Abstract
Mitochondrial calcium has been postulated to regulate a wide range of processes from bioenergetics to cell death. Here, we characterize a mouse model that lacks expression of the recently discovered mitochondrial calcium uniporter (MCU). Mitochondria derived from MCU-/- mice have no apparent capacity to rapidly uptake calcium. While basal metabolism appears unaffected, the skeletal muscle of MCU-/- mice exhibited alterations in the phosphorylation and activity of pyruvate dehydrogenase. In addition, MCU-/- mice exhibited marked impairment in their ability to perform strenuous work. We further show that mitochondria from MCU-/- mice lacked evidence for calcium-induced permeability transition pore (PTP) opening. The lack of PTP opening does not appear to protect MCU-/- cells and tissues from cell death, although MCU-/- hearts fail to respond to the PTP inhibitor cyclosporin A (CsA). Taken together, these results clarify how acute alterations in mitochondrial matrix calcium can regulate mammalian physiology.
Calcium plays a central role in a diverse array of cellular processes including signal transduction, secretion of bioactive molecules, muscle contraction and gene expression. Over fifty years ago, it was demonstrated that fully energized mitochondria could rapidly sequester a large, sudden increase in intracellular calcium1,2. Calcium entry into this organelle requires that the ion traverses both the outer and inner mitochondrial membrane (IMM). Subsequent studies have demonstrated that passage of calcium through the ion-impermeable IMM requires the large membrane potential difference generated by the action of the electron transport chain3. Subsequent physiological and biophysical studies identified that large amounts of calcium could rapidly enter the mitochondrial matrix through this transport mechanism4,5. These observations, along with observations that entry of calcium was not directly coupled to the movement of another ion6, established that mitochondrial calcium uptake occurred through a specific channel termed the mitochondrial calcium uniporter (MCU), that could bind calcium with nanomolar affinity7. While it was well known that the entry of calcium could be inhibited by the cell-impermeant compound ruthenium red8, for nearly four decades the identification of this ruthenium red sensitive mitochondrial uniporter remained elusive. That situation changed when two groups recently reported the existence of a transmembrane protein CCDC109A that appeared to fulfill the requirement of the long elusive MCU protein9,10. These groups identified that MCU is a protein of approximately 40-kDa that is widely expressed and localizes, as expected, to the IMM9,10.
Although the molecular identity of MCU was unknown until recently, the role of mitochondrial calcium has been intensively studied over the last four decades. These studies have collectively demonstrated that mitochondrial calcium acutely regulates a range of mitochondrial enzymes involved in either the supply of reducing equivalents 11, metabolic substrates 12 or electron transport13. Together, these observations supported the notion that MCU-dependent entry of calcium represented a central component of metabolic regulation. Indeed, it had been known that cells and tissues appear capable of exquisitely matching the rate of ATP production with ATP utilization such that even with large fluctuations in power output, levels of metabolic intermediates such as ATP, ADP and Pi appear unchanged14,15. This has been extensively studied in tissues such as the heart or skeletal muscle that see large and acute changes in their energy utilization when, for instance, the organism goes from a resting state to a full speed sprint. Under these conditions, it has been widely believed that the entry of mitochondrial calcium augments mitochondrial ATP production to acutely match the rapid increase in ATP demand11,16-18.
While the entry of small amounts of calcium may have beneficial effects for metabolic homeostasis, there is a significant amount of data demonstrating that the uptake of large amounts of Ca2+ can induce cell death 19,20. The basis for this phenomenon involves opening of the permeability transition pore (PTP). While the precise molecular makeup of the PTP has remained elusive, evidence suggest that the entry of calcium through an MCU-dependent mechanism is the central mediator of PTP opening 21-23. Once opened, the PTP results in depolarization of the IMM leading to collapse of the mitochondrial membrane potential and thus inhibition of electron transport and mitochondrial-dependent ATP production. This has led to the widespread belief that targeting this pathway, including the development of potential inhibitors of MCU, might be a robust strategy to block injury that occurs in a wide array of clinically important disease processes from ischemia to neurodegeneration 19,24.
Taken together, there is an long and extensive literature suggesting that dynamic alterations in mitochondrial calcium plays a central role in a wide range of physiological conditions from acute metabolic regulation to determining the threshold for cell death. Nonetheless, the vast majority of these studies have been hampered by the inability to directly alter in vivo mitochondria calcium uptake. While ruthenium red and some of its derivatives readily block MCU function, these agents cannot reliably enter cells, thus limiting this as an approach to dynamically regulate calcium uptake in intact tissues 25. However, the recent identification of MCU provides a potential genetic approach to directly test many of these concepts. Here, we have generated and characterized mice lacking MCU expression to gain further insight into the role of mitochondrial calcium in mammalian physiology.
