Abstract
A vast number of apicobasal polarity proteins play essential roles in the polarization and morphogenesis of the neuroepithelia. Crumbs (Crb) type I transmembrane cell-cell adhesion proteins are among these proteins. Five crb genes have been identified in zebrafish. However, their expressional and functional differences during early neural development remain to be fully elucidated. Here, we study the spatial-temporal expression patterns and functions of Crb1, Crb2a, and Crb2b in the central nervous system (CNS) during the neurulation period. We show that: 1, the optic vesicle and undifferentiated retinal neuroepithelium only express Crb2a; 2, Crb1 and Crb2a expressions overlap extensively in the undifferentiated neural tube epithelium; 3, Crb2b expression is the weakest of the three and is restricted to the ventral-most regions of the anterior CNS; and 4, Nok and Crb proteins require each other for their apical localization in neuroepithelium. The commencements of Crb1, Crb2a, and Crb2b expressions follow a spatial-temporal spread from anterior to posterior and from ventral to dorsal and lag behind that of adherens junction components, such as ZO-1 and actin bundles. Genetic and morpholino suppression analyses suggest that in regions where these Crb expressions overlap, they are functionally redundant in maintaining apicobasal polarity of the undifferentiated neuroepithelium.
Keywords: Crumbs, Nagie oko, Lin7c, apicobasal polarity, neuroepithelium
1. Introduction
During neurulation dorsal ectoderm cells reorganize to form the neural tube, which is composed of a single layer of undifferentiated and polarized neuroepithelial cells. The progression of neurulation follows the anterior-to-posterior and ventral-to-dorsal directions (Lowery and Sive, 2004). Both apicobasal and planar polarization are essential for neural tube morphogenesis (Montcouquiol et al., 2006; Copp et al., 2003).The formation and maintenance of the apicobasal polarity of neuroepithelial cells require a complex set of apicobasal polarity proteins (Hildebrand and Soriano, 1999; Pujic and Malicki, 2001; Lele et al., 2002; Wei and Malicki, 2002; Murdoch et al., 2003; Lowery and Sive 2005; Hong and Brewster, 2006; Wei et al., 2004; Wei et al., 2006a; Tawk et al., 2007; Munson et al., 2008; Yang et al., 2009). Many of these proteins, such as Cadherin, ZO-1, Lin7c, Crumbs (Crb), Nagie oko (Nok), Pard3, Pard6, Scribble, Shroom, etc., are also required for the proper morphologies and functions of other types of epithelium (Knust and Bossinger, 2002). Eventually, the neural tube differentiates into the complex central nervous system (CNS) (Lowery and Sive, 2004; Colas and Schoenwolf, 2001).
Among these various apicobasal polarity proteins, Crb proteins form evolutionarily conserved protein complexes with Nok and Lin7c homologs (Kamberov et al., 2000; Hong et al., 2001; Bachmann et al., 2001; Hsu et al., 2006; Yang et al., 2009). The Crb proteins are type I transmembrane cell-cell adhesion proteins (Tepass et al., 1990; Zou et al., 2012) and are essential for the apicobasal polarity of many types of epithelium (Izaddoost et al., 2002; Knust and Bossinger, 2002; Makarova et al., 2003; Macara, 2004). The vertebrate Crb homologs can be divided into two groups: the Crb1/Crb2 group, which contain a large extracellular domain, and the Crb3 group, which has a very short extracellular domain (Makarova et al., 2003; Omori and Malicki, 2006). In zebrafish, five crb genes have been identified: crb1, crb2a, crb2b, crb3a, and crb3b (Omori and Malicki, 2006). The crb2b gene is transcribed as a longer form (crb2b-lf) as well as a shorter form (crb2b-sf), with Crb2b-sf lacking the N-terminal half of the extracellular domain of Crb2b-lf (Zou et al., 2012). The structures of Crb1, Crb2a, and Crb2b-lf are very similar and they all have 19-21 EGF-like and three laminin G-like motifs in their extracellular domains (Omori and Malicki, 2006). In situ hybridization analyses indicate that crb1, crb2a, and crb2b genes are expressed in early CNS, crb3a in the otic vesicle and digestive system, and crb3b only in the digestive system (Omori and Malicki, 2006). However, it remains to be seen whether the temporal and spatial expression patterns of Crb1, Crb2a and Crb2b differ during the development of the CNS.
In this study, we generated antibodies that specifically recognize Crb1. Previously, we have reported the generation of specific antibodies against Crb2a and Crb2b (Zou et al., 2012). With these antibodies, we revealed the distinct yet overlapping spatial-temporal expressions of Crb1, Crb2a, and Crb2b in zebrafish CNS during its early development. These Crb proteins carry redundant polarity-maintaining functions in the regions of the neuroepithelium where their expressions overlap. We also showed that Nok is likely the sole partner that binds to the C-termini of these Crb proteins in undifferentiated neuroepithelium. Furthermore, we showed that proper subcellular localizations of Crb and Nok in the neuroepithelium rely on each other.
2. Experimental procedures
2.1 Animal care
AB wildtype, nokm227, nokm520 and crb2am289 zebrafish embryos were raised at 28.5 °C in E3 egg water until desired developmental stages. Care of experimental animals was in accordance with University Pittsburgh guidelines.
2.2 Antibody production
The region of Crb1amino acids 952-1258 was PCR amplified with a forward primer (5′gaattcatttcaaacgtgactttcca3′) and a reverse primer (5′aagcttcttgcaaagtggcccagtaa3′) and cloned into the His-tag expression vector pET32a+ (Novagen). The Crb1amino acids952-1258 region covers the third Laminin A G-like domain and some flanking sequences. The construct was transformed into BL21 competent cells (Invitrogen) and to express the Crb1952-1258-His fusion protein. Two milligrams of His fusion protein was purified with a His-trap column (Amersham) and used to immunize rabbits (service provided by Proteintech Group, Inc.). Antibodies that recognize Crb1 were affinity purified using an Aminolink Plus immobilization affinity column (Pierce) that was conjugated with 1 milligram of GST-Crb1952 fusion protein, which was expressed and purified with the pGex-5x-1 system (Amersham). The generations of antibodies against Crb2aamino acids97-457 and Crb2b-lf amino acids 466-773, Nok, and Ponli were described previously (Wei and Malicki, 2002; Zou et al., 2010; Zou et al., 2012).
2.3 Immunohistochemistry
Crb, Nok, and Ponli antibodies were used at a 1:350 dilution (Wei and Malicki, 2002; Zou et al., 2010). The mouse anti-ZO-1 antibody (Invitrogen) was used at 1:300. The Alexa Fluor 488 Phalloidin (Invitrogen) was used at 1:200. Immunostained sections were examined and photographed as single optical section images under a confocal microscope, which has a maximal axial resolution of 0.8 ! m and an x, y plane resolution of 0.25 ! m (Fluoview FV1000; Olympus, Tokyo, Japan).
