doi: 10.2337/dc09-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sfikakis PP, Markomichelakis N, Theodossiadis GP, Grigoropoulos V, Katsilambros N, et al. Regression of sight-threatening macular edema in type 2 diabetes following treatment with the anti-tumor necrosis factor monoclonal antibody infliximab. Diabetes Care. 2005;28:445–447. doi: 10.2337/diacare.28.2.445. [DOI] [PubMed] [Google Scholar]
- 156.Wu L, Hernandez-Bogantes E, Roca JA, Arevalo JF, Barraza K, et al. intravitreal tumor necrosis factor inhibitors in the treatment of refractory diabetic macular edema: a pilot study from the Pan-American Collaborative Retina Study Group. Retina. 2011;31:298–303. doi: 10.1097/IAE.0b013e3181eac7a6. [DOI] [PubMed] [Google Scholar]
- 157.Giganti M, Beer PM, Lemanski N, Hartman C, Schartman J, et al. Adverse events after intravitreal infliximab (Remicade) Retina. 2010;30:71–80. doi: 10.1097/IAE.0b013e3181bcef3b. [DOI] [PubMed] [Google Scholar]
- 158.Zhang H, Park Y, Wu J, Chen Xp, Lee S, et al. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 2009;116:219–230. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Qin L, Wu X, Block ML, Liu Y, Breese GR, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zubair M, Malik A, Ahmad J. Plasma adiponectin, IL-6, hsCRP, and TNF-α levels in subject with diabetic foot and their correlation with clinical variables in a North Indian tertiary care hospital. Indian J Endocrinol Metab. 2012;16:769–776. doi: 10.4103/2230-8210.100672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ahmad J, Zubair M, Malik A, Siddiqui MA, Wangnoo SK. Cathepsin-D, adiponectin, TNF-alpha, IL-6 and hsCRP plasma levels in subjects with diabetic foot and possible correlation with clinical variables: a multicentric study. Foot (Edinb) 2012;22:194–199. doi: 10.1016/j.foot.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 162.Nwanyanwu KH, Talwar N, Gardner TW, Wrobel JS, Herman WH, et al. Predicting Development of Proliferative Diabetic Retinopathy. Diabetes Care. 2013 doi: 10.2337/dc12-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang W, Liu H, Al-Shabrawey M, Caldwell RW, Caldwell RB. Inflammation and diabetic retinal microvascular complications. J Cardiovasc Dis Res. 2011;2:96–103. doi: 10.4103/0975-3583.83035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 165.Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- 166.Holms TH, Draeby D, Owens T. Microglia are required for astroglial Toll-like receptor 4 response and for optimal TLR2 and TLR3 response. Glia. 2012;60:630–638. doi: 10.1002/glia.22296. [DOI] [PubMed] [Google Scholar]
- 167.Graeber MB, Li W, Rodriguez ML. Role of microglia in CNS inflammation. FEBS Lett. 2011;585:3798–3805. doi: 10.1016/j.febslet.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 168.Vrabec F. Activated human retinal microglia under pathological conditions. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1975;196:49–60. doi: 10.1007/BF00410026. [DOI] [PubMed] [Google Scholar]
- 169.Giulian D. Ameboid microglia as effectors of inflammation in the central nervous system. J Neurosci Res. 1987;18:155–171. 132–3. doi: 10.1002/jnr.490180123. [DOI] [PubMed] [Google Scholar]
- 170.Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. Glia. 1993;7:111–118. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- 171.Colton C, Wilcock DM. Assessing activation states in microglia. CNS Neurol Disord Drug Targets. 2010;9:174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- 172.McVicar CM, Hamilton R, Colhoun LM, Gardiner TA, Brines M, et al. Intervention with an erythropoietin-derived peptide protects against neuroglial and vascular degeneration during diabetic retinopathy. Diabetes. 2011;60:2995–3005. doi: 10.2337/db11-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tsai GY, Cui JZ, Syed H, Xia Z, Ozerdem U, et al. Effect of N-acetylcysteine on the early expression of inflammatory markers in the retina and plasma of diabetic rats. Clin Experiment Ophthalmol. 2009;37:223–231. doi: 10.1111/j.1442-9071.2009.02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Silva KC, Pinto CC, Biswas SK, de Faria JB, de Faria JM. Hypertension increases retinal inflammation in experimental diabetes: a possible mechanism for aggravation of diabetic retinopathy by hypertension. Curr Eye Res. 2007;32:533–541. doi: 10.1080/02713680701435391. [DOI] [PubMed] [Google Scholar]
- 175.Zeng XX, Ng YK, Ling EA. Neuronal and microglial response in the retina of streptozotocin-induced diabetic rats. Vis Neurosci. 2000;17:463–471. doi: 10.1017/s0952523800173122. [DOI] [PubMed] [Google Scholar]
- 176.Gaucher D, Chiappore JA, Pâques M, Simonutti M, Boitard C, et al. Microglial changes occur without neural cell death in diabetic retinopathy. Vision Res. 2007;47:612–623. doi: 10.1016/j.visres.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 177.Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, et al. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- 178.Kezic JM, Chen X, Rakoczy EP, McMenamin PG. The effects of age and Cx3cr1 deficiency on retinal microglia in the Ins2(Akita) diabetic mouse. Invest Ophthalmol Vis Sci. 2013;54:854–863. doi: 10.1167/iovs.12-10876. [DOI] [PubMed] [Google Scholar]
- 179.Ibrahim AS, El-Remessy AB, Matragoon S, Zhang W, Patel Y, et al. Retinal microglial activation and inflammation induced by amadori-glycated albumin in a rat model of diabetes. Diabetes. 2011;60:1122–1133. doi: 10.2337/db10-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Koistinaho M, Koistinaho J. Role of p38 and p44/42 mitogen-activated protein kinases in microglia. Glia. 2002;40:175–183. doi: 10.1002/glia.10151. [DOI] [PubMed] [Google Scholar]
- 181.Cao T, Thomas TC, Ziebell JM, Pauly JR, Lifshitz J. Morphological and genetic activation of microglia after diffuse traumatic brain injury in the rat. Neuroscience. 2012;225:65–75. doi: 10.1016/j.neuroscience.2012.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stence N, Waite M, Dailey ME. Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia. 2001;33:256–266. [PubMed] [Google Scholar]
- 183.Soulet D, Rivest S. Bone-marrow-derived microglia: myth or reality? Curr Opin Pharmacol. 2008;8:508–518. doi: 10.1016/j.coph.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 184.Hinze A, Stolzing A. Differentiation of mouse bone marrow derived stem cells toward microglia-like cells. BMC Cell Biol. 2011;12:35. doi: 10.1186/1471-2121-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Weller M, Esser P, Heimann K, Wiedemann P. Retinal microglia: a new cell in idiopathic proliferative vitreoretinopathy? Exp Eye Res. 1991;53:275–281. doi: 10.1016/0014-4835(91)90084-r. [DOI] [PubMed] [Google Scholar]
- 186.Zeng HY, Green WR, Tso MO. Microglial activation in human diabetic retinopathy. Arch Ophthalmol. 2008;126:227–232. doi: 10.1001/archophthalmol.2007.65. [DOI] [PubMed] [Google Scholar]
- 187.Devitt A, Marshall LJ. The innate immune system and the clearance of apoptotic cells. J Leukoc Biol. 2011;90:447–457. doi: 10.1189/jlb.0211095. [DOI] [PubMed] [Google Scholar]