Results
MCU regulates calcium uptake
To determine the physiological functions of MCU, we constructed a mouse model using a gene trap strategy in which a retroviral trapping vector was integrated into the first intron of the CCDC109A locus (Supplemental Fig. S1a,b). While slightly smaller (Fig. 1a,b), organ weight was proportional to body size and overall body composition was indistinguishable between young and old WT and MCU-/- mice (Supplemental Fig. S1c,d). Further examination of MCU-/- embryonic and adult tissues by electron microscopy revealed no obvious abnormalities in mitochondrial number or morphology (Supplemental Fig. S2a). Although mice generated using a gene trap strategy sometimes leads to hypomorphic expression of the targeted gene, there was little evidence of MCU mRNA expression (Fig. 1c). Similarly, Western blot analysis using antibodies recognizing either N-terminal or C-terminal regions of the protein failed to detect any MCU protein expression in tissues derived from MCU-/- mice (Fig. 1d and Supplemental Fig. S2b,c).
Figure 1.
MCU-/- mice lack MCU expression and evidence for rapid mitochondrial calcium uptake. a) Besides their size, MCU-/- mice (on right) lack a discernible phenotype. b) MCU-/- mice are smaller than WT mice (mean +/- S.E.M., p<0.01 by ANOVA; n=14 female WT and n=13 female MCU-/- mice). c) MCU mRNA expression by RT-PCR analysis in various tissues of WT, heterozygous and MCU-/- mice (n=3 animals per genotype, mean +/- S.E.M., *p<0.05, **p<0.01 by ANOVA compared to WT expression). d) MCU protein expression in various tissues using a rabbit polyclonal antibody generated against the C-terminus of MCU. Tubulin is used as a loading control. e) Assessment of mitochondrial calcium levels using the fluorescent mitochondrial calcium sensor Fluo-4FF. Calcium addition over the physiological (micromolar) range results in increasing calcium levels in mitochondria isolated from WT skeletal muscle. This uptake in WT mitochondria is inhibited by Ru360 addition. MCU-/- mitochondria lack any demonstrable uptake. Shown is one experiment that is representative of three similar experiments. Inset-Western blot analysis of MCU expression in purified WT and MCU-/- mitochondria with cytochrome C oxidase subunit IV (isoform 1) used as a loading control. f) Similar experiment performed using cardiac mitochondria. At higher Ca2+ concentrations, there is a small, non Ru360-inhibitable, increase in Fluo-4FF fluorescence in MCU-/- mitochondria observed. g) Parallel assessment of extramitochondrial calcium measurements demonstrating that only WT cardiac mitochondria appear capable of calcium uptake.
We next sought to assess whether the absence of MCU expression altered mitochondrial calcium uptake. When purified mitochondria isolated from skeletal muscle of adult WT and MCU-/- mice were loaded with the calcium sensitive dye Fluo-4FF, wild type mitochondria exhibited robust calcium uptake over a range of physiological relevant extramitochondrial calcium concentrations (Fig. 1e). This uptake was inhibited in wild type mitochondria by the addition of the potent ruthenium red subcomponent, Ru360. However, MCU-/- skeletal muscle mitochondria, that lacked detectable MCU expression (Fig. 1e inset), exhibited no appreciable calcium uptake over a range of physiological calcium concentrations. Additional experiments performed with heart mitochondria revealed similar findings, although with cumulative additions of micromolar concentrations of calcium, we did note that MCU-/- cardiac mitochondria marginally increased their Fluo-4FF fluorescence (Fig. 1f), although this appeared to represent non-specific leakage of dye out of the mitochondrial matrix as indicated by rapid quenching of the fluorescent signal with EGTA (Supplemental Fig. S3a,b). We performed parallel experiments using Calcium Green-5N to measure extramitochondrial calcium levels (Fig. 1g). In these experiments, calcium addition to WT cardiac mitochondria resulted in a rapid increase in Calcium Green-5N fluorescence followed by a slower decline in the fluorescence intensity of the calcium sensor (Fig. 1g). This slower decrease in fluorescence after calcium addition is consistent with the known buffering capacity of mitochondria to uptake cytosolic calcium. At higher concentrations of calcium (≥15 μM), buffering capacity is eventually exceeded and stored mitochondrial calcium is released into the extramitochondrial space. Over the same range of calcium concentration, MCU-/- mitochondria did not demonstrate evidence for either mitochondrial uptake or release.