2.4 Morpholino knockdown
To block the translation of crb1, crb2a, crb2b-lf, and crb2b-sf, about 2 nanogram of each morpholino (Gene-Tools) was injected into each AB wildtype embryo at 1 cell stage. The morpholino was dissolved in distilled water (DNase, RNase free) at 1.5 mg/ml. The sequences of the translation-blocking morpholinos are as follows: MO-crb1, 5′CCCAAACCGCTAGATCCATATCCAT3′; MO-crb2a, 5′CAGATGCACTTTCCTGATCTCCATG3′; MO-crb2b-lf, 5′ACATCCGTCCAAAATCCATGTCCTG3′; MO-crb2b-sf, 5′TCACTTTCACTATTAGTCCTCTCAT3′. These morpholinos are complementary to regions of the translation start sites of their cognate crb mRNA molecules. BLAST searches of the entire zebrafish genomic sequence confirmed that these morpholinos match only to their cognate crb genes. In addition, pair-wise alignments of the morpholinos did not reveal significant similarities that would raise the concern of cross-suppression. The specificity of MO-crb2a and MO-crb2b-lf were previously verified with other splicing-blocking morpholinos (Omori and Malicki, 2006). For triple suppression of Crb1, Crb2a and Crb2b, each crb morpholino was dissolved at 1.5 mg/ml, giving a combined concentration of 6 mg/ml. For ponli morphants controls, the ponli morpholino (gccaacacggtacacgttcagagca) (Zou et al., 2010) was injected at 6 mg/ml.
2.5 Western blotting analyses of Crb expression
The Crb antibodies used in this study are very sensitive for Western blotting conditions, presumably due to their requirement of proper three-dimensional conformation of the extracellular domains of Crb protein antigens. The following special technical details should be integrated into standard Western blotting procedures to detect Crb1 and Crb2a signals: nokm520, crb2am289 mutant embryos as well as wildtype embryos were collected at 30hpf. The yolks were manually removed with forceps. The de-yolked embryos were homogenized on ice in a lysis buffer at 1.5! l/embryo for 30 minutes (1x PBS with 1% Triton X-100, 1mM proteinase inhibitor Phenylmethylsulfonyl fluoride (PMSF), 1 x Roche protease inhibitor cocktail, pH 7.4). The lysates were centrifuged at 14000 rpm at 4 °C for 15minutes to collect the supernatants, which were then mixed with 1/5 volume of 6X SDS loading buffer (375 mM Tris-HCl pH 6.8, 6% SDS, 48% glycerol, and 0.03% bromophenol blue), followed by a 5-min incubation at 80 °C (boiling temperature and reducing agents should be avoided to preserve the conformation of the EGF-like domains of the Crb proteins and a brief centrifugation to collect supernatants). The protein extracts from 15 embryos were then separated on 10% SDS PAGE and transferred to PVDF membranes for blotting analyses. The top halves of the blots were incubated with anti-Crb2a (1:5000) and anti-Crb1 (1:3000) at 4 °C for 20 hrs after a 1-hr of blocking with 5% non-fat milk solution. To detect Crb1 antibodies, the blots were incubated with phosphatase conjugated anti-rabbit IgG (1:3000) for 12hrs at 4 °C. To detect bound Crb2a antibodies, the blots were additionally incubated with mouse-anti-rabbit IgG (1:2000) at RT for 5 hrs, followed by extensive washes and then a subsequent 12-hr incubation with alkaline phosphatase conjugated anti-mouse IgG (1:3000) at 4 °C. For loading controls, the bottom halves of the blots were incubated with ! -tubulin antibodies as previously (Zou et al., 2012).
3. Results
3.1 Zebrafish Crb1, Crb2a and Crb2b display distinct and overlapping spatial-temporal expression patterns during neurulation
In order to evaluate the functions and expression patterns of Crb1, Crb2a, and Crb2b in the CNS during neurulation period, we have generated antibodies that specifically recognize distinct regions of the following Crb proteins: Crb1amino acid 952-1258, Crb2aamino acid 97-457, and Crb2b-lfamino acid 466-773 (which is also shared in Crb2b-sf) (Zou et al., 2012). Because the Crb2b antibodies cannot distinguish Crb2b-lf from Crb2b-sf, we use Crb2b to represent the two proteins collectively. To ensure that we were comparing the developmental expressions of Crb1, Crb2a and Crb2b in identical regions of the CNS, we focused our comparisons on two CNS regions that can be conveniently defined along the anterior-posterior axis: an anterior region that contains the optic vesicle/cup and the diencephalon, and a posterior region that contains rhombomeres 5 and 6 of the hindbrain that is adjacent to the otic vesicles (Barald and Kelley, 2004).
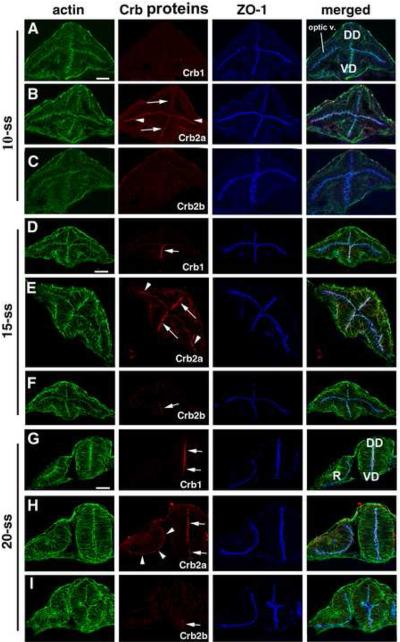
At the anterior CNS, Crb2a expression was detectable at the apical surface of the optic vesicle as well as of the ventral diencephalon neuroepithelium at 10-somite stage (-ss), whereas Crb1 and Crb2b were not detectable at the same stage (Fig. 1A-C). By 15-ss, Crb2a expression was extended to the dorsal diencephalon (Fig. 1E), whereas Crb1 emerged at the ventral diencephalon neuroepithelium (Fig. 1D). Meanwhile, very weak Crb2b was detected in the ventral-most diencephalon neuroepithelium (Fig 1F). By 20-ss, both Crb1 and Crb2a expressions extended to the apical surfaces of the entire diencephalon neuroepithelium, and Crb2b remained restricted to the ventral-most diencephalon neuroepithelium at a much weaker level (Fig. 1G-I). Meanwhile, Crb2a expression persists at the apical surface of the retinal neuroepithelium, whereas Crb1 and Crb2b were not detectable in the retina at all (Fig. 1G-I).
Figure 1.
