Abstract
Background
We investigated the relationship between self-reported sleep characteristics and brachial artery flow-mediated dilation (FMD) in a community-based population. Prior studies document that sleep apnea may be related to endothelial dysfunction but disagree whether subjective reports of sleep may also reflect such associations.
Methods
In 684 subjects (32% male) between 37 and 60 years enrolled in the Emory-Georgia Tech Predictive Health Institute study, we measured reported sleep characteristics using the Epworth Sleepiness Scale (ESS) and the Pittsburgh Sleep Quality Index (PSQI) along with cardiovascular risk factors. Endothelial function was assessed using brachial artery FMD. Multivariate analysis of covariance was used to adjust for various cardiovascular risk factors including age, race, gender, smoking, hypertension, diabetes, and body mass index.
Results
Lower brachial artery FMD values were correlated with higher ESS scores (p = 0.0275), even after adjustment for risk factors (p = 0.03). Total PSQI score was unrelated to brachial artery FMD. However, lower sleep quality (PSQI component 1) was associated with lower brachial artery FMD (multivariate p = 0.038), and participants who coughed or snored during sleep also had lower brachial artery FMD (6.24±3.42%) compared to those who did not (6.92±4.30%) (p = 0.056). This difference remained significant after adjustment for risk factors (p = 0.03).
Conclusion
In a community-based population, our analysis indicates a significant association between sleepiness and snoring assessed by questionnaires and endothelial function. Simple subjective reports about individuals’ sleep may be highly revealing indicators of endothelial function impairment and thus important indicators of cardiovascular disease risk.
Keywords: flow mediated dilation, survey-based sleep assessment, proxy sleep apnea symptoms
INTRODUCTION
Abnormal endothelial function has been associated with sleep apnea using biochemical markers such as vascular endothelial growth factor (VEGF)1, declines in nitric oxide bioavailability2, monocyte activation3, and with radiologic or dynamic vascular measures such as coronary artery calcification4, carotid intima-media thickness5, and flow mediated vasodiliation6. Some of these alterations may even be reversible with nasal continuous positive airway pressure (CPAP) therapy1,2,7. Far more equivocal are associations between endothelial function and subjective reports of sleep duration or other aspects such as snoring or low sleep quality. For example, although laboratory-based studies of experimental sleep deprivation may increase adhesion molecules and E-selectin8 or result in elevated blood pressures9, whether such relationships are also reflected in subjects’ verbal reports about their own short duration of sleep remain far more dubious. Low reported sleep quality in a large population based sample10 was reported to be unrelated to altered FMD and short reported sleep durations were unrelated to levels of endothelial progenitor cells11, but other studies suggest that reported (or actigraphically assessed) short sleep durations may be associated with increased carotid intima-media thickness12,13 or coronary artery calcification14. In this study, we examined self-reported, sleep-related symptoms in a community-based, non-clinic population with commonly used standardized scales and related these results to brachial artery FMD – long recognized to be the gold standard of abnormal endothelial dysfunction15.
METHODS
Study Sample
The sample was derived from the “Predictive Health Institute: The Center for Health Discovery and Well Being,” which consists of participating Emory University and Georgia Tech University employees (mean age 48±11 years, 68% female, and 71% white.) History of diabetes and hypertension were defined with the use of anti-diabetic and anti-hypertensive medications respectively. Smoking history, obtained using standardized questionnaires, was defined as current or never/former (no cigarettes within the past 30 days). Height and weight were measured. Blood pressure was monitored with an automatic blood pressure monitor. The study was approved by the Emory University and Georgia Tech University Institutional Review Committees. Informed consent was obtained from all participants.
Measurement of Reported Sleep Characteristics
Participants enrolled in the study completed two questionnaires collecting data related to their sleep: the Pittsburgh Sleep Quality Index (PSQI) and the Epworth Sleepiness Scale (ESS). The PSQI is a self-administered validated 19-item scale that assesses overall sleep-quality and a wide range of sleep-related symptoms experienced during the previous 1 month16. The 19 items of the PSQI yield 7-component scores that reflect the frequency of sleep problems. The sum of the 7 components yields a global score that ranges from 0 to 21, with higher scores indicating poorer sleep quality. We utilized total PSQI scores, the 7 component scores, and selected item analysis of specific sleep disturbance complaints as done previously17. These items included self-reports of cough / snore, sleep durations, and reports of getting up at night to use the restroom – all symptoms that have been associated with sleep apnea.
