Abstract
The structure and composition of the endothelial cell (EC) glycocalyx reflects a balance of the biosynthesis of glycans and their shear dependent removal. Shedding of glycans from the EC surface has been shown to occur in response to reactive oxygen species (ROS) and inflammatory mediators. Using sub-antimicrobial doses of doxycycline, a broad spectrum matrix metalloprotease (MMP) inhibitor, inhibition of chemoattractant induced glycan shedding has suggested that MMPs may be a major effector of the loss of glycans. However, it has also been reported that doxycycline is a scavenger of ROS that may also activate MMPs. To clarify the basis for doxycycline as an inhibitor of glycan shedding, the present studies were undertaken to determine its effect on ROS induced shedding in post-capillary venules of the exteriorized mesentery of the rat. To this end, hypoxanthine (HX) and xanthine oxidase (XO) were rapidly mixed on the mesenteric surface for a 2 min period to generate superoxide anion ( ) and the time course of glycan shedding was monitored in post-capillary venules over a 30 min period. Glycan shedding was quantitated by loss of adherent fluorescently labeled lectin coated microspheres (FLMs, 0.1 μm diameter) that were systemically infused. It was found that HX/XO caused FLM adhesion to decrease 45% within 30 min. This effect could be inhibited in a dose dependent manner by the addition of superoxide dismutase to the superfusion solution, thus confirming the role of . In contrast, 0.5 μM doxycycline had no effect on FLM shedding in response to HX/XO, contrary to its ability to attenuate shedding in response to the chemoattractant fMLP. Thus it is suggested that the efficacy of doxycycline as an inhibitor of glycan shedding during inflammation arises from its ability to inhibit MMP activation.
Keywords: Glycocalyx, Glycan Shedding, Lectin Binding, Reactive Oxygen Species, Matrix Metalloproteases
Introduction
It is well established that the endothelial surface layer (ESL) is composed of a matrix of proteoglycans synthesized by the endothelial cell (EC) and an adsorbed layer of proteins derived from the blood stream (Pries et al., 2000; Reitsma et al., 2007; Weinbaum et al., 2007). The role of the ESL in microvascular function has been studied in light of its multiple roles as: (1) a barrier to transvascular exchange of water and solutes (Adamson, 1990; Henry and Duling, 1999) and sieving of plasma-borne macromolecules (Huxley and Williams, 2000; van Haaren et al., 2003; Vink and Duling, 2000), (2) as a repository for bioactive agents such as antithrombin III, tissue factor pathway inhibitors, lipoprotein lipase, vascular endothelial growth factor, fibroblast growth factor, and extracellular superoxide dismutase (Reitsma et al., 2007), (3) a barrier to leukocyte-endothelium adhesion (Mulivor and Lipowsky, 2002), (4) shear stress sensor and regulator of mechanotransduction (Florian et al., 2003), and (5) a determinant of capillary hematocrit (Desjardins and Duling, 1990) and the resistance to flow (Lipowsky et al., 2011a; Pries et al., 1997). Studies of the composition of the endothelial glycocalyx have revealed proteoglycans with core proteins of the transmembrane syndecan family and lipid linked glypicans, both decorated with the glycosaminoglycans (GAGs) heparan sulfate, chondroitin sulfate and hyaluronan (Chappell et al., 2009b; Gotte, 2003; Pries et al., 2000; Reitsma et al., 2007; Weinbaum et al., 2007). It has been demonstrated that the composition of the microvascular glycocalyx reflects a balance between the continued biosynthesis of glycans and their shedding in response to ischemic and inflammatory stimuli (Mulivor and Lipowsky, 2009) and can be modified by shear dependent processes (Arisaka et al., 1995; Grimm et al., 1988). The endothelial glycocalyx has been shown to be shed in response to inflammation (Henry and Duling, 2000; Mulivor and Lipowsky, 2004), hyperglycemia (Zuurbier et al., 2005), endotoxemia and septic shock (Hofmann-Kiefer et al., 2009), presence of oxidized LDL (Constantinescu et al., 2001), TNFα (Chappell et al., 2009a), atrial natriuretic peptide (Bruegger et al., 2005), abnormal blood shear stress (Gouverneur et al., 2006; Haldenby et al., 1994), ischemia-reperfusion injury(Mulivor and Lipowsky, 2004), light induced production of free radicals (Vink and Duling, 1996) and during by-pass surgery (Rehm et al., 2007; Svennevig et al., 2008). Shedding of the glycocalyx in response to cytokines and chemoattractants has been found to occur in arterioles (Henry and Duling, 2000), capillaries (Constantinescu et al., 2001; Henry and Duling, 2000) and venules (Henry and Duling, 2000; Mulivor and Lipowsky, 2004).
The primary effector of the shedding of glycans from the EC surface has been suggested to be either a member of the family of matrix metalloproteases that cleaves core proteins from the EC surface (Mulivor and Lipowsky, 2009), or endoglycosidases such heparanase, produced by the EC (Vlodavsky et al., 2007) or parenchymal tissue. In the case of the later, it has been shown that in the human myocardium, resident mast cells are a major source of heparanase, in contrast to the vascular EC (Becker et al., 2010). Matrix metalloproteinases represent a family of over two dozen zinc dependent proteases that play a role in normal tissue remodeling during bone growth, wound healing, reproduction, cancer, inflammation and cardiovascular disease (Spinale, 2007). MMPs can be stored within vesicles in the endothelium in their active and proactive forms, and rapidly released under the appropriate stimulus (Taraboletti et al., 2002). It has been shown that MMP-7, has a high affinity for and binds to heparan sulfate (Yu and Woessner, Jr., 2000), and MMP-2, MMP-7 and MMP-9 are capable of directly cleaving chondroitin sulfate (Gronski, Jr. et al., 1997). In addition, MMP-1 was shown to cleave the heparan sulfate proteoglycan syndecan-1 (Endo et al., 2003). The putative role of MMPs in cleaving glycans from the EC surface is supported by studies of in situ microzymography to quantify MMP activation on the surface of post-capillary venules in response to the chemoattractant fMLP (Mulivor and Lipowsky, 2009). Hence, it is likely that cleavage of GAG bearing proteoglycans by either membrane bound or cytosolic MMPs in the endothelial cell may be responsible for shedding of the glycocalyx.