Differential expression of Crb1, Crb2a and Crb2b in the diencephalon and the optic vesicle during early development of the CNS. A-C. At 10-somite stage (-ss), only Crb2a (B) was detectable at the apical surfaces of the optic vesicle (B, arrowheads) and the diencephalon epithelium (arrows), with weaker Crb2a expression in the dorsal diencephalon neuroepithelium than in the ventral region (B, arrow). By contrast, Crb1 (A) and Crb2b (C) expressions were not detectable. D-F. At 15-ss, Crb1 expression was detectable at the ventral diencephalon (D, arrow), and Crb2a expression was detectable throughout the apical surfaces of the diencephalon (arrows) and the optic vesicle (E, arrowheads). Weak Crb2b expression was detected at ventral diencephalon (F, arrow). G-I. At 20-ss, the Crb1 expression extended to the entire apical surface of the diencephalon epithelium (G; arrows), Crb2a expression persisted in the retina (H, arrowheads) and the diencephalon (H, arrows). Crb2b expression remained restricted to the most ventral region of the diencephalon (I, arrow). The ventral diencephalon (VD); dorsal diencephalon (DD); optic vesicle (optic v.); and the retina (R) are marked in panel A and G. Scale bars, 50 ! m.
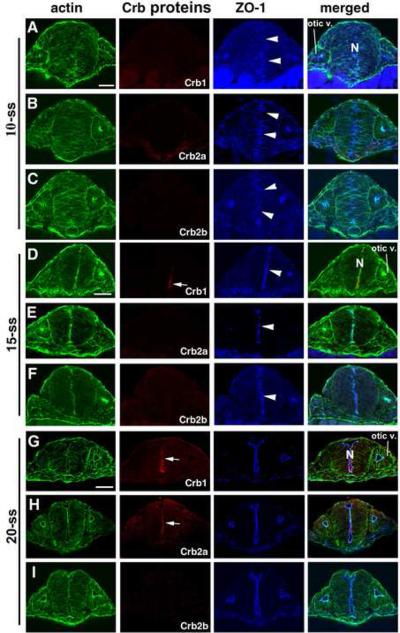
In the hindbrain near the otic vesicles, none of Crb1, Crb2a and Crb2b was detectable at 10-ss. By contrast, ZO-1 expression was already roughly aggregated at the midline region (Fig. 2 A-C), even though the midline alignment of ZO-1 signals is not as neat and narrow as they are at 15-ss and 20-ss stages (Fig. 2 D-I), illustrating a dynamic ongoing progress of finalizing the apical junctional integrity at this region and time. By 15-ss, Crb1 expression was detectable at the ventral hindbrain neural rod (Fig. 2D), whereas neither Crb2a nor Crb2b was detectable (Fig. 2E-F). By 20-ss, both Crb1 and Crb2a were detectable at the apical surfaces of the hindbrain neural rod (Fig. 2G and 2H), while the expression of Crb2b remained undetectable (Fig. 2I). By contrast, Crb1, Crb2a and Crb2b were not detectable in the otic vesicle at any of the three stages examined (Fig. 2), consistent with previous published in situ hybridization data (Omori and Malicki, 2006). Thus, Crb1, Crb2a and Crb2b are expressed in the CNS with distinct yet overlapping temporal and spatial expression patterns.
Figure 2.
The differential expressions of Crb1, Crb2a and Crb2b in the hindbrain at the otic vesicle region during early development. A-C. At the 10-ss, Crb1, Crb2a, Crb2b were all not detectable. However, ZO-1 expression (arrowheads) was detectable in the midline region, although not yet neatly aligned. D-F. By the 15-ss, Crb1 expression was detectable at the ventral neural rod (D, arrow). By contrast, Crb2a (E), Crb2b (F) expressions were still not detectable. ZO-1 expression (arrowheads) and the actin bundles were more smoothly aligned at the midline region at this stage than at the 10-ss. G-I. At the 20-ss, Crb1 (G) and Crb2a (H) were detectable at the entire apical surface of the neural rod (arrows). However, Crb2b (I) expression remained undetectable at 20-ss. The otic vesicle (otic v.) and the neural rod (N) are indicated in panels A, D, G for 10-ss, 15-ss, and 20-ss, respectively. Scale bars, 50 ! m.
3.2 Nok expression patterns resemble the combined patterns of Crb1, Crb2a and Crb2b expression
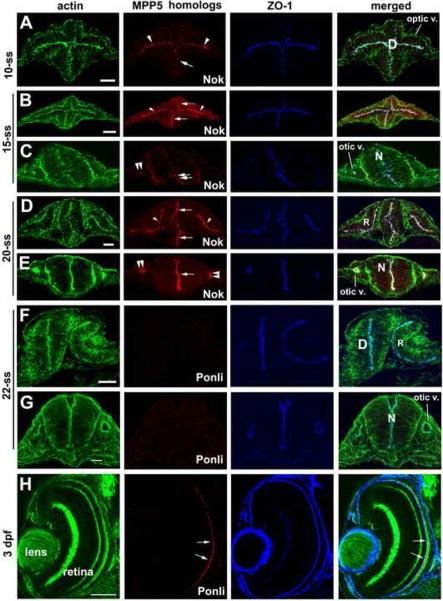
Crb proteins form complexes with PDZ domain-containing MPP5 homologs through their C-termini (Hong et al., 2001; Bachmann et al., 2001; Hsu et al., 2006). The MPP5 proteins (MPP for membrane palmitoylated protein) constitute a subfamily of the membrane associated guanylate kinase (MAGUK) protein family (Gosens et al., 2008). We thus ask whether the temporal and spatial expression of Nok, a zebrafish MPP5 homolog (Wei and Malicki, 2002), parallels with any of the three Crb proteins in the neural tissue. We found that the expression patterns of Nok resemble the combined patterns of Crb1, Crb2a, and Crb2b expressions, except that Nok is also expressed in the otic vesicles (Fig. 3A-E, compare with Fig. 1 and Fig. 2). This suggests that Crb1, Crb2a, and Crb2b all likely interact with Nok to form functional complexes in the CNS. Supporting this notion, the other of the two known zebrafish MPP5 homolog, Ponli, which is restrictively expressed in green, red and blue cones (Zou et al., 2010; Fig. 3H), is, however, not expressed in the retina, the neural tube, and the otic vesicles at 10-, 15-, and 20-ss (Fig. 3F and G). Because Ponli and Nok are the only known MPP5 homologs in zebrafish, these data suggest that the Nok is likely the sole MPP5 protein that binds to Crb C-termini in undifferentiated neuroepithelium.
Figure 3.
