Abstract
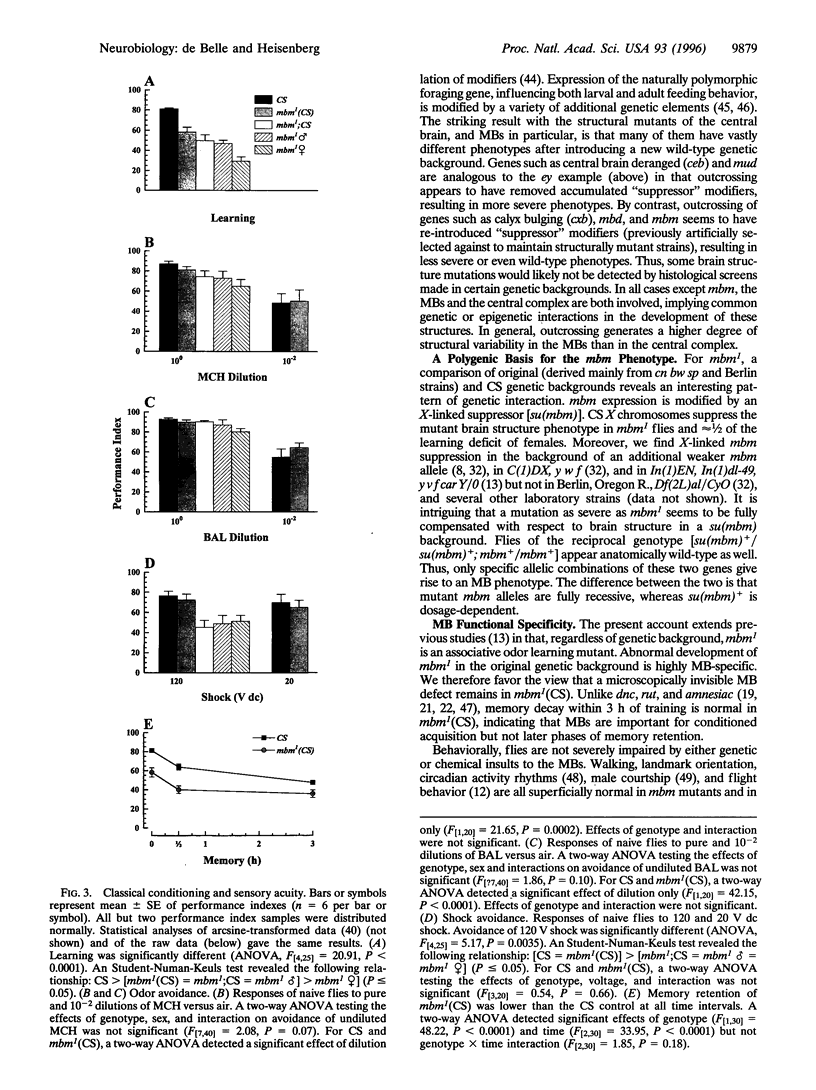
Mutations in 12 genes regulating Drosophila melanogaster mushroom body (MB) development were each studied in two genetic backgrounds. In all cases, brain structure was qualitatively or quantitatively different after replacement of the "original" genetic background with that of the Canton Special wild-type strain. The mushroom body miniature gene (mbm) was investigated in detail. mbm supports the maintenance of MB Kenyon cell fibers in third instar larvae and their regrowth during metamorphosis. Adult mbm1 mutant females are lacking many or most Kenyon cell fibers and are impaired in MB-mediated associative odor learning. We show here that structural defects in mbm1 are apparent only in combination with an X-linked, dosage-dependent modifier (or modifiers). In the Canton Special genetic background, the mbm1 anatomical phenotype is suppressed, and MBs develop to a normal size. However, the olfactory learning phenotype is not fully restored, suggesting that submicroscopic defects persist in the MBs. Mutant mbm1 flies with full-sized MBs have normal retention but show a specific acquisition deficit that cannot be attributed to reductions in odor avoidance, shock reactivity, or locomotor behavior. We propose that polymorphic gene interactions (in addition to ontogenetic factors) determine MB size and, concomitantly, the ability to recognize and learn odors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aszódi A., Müller U., Friedrich P., Spatz H. C. Signal convergence on protein kinase A as a molecular correlate of learning. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5832–5836. doi: 10.1073/pnas.88.13.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balling A., Technau G. M., Heisenberg M. Are the structural changes in adult Drosophila mushroom bodies memory traces? Studies on biochemical learning mutants. J Neurogenet. 1987 Apr;4(2-3):65–73. [PubMed] [Google Scholar]
- Benzer S. BEHAVIORAL MUTANTS OF Drosophila ISOLATED BY COUNTERCURRENT DISTRIBUTION. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1112–1119. doi: 10.1073/pnas.58.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton S., Tully T. latheo, a new gene involved in associative learning and memory in Drosophila melanogaster, identified from P element mutagenesis. Genetics. 1992 Jul;131(3):655–672. doi: 10.1093/genetics/131.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L. Mushroom bodies and Drosophila learning. Neuron. 1993 Jul;11(1):1–14. doi: 10.1016/0896-6273(93)90266-t. [DOI] [PubMed] [Google Scholar]
- Drain P., Folkers E., Quinn W. G. cAMP-dependent protein kinase and the disruption of learning in transgenic flies. Neuron. 1991 Jan;6(1):71–82. doi: 10.1016/0896-6273(91)90123-h. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Neurogenetic dissection of learning and short-term memory in Drosophila. Annu Rev Neurosci. 1988;11:537–563. doi: 10.1146/annurev.ne.11.030188.002541. [DOI] [PubMed] [Google Scholar]