Doxycycline, a member of the tetracycline family of antibiotics, has been shown to be a broad spectrum inhibitor of MMPs, scavenge divalent cations and reactive oxygen species, indirectly inhibit serine proteinases, inhibit the secretion of inflammatory cytokines, and block nitric-oxide synthase (Golub et al., 1998). Inhibition of MMP activity with doxycycline in animal models of diabetes have suggested that it acts through mechanisms other than scavenging ROS (Yaras et al., 2008). It has also been suggested that the inhibitory activity of doxycycline on shedding results from its direct effect on MMP activation and not by its ability to chelate divalent cations, as evidenced by inhibition of MMP activation by the zinc chelator GM6001, and lack of inhibition by chelation of cations with EDTA (Lipowsky et al., 2011b).
Within this framework, the present study was undertaken to quantitate the extent to which doxycycline may inhibit shedding of the glycocalyx that is induced by reactive oxygen species. To that end, shedding of glycans from the endothelium in post-capillary venules of mesentery (rat) was induced by the topical application of a mixture of hypoxanthine and xanthine oxidase to generate superoxide anion, and the shedding of lectin laden microspheres from the EC surface was measured, with and without the presence of doxycycline. The generation of superoxide anion was confirmed by the addition of superoxide dismutase (SOD), which nullified the effect of HX/XO. To facilitate a comparison of FLM shedding with that incurred by application of the chemoattractant fMLP, separate studies were conducted to verify that SOD had no effect on fMLP induced shedding of FLMs.
Materials and Methods
Animal Preparation and Intravital Microscopy
All animal studies conformed to the Guiding Principles in the Care and Use of Animals established by the American Physiological Society and all protocols have been approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University.
Male Wistar rats, weighing 250–400g were anesthetized with Inactin (120 mg/kg, i.p), tracheostomized, and allowed to breathe under spontaneous respiration. The right jugular vein and its paired carotid artery were cannulated with polyethylene tubing (PE-50). Supplemental anesthetic was administered via the jugular catheter, as needed, to maintain a surgical plane of anesthesia. The carotid catheter was connected to a strain-gage pressure transducer to monitor central arterial pressure, which averaged 111.5 ± 7.7 SD mmHg, for 15 animals. Core temperature was monitored by a rectal probe and was maintained between 36–37 °C with the aid of a heating pad.
The intestinal mesentery was exteriorized through a midline abdominal incision and placed on a glass pedestal to permit viewing under brightfield microscopy by either trans- or incident-illumination. The tissue was superfused with HEPES-buffered Ringer’s solution (pH = 7.4) at a temperature of 37.0 °C. Fluorescence microscopy was performed under incident illumination (Hg arc lamp) using a dichroic mirror and filters appropriate for fluorescein excitation and emission spectra. Post-capillary venules were selected for microscopic observation under bright field or fluorescent epi-illumination, using a Zeiss 40×/0.75NA water immersion objective. Visual recordings of post-capillary venules were digitized with a PCO 1600 digital CCD camera (PCO Imaging, Germany) at a spatial resolution of 1600×1200 pixels with a depth of 14 bits for subsequent analysis. The effective magnification yielded a pixel spacing of 17.4 pixels/μm and a width of the video field of 92 μm. Transmitted light images were made with tungsten illumination.
Alterations in the structure and glycan content of the endothelial (EC) surface layer were elucidated by the binding of fluorescently labeled microspheres (FLMs) coated with the lectin Bandeiraea Simplificifolia (BS1; Sigma, St. Louis, MO), as detailed previously (Mulivor and Lipowsky, 2009). In brief, a bolus of coated FLMs was infused at 2 × 1012 spheres/ml/kg via the jugular vein to obtain a circulating concentration of 106 FLM/mm3 that was maintained by intravenous infusion of 2 × 1010 spheres/kg/min via the jugular vein. Images of fluorescently labeled microspheres (FLMs) adhered to the endothelium in a 100 μm length of venule were recorded while focusing above and below the microvessel diametral plane and the number of FLMs adhered were counted during off-line video analysis. Fluorescent (yellow-green) carboxylate-modified polystyrene microspheres, 0.1 μm in diameter (FLMs, Fluospheres; Molecular Probes, Eugene, OR), were labeled by covalent linkage (carbodiimide reaction) of the lectin which preferentially binds to galactose residues on the endothelial cell surface.
Previous studies have validated the assumption that the concentration of BS-1 coated FLMs is proportional to GAG concentration on the EC surface. Comparison of the shedding of FLMs and fluorescently labeled BS-1 lectin bound to the EC surface revealed similar trends in response to activation of the endothelium with fMLP, and in response to prolonged ischemia (Mulivor and Lipowsky, 2004). Reductions in fluorescently labeled BS-1 in response to heparinase, chondroitinase, hyaluronidase and fMLP were shown to be proportional to reductions in thickness of the glycocalyx (Gao and Lipowsky, 2010). The binding of BS-1 coated FLMs was shown to be proportional to the surface concentration of chondroitin sulfate that was covalently linked to glass slides, and was invariant with shear stresses over the physiological range (Lipowsky and Haynes, 2005).
Leukocyte-endothelium adhesion was monitored by capturing images of post-capillary venules with transmitted light using tungsten illumination. The number of WBCs adhering per 100 μm length of venule was acquired while focusing from top to bottom of each venule using a Zeiss water-immersion 40×/0.75 NA objective.