The expressions of Nok and Ponli in the diencephalon, retina and hindbrain during early development. A. At 10-ss, Nok expression was detectable in the diencephalon (arrow), and optic vesicle (arrowheads). B-C. At 15-ss, Nok expression was detectable in the optic vesicle (arrowheads), diencephalon (arrows), neural rod (double arrows), and otic vesicles (double arrowheads). D-E. At 20-ss, Nok expression was detectable in the retina (arrowheads), diencephalon (arrows, D), otic vesicles (double arrowheads) and hindbrain (arrow, E). F-G. At 22-ss, Ponli expression was not detectable in the retina, diencephalon, or otic vesicles. H. Ponli expression in 3-dpf retina at the region immediate apical to the outer limiting membrane (arrows) was visualized as a positive control for immunohistochemistry of panels F and G. The retina, R; neural rod, N; and diencephalon, D are marked in the merged image panels. Scale bars, 50 ! m.
3.3 Crb1, Crb2a, and Crb2b appear to carry redundant function in regions where their expressions overlap
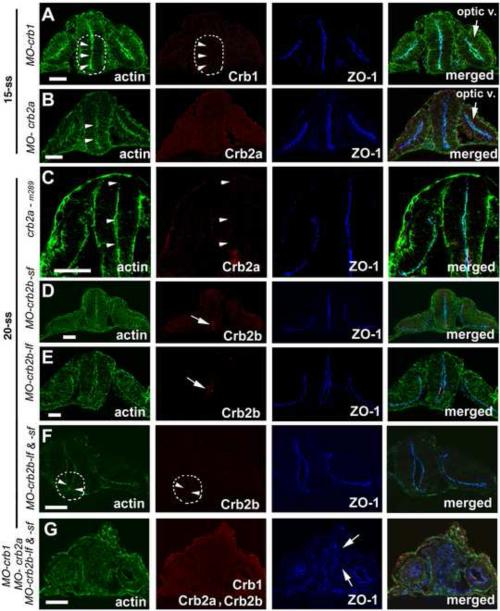
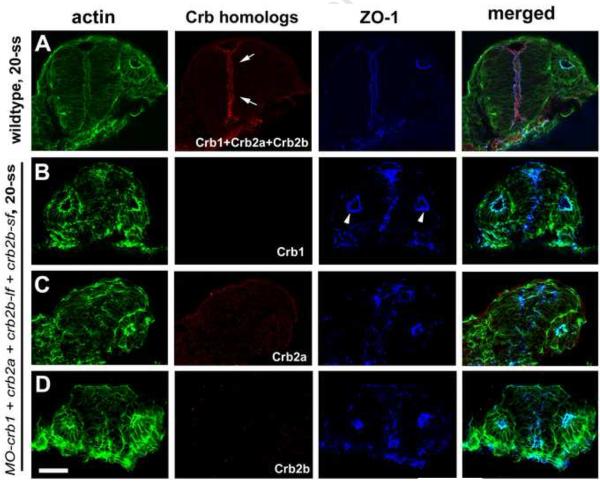
Since zebrafish Crb1, Crb2a and Crb2b proteins are very similar in their amino acid sequences and protein domain structures (Omori and Malicki, 2006; Zou et al., 2012), we thus asked if Crb proteins are functionally redundant in the CNS regions where their expressions overlap. To address this question, we utilized specific morpholinos to individually knock down the expressions of Crb1, Crb2a and Crb2b. We found that the loss of function of each Crb protein did not affect the apicobasal epithelial polarity of the diencephalon and the posterior neural rod at the hindbrain region, as evidenced by the smooth apical alignment of apical makers ZO-1 and actin bundles (Fig. 4A-F). The suppression of Crb in these single morphants is homolog-specific because non-cognate Crb homologs were not suppressed (Supplementary Fig. 1). Interestingly, for unknown reasons, about 50% MO-crb2a and MO-Crb2b morphants displayed otic apical expression of Crb1, which was not observed in wildtype embryos (Fig. 2G; Supplementary Fig. 1C and E). Although loss of individual Crb did not affect the polarity of diencephalon neuroepithelium, when Crb1, Crb2a and Crb2b were knocked down simultaneously with a mixture of morpholinos, apicobasal epithelial polarization of brain neuroepithelium but not otic epithelium was severely affected, as evidenced by misalignment of apical markers ZO-1 and actin bundles in the diencephalon and hindbrain neuroepithelia (Fig. 4G; Fig. 5B-D). This polarity defect was unlikely due to the high concentration of the injected morpholino mixture because the same concentration of ponli morpholino did not disrupt neuroepithelial polarity (Supplementary Fig. 2). Although some levels of cell death were observed in both ponli and crb morphants, the fact that the disruption of cell polarity in crb morphants was also observed in non-dying cells, together with the lack of polarity defect in ponli morphants suggest that the cellular polarity defect of the crb morphants was unlikely a secondary effect of cell death (Supplementary Fig. 2). It is worth noting that Crb2b immunostaining signals were observed in crb2b-lf and crb2b-sf single morphants; by contrast, Crb2b signals were completely abolished in crb2b-lf and crb2b-sf double morphants (Fig. 4DF), suggesting both Crb2b-lf and Crb2b-sf are expressed in the neuroepithelium. This is consistent with our previous RT-PCR analyses that showed both crb2b-lf and crb2b-sf are specifically expressed in the eye at the mRNA level (Zou et al., 2012). Collectively, these data suggest that Crb1, Crb2a and Crb2b play a redundant role in maintaining apicobasal epithelial polarity of undifferentiated CNS neuroepithelium, and that they do not function in the otic vesicle, which expresses Crb3a (Omori and Malicki, 2006).
Figure 4.
Individual loss of Crb1, Crb2a, and Crb2b function did not disrupt the neuroepithelial polarity at 15-ss and 20-ss as evidenced by proper localization of apical makers ZO-1 and the actin bundles (arrowheads). A-B. Morpholino suppression of either Crb1 (A) or Crb2a (B) expression did not interrupt the proper apicobasal polarization of diencephalon neuroepithelium at 15-ss. The dashed line circles the ventral brain region where Crb1 should otherwise be expressed in wildtype. C. crb2am289 mutational deficiency of Crb2a did not affect the apicobasal polarity of the diencephalon (C) at 20-ss. D-F. Morpholino suppression of Crb2b-lf (E) and Crb2b-sf (D) or both Crb2b-lf and Crb2b-sf (F) did not affect the apicobasal polarity of the ventral diencephalon neuroepithelium where Crb2b-lf and Crb2b-sf are otherwise expressed (encircled by a dashed line) at 20-ss in wildtype. Arrows indicate the supposed expression of Crb2b-lf and Crb2b-sf in corresponding embryos treated with individual morpholinos against crb2b-sf and crb2b-lf, respectively. G. Simultaneous suppression of Crb1, Crb2a, and Crb2b led to the mislocalization of apical markers ZO-1 (arrows) and actin bundles in the diencephalon at 20-ss. The lack of Crb1, Crb2a and Crb2b immunostaining demonstrated the effectiveness of expression suppression by the morpholinos. “Optic V.” for optical vesicle; Scale bars, 50 ! m.
Figure 5.