To further analyze the function of MCU within intact cells, we generated primary mouse embryonic fibroblasts (MEFs) from wild type and MCU-/- embryos. Functional assessment revealed that when calcium was added to permeabilized MEFs, there was an initial rapid spike in Calcium Green-5N fluorescence intensity (Fig. 2a), which was followed by a slower decline in the fluorescence intensity of the cytosolic calcium sensor. As described above, this decline in fluorescence corresponds to mitochondrial calcium uptake. In permeablized wild type MEFs, in the presence of ER calcium uptake inhibition, mitochondrial uptake continued until the calcium retention capacity of the mitochondria was exceeded. At this point (e.g. the fourth addition of calcium in Fig. 2a), any further increase in extramitochondrial calcium resulted in a large rise in measured fluorescence. This terminal, large fluorescent increase indicates the irreversible release of mitochondria calcium stores and is commonly used as an index of PTP opening (similar to Fig. 1g). In contrast, calcium addition to MCU-/- MEFs resulted in a staircase profile consistent with the inability of MCU-/- mitochondria to rapidly uptake calcium (Fig. 2a). The profile seen in MCU-/- MEFs was similar to what is observed when wild type MEFs were treated with ruthenium red (Fig. 2b). Retroviral-mediated reconstitution of wild type MCU expression restored mitochondrial calcium uptake in MCU-/- MEFs (Fig. 2c,d). In contrast, a mutant of MCU where the critical acidic linker region was mutated at amino acid 261 and 264 was unable to reconstitute calcium uptake when expressed in MCU-/- cells.
Figure 2.
MCU regulates mitochondrial calcium uptake in permeabilized MEFs. a) Comparison of cytosolic calcium levels in permeabilized WT and MCU-/- MEFs. Arrows indicate calcium addition. Increasing cytosolic calcium results in a rapid increase in the fluorescent signal in both cell types but the subsequent decline in the fluorescent signal, representing mitochondrial calcium uptake, is only observed in WT cells. Shown is one tracing that is representative of three similar experiments. b) Ruthenium red (Ru360; 3 μM) inhibits mitochondrial calcium uptake in permeabilized WT MEFs. c) Western blot analysis of MCU expression. MCU-/- MEFs were infected with retroviruses encoding an epitope-tagged form of either WT MCU or MCUmut containing two amino acid substitutions (at amino acid 261 and 264) known to abrogate uniporter activity. The tagged constructs migrate slightly slower than endogenous MCU but were expressed at roughly endogenous levels. d) Cytosolic calcium measurements in permeabilized WT MEFs, MCU-/- MEFs, MCU-/- MEFs reconstituted with wild type MCU and MCU-/- MEFs reconstituted with MCUmut.
To assess mitochondrial calcium uptake in a more physiological context, we isolated adult cardiac myocytes from WT and MCU-/- mice. Consistent with previous results26, in WT myocytes loaded with the mitochondrial-targeted calcium sensitive dye Rhod-2 in the presence of manganese added to quench any cytosolic signal, isoproterenol addition stimulated a measurable rise in mitochondrial calcium levels (Fig. 3a). This response was absent in cardiac myocytes obtained from MCU-/- mice. Mitochondrial calcium levels were also unchanged in MCU-/- myocytes treated with agents (KCl and caffeine) that have been shown to directly increase cytosolic calcium (Supplemental Fig. S3c,d). Similarly, analysis of MEFs engineered to express a mitochondrial-targeted aequorin construct and then stimulated with histamine revealed that ligand-stimulated mitochondrial calcium fluxes were markedly diminished in MCU-/- cells (Fig. 3b). In contrast, histamine-induced cytosolic calcium changes were similar (Fig. 3c). Thus, as we observed with isolated mitochondria, cells derived from MCU-/- mice appear unable to rapidly uptake calcium.
Figure 3.
MCU regulates ligand-stimulated mitochondrial calcium uptake. a) Isoproterenol stimulates an increase in mitochondrial calcium in WT cardiac myocytes. Adult WT and MCU-/- adult cardiac myocytes were freshly isolated, loaded with mitochondrial calcium sensitive probe Rhod-2 and stimulated with 1 μM isoproterenol at time zero (n=10 random fields per genotype). b) WT or MCU-/- MEFs were infected with an adenovirus encoding a mitochondrial targeted aequorin construct. Levels of mitochondrial calcium were assessed by aequorin luminescence following histamine stimulation (100 μM) for WT (n=10) and MCU-/- MEFs (n=12). Values were normalized to maximal aequorin luminescence observed in permeabilzed cells exposed to exogenous calcium. C) Cytosolic calcium levels in WT (n=14) or MCU-/- MEFs (n=17) as measured by Fluro-4 fluorescence following histamine stimulation. All pooled data represents mean +/- S.E.M.