Hemodynamic Measurements
To obtain a measure of hemodynamic shear rates on the EC surface, red cell velocities and microvessel diameters were measured in postcapillary venules ranging in width from 10 to 50 μm using a Zeiss water-immersion 40×/0.75 NA objective under transillumination. The image of a microscopic field was switched to project onto a CCD camera (Panasonic, WV-51CCD) for an effective width of the video field of 100 μm. Red cell velocity (VRBC) along the centerline of post-capillary venules was measured with the two-slit photometric technique using a self-tracking correlator (IPM, San Diego). The mean velocity of blood (VMEAN) was calculated from the relationship VMEAN = VRBC/1.6 (Lipowsky and Zweifach, 1978). Vessel diameter (D) was measured by the video image shearing technique using an image shearing monitor (IPM, San Diego). Wall shear rates were estimated from the Newtonian-flow relationship for flow in a cylindrical tube, viz. γ̇ = 8VMEAN/D.
Experiment Protocols
Following exteriorization of the intestinal mesentery, the tissue was allowed to stabilize for a period of 20 min while fluorescently labeled microspheres (FLMs) were systemically infused and allowed to reach a steady state concentration. Baseline measurements of FLM adhesion count and red cell velocities were obtained. The Ringer’s irrigation superfusion solution was turned off and a mixture of hypoxanthine (HX, 1.8 mM, Sigma) and xanthine oxidase (XO, 0.05 U/ml, Sigma) were rapidly expelled from two separate 5 ml syringes, respectively, and mixed on the surface of the mesentery, similar to the techniques of Del Maestro et al. (Del Maestro et al., 1982). After two minutes, the tissue was flooded with Ringer’s solution to wash off the HX/XO mixture and the normal superfusate was resumed. To validate the ability to generate superoxide anion, separate experiments were conducted with the addition of superoxide dismutase (SOD, Sigma), prior to and following application of the HX/XO mixture, following the protocol of Vink and Duling (Vink and Duling, 1996). An initial bolus of 250 U/0.1 ml was given i.v. that was followed by a continuous infusion of 29U/ml (added to the FLM maintenance infusion, 1 ml/hr) with 50 U/ml added to the superfusate. To explore the effects of increased concentration of SOD, in separate experiments these concentrations (termed 1X herein) were doubled (2X). To test the efficacy of doxycycline, 0.5 μM was added to the Ringer’s superfusate prior to and following application of the HX/XO. Separate experiments were conducted to establish that SOD had no effect on the shedding of FLMs in response to superfusion of the mesentery with 10−7M fMLP (Sigma).
Statistics
Statistical analyses were performed using SigmaStat (Systat, Inc. San Jose, CA) with either Student’s t-test for paired measurements or the Holm-Sidak method for ANOVA of multiple comparisons. Non-parametric Kruskal-Wallis ANOVA on ranks was used when a test for normality failed. Analysis of the response to each treatment was performed on measurements normalized with respect to values obtained during the control period for each venule and based on the number of venules for each treatment, as indicated in Table 1.
Table 1.
Control values for hemodynamic and adhesion data.
| Treatment
| |||||
|---|---|---|---|---|---|
| Parameter | Ringer’s | HX/XO | HX/XO + Doxy | HX/XO + SOD 1X | HX/XO + SOD 2X |
| N | 12 | 12 | 13 | 13 | 9 |
| Dia (μm) | 32.0±8.0 | 26.7 ±7.0 | 33.4 ±7.4 | 30.9 ±6.2 | 30.9±7.7 |
| VRBC (mm/s) | 2.3±1.3 | 1.4 ±0.3 | 2.1 ±0.7 | 1.7 ±0.7 | 2.3±1.2 |
| γ̇ (sec−1) | 347±129 | 279 ±65 | 330 ±156 | 273 ±115 | 426±377 |
| FLM (#/100μm) | 10.8±2.6 | 13.0 ±5.5 | 10.1 ±6.0 | 9.3 ±3.0 | 11.6±3.3 |
| WBC (#/100um) | 1.7±1.9 | 1.5 ±1.7 | 1.5 ±1.5 | 1.8 ±1.9 | 1.2±1.4 |
Given above are the mean ± SD of data for N venules for superfusion of mesentery with each treatment. There were no statistically significant differences amongst the five treatments. Key: HX/XO, mixture of hypoxanthine and xanthine oxidase; Doxy, doxycycline; SOD, superoxide dismutase; Dia, venule diameter; VRBC, red cell velocity; γ̇, wall shear rate; FLM, number of adherent fluorescently labeled microspheres coated with lectin BS-1; WBC, leukocyte adhesion count.
Results
Values of hemodynamics, FLM and leukocyte (WBC) adhesion are presented in Table 1 for the control conditions prior to the five treatments examined: Ringer’s superfusate alone, Ringer’s following application of HX/XO, and Ringer’s following HX/XO with the addition of doxycycline or one of two concentrations of SOD. Measurements were obtained in 4–6 venules in each of 2–3 animals for each treatment, with a total of 15 animals that yielded 9–13 venules for each treatment, as indicated in Table 1. No significant differences were apparent during the control conditions prior to each treatment.
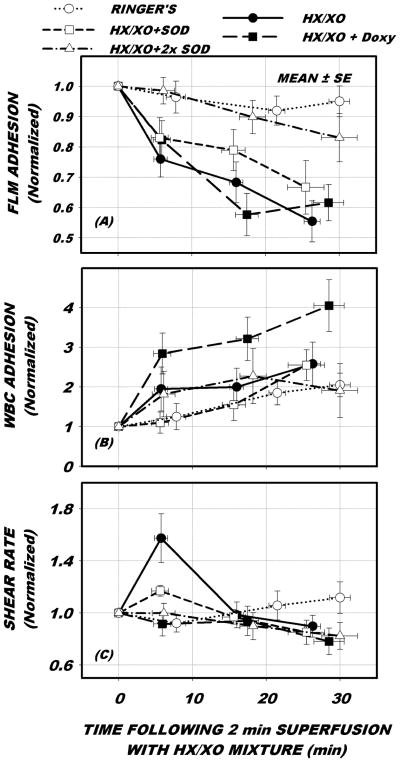
The effect of each treatment on the time duration of hemodynamics and adhesion events are presented in Fig. 1. All data are presented normalized with respect to control conditions acquired prior to application of the HX/XO mixture. With Ringer’s alone, no significant change in FLM (p=0.772) or WBC adhesion (p=0.053) occurred over the 30 min observation period. With the application of HX/XO, FLMs bound to the EC fell significantly (p<0.001) but WBC adhesion did not rise significantly, p=0.166. The addition of doxycycline had no effect on the shedding of FLMs, which fell significantly (p < 0.001), but WBC adhesion increased significantly, p < 0.001. The addition of SOD to HX/XO at the 1X dose lessened the fall in FLM adhesion but it still fell significantly, p<0.008. At 2X, SOD completely inhibited the fall in FLM adhesion, which did not fall significantly over the 30 min period, p=0.207. WBC adhesion rose significantly in response to HX/XO with 1X SOD, p<0.038, but did not rise significantly with 2X SOD, p=0.505. The addition of doxycycline caused a significant rise in WBC-EC, similar to previous studies, as noted in the Discussion.