- Dura J. M., Preat T., Tully T. Identification of linotte, a new gene affecting learning and memory in Drosophila melanogaster. J Neurogenet. 1993 Aug;9(1):1–14. doi: 10.3109/01677069309167272. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 1996 May;19(5):177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- Hammer M., Menzel R. Learning and memory in the honeybee. J Neurosci. 1995 Mar;15(3 Pt 1):1617–1630. doi: 10.1523/JNEUROSCI.15-03-01617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P. L., Levin L. R., Reed R. R., Davis R. L. Preferential expression of the Drosophila rutabaga gene in mushroom bodies, neural centers for learning in insects. Neuron. 1992 Oct;9(4):619–627. doi: 10.1016/0896-6273(92)90026-a. [DOI] [PubMed] [Google Scholar]
- Heisenberg M., Borst A., Wagner S., Byers D. Drosophila mushroom body mutants are deficient in olfactory learning. J Neurogenet. 1985 Feb;2(1):1–30. doi: 10.3109/01677068509100140. [DOI] [PubMed] [Google Scholar]
- Heisenberg M., Heusipp M., Wanke C. Structural plasticity in the Drosophila brain. J Neurosci. 1995 Mar;15(3 Pt 1):1951–1960. doi: 10.1523/JNEUROSCI.15-03-01951.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilius M., Wolf R., Heisenberg M. The central complex of Drosophila melanogaster is involved in flight control: studies on mutants and mosaics of the gene ellipsoid body open. J Neurogenet. 1994 Jul;9(3):189–206. doi: 10.3109/01677069409167279. [DOI] [PubMed] [Google Scholar]
- Ito K., Hotta Y. Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev Biol. 1992 Jan;149(1):134–148. doi: 10.1016/0012-1606(92)90270-q. [DOI] [PubMed] [Google Scholar]
- Levin L. R., Han P. L., Hwang P. M., Feinstein P. G., Davis R. L., Reed R. R. The Drosophila learning and memory gene rutabaga encodes a Ca2+/Calmodulin-responsive adenylyl cyclase. Cell. 1992 Feb 7;68(3):479–489. doi: 10.1016/0092-8674(92)90185-f. [DOI] [PubMed] [Google Scholar]
- Nighorn A., Healy M. J., Davis R. L. The cyclic AMP phosphodiesterase encoded by the Drosophila dunce gene is concentrated in the mushroom body neuropil. Neuron. 1991 Mar;6(3):455–467. doi: 10.1016/0896-6273(91)90253-v. [DOI] [PubMed] [Google Scholar]
- Ogaki M. Reversal effect of the genetic background on some small-eye mutants, with special reference to gene action of morphogenetic mutants in Drosophila. Genetica. 1966;37(3):391–402. doi: 10.1007/BF01547144. [DOI] [PubMed] [Google Scholar]
- Pereira H. S., MacDonald D. E., Hilliker A. J., Sokolowski M. B. Chaser (Csr), a new gene affecting larval foraging behavior in Drosophila melanogaster. Genetics. 1995 Sep;141(1):263–270. doi: 10.1093/genetics/141.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A., Technau G. M. Normal function of the mushroom body defect gene of Drosophila is required for the regulation of the number and proliferation of neuroblasts. Dev Biol. 1994 Feb;161(2):321–337. doi: 10.1006/dbio.1994.1034. [DOI] [PubMed] [Google Scholar]