MCU and metabolism
We next sought to ascertain the role of MCU in basal metabolism. Somewhat surprisingly, when we analyzed oxygen consumption of MCU-/- MEFs under basal conditions, we noted no detectable difference from WT MEFs using a variety of potential metabolic substrates (Fig. 4a and Supplemental Fig. S4a). This lack of a discernible difference may reflect the low basal energetic requirement for MEFs in culture, although treatment with mitochondrial membrane depolarizing agents such as FCCP, suggests WT and MCU-/- MEFs had a similar maximal oxidative capacity. To further pursue the absence or presence of a basal metabolic phenotype, we isolated mitochondria from wild type and MCU-/- mice. When purified mitochondria were given glutamate and malate as a substrate, we noted that MCU-/- and wild type mitochondria exhibited similar levels of respiration (Fig. 4b). Similarly, the response to ADP and the respiratory control ratio (State 3/State 4) were indistinguishable (Fig. 4b,c). Furthermore, metabolic assessment of total body basal oxygen consumption was not altered in MCU-/- mice (Fig. 4d). Finally, in contrast to what has been observed in cell culture models using primarily immortalized and transformed cells27, we found no clear evidence of increased levels of autophagy in primary cells or tissues lacking MCU expression (Supplemental Fig. S4b,c). Taken together, these results demonstrate that basal metabolism is not markedly altered in the absence of MCU expression.
Figure 4.
The role of MCU in basal metabolism. a) Seahorse X-24 analysis of oxygen consumption rate (OCR) in WT and MCU-/- MEFs under basal conditions or following the addition of oligomycin, the uncoupler FCCP or the electron transport inhibitor antimycin A (n=5). b) Representative tracings of the oxygen consumption observed in isolated hepatic mitochondria given glutamate/malate as a substrate (G/M) followed by ADP (State 3). c) Quantification of the respiratory control ratio (RCR; State 3/State 4 respiration) in WT and MCU-/- mitochondria (p=NS; n=3 independent experiments). d) Average total body oxygen consumption in WT and MCU-/- mice during day and night conditions (n=5 WT and n=6 MCU-/- mice; p=NS between genotypes). All pooled data represents mean +/- S.E.M.
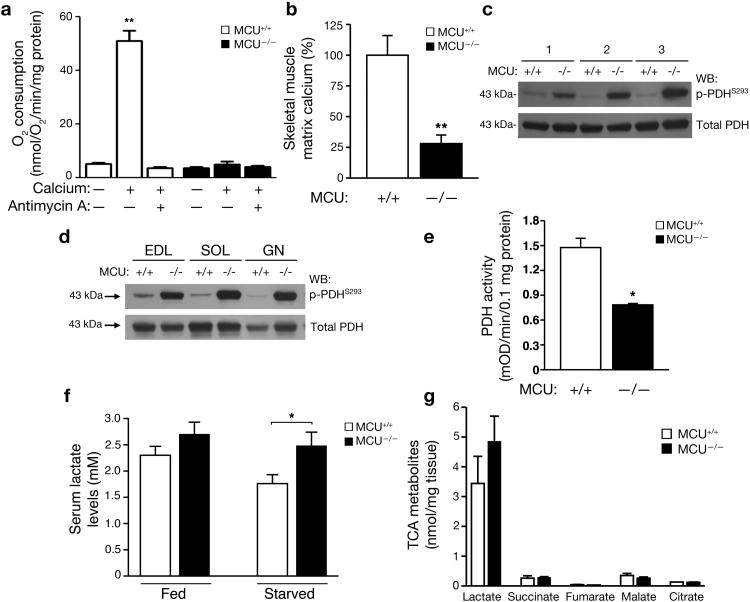
It has been known for nearly 50 years that calcium, similar to ADP and FCCP, can induce mitochondrial membrane depolarization and thereby transiently stimulate oxygen consumption28. Consistent with these past observations, in WT mitochondria, calcium addition resulted in nearly a 10-fold increase in mitochondrial respiration (Fig. 5a). In contrast, no calcium-dependent stimulation was observed in the respiration of mitochondria obtained from MCU-/- mice. While informative, such experiments using isolated mitochondria and high concentrations of calcium are by nature non-physiological. Thus, to better understand the physiological importance of mitochondrial calcium, we concentrated on the role of MCU in skeletal muscle physiology in vivo. One postulated effect of intramitochondrial calcium is the regulation of key mitochondrial enzymes involved in bioenergetics and metabolic flux. In particular, the phosphorylation of pyruvate dehydrogenase (PDH) is thought to be modulated by the calcium-sensitive phosphatase PDP129. We reasoned that MCU-/- mice should allow a direct test of whether PDH is indeed regulated in vivo by these mechanisms. We first measured resting matrix calcium levels in mitochondria isolated from the skeletal muscle of WT and MCU-/- mice. We noted an approximate 75% reduction in basal matrix calcium levels in the mitochondria of MCU-/- mice (Fig. 5b). Using a phospho-specific PDH E1 subunit antibody, we next analyzed the level of PDH phosphorylation in three pairs of WT and MCU-/- mice. Consistent with the role for the calcium-sensitive phosphatase PDP1 regulating PDH phosphorylation, lower levels of matrix calcium in the MCU-/- mice led to markedly increased levels of PDH phosphorylation in the MCU-/- mice (Fig. 5c). We noted these changes across a variety of muscle types including skeletal muscle groups that were either fast twitch, slow twitch or mixed fibers (Fig. 5d). These phosphorylation differences were most evident under starved conditions and largely disappeared when the animals were re-fed (Supplemental Fig. S5a-c). Similarly, direct addition of calcium to WT mitochondria led to a rapid reduction in PDH E1 phosphorylation, a response not evident in isolated MCU-/- mitochondria (Supplemental Fig. S5d). Previous results have indicated that this phosphorylation change on the E1 subunit is known to negatively regulate PDH activity30. Consistent with these past results, PDH activity was significantly reduced in the skeletal muscle of starved MCU-/- mice, while citrate synthase activity was not altered (Fig. 5e and Supplemental Fig. S5e). Finally, a decrease in PDH activity often correlates with a rise in serum lactate levels. We noted that MCU-/- mice indeed had a significantly elevated serum lactate level (Fig. 5f). Direct measurement of muscle lactate levels demonstrated a similar trend, while other TCA metabolites were not altered (Fig. 5g).