Figure 1.
Venular time course of adhesion of lectin coated fluorescently labeled microspheres (FLMs) and leukocytes (WBC), and wall shear rate in response to baseline conditions (○, Ringers alone), following 2 min exposure to mixture of hypoxanthine and xanthine oxidase (•, HX/XO), HX/XO exposure preceded by and following the addition of 0.5 μM doxycycline (■, HX/XO + Doxy), and HX/XO preceded and followed by superfusion with super oxide dismutase (SOD) at 1X (□) and 2X (Δ) concentrations. All data were normalized with respect to control period conditions given in Table 1. As shown in panel A, FLM adhesion fell significantly 45% in 30 min following exposure to HX/XO and was not affected by the addition of Doxy. Introduction of SOD significantly mitigated FLM shedding, in a dose dependent manner. WBC-EC adhesion (panel B) did not rise significantly in response to HX/XO. With the addition of SOD, WBC-EC adhesion rose significantly with SOD at 1X concentration, but not with SOD at 2X. Doxycycline caused a significant rise in WBC-EC adhesion. Shear rates (panel C) remained mostly constant with all treatments, except for a transient rise in response to HX/XO within the first 5 min.
During the five treatments, wall shear rate remained largely invariant over the 30 min observation period with two exceptions. Under control (Ringer’s solution alone) shear rates remained invariant with time, p = 0.455. Following application of HX/XO shear rate rose significantly within 5 min and returned to normal over the 30 min period, p<0.017. Application of 1X SOD attenuated the initial rise in shear rate but it was still significant, p<0.010. The addition of SOD at 2X concentration completely abolished the shear rate response to HX/XO (p=0.456). With the addition of doxycycline, shear rates remained invariant throughout the entire observation period, p=0.564.
In separate experiments, the adhesion of FLMs was observed in response to topical superfusion of the mesentery with 10−7 fMLP during treatment with SOD. For 10 venules, FLM adhesion fell significantly by 31% to 0.69 ± 0.19 SD of control within 10 min exposure to fMLP, p<0.001. Leukocyte adhesion rose significantly two-fold from 1.0 ± 1.2 SD WBCs/100 μm to 2.2 ± 1.8 SD WBCs/100 μm, p=0.02.
Discussion
Disruption of the in vivo ESL by either experimental manipulations using enzymatic treatments (e.g. infusion of heparinase, chondroitinase and/or hyaluronidase) that target specific components of the glycocalyx has been extensively studied to elucidate the response to pathological disturbances such as inflammation and ischemia (Adamson, 1990; Desjardins and Duling, 1990; Mulivor and Lipowsky, 2002; Mulivor and Lipowsky, 2004; Pries et al., 1997; Vink and Duling, 1996; Williams, 2007). In models of the inflammatory process, topical stimulation of the endothelium for prolonged periods (20–120 min) with the cytokine TNF-α resulted in an increased porosity of the glycocalyx in the absence of WBC-EC adhesion (Henry and Duling, 2000). Significant shedding of components of the glycocalyx in coronary vessels has been observed following perfusion of isolated hearts for 20 min with TNF-α, which was lessened by the serine protease inhibitor antithrombin III (Chappell et al., 2009a). Acute activation of the endothelium in post-capillary venules with the chemoattractant fMLP has been found to induce a rapid (< 5 min) shedding of glycans from the EC surface as evidenced by a loss of lectin laden microspheres bound to the EC surface (Mulivor and Lipowsky, 2004). Shedding of proteoglycans and GAGs from cultured endothelial cells, or their analogs, occurs in response to a broad spectrum of agonists (Colburn et al., 1994; Fitzgerald et al., 2000; Fux et al., 2009; Ihrcke et al., 1993; Park et al., 2000; Platt et al., 1990; Platt et al., 1991). Shedding of heparan sulfate proteoglycans (namely the ectodomain of syndecans 1–4) occurs in response to endotoxin (Colburn et al., 1994), serine and/or cystein proteinases (Ihrcke and Platt, 1996), complement activation (Platt et al., 1991), thrombin and growth factors (Subramanian et al., 1997) and activation of protein tyrosine kinase by phorbol ester (Fitzgerald et al., 2000). Using hydroxamic acid inhibitors of matrix metalloproteinases, it has been shown that proteolytic cleavage of the syndecan ectodomain results from the convergence of multiple intracellular pathways that activate a cell surface metalloproteinase (Fitzgerald et al., 2000). In recent studies, it has been shown that doxycycline, a broad spectrum inhibitor of MMPs effectively inhibits MMP activation and shedding of the glycocalyx in response to fMLP (Mulivor and Lipowsky, 2009).
Previous studies of the shedding of glycans in response to fMLP have demonstrated that chelation of divalent cations with EDTA did not inhibit shedding, thus suggesting that the inhibition by doxycycline may arise from its ability to scavenge reactive oxygen species (Lipowsky et al., 2011b). To address this possibility, the present studies clearly reveal that doxycycline had no effect on shedding of glycans caused by superoxide anion (Fig. 1A).
