Abstract
Introduction
Outcomes for patients undergoing intervention for restenosis after prior ipsilateral carotid endarterectomy (CEA) in the era of carotid stenting (CAS) are unclear. We compared perioperative results and durability of CAS versus CEA in patients with symptomatic or asymptomatic restenosis after prior CEA and investigated the risk of re-intervention compared to primary procedures.
Methods
Patients undergoing CAS and CEA for restenosis between January 2003 and March 2012 were identified within the Vascular Study Group of New England (VSGNE) database.Endpoints included any stroke, death or myocardial infarction (MI) within 30 days, cranial nerve injury at discharge and restenosis ≥70% at 1-year follow-up. Multivariable logistic regression was done to identify whether prior ipsilateral CEA was an independent predictor for adverse outcome.
Results
Out of 9305 CEA procedures, 212 patients (2.3%) underwent redo-CEA (36% symptomatic). Of 663 CAS procedures, 220 patients (33%) underwent CAS after prior ipsilateral CEA (31% symptomatic). Demographics of patients undergoing redo-CEA were comparable to patients undergoing CAS after prior CEA. Stroke/death/MI rates were statistically similar between redo-CEA vs CAS after prior CEA in both asymptomatic (4.4% vs 3.3%, P=0.8) and symptomatic patients (6.6% vs 5.8%, P=1.0). No significant difference in restenosis ≥70% was identified between redo-CEA and CAS after prior CEA (5.2% vs. 3.0%, P = 0.5). Redo-CEA vs primary CEA had increased stroke/death/MI rate in both symptomatic (6.6% vs 2.3%, P=0.05) and asymptomatic patients 4.4% vs 1.7%, P=0.03). Prior ipsilateral CEA was an independent predictor for stroke/death/MI among all patients undergoing CEA (OR 2.1, 95% CI 1.3 – 3.5). No difference in cranial nerve injury was identified between redo-CEA and primary CEA (5.2% vs 4.7%, P=0.8).
Conclusions
In the VSGNE, CEA and CAS showed statistically equivalent outcomes in asymptomatic and symptomatic patients treated for restenosis after prior ipsilateral CEA.However, regardless of symptom status, the risk of re-intervention was increased compared to patients undergoing primary CEA.
INTRODUCTION
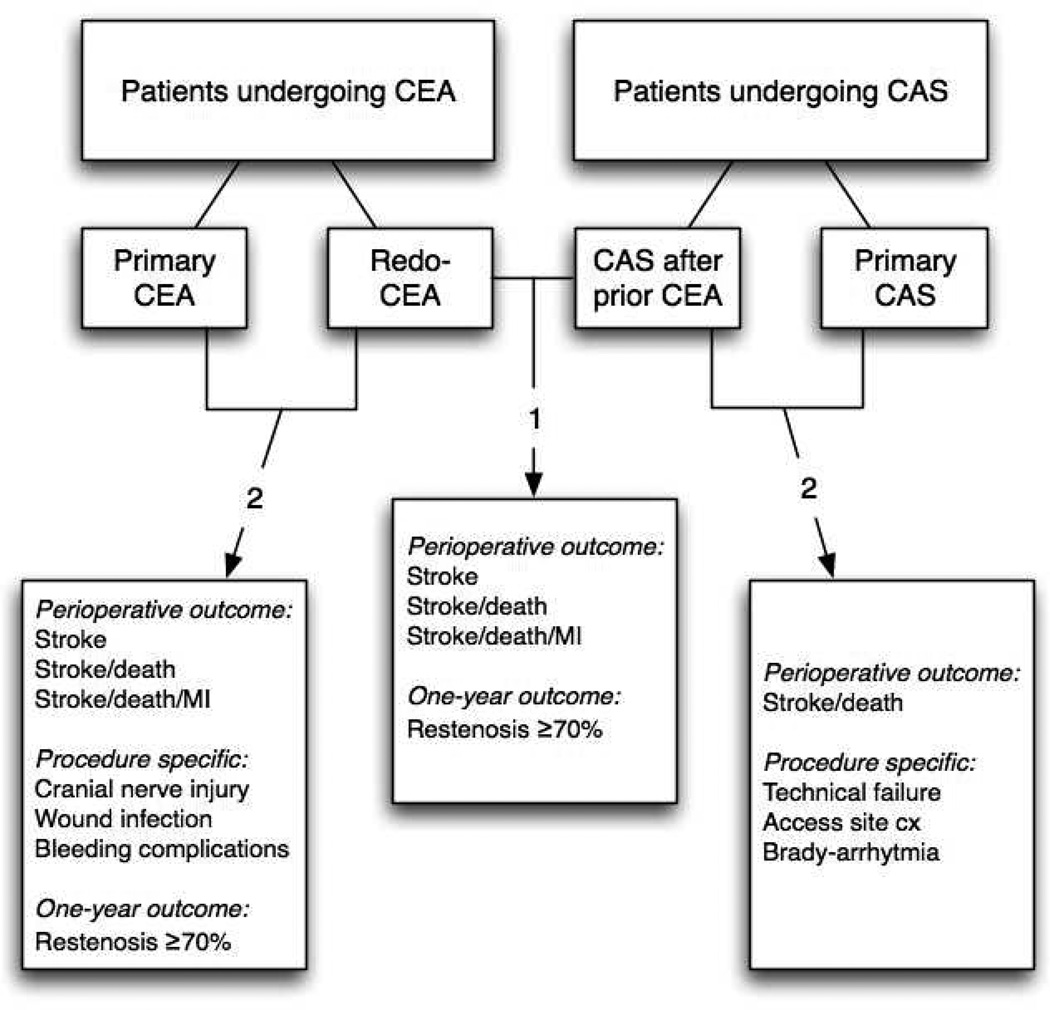