Figure 5.
Altered in vivo skeletal muscle metabolism and PDH activity in MCU-/- mice. a) Mitochondrial oxygen consumption following depolarization induced by the addition of 500 μM calcium with and without the respiratory chain inhibitor Antimycin A (15 μM). The average +/- SEM of three independent experiments is shown. **p<0.01 by t-test compared to without calcium. b) Levels of matrix calcium measured in WT and MCU-/- mitochondria derived from skeletal muscle following an overnight 16 hour fast (**p<0.01 by t-test; n=3 WT and N=4 MCU-/- mice). c) Western blot determination of the levels of phospho-PDH (serine 293 of the E1-α subunit) and total PDH levels in the skeletal muscle of three pairs of WT and MCU-/- mice starved for 16 hours. d) Under starved conditions, altered PDH phosphorylation is seen various muscle types including the extensor digitorum longus (EDL) representing glycolytic/fast twitch fibers, the soleus (SOL) that is predominantly oxidative/slow twitch and the gastrocnemius (GN) that is a mix of fast and slow twitch. e) Skeletal muscle PDH activity in WT and MCU-/- mice after a 16 hour fast (*p< 0.05; n=3 mice per genotype). f) Serum lactate levels in WT and MCU-/- male mice under fed conditions or after 16 hours of starvation (n=7 WT and n=6 MCU-/-, *p<0.05 by t-test). g) Metabolomic analysis of skeletal muscle demonstrating levels of various TCA cycle intermediates in mice that were starved overnight (n=3 per genotype). There was a trend for increased lactate levels in the MCU-/- muscle. All pooled data represents mean +/- S.E.M.
MCU regulates skeletal muscle work
The entry of calcium into the mitochondria during various physiological stresses is thought to increase mitochondrial energy production11. We next designed a number of functional tests that required a rapid increase in skeletal muscle work load. When we assessed skeletal muscle peak performance by placing mice on a rapid, steep treadmill, we observed a statistically significant impairment in the exercise capacity of MCU-/- mice (Fig. 6a). Similarly, when we assessed forearm grip strength, a measure of predominantly isometric muscle contraction, we also noted a small, but significant decrease in the strength of MCU-/- mice (Fig. 6b). This defect was more dramatic when we measured the ability of the mice to perform a pull up maneuver that requires concentric muscle contraction and hence increased power output (Fig. 6c). These observed defects in the ability of MCU-/- animals to generate maximal power output were not due to any apparent alterations in skeletal muscle fiber composition (Fig 6d and Supplemental Fig. S6).
Figure 6.
MCU regulates skeletal muscle peak performance. a) Assessment of skeletal muscle function using maximal work performed on an inclined treadmill test (n=6 WT and n=11 MCU-/- mice). b) Grip strength assessment for WT and MCU-/- mice (n=5 WT and n=5 MCU-/- mice). c) Forelimb strength during a modified vertical pull up test (n=11 WT and n=12 MCU-/- mice). All mice assessed for physiological responses were female. *p<0.05 and **p<0.01 by t-test. d) Analysis of fiber-specific mRNA abundance in the gastrocnemius of WT and MCU-/- mice (n=3 mice per genotype, p=NS between genotypes). All pooled data represents mean +/- S.E.M.