In contrast, it has been shown that doxycycline could significantly inhibit the production of in a mixture of HX and XO by about 50% at a concentration of about 5 μM, and that it could inhibit generation by neutrophils by about 50% at a concentration of 10 μM (Akamatsu et al., 1992). Doxycycline did not inhibit collagenase activity induced by ROS (from sodium hypochlorite) in an osteogenic cell line at concentrations ranging from 6–25 μM (Ramamurthy et al., 1993). Examination of glycan shedding in mesenteric venules in response to fMLP revealed that doxycycline could inhibit WBC-EC adhesion at an EC50 of 0.15 μM and completely inhibited lectin (BS-1) shedding at 0.5 μM (Mulivor and Lipowsky, 2009). Thus, the levels of doxycycline herein were well below those of other studies aimed at elucidating its role in scavenging ROS. Hence, the present results are consistent with the conclusion that MMP inhibition as opposed to ROS scavenging may be the basis for its protective effect. Similar findings were noted with sodium hypochlorite activation of pro-collagenase in a model of diabetes-induced cardiomyopathy (Yaras et al., 2008), and peroxynitrite activation of MMPs in a model of endotoxin induced vascular hyporeactivity (Cena et al., 2010).
The present approach of generating by exposing the tissue to HX/XO mixture has relied on an established tool for elucidating the effects of ROS on microvascular dysfunction. Del Maestro et al. (Del Maestro et al., 1981a; Del Maestro et al., 1981b) employed its topical superfusion of tissue to examine changes in microvessel permeability, Parks et al (Parks et al., 1984) infused the mixture of HX/XO into the circulation to study the effects of oxygen radicals on intestinal vascular permeability, and Gaboury et al. (Gaboury et al., 1993) employed infusions to elucidate the effects of NO on WBC-EC adhesion. The magnitude of WBC-EC adhesion (Fig. 1B) and hemodynamics (shear rate, Fig. 1C) are similar to the trends observed by Del Maestro et al. (Del Maestro et al., 1982). The transient rise in red cell velocities and hence shear rates (Fig. 1C) at five min following application of HX/XO was consistent with the hyperemia noted therein. In contrast to their significant rise in WBC adhesion, the present results showed only a modest and insignificant rise in WBC-EC adhesion (Fig. 1B). This disparity may have arisen due to methodological differences, in that in the present study each venule was monitored periodically and the results normalized to control values within each microvessel. In contrast, Del Maestro et al. sampled fewer venules and compared pooled absolute data, thus possibly introducing effects of spatial heterogeneity within the network. Nonetheless, the maximum number of adherent WBCs was comparable. A substantially 10-fold greater amount of WBC adhesion was observed by Gaboury et al. (Gaboury et al., 1993) who infused a five-fold greater concentration of HX/XO for 30 min into the rat mesenteric microcirculation. In both cases, as herein, SOD served to completely negate the effects of the HX/XO treatment, thus signifying the specificity of generation of superoxide anion as the principal ROS in this model.
The significant rise in WBC adhesion found here with the addition of doxycycline during the HX/XO treatment was consistent with previous studies of the effects of doxycycline alone (Lipowsky et al., 2011b). In both studies, WBC adhesion rose about two-fold in response to 30 min superfusion with 0.5 μM doxycycline, presumably due to sustained inhibition of sheddase activity on the surface of the EC that normally maintains a lower level of ligands that promote rolling of WBCs on the EC. As shown in the previous study, an increased rolling flux of WBCs yielded a greater number of WBCs in rolling contact with the EC at any instant, thus leading to a greater probability of contact and adhesion. The apparent stickiness of the EC surface was also increased by doxycycline as evidenced by a significantly reduced ratio (compared with control) of WBC rolling velocity to γ̇. The additional WBC adhesion concomitant to superfusion with doxycycline prior to and following the HX/XO mixture appears to have increased venular hemodynamic resistance that in turn mitigated the sharp rise in shear rates immediately following the HX/XO (Fig. 1C).
While the present studies demonstrate the specific ability of superoxide anion, , to cause shedding of glycans from the EC surface, other species such as hydrogen peroxide ( H2O2 ) may play a role in shedding of glycans from the EC in response to various stimuli. For example, Vink and Duling found complete inhibition of degradation of the glycocalyx stimulated by UV illumination of the tissue by concurrent infusion and superfusion of a mixture of SOD and catalase (Vink and Duling, 1996), where the latter scavenges H2O2. In these studies, it was shown that degradation of the glycocalyx was manifest by an 80% reduction of its thickness (assessed by exclusion of 70 kDa dextran from the capillary EC membrane). The loss of glycans shown here by shedding of lectin coated microspheres was comparable to that incurred by fMLP induced shedding, where a 40% reduction of lectins bound to venular ECs was induced by superfusion of the tissue with 10−7M fMLP (Gao and Lipowsky, 2010). In these latter studies, it was shown that shedding corresponded to a 30% reduction in thickness of the glycocalyx, as measured by dextran exclusion. Thus, the shedding induced by the present HX/XO protocol are similar in magnitude to those induced by UV radiation and fMLP. However, whereas shedding in response to fMLP could be mitigated by inhibition of MMPs with doxycycline, that due to ROS appears to be caused by other mechanisms.
To verify that the shedding of glycans in response to fMLP were not caused by superoxide anion, it was established that FLM shedding and WBC adhesion responses to fMLP were not affected by the presence of SOD. Although it has been shown that SOD may mitigate the effects of ROS arising from fMLP stimulation of adherent WBCs (Zhu and He, 2006), it does not appear to affect the shedding of EC glycans and WBC adhesion induced by fMLP. This response is consistent with the hypothesis that fMLP induces shedding of EC glycans by stimulation of an endothelial G-protein coupled receptor (Mulivor and Lipowsky, 2004).