Simultaneous suppression of Crb1, Crb2a, and Crb2b severely affected the polarity of the hindbrain neuroepithelium but not that of the otic vesicle. A. The combined expression profiles of Crb1, Crb2a and Crb2b in the hindbrain at 20-ss was revealed by using a mixture of Crb antibodies for confocal immunohistochemistry. B-D. The suppression of Crb1 (B), Crb2a (C), Crb2b (D) in embryos treated with morpholinos against these Crb proteins was verified with immnohistochemistry using individual antibodies. The polarity of the neuroepithelium is affected in the morphants as evidenced by mislocalization/misalignment of apical marker ZO-1 and actin bundles. By contrast, the apicobasal polarity of the otic vesicle remains unaffected (arrowheads). The lack of Crb1, Crb2a and Crb2b immunostaining demonstrated the effectiveness of morpholino suppression. Scale bar, 50 ! m.
3.4 Crb proteins are required for apical localization of Nok in neuroepithelial cells
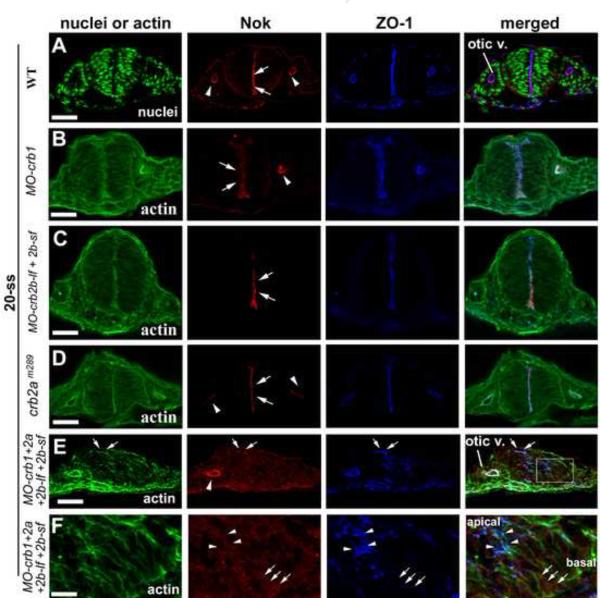
Because the expression patterns of Nok resemble the combined expression patterns of Crb proteins and because no MPP5 homologs other than Nok have been found to be expressed in undifferentiated CNS (Fig 3), we thus next investigated the possibility of interdependency of Nok and Crb proteins for their apical localization and functions in brain neuroepithelium with Nok or Crb deficient embryos. We found that loss of individual Crb1, Crb2a, or Crb2b did not affect the apical localization of Nok (Fig. 6BD). However, loss of all three Crb proteins largely abolished the apical localization of Nok in the hindbrain neuroepithelium but not in the otic vesicle (Fig. 6E), indicating that Crb1, Crb2a, and Crb2b function redundantly in targeting Nok to the apical surface in the neuroepithelium. Moreover, as shown at higher magnification (Fig. 6F), ZO-1 signals were concentrated as irregular spots (arrowheads) in the apical half of the neural epithelial cells in these triple compound morphants, suggesting the apical basal polarity was not completely lost in these triple crb morphants. However, even in the presence of this partial apicobasal polarity, very minimal Nok signals are found to be colocalizing with small number of ZO-1 positive structures (Fig. 6F, arrowheads; also see Fig. 6E, arrows). The Nok signal strength in such places is much weaker than in wildtype neuroepithelium or than in the apical surface of the otic epithelium in the same embryo (Fig. 6E), suggesting that loss of Crb1/Crb2a/Crb2b expression significantly diminished the apical localization of Nok. By contract, Nok signals appeared to be more diffusely distributed throughout the neural epithelial cells, showing no preferred co-localization with ZO-1 in majority of the morphants neuroepithelial cells (Fig. 6F; arrows indicate basally-localized Nok signals where no ZO-1 signals were detected.). Although we could not state in a quantitative term about the stability of Nok in crb triple morphants (also see discussion), these Nok immuno-fluorescence signals suggest the presence of Nok protein in these morphants (Fig. 6E and F). If we could regard the ZO-1 positive structures as residual apical domain of neural epithelial cells in these morphants (Fig. 6F), the above phenomena would suggest that the lack of apical localization of Nok in Crb triple morphants is unlikely a non-specific consequence of the polarity defects; rather, it would suggest that Crb proteins are required for apical targeting of Nok.
Figure 6.
The localization of Nok and Crb1, Crb2a, and Crb2b relies on each other in the central nervous system but not in the otic vesicle. A. Nok localizes to the apical surfaces of the hindbrain neuroepithelium (arrows) and the otic vesicle (arrowheads) in wildtype at 20-ss. B-D. Individual loss of Crb1 (B), Crb2b (C), and Crb2a (D) did not affect the apical localization of Nok in the hindbrain (arrows) and the otic vesicle (arrowheads). E. Simultaneous suppression of Crb1, Crb2a, and Crb2b disrupted proper apicobasal polarization of the hindbrain neuroepithelium and Nok localized diffusely in the neural rod. By contrast, the apical localization of Nok in the otic vesicle (arrowhead) was not affected. Arrows indicate some occasional ZO-1 positive structures that also show some low level of Nok signals. F. A boxed region in panel E was magnified four times to show the biased distribution of ZO-1 in the apical end of the epithelium (arrowheads) compared to the basal ends (arrows). By contrast, there was no clear difference in Nok distribution between apical end and the basal end. “Otic V.” for otic vesicle; Scale bar, 50 ! m (A-E) and 12 ! m (F).
3.5 Nok is required for apical localization of Crb proteins in neuroepithelial cells
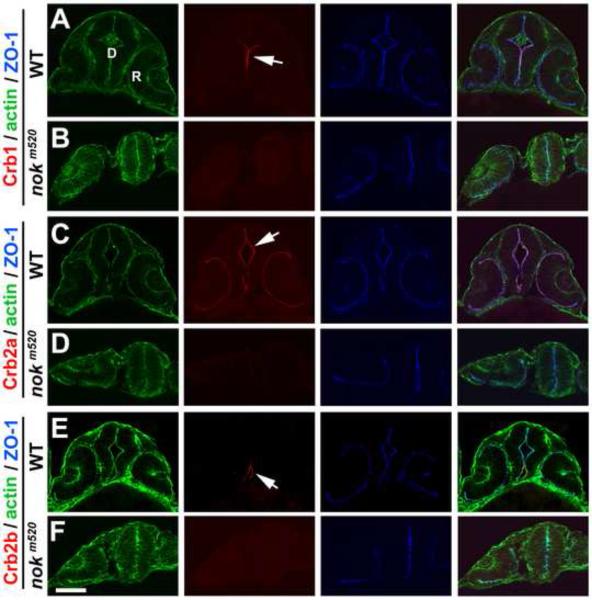
To examine the role of Nok for Crb localization, we next investigated the distribution patterns of Crb in nok mutants. We found that all three Crb proteins lost their apical localization in the diencephalon neuroepithelium in nokm520 mutants at 26-ss (Fig. 7). However, in these Nok deficient embryos, the polarization of ZO-1 and actin bundles in the diencephalon neuroepithelial cells the was largely unaffected; total rounding of neuroepithelial cells was rarely observed and ZO-1 signals were still largely concentrated in the midline vicinity, suggesting that in the absence of Nok, additional layers of polarity maintenance mechanisms, such as adherens junction, are at least partially at play in the brain neuroepithelium. Nevertheless, the apical markers were not smoothly aligned in mutants compared to wildtype and the diencephalon and hindbrain ventricle was not developed as well (Figs. 7). Thus, Nok is required for all three Crb proteins to localize properly to the apical surface diencephalon neuroepithelium at 26-ss.