The role of MCU in cell death
The opening of the PTP causes a rapid inflow of solutes inducing mitochondrial swelling and can be readily monitored by a number of approaches including a rapid fall in absorbance31. Consistent with previous results, the addition of extramitochondrial calcium to WT cardiac mitochondria induced PTP opening (Fig. 7a). For WT mitochondria, this opening was inhibited by addition of either cyclosporin A or Ru360. In contrast, MCU-/- mitochondria isolated from either liver or heart exhibited no evidence of PTP opening even after exposure to high levels of calcium for prolonged periods of time (Fig. 7a,b and Supplemental Fig. S7a). As such, these observations are consistent with our previous observations in permeabilized cells (see Fig. 2a) and demonstrate that MCU is required for calcium-induced PTP opening.
Figure 7.
MCU expression is necessary for calcium-induced PTP opening but not required for cell death. a) Only WT mitochondria undergo PTP opening after calcium addition (500 μM) as evidence by a rapid drop in absorbance. Shown is one experiment using heart mitochondria that is representative of three similar experiments. b) Average change in absorbance from three independent experiments using isolated cardiac mitochondria in the presence or absence of CsA (0.2 μM) and Ru360 (3.0 μM). c) Cell viability as measured by Annexin V/PI staining in WT and MCU-/- MEFs following a wide array of challenges including hydrogen peroxide exposure (1 mM), tunicamycin (2 μg/ml), doxorubicin (2 μM), C2-ceramide (100 μM) and thapsigargin (1 μM). The time course and magnitude of cell death was not altered by the absence of MCU expression (p=NS, n=3 per genotype). d) Cytosolic cytochrome C levels in WT or MCU-/- MEFs following the addition of hydrogen peroxide. Tubulin is shown as a loading control. e) Caspase-3 activity was measured under basal conditions or 24 hours after treatment with tunicamycin or doxorubicin (p=NS; n=3). All pooled data represents mean +/- S.E.M.
We next sought to understand the physiological importance of these observations. We took MCU-/- or WT MEFs and exposed them to a range of potential inducers of cell death. These agents included hydrogen peroxide as a prototypical example of oxidative stress, the ER stress inducing agent tunicamycin, the chemotherapeutic and DNA damaging agent doxorubicin, C2-ceramide an agent that can activate both apoptotic and necrotic pathways and thapsigargin, which triggers cell death by interfering with ER calcium uptake. We monitored cell death using the combination of annexin V and propidium iodine staining. While all of these agents were effective at inducing cell death, we noted no difference in the kinetics or magnitude of cell death in MEFs with or without MCU expression (Fig. 7c). We also noted that the release of cytochrome C from the mitochondria into the cytosol was not affected by MCU expression, nor did we observe a significant difference in cytosolic calcium levels during cell death (Fig. 7d and Supplemental Fig. S7b,c). For both tunicamycin and doxorubicin we also measured the degree of Caspase-3 activity. Again, we noted indistinguishable levels of Caspase-3 activation in wild type and MCU-/- cells (Fig. 7e).
Finally, to assess whether similar effects were observed in tissues and organs, we subjected heart of wild type and MCU-/- mice to ischaemia-reperfusion (I/R) injury. When the degree of injury was assessed by functional measurements such as the post-ischemic recovery in the rate pressure product (RPP) or by pathological measurements such as direct assessment of the infarct area, our analysis revealed that MCU-/- mice exhibited no evidence for protection from I/R-mediated injury (Fig. 8a,b). Similarly, the level of apoptosis and the magnitude of ischemic contracture was equivalent in WT and MCU-/- hearts subjected to I/R injury (Supplemental Fig. S8a,b). Interestingly, while as expected the PTP inhibitor cyclosporin A (CsA) provided significant protection to WT hearts32, this agent had no demonstrable effect on MCU-/- hearts (Fig. 8a,b).
Figure 8.
Role of MCU in ischaemia-reperfusion injury. a) Assessment of the rate pressure product (RPP, heart rate times systolic blood pressure) after ischaemia-reperfusion injury in the hearts of WT or MCU-/- mice with and without Cyclosporin A (CsA, 0.2 μM) for five minutes prior to ischemia. g) Infarct size in WT and MCU-/- mice following 20 minutes of global ischemia and 90 minutes of reperfusion. *p<0.05, **p<0.01 by ANOVA; all pooled data represents mean +/- S.E.M.