The reported incidence of restenosis after carotid endarterectomy (CEA) ranges from 6 to 15%, depending on the duration of follow-up and its measurement criteria1,2. Although most lesions remain asymptomatic, results from the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) showed the clinical significance of recurrent stenosis ≥70%, with increased risk of ipsilateral stroke within two years of surgery.3 The management of restenotic lesions remains unclear4–6. Since redo-surgery after prior ipsilateral CEA potentially leads to a more challenging operation, prior CEA has been considered a ‘high-risk’ condition for CEA with increased risk of cranial nerve injury (CNI) and other local complications7,8. Yet, only few studies also report an increased stroke7 risk for redo-CEA compared to primary CEA9,10. In patients for whom re-intervention is indicated, carotid angioplasty and stenting (CAS) might be a suitable alternative to re-operation. CAS has been increasingly performed in restenotic lesions after the Centers for Medicare and Medicaid Services (CMS) approved reimbursement for CAS in patients with symptomatic restenosis after CEA.11 Relative safety has been shown in early results,12 but long-term outcome remains undefined.13 Few analyses have directly compared outcomes of redo-CEA versus CAS in patients with restenosis after prior CEA14–17. Most studies that reported on outcome after CAS and/or CEA in restenotic lesions have been limited to single institution series with insufficient power to detect differences in outcome.13 Further, these studies did not distinguish symptomatic from asymptomatic disease. Nor did they report on the benefit of intervention beyond the perioperative period. In a recent study by the Vascular Study Group of New England (VSGNE), a history of prior ipsilateral CEA predicted stroke or death following carotid revascularization.17 In the current study, we aimed to further investigate this observation. Our primary goal was to compare perioperative major adverse events and one year patency between redo-CEA and CAS for atients with restenosis after prior ipsilateral CEA, stratified by symptom status. Secondly, we investigated the risk of re-intervention compared to primary procedures. (Figure 1)
Figure 1. Overview of study groups and outcome.