Figure 7.
The localization of Crb1, Crb2a, and Crb2b requires Nok in the diencephalon at 26-ss. A, C, E. Crb1, Crb2a, and Crb2b localized to the apical surface of the diencephalon (arrows) in wildtype embryos. B, D, F. Unlike in wildtype embryos, Crb1, Crb2a and Crb2b could not localize to the apical surface of the diencephalon in nokm520 mutants. R, retina; D, diencephalon. Scale bars, 50 ! m.
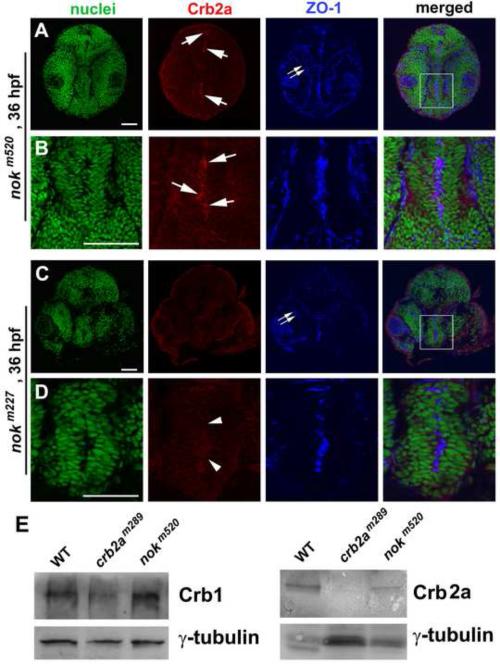
Interestingly, at later developmental stages, such as at 24 hpf and 36 hpf, when cell division activity slows down in certain brain neuroepithelial regions, certain levels of Crb proteins localize to their apical surfaces in nokm520 mutants but not in nokm227 mutants (Fig. 8 for Crb2a; data for Crb1 and Crb2b not shown). Because nokm227mutation disrupts Nok’s Crb-binding PDZ domain by a single amino acid replacement and nokm520 mutation truncates its C-terminal GUK domain and leaves its PDZ domain intact (Wei and Malicki, 2002), the observation of apical localization of Crb at certain brain apical surfaces in nokm520 but not in nokm227 mutants has two implications: The first implication is that nok mutations do not lead to a complete wipeout of Crb expression during early development stages. This observation, however, does not agree with the conclusion of the complete lack of Crb2a expression in embryonic protein extracts of 30-hpf nokm520 mutants, which was reached via Western blotting analyses using pan-Crb antibodies that recognize short common amino acid sequences of the intracellular domains of all types of Crb proteins (Hsu et al., 2006). To further verify the presence of Crb1 and Crb2a in nokm520 mutants at 30 hpf, we also performed Western blotting analyses using our Crb antibodies which specifically recognize the extracellular domains of Crb proteins; we confirmed the presence of Crb1 and Crb2a expression in nokm520 mutants at 30 hpf (Fig. 8E), suggesting that lack of detection of Crb in nokm520 mutants by Hsu et al. (2006) was likely due to the low sensitivity of the pan-Crb antibodies. The detection of Crb protein expression in nok mutants is not unique to early developmental stages; previously, high levels of Crb proteins were also detected within photoreceptors in nok mutants at 4-5 dpf (Wei et al., 2006b; Zou et al., 2012). The second implication of the apical expression of Crb proteins in nokm520 mutants is that a mutant Nok protein such as Nokm520 is still partially capable of targeting Crb to the apical surface of the certain regions of brain neuroepithelium between 24 and 36 hpf, as long as Nok’s PDZ domain is still functional.
Figure 8.
Crb2a localized to the apical surface of certain regions of the diencephalon neuroepithelium in nokm520 mutants but not in nokm227 mutants at 36 hpf. A, B. Arrows indicate the local enrichment of Crb2a at the apical surfaces of certain regions of the diencephalon in nokm520 mutants. A boxed region in panel A is enlarged in panel B. C, D. Arrowheads indicate the corresponding apical surfaces of the diencephalon in nokm227mutants, showing the lack of apical enrichment of Crb2a. A boxed region in panel C is enlarged in panel D. The apical markers ZO-1 localized ectopically to the interior of the retina (double arrows), as expected in nok mutants (A, C). The nuclei were stained with TO-PRO (green). Scale bar, 50 ! m. E. Western blotting analyses of protein expression at 30 hpf detected Crb1 in both nokm520 and crb2am289 mutants as well as Crb2a in WT and nokm520 but not in crb2am289 mutants. ! -tubulin blotting was used as loading controls. Please note that even though there are variations in ! -tubulin loading control signals for the Crb2a blotting (likely due to sample lose during preparation), our conclusion that certain level of Crb2a is present in nokm520 mutant embryos still stands.
In summary, the proper apical localizations of Crb proteins in the CNS depend on functional Nok. Our data suggest that Nok is the sole PDZ domain-containing Crb MPP5 partner in the undifferentiated CNS that binds to the C-termini of the Crb proteins. This notion is consistent with the previous observation of direct physical interaction between Nok and Crb proteins in zebrafish (Hsu et al., 2006).
4. Discussion
In this study, we show that the undifferentiated CNS expresses Crb1, Crb2a, and Crb2b in distinct spatial and temporal patterns, with Crb2a and Crb1 expressed more broadly than Crb2b. Specifically, Crb2a is expressed earlier than Crb1 in the diencephalon. By contrast, Crb1 is expressed slightly earlier than Crb2a in the hindbrain region. The optic vesicle and undifferentiated retina express Crb2a alone. Finally, Crb2b expression is restricted to the ventral neuroepithelium in the anterior CNS.