The ESS measures daytime sleepiness. A summary score is derived from the likelihood of falling asleep during the daytime in eight different situations,18 which the subject rates on a scale of 0–3 how likely they would be to doze off or fall asleep. Scores range from 0 to 24, with higher scores indicating greater daytime sleepiness.
Assessment of endothelial function
The assessment of endothelial function in this study was done via FMD. Endothelium-dependent brachial artery FMD was determined as previously described19,20. Briefly, ultrasound images were obtained at baseline under standardized conditions and ≈60 seconds after induction of reactive hyperemia by 5-minute cuff occlusion of the forearm. After a 15-minute period to re-establish baseline conditions, endothelium-independent dilation of the brachial artery was assessed from images obtained before and 3 to 5 minutes after administration of 0.4 mg of sublingual nitroglycerin. Images were digitized online, and arterial diameters were measured with customized software (Medical Imaging Applications, Inc) by individuals blinded to the clinical status and laboratory status of the subjects. FMD and endothelium-independent vasodilation were expressed as the percentage increase in diameter from baseline.
Statistical methods
Study variables are described as the mean ± SD (unless otherwise specified) for continuous variables or as counts or proportions for categorical variables. Age, body mass index, and FMD were treated as continuous variables. Smoking, gender, and medication use (anti-hypertensive drugs, glucose lowering agents) were categorical variables. Continuous variables were tested for normality with the Kolmogorov-Smirnov criterion. Skewed variables were log transformed and tested again for normality before any parametric analysis.
Univariate correlations between brachial artery FMD and measured sleep parameters were performed with Pearson correlation. Multivariate linear regression models were constructed to determine relationships between sleep and vascular function parameters before and after adjustment for age, race, gender, smoking, hypertension (defined by medication use), diabetes (defined by medication use), and body mass index. Group differences were evaluated by Student t-tests or 1-way analysis of covariance. Statistical significance was based on 2-tailed tests, and p-values ≤0.05 were considered significant. Analyses were performed with SPSS (version 17.0, SPSS, Inc., Chicago, Illinois).
RESULTS
Subject characteristics