Discussion
In summary, we provide the first in vivo description of the physiological effects seen in the absence of MCU expression. Remarkably, although mitochondria and cells from MCU-/- mice lack evidence for high capacity calcium uptake, these animals are grossly normal. While this result is somewhat unexpected, it should be noted that a previous study found no significant organ pathology following continuous siRNA-mediated knockdown of MCU in the liver9. Furthermore, few discernable phenotypes were observed in mice genetically deficient for myoglobin, creatine kinase or creatine, molecules that were all originally thought to be absolutely essential for cardiac and skeletal muscle bioenergetics33-35. Indeed, in the context of the whole animal, the absence of MCU produces relatively minor alterations in basal energetics. However, we should note that we found a significant reduction, but not a complete absence, of mitochondrial matrix calcium in mice lacking MCU. For instance, in our analysis, matrix calcium appeared to be reduced to about 25% of wild type levels in the skeletal muscle mitochondria of starved MCU-/- mice. This non-zero value suggests that alternative mechanisms must exist for calcium entry, although based on our cellular and isolated mitochondrial studies; the high capacity, rapid entry mode that occurs through the uniporter is clearly absent. It is conceivable that these slower and presumably low capacitance mechanisms might allow for some physiological adaptation over time.
We also observed that while MCU-/- mitochondria lacked evidence for calcium-induced PTP opening, the absence of MCU expression did not result in a demonstrable alteration in the magnitude of in vitro or in vivo cell death. Previous results with transient manipulation had suggested that altering MCU expression modulated the sensitivity to cell death in many 10,36 but not all37 cellular systems. Interestingly, we observed that while the absence of MCU did not alter the magnitude of cell death, MCU-/- hearts were insensitive to CsA treatment. One interpretation is that in the absence of MCU, additional CsA-independent and calcium-independent cell death pathways emerge and predominate. Interestingly, yeast mitochondria lack an MCU equivalent but still appear to undergo PTP opening, although this event appears to be insensitive to both calcium and CsA38.
Finally, while there is unequivocal evidence for the in vitro regulation of mitochondrial dehydrogenase function by calcium11, the observation that MCU-/- mice have limited defects in basal metabolism suggests that the in vivo effects of altering matrix calcium may be most important under certain stress conditions. Our results demonstrate that in tissues such as skeletal muscle, that exhibit a large dynamic energetic range, mitochondrial calcium regulates the intrinsic metabolism of the tissue as well as the peak performance. The observation that the defect in maximal skeletal muscle power output of MCU-/- mice is qualitatively similar to what was observed in mice deficient in creatine kinase34 suggests that this property of skeletal muscle, i.e the ability to rapidly boost and maintain peak power output, is for obvious reasons, under intense evolutionary pressure.
Supplementary Material
Acknowledgments
We are grateful to C. Brantner, P.S. Connelly and M.P. Daniels of the NHLBI Electron Microscopy Core Facility for assistance with electron microscopy, C. Petucci of the Metabolomics Core Facility Sanford-Burnham Medical Research Institute for aiding in the metabolomic profiling, C. Combs and the NHLBI Microscopy Core for help with the Rhod-2 fluorescent measurements and A. Wiederkehr for the original mito-aequorin adenovirus. This work was supported by NIH Intramural funds.
Footnotes
The authors declare no competing financial interest.
Contributions: X.P, J.L and T.N. designed, performed and analyzed the experiments and aided in writing the manuscript, C.L. help construct the mouse model, J.S., Y.T., M.M.F., I.I.R., M.A., D.A.S., A.M.A, and M.G. contributed to the completion of various experiments, R.S.B, E.M and T.F. conceived the study, supervised the research and contributed to the writing the manuscript.
References
- 1.Deluca HF, Engstrom GW. Calcium uptake by rat kidney mitochondria. Proc Natl Acad Sci U S A. 1961;47:1744–1750. doi: 10.1073/pnas.47.11.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasington FD, Murphy JV. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J Biol Chem. 1962;237:2670–2677. [PubMed] [Google Scholar]
- 3.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 4.Gunter TE, Sheu SS. Characteristics and possible functions of mitochondrial Ca(2+) transport mechanisms. Biochim Biophys Acta. 2009;1787:1291–1308. doi: 10.1016/j.bbabio.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raffaello A, De Stefani D, Rizzuto R. The mitochondrial Ca(2+) uniporter. Cell Calcium. 2012;52:16–21. doi: 10.1016/j.ceca.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Selwyn MJ, Dawson AP, Dunnett SJ. Calcium transport in mitochondria. FEBS Lett. 1970;10:1–5. doi: 10.1016/0014-5793(70)80402-1. [DOI] [PubMed] [Google Scholar]