In summary, the present experiments suggest that doxycycline inhibits the shedding of glycans on the EC surface by mechanisms other than scavenging of reactive oxygen species. Although it has been shown that ROS may cleave either the core proteins or GAGs of proteoglycans (Raats et al., 1997), the loss of glycans shown here most likely was not a result of MMP activation. Further, while shedding of glycans from the EC surface of venules in response to fMLP is inhibited by doxycycline, the precise target of the MMPs remains uncertain. It is not clear if MMPs cleave GAG bearing core proteins (e.g. syndecans) or activate the endothelium to liberate endoglycosidases, such as heparanase, that cleave the GAG chains. There appears to be an indirect association between heparanase and MMP expression (Purushothaman et al., 2010; Zcharia et al., 2009). Heparanase induced shedding of syndecan-1 in myeloma cells may be inhibited by blocking activation of MMP-9 (Purushothaman et al., 2010), and over expression of heparanase in cultured human mammary carcinoma cells resulted in diminished expression of MMP-2, −9 and 14 (Zcharia et al., 2009). While it has been demonstrated that superoxide may activate MMPs, through multiple pathways (Yaras et al., 2008), the superoxide stimulated shedding of glycans found here is consistent with enhanced activation of heparanase in contrast to MMPs, as suggested previously for the growth and invasion of breast cancer cells (Teoh et al., 2009). Thus, the specificity of doxycycline as a tool for studying the effects of MMPs on the microvascular glycocalyx is supported and warrants further exploration into its effect on the cellular signaling mechanisms that lead to glycan shedding.
Highlights.
Explored reactive oxygen species vs metalloproteases in shedding of the glycocalyx
Reactive oxygen species (ROS) were generated on the surface of rat mesentery
Doxycycline, an MMP inhibitor, had no effect on ROS induced shedding
SOD inhibited shedding due to ROS but not fMLP
Thus doxycycline inhibits fMLP induced shedding by inhibition of MMPs and not ROS
Acknowledgments
This study was supported in part by NIH R01 HL39286-20.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamson RH. Permeability of frog mesenteric capillaries after partial pronase digestion of the endothelial glycocalyx. J Physiol. 1990;428:1–13. doi: 10.1113/jphysiol.1990.sp018197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akamatsu H, Asada M, Komura J, Asada Y, Niwa Y. Effect of doxycycline on the generation of reactive oxygen species: a possible mechanism of action of acne therapy with doxycycline. Acta Derm Venereol. 1992;72:178–179. [PubMed] [Google Scholar]
- Arisaka T, Mitsumata M, Kawasumi M, Tohjima T, Hirose S, Yoshida Y. Effects of shear stress on glycosaminoglycan synthesis in vascular endothelial cells. Ann N Y Acad Sci. 1995;748:543–554. doi: 10.1111/j.1749-6632.1994.tb17359.x. [DOI] [PubMed] [Google Scholar]
- Becker BF, Chappell D, Jacob M. Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic Res Cardiol. 2010;105:687–701. doi: 10.1007/s00395-010-0118-z. [DOI] [PubMed] [Google Scholar]
- Bruegger D, Jacob M, Rehm M, Loetsch M, Welsch U, Conzen P, Becker BF. Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of guinea pig hearts. Am J Physiol Heart Circ Physiol. 2005;289:H1993–H1999. doi: 10.1152/ajpheart.00218.2005. [DOI] [PubMed] [Google Scholar]
- Cena JJ, Lalu MM, Cho WJ, Chow AK, Bagdan ML, Daniel EE, Castro MM, Schulz R. Inhibition of matrix metalloproteinase activity in vivo protects against vascular hyporeactivity in endotoxemia. Am J Physiol Heart Circ Physiol. 2010;298:H45–H51. doi: 10.1152/ajpheart.00273.2009. [DOI] [PubMed] [Google Scholar]
- Chappell D, Hofmann-Kiefer K, Jacob M, Rehm M, Briegel J, Welsch U, Conzen P, Becker BF. TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res Cardiol. 2009a;104:78–89. doi: 10.1007/s00395-008-0749-5. [DOI] [PubMed] [Google Scholar]
- Chappell D, Jacob M, Paul O, Rehm M, Welsch U, Stoeckelhuber M, Conzen P, Becker BF. The glycocalyx of the human umbilical vein endothelial cell: an impressive structure ex vivo but not in culture. Circ Res. 2009b;104:1313–1317. doi: 10.1161/CIRCRESAHA.108.187831. [DOI] [PubMed] [Google Scholar]
- Colburn P, Kobayashi E, Buonassisi V. Depleted level of heparan sulfate proteoglycan in the extracellular matrix of endothelial cell cultures exposed to endotoxin. J Cell Physiol. 1994;159:121–130. doi: 10.1002/jcp.1041590116. [DOI] [PubMed] [Google Scholar]
- Constantinescu AA, Vink H, Spaan JA. Elevated capillary tube hematocrit reflects degradation of endothelial cell glycocalyx by oxidized LDL. Am J Physiol Heart Circ Physiol. 2001;280:H1051–H1057. doi: 10.1152/ajpheart.2001.280.3.H1051. [DOI] [PubMed] [Google Scholar]
- Del Maestro RF, Bjork J, Arfors KE. Increase in microvascular permeability induced by enzymatically generated free radicals. I. In vivo study. Microvasc Res. 1981a;22:239–254. doi: 10.1016/0026-2862(81)90095-9. [DOI] [PubMed] [Google Scholar]
- Del Maestro RF, Bjork J, Arfors KE. Increase in microvascular permeability induced by enzymatically generated free radicals. II. Role of superoxide anion radical, hydrogen peroxide, and hydroxyl radical. Microvasc Res. 1981b;22:255–270. doi: 10.1016/0026-2862(81)90096-0. [DOI] [PubMed] [Google Scholar]
- Del Maestro RF, Planker M, Arfors KE. Evidence for the participation of superoxide anion radical in altering the adhesive interaction between granulocytes and endothelium, in vivo. Int J Microcirc Clin Exp. 1982;1:105–120. [PubMed] [Google Scholar]