- Qiu Y. H., Chen C. N., Malone T., Richter L., Beckendorf S. K., Davis R. L. Characterization of the memory gene dunce of Drosophila melanogaster. J Mol Biol. 1991 Dec 5;222(3):553–565. doi: 10.1016/0022-2836(91)90496-s. [DOI] [PubMed] [Google Scholar]
- Skoulakis E. M., Kalderon D., Davis R. L. Preferential expression in mushroom bodies of the catalytic subunit of protein kinase A and its role in learning and memory. Neuron. 1993 Aug;11(2):197–208. doi: 10.1016/0896-6273(93)90178-t. [DOI] [PubMed] [Google Scholar]
- Strauss R., Hanesch U., Kinkelin M., Wolf R., Heisenberg M. No-bridge of Drosophila melanogaster: portrait of a structural brain mutant of the central complex. J Neurogenet. 1992 Sep;8(3):125–155. doi: 10.3109/01677069209083444. [DOI] [PubMed] [Google Scholar]
- Strauss R., Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. J Neurosci. 1993 May;13(5):1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabad J., Máthé E., Puro J. Horka, a dominant mutation of Drosophila, induces nondisjunction and, through paternal effect, chromosome loss and genetic mosaics. Genetics. 1995 Apr;139(4):1585–1599. doi: 10.1093/genetics/139.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Technau G. M. Fiber number in the mushroom bodies of adult Drosophila melanogaster depends on age, sex and experience. J Neurogenet. 1984 Apr;1(2):113–126. doi: 10.3109/01677068409107077. [DOI] [PubMed] [Google Scholar]
- Technau G., Heisenberg M. Neural reorganization during metamorphosis of the corpora pedunculata in Drosophila melanogaster. Nature. 1982 Feb 4;295(5848):405–407. doi: 10.1038/295405a0. [DOI] [PubMed] [Google Scholar]
- Tully T., Boynton S., Brandes C., Dura J. M., Mihalek R., Preat T., Villella A. Genetic dissection of memory formation in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1990;55:203–211. doi: 10.1101/sqb.1990.055.01.022. [DOI] [PubMed] [Google Scholar]
- Tully T., Gold D. Differential effects of dunce mutations on associative learning and memory in Drosophila. J Neurogenet. 1993 Aug;9(1):55–71. doi: 10.3109/01677069309167275. [DOI] [PubMed] [Google Scholar]
- Tully T., Preat T., Boynton S. C., Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994 Oct 7;79(1):35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Tully T., Quinn W. G. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A. 1985 Sep;157(2):263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Yang M. Y., Armstrong J. D., Vilinsky I., Strausfeld N. J., Kaiser K. Subdivision of the Drosophila mushroom bodies by enhancer-trap expression patterns. Neuron. 1995 Jul;15(1):45–54. doi: 10.1016/0896-6273(95)90063-2. [DOI] [PubMed] [Google Scholar]
- de Belle J. S., Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994 Feb 4;263(5147):692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]