- 7.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 8.Moore CL. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971;42:298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- 9.Baughman JM, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta. 2009;1787:1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Satrustegui J, Pardo B, Del Arco A. Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol Rev. 2007;87:29–67. doi: 10.1152/physrev.00005.2006. [DOI] [PubMed] [Google Scholar]
- 13.Territo PR, Mootha VK, French SA, Balaban RS. Ca(2+) activation of heart mitochondrial oxidative phosphorylation: role of the F(0)/F(1)-ATPase. Am J Physiol Cell Physiol. 2000;278:C423–435. doi: 10.1152/ajpcell.2000.278.2.C423. [DOI] [PubMed] [Google Scholar]
- 14.Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. Journal of molecular and cellular cardiology. 2002;34:1259–1271. doi: 10.1006/jmcc.2002.2082. [DOI] [PubMed] [Google Scholar]
- 15.Hochachka PW, McClelland GB. Cellular metabolic homeostasis during large-scale change in ATP turnover rates in muscles. J Exp Biol. 1997;200:381–386. doi: 10.1242/jeb.200.2.381. [DOI] [PubMed] [Google Scholar]
- 16.Denton RM, McCormack JG. Ca2+ as a second messenger within mitochondria of the heart and other tissues. Annu Rev Physiol. 1990;52:451–466. doi: 10.1146/annurev.ph.52.030190.002315. [DOI] [PubMed] [Google Scholar]
- 17.Glancy B, Balaban RS. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry. 2012;51:2959–2973. doi: 10.1021/bi2018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu T, O'Rourke B. Regulation of mitochondrial Ca2+ and its effects on energetics and redox balance in normal and failing heart. J Bioenerg Biomembr. 2009;41:127–132. doi: 10.1007/s10863-009-9216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasola A, Bernardi P. Mitochondrial permeability transition in Ca(2+)-dependent apoptosis and necrosis. Cell Calcium. 2011;50:222–233. doi: 10.1016/j.ceca.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 21.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 22.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spat A, Szanda G, Csordas G, Hajnoczky G. High- and low-calcium-dependent mechanisms of mitochondrial calcium signalling. Cell Calcium. 2008;44:51–63. doi: 10.1016/j.ceca.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffiths EJ. Mitochondrial calcium transport in the heart: physiological and pathological roles. Journal of molecular and cellular cardiology. 2009;46:789–803. doi: 10.1016/j.yjmcc.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Chacon E, et al. Mitochondrial free calcium transients during excitation-contraction coupling in rabbit cardiac myocytes. FEBS Lett. 1996;382:31–36. doi: 10.1016/0014-5793(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 27.Mallilankaraman K, et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol. 2012;14:1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chance B. The Energy-Linked Reaction of Calcium with Mitochondria. J Biol Chem. 1965;240:2729–2748. [PubMed] [Google Scholar]
- 29.Karpova T, Danchuk S, Kolobova E, Popov KM. Characterization of the isozymes of pyruvate dehydrogenase phosphatase: implications for the regulation of pyruvate dehydrogenase activity. Biochim Biophys Acta. 2003;1652:126–135. doi: 10.1016/j.bbapap.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Holness MJ, Sugden MC. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem Soc Trans. 2003;31:1143–1151. doi: 10.1042/bst0311143. [DOI] [PubMed] [Google Scholar]
- 31.Blattner JR, He L, Lemasters JJ. Screening assays for the mitochondrial permeability transition using a fluorescence multiwell plate reader. Anal Biochem. 2001;295:220–226. doi: 10.1006/abio.2001.5219. [DOI] [PubMed] [Google Scholar]
- 32.Hausenloy DJ, Boston-Griffiths EA, Yellon DM. Cyclosporin A and cardioprotection: from investigative tool to therapeutic agent. Br J Pharmacol. 2012;165:1235–1245. doi: 10.1111/j.1476-5381.2011.01700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garry DJ, et al. Mice without myoglobin. Nature. 1998;395:905–908. doi: 10.1038/27681. [DOI] [PubMed] [Google Scholar]
- 34.van Deursen J, et al. Skeletal muscles of mice deficient in muscle creatine kinase lack burst activity. Cell. 1993;74:621–631. doi: 10.1016/0092-8674(93)90510-w. [DOI] [PubMed] [Google Scholar]
- 35.Lygate CA, et al. Living Without Creatine: Unchanged Exercise Capacity and Response to Chronic Myocardial Infarction in Creatine-Deficient Mice. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.112.300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu J, et al. Mitochondrial calcium uniporter Mcu controls excitotoxicity and is transcriptionally repressed by neuroprotective nuclear calcium signals. Nat Commun. 2013;4:2034. doi: 10.1038/ncomms3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curry MC, Peters AA, Kenny PA, Roberts-Thomson SJ, Monteith GR. Mitochondrial calcium uniporter silencing potentiates caspase-independent cell death in MDA-MB-231 breast cancer cells. Biochem Biophys Res Commun. 2013;434:695–700. doi: 10.1016/j.bbrc.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 38.Jung DW, Bradshaw PC, Pfeiffer DR. Properties of a cyclosporin-insensitive permeability transition pore in yeast mitochondria. J Biol Chem. 1997;272:21104–21112. doi: 10.1074/jbc.272.34.21104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.