Baseline characteristics of the 684 subjects are shown in Table 1. Of the participants (mean age 48±11 years), 68% were female, and 71% were white. This was a relatively healthy population, as indicated by relative low rates of hypertension, diabetes, and tobacco use.
Table 1. Participant Demographics.
Demographic characteristics for the entire study cohort, subjects who report coughing or snoring during sleep, and those who did not.
| All participants | Denies Cough / Snore | Reports Cough / Snore | ||
|---|---|---|---|---|
| n = 684 | n = 477 | n = 207 | p-value | |
| Age | 48 ± 11 | 48 ± 11 | 51 ± 9 | 0.002 |
| Male Gender (%) | 219 (32.0%) | 169 (31.3%) | 46 (36.2%) | 0.2927 |
| Whites (%) | 483 (70.6%) | 392 (58.8%) | 80 (12.0%) | 0.0242 |
| Blacks (%) | 162 (23.7%) | 115 (17.2%) | 43 (6.45%) | 0.0242 |
| Asians (%) | 35 (5.1%) | 29 (4.35%) | 4 (0.6%) | 0.0242 |
| Hypertension (%) | 88 (12.9%) | 58 (8.7%) | 29 (4.4%) | <0.001 |
| Diabetes (%) | 12 (1.8%) | 7 (1.15) | 4 (0.6%) | 0.2348 |
| Tobacco Use | 37 (5.4%) | 25 (3.9%) | 12 (1.9%) | 0.0477 |
| BMI | 28 ± 6.5 | 27 ± 6 | 31 ± 7 | <.0001 |
| Epworth Sleepiness Score | 6.7 ± 4.1 | 6.3 ± 4.0 | 8.0 ± 4.4 | <.0001 |
| FMD % | 6.8 ± 4.2 | 6.9 ± 4.3 | 6.2 ± 3.4 | 0.0557 |
Reported Sleep
PSQI
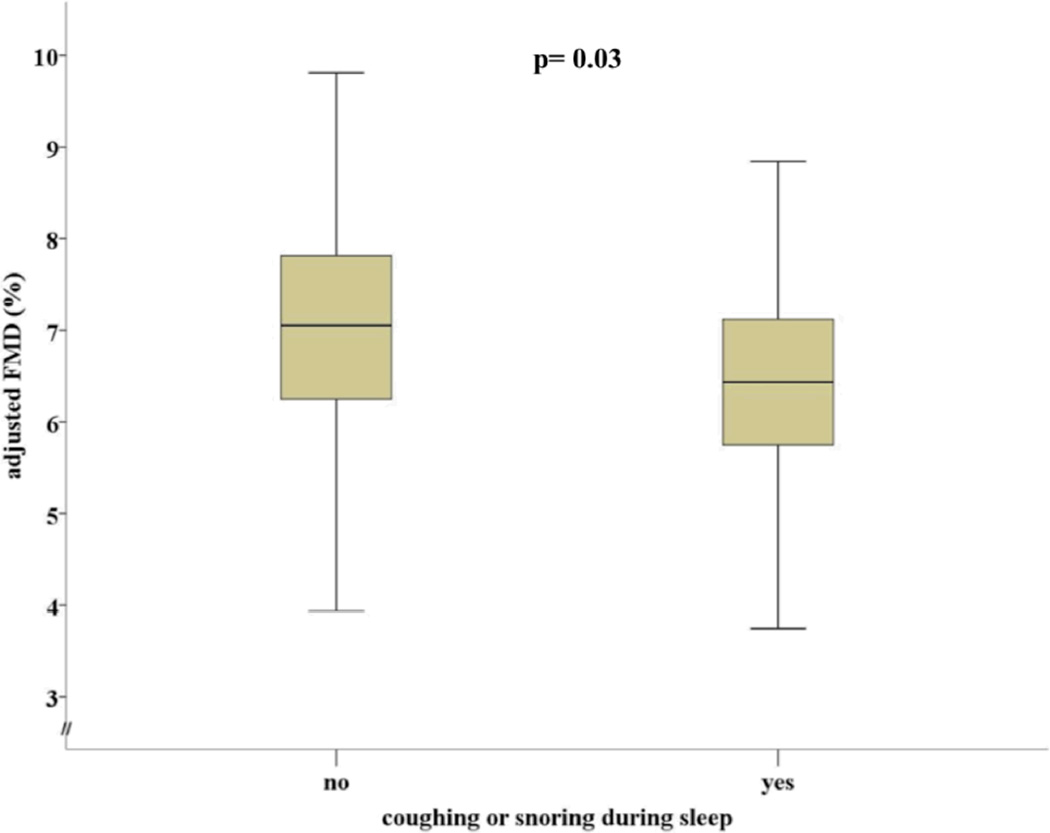
Total PSQI scores were unrelated to brachial artery FMD (multivariate p= 0.1223), suggesting no relationship to endothelial function. Of the 7 PSQI component scores, only component 1 (sleep quality) was related to FMD and indicated that poorer subjective sleep quality was associated with lower FMD (multivariate p= 0.038). Additionally, subjects who reported coughing or snoring at least once a week had lower FMD values (6.2±3.4) when compared to those individuals who reported no such symptoms over the last month (6.9±4.3) (univariate p= 0.056) (effect size d = .20). This value maintained statistical significance after multivariate adjustment for age, gender, race, body mass index (BMI), smoking history, history of hypertension, and history of diabetes (multivariate p= 0.03) (Figure 1). When examining sleep duration as a continuous variable, there was a near significant association (multivariate p= 0.0671) with those subjects who obtain less sleep and lower FMD measurements. Getting up at night to use the bathroom and FMD were unrelated (multivariate p= 0.6133).
Figure 1.
Difference in multivariate-adjusted FMD by report of coughing and/or snoring during sleep. FMD: Flow mediated dilation (%).