- Desjardins C, Duling BR. Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. Am J Physiol. 1990;258:H647–H654. doi: 10.1152/ajpheart.1990.258.3.H647. [DOI] [PubMed] [Google Scholar]
- Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M, Sato H. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J Biol Chem. 2003;278:40764–70. doi: 10.1074/jbc.M306736200. [DOI] [PubMed] [Google Scholar]
- Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M. Shedding of syndecan-1 and −4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J Cell Biol. 2000;148:811–824. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93:e136–e142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- Fux L, Ilan N, Sanderson RD, Vlodavsky I. Heparanase: busy at the cell surface. Trends Biochem Sci. 2009;34:511–519. doi: 10.1016/j.tibs.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaboury J, Woodman RC, Granger DN, Reinhardt P, Kubes P. Nitric oxide prevents leukocyte adherence: role of superoxide. Am J Physiol. 1993;265:H862–H867. doi: 10.1152/ajpheart.1993.265.3.H862. [DOI] [PubMed] [Google Scholar]
- Gao L, Lipowsky HH. Composition of the endothelial glycocalyx and its relation to its thickness and diffusion of small solutes. Microvasc Res. 2010;80:394–401. doi: 10.1016/j.mvr.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- Gotte M. Syndecans in inflammation. FASEB J. 2003;17:575–591. doi: 10.1096/fj.02-0739rev. [DOI] [PubMed] [Google Scholar]
- Gouverneur M, Spaan JA, Pannekoek H, Fontijn RD, Vink H. Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2006;290:H458–2. doi: 10.1152/ajpheart.00592.2005. [DOI] [PubMed] [Google Scholar]
- Grimm J, Keller R, de Groot PG. Laminar flow induces cell polarity and leads to rearrangement of proteoglycan metabolism in endothelial cells. Thromb Haemost. 1988;60:437–441. [PubMed] [Google Scholar]
- Gronski TJ, Jr, Martin RL, Kobayashi DK, Walsh BC, Holman MC, Huber M, Van Wart HE, Shapiro SD. Hydrolysis of a broad spectrum of extracellular matrix proteins by human macrophage elastase. J Biol Chem. 1997;272:12189–94. doi: 10.1074/jbc.272.18.12189. [DOI] [PubMed] [Google Scholar]
- Haldenby KA, Chappell DC, Winlove CP, Parker KH, Firth JA. Focal and regional variations in the composition of the glycocalyx of large vessel endothelium. J Vasc Res. 1994;31:2–9. doi: 10.1159/000159025. [DOI] [PubMed] [Google Scholar]
- Henry CB, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol. 1999;277:H508–H514. doi: 10.1152/ajpheart.1999.277.2.H508. [DOI] [PubMed] [Google Scholar]
- Henry CB, Duling BR. TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2000;279:H2815–H2823. doi: 10.1152/ajpheart.2000.279.6.H2815. [DOI] [PubMed] [Google Scholar]
- Hofmann-Kiefer KF, Kemming GI, Chappell D, Flondor M, Kisch-Wedel H, Hauser A, Pallivathukal S, Conzen P, Rehm M. Serum Heparan Sulfate Levels are Elevated in Endotoxemia. Eur J Med Res. 2009;14:526–531. doi: 10.1186/2047-783X-14-12-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley VH, Williams DA. Role of a glycocalyx on coronary arteriole permeability to proteins: evidence from enzyme treatments. Am J Physiol Heart Circ Physiol. 2000;278:H1177–H1185. doi: 10.1152/ajpheart.2000.278.4.H1177. [DOI] [PubMed] [Google Scholar]
- Ihrcke NS, Platt JL. Shedding of heparan sulfate proteoglycan by stimulated endothelial cells: evidence for proteolysis of cell-surface molecules. J Cell Physiol. 1996;168:625–637. doi: 10.1002/(SICI)1097-4652(199609)168:3<625::AID-JCP15>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Ihrcke NS, Wrenshall LE, Lindman BJ, Platt JL. Role of heparan sulfate in immune system-blood vessel interactions. Immunol Today. 1993;14:500–505. doi: 10.1016/0167-5699(93)90265-M. [DOI] [PubMed] [Google Scholar]
- Lipowsky HH, Gao L, Lescanic A. Shedding of the Endothelial Glycocalyx in Arterioles, Capillaries and Venules and its Effect on Capillary Hemodynamics During Inflammation. Am J Physiol Heart Circ Physiol. 2011a;301:H2235–H2245. doi: 10.1152/ajpheart.00803.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowsky HH, Haynes CA. Synthesis of an artificial glycocalyx for studies of leukocyte adhesion. Proceedings of the 2005 Summer Bioengineering Conference; Vail, CO. June 22–26; 2005. p. abstract 247851. [Google Scholar]
- Lipowsky HH, Sah R, Lescanic A. Relative roles of doxycycline and cation chelation in endothelial glycan shedding and adhesion of leukocytes. Am J Physiol Heart Circ Physiol. 2011b;300:H415–H422. doi: 10.1152/ajpheart.00923.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowsky HH, Zweifach BW. Application of the “two-slit” photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res. 1978;15:93–101. doi: 10.1016/0026-2862(78)90009-2. [DOI] [PubMed] [Google Scholar]
- Mulivor AW, Lipowsky HH. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol. 2002;283:H1282–H1291. doi: 10.1152/ajpheart.00117.2002. [DOI] [PubMed] [Google Scholar]
- Mulivor AW, Lipowsky HH. Inflammation- and ischemia-induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol. 2004;286:H1672–H1680. doi: 10.1152/ajpheart.00832.2003. [DOI] [PubMed] [Google Scholar]
- Mulivor AW, Lipowsky HH. Inhibition of Glycan Shedding and Leukocyte-Endothelial Adhesion in Postcapillary Venules by Suppression of Matrixmetalloprotease Activity with Doxycycline. Microcirculation. 2009;16:657–666. doi: 10.3109/10739680903133714. [DOI] [PubMed] [Google Scholar]