4.1 Spatial and temporal regulation of Crb protein expression for stepwise neural epithelial development
The spatial and temporal divergence of the expressions of different Crb homologs may reflect a requirement for quantitative balance of the overall Crb expression level. Although it is unclear whether the slight temporal variations in the apical accumulation of Crb1 and Crb2a in the diencephalon and hindbrain reflect differences in the timing of their mRNA expressions, protein translation, or protein trafficking, the apical expression of Crb2a but not Crb1 and Crb2b in the optic vesicle and undifferentiated retina should be largely regulated at mRNA expression level because crb2a mRNA but not that of crb1 and crb2b is expressed at early developmental stages in the retina (Omori and Malicki, 2006). However, it is yet to know if crb mRNA molecules are directly targeted to the apical ends of the epithelial cells to facilitate the subsequent apical location of their translation protein products.
This study reconfirms that the apical localization of adherens junction components such as actin bundles and ZO-1 precede that of the Crb/Nok/Lin7c complex (Fig. 2; Yang et al., 2009). Previously, we showed that temporal regulations of polarity protein expression play important roles in stepwise maturation of neuroepithelial polarity and proper neurulation (Yang et al., 2009). It would be exciting to generate additional antibodies against various zebrafish polarity proteins to further dissect the maturation process of the neuroepithelium. To this end, with genetic, transgenic and various imaging approaches, the quick and external development of the transparent zebrafish embryos make them a much better system than other vertebrates in order to study how a vast number of apicobasal polarity proteins interplay with each other to regulate neuroepithelial polarity and neurulation.
4.2 Interdependency of Nok and Crb proteins for their apical localizations in epithelia
The interdependency of Nok and Crb proteins for their localizations to the apical surface of the neuroepithelium is consistent with the notions that they are the central components of an evolutionarily conserved protein complex (Macara, 2004), and that their physical association is required for their proper locations and functions. Although they may act as equal partners, and although there is no particular hierarchical relationship between them regarding subcellular localizations and functions, we could not rule out the possibility that one protein is expressed slightly earlier and plays a more upstream role than the other. Unfortunately, our study could not test this latter possibility because our rabbit Nok and Crb antibodies are not suited for such a high-resolution co-localization study.
4.3 Physical stability of Nok and Crb proteins
Unlike what is suggested previously (Hsu et al., 2006), our Western blotting analyses show that loss of Nok function does not cause complete loss of Crb proteins (Fig. 8E). This disparity is like due to the sensitivity and specificity differences between our Crb paralog-specific antibodies and the pan-Crb antibody used in the Hsu study (Hsu et al., 2006). Although it is relatively easy to confirm the presence of the presumably diffuse distribution of Crb proteins in nok mutants by Western blotting analyses, it is impractical to do a similar Western blotting analysis to verify that loss of Crb does not lead to physical degradation of Nok. The reasons for this difficulty are: First, it would be impractical to dissection Crb1, Crb2a, Crb2b expressing tissues from the rest of the body that express Crb3a and Crb3b as well as Nok. Thus, if protein extracts of whole Crb1/Crb2a/Crb2b triple morphants embryos were analyzed by Western, a positive result of Nok signals would not be conclusive. For example, as shown in Fig 6E (arrowhead), Nok apical expression remained strong in the otic vesicle of triple crb morphants, positive Nok Western signals would not distinguish whether the signals come from the otic vesicle or from the neuroepithelium. Second, there are five crb genes, to completely knockdown all of them (Crb1, Crb2a, Crb2b-lf, Crb2b-sf, Crb3a, Crb3b) with a mixture of six morpholinos for such a Western blotting analysis, the super high concentration of morpholino become toxic to embryos and embryos die before neurulation (already, embryos injected with four morpholinos showed many dying cells Supplementary Fig 2B).
4.4 Differences between retinal neuroepithelium and brain neuroepithelium
Although retinal neuroepithelium is derived from the neuroepithelium of the anterior neural tube, deficiency of Nok or Crb proteins affect apicobasal polarity of the neural retina and diencephalon and hindbrain neuroepithelia differently. As illustrated in Figure 8A and C, apical marker ZO-1 and actin bundles localized ectopically to the interior of the retina at 36 hpf, whereas they largely remained at the apical region in the diencephalon, albeit much less neatly organized than in wildtype (also see Wei and Malicki, 2002; Wei et al., 2006b). Similar observations were also made with crb2a deficiency (Malicki and Driever, 1999). It is also interesting that such tissue difference does not emerge until later developmental stages. For example, at 26-ss, retinal polarity remains intact in nok mutants (Fig. 7B, D, and F; Zou et al., 2008). Thus, there must be some mechanistic differences in the roles of Nok and Crb in regulating epithelial polarity in different biological contexts in terms of both space and time. Identification of such differences would provide critical insight into the regulations of epithelial polarity during embryogenesis.
Supplementary Material
Highlights.
-
! !
Temporal and spatial expression patterns of the Crb1, Crb2a and Crb2b proteins in the early CNS.
-
! !
Functional redundancy among Crb1, Crb2a, and Crb2b in maintaining the polarity of undifferentiated neuroepithelium.
-
! !
Interdependency of Crb and Nok in subcellular localizations and polarity maintenance.
Acknowledgements
This work was supported by an NIH Core Grant (P30EY008098), Eye and Ear Foundation of Pittsburgh, an unrestricted grant from Research to Prevent Blindness (RPB), and a NIH R01 grant (EY016099) and a RPB Wasserman Merit Award to XW. We thank Mrs. Lynne Sunderman for proofreading the English of the manuscript.