ESS
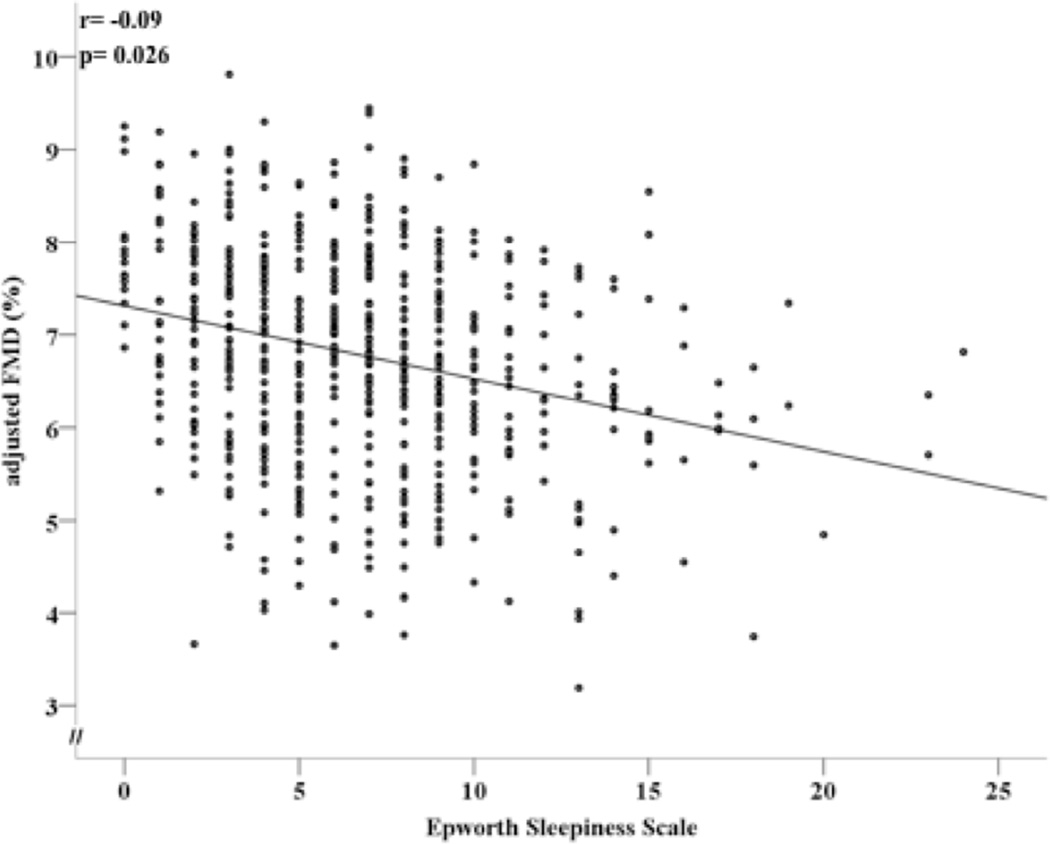
Using multivariate models comparable to those used for PSQI, higher ESS scores were associated with lower brachial artery FMD (p= 0.026) (Figure 2). Interestingly, those who reported cough / snore at night had higher ESS scores (8.0±4.4) compared to those who did not (6.3±4.0) (multivariate p< 0.0001). (Table 1). The mean ESS score in this population is similar to those reported in other biracial populations, such as the Coronary Artery Risk Development in Young Adults (CARDIA) study21, although the distribution (Figure 2) suggests a substantial number of individuals reporting high levels of subjective daytime sleepiness, perhaps compatible with a degree of relatively restricted within the sleep population generally22–24.
Figure 2.
The relationship between the Epworth Sleepiness Scale and FMD adjusted for CVD risk factors. FMD: Flow mediated dilation (%). r: Pearson’s correlation coefficient; p-value is for 2-tailed test of significance.
DISCUSSION
Using a “gold standard” assessment of endothelial function in a relatively large, community-based population with a relatively low risk factor burden, we demonstrated an association between brachial artery FMD and selected aspects of reported sleep, including subjective quality, coughing/snoring during sleep, and daytime sleepiness even after adjustment for common CVD risk factors. It may well be that reports of relatively poor sleep quality, coughing/snoring and daytime sleepiness in this study were related to lower FMD values because they serve as proxy symptoms of sleep apnea25. One weakness of this study is that we did not obtain measurements of sleep physiology to confirm whether this may indeed be the case. However, the apparent interaction of reported snoring and daytime sleepiness suggests that these symptoms may have additive risk. Epidemiologic studies examining sleep-related cardiovascular risk factors may well consider viewing these symptoms in tandem. The association observed between brachial artery FMD and coughing/snoring was of small magnitude (d = .20), although this effect was at least comparable to, if not larger, than the effect size noted (r2 = .012) in a study relating brachial artery FMD to polysomnographically assessed AHI6. Brachial artery FMD is known to be affected beneficially by beta-blockers, perhaps by increasing nitric oxide bioavailability26,27, although the relatively small number of number of patients using this drug class (n = 62) makes it unlikely this had a major impact on our results.