- Park PW, Reizes O, Bernfield M. Cell surface heparan sulfate proteoglycans: selective regulators of ligand-receptor encounters. J Biol Chem. 2000;275:29923–29926. doi: 10.1074/jbc.R000008200. [DOI] [PubMed] [Google Scholar]
- Parks DA, Shah AK, Granger DN. Oxygen radicals: effects on intestinal vascular permeability. Am J Physiol. 1984;247:G167–G170. doi: 10.1152/ajpgi.1984.247.2.G167. [DOI] [PubMed] [Google Scholar]
- Platt JL, Dalmasso AP, Lindman BJ, Ihrcke NS, Bach FH. The role of C5a and antibody in the release of heparan sulfate from endothelial cells. Eur J Immunol. 1991;21:2887–2890. doi: 10.1002/eji.1830211135. [DOI] [PubMed] [Google Scholar]
- Platt JL, Vercellotti GM, Lindman BJ, Oegema TR, Jr, Bach FH, Dalmasso AP. Release of heparan sulfate from endothelial cells. Implications for pathogenesis of hyperacute rejection. J Exp Med. 1990;171:1363–1368. doi: 10.1084/jem.171.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440:653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- Pries AR, Secomb TW, Jacobs H, Sperandio M, Osterloh K, Gaehtgens P. Microvascular blood flow resistance: role of endothelial surface layer. Am J Physiol. 1997;273:H2272–H2279. doi: 10.1152/ajpheart.1997.273.5.H2272. [DOI] [PubMed] [Google Scholar]
- Purushothaman A, Uyama T, Kobayashi F, Yamada S, Sugahara K, Rapraeger AC, Sanderson RD. Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood. 2010;115:2449–2457. doi: 10.1182/blood-2009-07-234757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raats CJ, Bakker MA, van den Born J, Berden JH. Hydroxyl radicals depolymerize glomerular heparan sulfate in vitro and in experimental nephrotic syndrome. J Biol Chem. 1997;272:26734–26741. doi: 10.1074/jbc.272.42.26734. [DOI] [PubMed] [Google Scholar]
- Ramamurthy NS, Vernillo AT, Greenwald RA, Lee HM, Sorsa T, Golub LM, Rifkin BR. Reactive oxygen species activate and tetracyclines inhibit rat osteoblast collagenase. J Bone Miner Res. 1993;8:1247–1253. doi: 10.1002/jbmr.5650081013. [DOI] [PubMed] [Google Scholar]
- Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Reichart B, Peter K, Becker BF. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–1906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- Subramanian SV, Fitzgerald ML, Bernfield M. Regulated shedding of syndecan-1 and −4 ectodomains by thrombin and growth factor receptor activation. J Biol Chem. 1997;272:14713–14720. doi: 10.1074/jbc.272.23.14713. [DOI] [PubMed] [Google Scholar]
- Svennevig K, Hoel T, Thiara A, Kolset S, Castelheim A, Mollnes T, Brosstad F, Fosse E, Svennevig J. Syndecan-1 plasma levels during coronary artery bypass surgery with and without cardiopulmonary bypass. Perfusion. 2008;23:165–171. doi: 10.1177/0267659108098215. [DOI] [PubMed] [Google Scholar]
- Taraboletti G, D’Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol. 2002;160:673–80. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh ML, Fitzgerald MP, Oberley LW, Domann FE. Overexpression of extracellular superoxide dismutase attenuates heparanase expression and inhibits breast carcinoma cell growth and invasion. Cancer Res. 2009;69:6355–6363. doi: 10.1158/0008-5472.CAN-09-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haaren PM, VanBavel E, Vink H, Spaan JA. Localization of the permeability barrier to solutes in isolated arteries by confocal microscopy. Am J Physiol Heart Circ Physiol. 2003;285:H2848–H2856. doi: 10.1152/ajpheart.00117.2003. [DOI] [PubMed] [Google Scholar]
- Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res. 1996;79:581–589. doi: 10.1161/01.res.79.3.581. [DOI] [PubMed] [Google Scholar]
- Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol. 2000;278:H285–H289. doi: 10.1152/ajpheart.2000.278.1.H285. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I, Ilan N, Naggi A, Casu B. Heparanase: structure, biological functions, and inhibition by heparin-derived mimetics of heparan sulfate. Curr Pharm Des. 2007;13:2057–2073. doi: 10.2174/138161207781039742. [DOI] [PubMed] [Google Scholar]
- Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- Williams DA. Change in shear stress (Deltatau)/hydraulic conductivity (Lp) relationship after pronase treatment of individual capillaries in situ. Microvasc Res. 2007;73:48–57. doi: 10.1016/j.mvr.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaras N, Sariahmetoglu M, Bilginoglu A, Aydemir-Koksoy A, Onay-Besikci A, Turan B, Schulz R. Protective action of doxycycline against diabetic cardiomyopathy in rats. Br J Pharmacol. 2008;155:1174–1184. doi: 10.1038/bjp.2008.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WH, Woessner JF., Jr Heparan sulfate proteoglycans as extracellular docking molecules for matrilysin (matrix metalloproteinase 7) J Biol Chem. 2000;275:4183–91. doi: 10.1074/jbc.275.6.4183. [DOI] [PubMed] [Google Scholar]
- Zcharia E, Jia J, Zhang X, Baraz L, Lindahl U, Peretz T, Vlodavsky I, Li JP. Newly generated heparanase knock-out mice unravel co-regulation of heparanase and matrix metalloproteinases. PLoS One. 2009;4:e5181. doi: 10.1371/journal.pone.0005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, He P. fMLP-stimulated release of reactive oxygen species from adherent leukocytes increases microvessel permeability. Am J Physiol Heart Circ Physiol. 2006;290:H365–H372. doi: 10.1152/ajpheart.00812.2005. [DOI] [PubMed] [Google Scholar]
- Zuurbier CJ, Demirci C, Koeman A, Vink H, Ince C. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. J Appl Physiol. 2005;99:1471–1476. doi: 10.1152/japplphysiol.00436.2005. [DOI] [PubMed] [Google Scholar]