Abbreviations
- Nok
Nagie oko
- Crb
Crumbs
- hpf
hours postfertilization
- CNS
the central nervous system
- -ss
-somite stage
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachmann A, Schneider M, Theilenberg E, Grawe F, Knust E. Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature. 2001;414:638–643. doi: 10.1038/414638a. [DOI] [PubMed] [Google Scholar]
- Barald KF, Kelley MW. From placode to polarization: new tunes in inner ear development. Development. 2004;131:4119–4130. doi: 10.1242/dev.01339. [DOI] [PubMed] [Google Scholar]
- Colas JF, Schoenwolf GC. Towards a cellular and molecular understanding of neurulation. Dev Dyn. 2001;221:117–145. doi: 10.1002/dvdy.1144. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4:784–793. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- Gosens I, den Hollander AI, Cremers FP, Roepman R. Composition and function of the Crumbs protein complex in the mammalian retina. Exp Eye Res. 2008;86:713–726. doi: 10.1016/j.exer.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD, Soriano P. Shroom, a PDZ domain-containing actin-binding protein, is required for neural tube morphogenesis in mice. Cell. 1999;99:485–497. doi: 10.1016/s0092-8674(00)81537-8. [DOI] [PubMed] [Google Scholar]
- Hong Y, Stronach B, Perrimon N, Jan LY, Jan YN. Drosophila Stardust interacts with Crumbs to control polarity of epithelia but not neuroblasts. Nature. 2001;414:634–638. doi: 10.1038/414634a. [DOI] [PubMed] [Google Scholar]
- Hong E, Brewster R. N-cadherin is required for the polarized cell behaviors that drive neurulation in the zebrafish. Development. 2006;133:3895–3905. doi: 10.1242/dev.02560. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Willoughby JJ, Christensen AK, Jensen AM. Mosaic Eyes is a novel component of the Crumbs complex and negatively regulates photoreceptor apical size. Development. 2006;133:4849–4859. doi: 10.1242/dev.02685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaddoost S, Nam SC, Bhat MA, Bellen HJ, Choi KW. Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature. 2002;416:178–183. doi: 10.1038/nature720. [DOI] [PubMed] [Google Scholar]
- Kamberov E, Makarova O, Roh M, Liu A, Karnak D, Straight S, Margolis B. Molecular cloning and characterization of Pals, proteins associated with mLin-7. Journal of Biological Chemistry. 2000;275:11425–11431. doi: 10.1074/jbc.275.15.11425. [DOI] [PubMed] [Google Scholar]
- Knust E, Bossinger O. Composition and formation of intercellular junctions in epithelial cells. Science. 2002;298:1955–1959. doi: 10.1126/science.1072161. [DOI] [PubMed] [Google Scholar]
- Lele Z, Folchert A, Concha M, Rauch GJ, Geisler R, Rosa F, Wilson SW, Hammerschmidt M, Bally-Cuif L. parachute/n-cadherin is required for morphogenesis and maintained integrity of the zebrafish neural tube. Development. 2002;129:3281–3294. doi: 10.1242/dev.129.14.3281. [DOI] [PubMed] [Google Scholar]
- Lowery LA, Sive H. Strategies of vertebrate neurulation and a re-evaluation of teleost neural tube formation. Mech Dev. 2004;121:1189–1197. doi: 10.1016/j.mod.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Lowery LA, Sive H. Initial formation of zebrafish brain ventricles occurs independently of circulation and requires the nagie oko and snakehead/atp1a1a.1 gene products. Development. 2005;132:2057–2067. doi: 10.1242/dev.01791. [DOI] [PubMed] [Google Scholar]
- Macara IG. Parsing the polarity code. Nat Rev Mol Cell Biol. 2004;5:220–231. doi: 10.1038/nrm1332. [DOI] [PubMed] [Google Scholar]
- Makarova O, Roh MH, Liu CJ, Laurinec S, Margolis B. Mammalian Crumbs3 is a small transmembrane protein linked to protein associated with Lin-7 (Pals1) Gene. 2003;302:21–29. doi: 10.1016/s0378111902010843. [DOI] [PubMed] [Google Scholar]
- Malicki J, Driever W. oko meduzy mutations affect neuronal patterning in the zebrafish retina and reveal cell-cell interactions of the retinal neuroepithelial sheet. Development. 1999;126:1235–1246. doi: 10.1242/dev.126.6.1235. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Crenshaw EB, 3rd, Kelley MW. Noncanonical Wnt signaling and neural polarity. Annu Rev Neurosci. 2006;29:363–386. doi: 10.1146/annurev.neuro.29.051605.112933. [DOI] [PubMed] [Google Scholar]
- Munson C, Huisken J, Bit-Avragim N, Kuo T, Dong PD, Ober EA, Verkade H, Abdelilah-Seyfried S, Stainier DY. Regulation of neurocoel morphogenesis by Pard6 gamma b. Dev Biol. 2008;324:41–54. doi: 10.1016/j.ydbio.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch JN, Henderson DJ, Doudney K, Gaston-Massuet C, Phillips HM, Paternotte C, Arkell R, Stanier P, Copp AJ. Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum Mol Genet. 2003;12:87–98. doi: 10.1093/hmg/ddg014. [DOI] [PubMed] [Google Scholar]
- Omori Y, Malicki J. oko meduzy and related crumbs genes are determinants of apical cell features in the vertebrate embryo. Curr Biol. 2006;16:945–957. doi: 10.1016/j.cub.2006.03.058. [DOI] [PubMed] [Google Scholar]
- Pujic Z, Malicki J. Mutation of the zebrafish glass onion locus causes early cell-nonautonomous loss of neuroepithelial integrity followed by severe neuronal patterning defects in the retina. Developmental Biology. 2001;234:454–469. doi: 10.1006/dbio.2001.0251. [DOI] [PubMed] [Google Scholar]
- Tawk M, Araya C, Lyons DA, Reugels AM, Girdler GC, Bayley PR, Hyde DR, Tada M, Clarke JD. A mirror-symmetric cell division that orchestrates neuroepithelial morphogenesis. Nature. 2007;446:797–800. doi: 10.1038/nature05722. [DOI] [PubMed] [Google Scholar]
- Tepass U, Theres C, Knust E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990;61:787–799. doi: 10.1016/0092-8674(90)90189-l. [DOI] [PubMed] [Google Scholar]
- Wei X, Malicki J. nagie oko, encoding a MAGUK-family protein, is essential for cellular patterning of the retina. Nature Genetics. 2002;31:150–157. doi: 10.1038/ng883. [DOI] [PubMed] [Google Scholar]
- Wei X, Cheng Y, Luo Y, Shi X, Nelson S, Hyde DR. The zebrafish Pard3 ortholog is required for separation of the eye fields and retinal lamination. Dev Biol. 2004;269:286–301. doi: 10.1016/j.ydbio.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Wei X, Luo Y, Hyde DR. Molecular cloning of three zebrafish lin7 genes and their expression patterns in the retina. Exp Eye Res. 2006a;82:122–131. doi: 10.1016/j.exer.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Wei X, Zou J, Takechi M, Kawamura S, Li L. Nok plays an essential role in maintaining the integrity of the outer nuclear layer in the zebrafish retina. Exp Eye Res. 2006b;83:31–44. doi: 10.1016/j.exer.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zou J, Hyde DR, Davidson LA, Wei X. Stepwise maturation of apicobasal polarity of the neuroepithelium is essential for vertebrate neurulation. J Neurosci. 2009;29:11426–11440. doi: 10.1523/JNEUROSCI.1880-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Lathrop KL, Sun M, Wei X. Intact retinal pigment epithelium maintained by Nok is essential for retinal epithelial polarity and cellular patterning in zebrafish. J Neurosci. 2008;28:13684–13695. doi: 10.1523/JNEUROSCI.4333-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Yang X, Wei X. Restricted localization of ponli, a novel zebrafish MAGUK-family protein, to the inner segment interface areas between green, red, and blue cones. Invest Ophthalmol Vis Sci. 2010;51:1738–1746. doi: 10.1167/iovs.09-4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Wang X, Wei X. Crb apical polarity proteins maintain zebrafish retinal cone mosaics via intercellular binding of their extracellular domains. Dev Cell. 2012;22:1261–1274. doi: 10.1016/j.devcel.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.