Our study has other weaknesses as well. Medication use served as a proxy for health conditions. Thus, a diagnosis of hypertension or diabetes was assigned on the basis of medication-use rather than by physical examination or laboratory findings. Utilization of such surrogate markers for disease is not uncommon in epidemiologic studies. In fact, several analyses have demonstrated that such reported medication use may serve as reasonable approximations for disease prevalence28,29. Finally, the questionnaires used here to assess for sleep apnea (PSQI, ESS) may not be best suited for this purpose. Currently, questionnaires such as the STOP-BANG30 or the Berlin Questionnaire31 see widespread use, target sleep apnea symptoms per se, and may be more valid measures.
CONCLUSION
Even in the absence of measured sleep apnea, specific subjective sleep-related symptoms may be important independent correlates of endothelial dysfunction as measured by brachial artery FMD.
Acknowledgments
Source of Funding: Information upon which this work is based is from the Emory Predictive Health Participant Database and supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: There are no conflicts of interest in publishing this manuscript.
REFERENCES
- 1.Lavie L, Kraiczi H, Hefetz A, Ghandour H, Perelman A, Hedner J, Lavie P. Plasma vascular endothelial growth factor in sleep apnea syndrome: effects of nasal continuous positive air pressure treatment. Am J Respir Crit Care Med. 2002;165:1624–1628. doi: 10.1164/rccm.20110-040OC. [DOI] [PubMed] [Google Scholar]
- 2.Lavie L, Hefetz A, Luboshitzky R, Lavie P. Plasma levels of nitric oxide and L-arginine in sleep apnea patients: effects of nCPAP treatment. J Mol Neurosci. 2003;21:57–63. doi: 10.1385/JMN:21:1:57. [DOI] [PubMed] [Google Scholar]
- 3.Minoguchi K, Tazaki T, Yokoe T, Minoguchi H, Watanabe Y, Yamamoto M, Adachi M. Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest. 2004;126:1473–1479. doi: 10.1378/chest.126.5.1473. [DOI] [PubMed] [Google Scholar]
- 4.Sorajja D, Gami AS, Somers VK, Behrenbeck TR, Garcia-Touchard A, Lopez-Jimenez F. Independent association between obstructive sleep apnea and subclinical coronary artery disease. Chest. 2008;133:927–933. doi: 10.1378/chest.07-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wattanakit K, Boland L, Punjabi NM, Shahar E. Relation of sleep-disordered breathing to carotid plaque and intima-media thickness. Atherosclerosis. 2008;197:125–131. doi: 10.1016/j.atherosclerosis.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Nieto FJ, Herrington DM, Redline S, Benjamin EJ, Robbins JA. Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. Am J Respir Crit Care Med. 2004;169:354–360. doi: 10.1164/rccm.200306-756OC. [DOI] [PubMed] [Google Scholar]
- 7.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–353. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 8.Sauvet F, Leftheriotis G, Gomez-Merino D, Langrume C, Drogou C, Van Beers P, Bourrilhon C, Florence G, Chennaoui M. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol. 2010;108:68–75. doi: 10.1152/japplphysiol.00851.2009. [DOI] [PubMed] [Google Scholar]
- 9.Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive subjects: A 24-h study. Am J Hypertens. 1999;12:63–68. doi: 10.1016/s0895-7061(98)00200-3. [DOI] [PubMed] [Google Scholar]
- 10.Strand LB, Laugsand LE, Skaug E-A, Ellingsen O, Madssen E, Wisloff U, Vatten L, Janszky I. Insomnia and endothelial function—The HUNT 3 Fitness Study. PLOS One. 2012;7:e50933. doi: 10.1371/journal.pone.0050933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weil BR, Maceneaney OJ, Stauffer BL, Desouza CA. Habitual short sleep duration and circulating endothelial progenitor cells. J Cardiovasc Dis Res. 2011;2:110–114. doi: 10.4103/0975-3583.83039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sands MR, Lauderdale DS, Liu K, Knutson KL, Matthews KA, Eaton CB, Linkletter CD, Loucks EB. Short sleep duration is associated with carotid intima-media thickness among men in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Stroke. 2012;43:2858–2864. doi: 10.1161/STROKEAHA.112.660332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolff B, Volzke H, Schwahn C, Robinson D, Kessler C, John U. Relation of self-reported sleep duration with carotid intima-media thickness in a general population sample. Atherosclerosis. 2008;196:727–732. doi: 10.1016/j.atherosclerosis.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 14.King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300:2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quyyumi AA. Prognostic value of endothelial function. Am J Cardiol. 2003;91:19H–24H. doi: 10.1016/s0002-9149(03)00430-2. [DOI] [PubMed] [Google Scholar]
- 16.Buysse DJ, Reynolds Iii CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiat Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 17.Stein MB, Chartier M, Walker JR. Sleep in nondepressed patients with panic disorder: I. Systematic assessment of subjective sleep quality and sleep disturbance. Sleep. 1993;16:724–726. doi: 10.1093/sleep/16.8.724. [DOI] [PubMed] [Google Scholar]
- 18.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 19.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 20.Prasad A, Tupas-Habib T, Schenke WH, Mincemoyer R, Panza JA, Waclawin MA, Ellahham S, Quyyumi AA. Acute and chronic angiotensin-1 receptor antagonism reverses endothelial dysfunction in atherosclerosis. Circulation. 2000;101:2349–2354. doi: 10.1161/01.cir.101.20.2349. [DOI] [PubMed] [Google Scholar]
- 21.Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS. Stability of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Questionnaires over 1 year in early middle-aged adults: The CARDIA Study. Sleep. 2006;29:1503–1506. doi: 10.1093/sleep/29.11.1503. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC) Perceived insufficient rest or sleep--four states, 2006. MMWR Morb Mortal Wkly Rep. 2008;57:200–203. [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC) Perceived insufficient rest or sleep among adults - United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1175–1179. [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC) Effect of short sleep duration on daily activities--United States, 2005–2008. MMWR Morb Mortal Wkly Rep. 2011;60:239–242. [PubMed] [Google Scholar]
- 25.Stradling JR, Crosby JH, Payne CD. Self reported snoring and daytime sleepiness in men aged 35–65 years. Thorax. 1991;46:807–810. doi: 10.1136/thx.46.11.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lekakis JP, Protogerou A, Papamichael C, Vamvakou G, Ikonomidis I, Fici F, Mavrikakis M. Effect of nebivolol and atenolol on brachial artery flow-mediated vasodilation in patients with coronary artery disease. Cardiovasc Drug Ther. 2005;19:277–281. doi: 10.1007/s10557-005-3117-9. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda Y, Akita H, Terashima M, Shiga N, Kanazawa K, Yokoyama M. Carvedilol improves endothelium-dependent dilatation in patients with coronary artery disease. Am Heart J. 2000;140:753–759. doi: 10.1067/mhj.2000.110093. [DOI] [PubMed] [Google Scholar]
- 28.Kehoe R, Wu SY, Leske MC, Chylack LT., Jr Comparing self-reported and physician-reported medical history. Am J Epidemiol. 1994;139:813–818. doi: 10.1093/oxfordjournals.aje.a117078. [DOI] [PubMed] [Google Scholar]
- 29.Paganini-Hill A, Ross RK. Reliability of recall of drug usage and other health-related information. Am J Epidemiol. 1982;116:114–122. doi: 10.1093/oxfordjournals.aje.a113386. [DOI] [PubMed] [Google Scholar]
- 30.Vasu TS, Doghramji K, Cavallazzi R, Grewal R, Hirani A, Leiby B, Markov D, Reiter D, Kraft WK, Witkowski T. Obstructive sleep apnea syndrome and postoperative complications: clinical use of the STOP-BANG questionnaire. Arch Otolaryngol Head Neck Surg. 2010;136:1020–1024. doi: 10.1001/archoto.2010.1020. [DOI] [PubMed] [Google Scholar]
- 31.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]