1. To compare outcome between redo-CEA and CAS in patients undergoing restenosis after prior ipsilateral CEA, 2. To investigate the risk of re-intervention compared to the primary intervention
METHODS
Database
Data collected by the VSGNE were used for this analysis. The VSGNE is a regional quality improvement initiative developed in 2002 and currently involves over 180 physicians at 30 centers (14 academic, 16 community). Preoperative clinical characteristics, imaging studies, operative outcome and follow-up data are collected and entered in the registry by trained nurses, r clinical data abstractors. Surgeons enter operative details. Research analysts are blinded to atient, surgeon, and hospital identity. Further details on this registry have been published previously and are available at http://www.vascularweb.org/regionalgroups/vsgne. VSGNE data have been validated for completeness using audits of discharge claims data from each participating institution.17 Additionally, we have not identified any mortality bias by cases not initially captured.18
Patients
Our study sample included all patients in the VSGNE registry who underwent CEA (January 2003 and December 2011) or CAS (July 2005 and March 2012).. Patients undergoing CEA with a concomitant coronary bypass procedure (CABG) were excluded (n = 221). If both the initial CEA procedure and the re-intervention (CAS or redo-CEA) were reported for one patient, the initial CEA was excluded (n = 52). In total, 9305 CEAs from 26 centers performed by 136 surgeons, and 663 CAS’ from 13 centers performed by 58 surgeons were available for analyses. Within this sample, patients with a prior ipsilateral CEA in their medical history were identified. This resulted in a ‘re-intervention group’ of 432 patients including 212 redo-CEAs and 220 CAS, and a ‘primary procedure group’ of 9536 patients including 9093 primary CEAs and 443 primary CAS procedures. In those who underwent a third ipsilateral carotid intervention (n=6), only the secondary intervention after the initial CEA was included for analyses.
Endpoints and Measurements
Our primary endpoints were any stroke, a composite of any stroke or death and a composite of stroke, death, or myocardial infarction (MI) at 30-days postoperatively. Secondary endpoints included restenosis ≥70% as assessed by duplex ultrasound (DUS) during follow-up. In addition, CEA and CAS specific perioperative outcomes were evaluated. For CEA these included any CNI (as assessed at discharge by the operating surgeon), wound infection and bleeding needing re-intervention. For CAS these included technical failure, access site complications and brady- arrhythmia requiring treatment during the procedure.
The definition of stroke included ipsilateral or contralateral major strokes (cortical, vertebrobasilar, or ocular disability resulting in non-independent living status, or blindness) and ipsilateral or contralateral minor stroke (other strokes not defined as major). Neurologists did not routinely examined patients postoperatively, though this is part of the protocol for CAS at several of the participating institutions. Myocardial infarctions included clinical, electrocardiogram, and troponin-only MI.
Indications for obtaining postoperative troponin are institution dependent and variable. Not all centers routinely screened all postoperative patients for MI with troponin.
For the evaluation of restenosis, we studied patients who had undergone DUS evaluation during follow-up. Among CAS patients, we were able to analyze 376 patients (56.7%) at a median follow-up of 254 days. Of the 287 patients (43.3%) without DUS information, 228 patients (34.4%) underwent stenting procedures in 2011 or 2012 and had therefore not completed one year follow-up yet at time of data-analysis. The remaining missing 59 patients (8.9%) were lost to follow-up or they did not undergo DUS imaging during follow-up. For CEA, 6189 patients 67%) were available for restenosis analyses at a median of 370 days. Of those without DUS information (n=3116, 33.4%), 1256 patients (13.5%) had undergone CEA in 2011 and had therefore not completed one year follow-up. The remaining 1860 patients (20%) were lost to follow-up or did not undergo DUS imaging at their follow-up consult. Results for primary outcome were stratified by preoperative symptom status. Symptomatic patients were defined as having an ipsilateral neurologic event, including any hemispheric or ocular transient ischemic attack, major or minor stroke preceding the intervention.
Statistical Analysis
Patient characteristics and outcome from patients who underwent redo-CEA or CAS after prior ipsilateral CEA were compared using χ2 or Fisher’s exact test for categorical variables and two tailed t test for continuous variables. Within the CAS and CEA group, patient characteristics and outcomes of re-intervention were also compared to primary procedures.
Multivariable logistic regression was performed to evaluate whether prior ipsilateral CEA was predictive for adverse outcome (stroke/death and stroke/death/MI) following CEA. Candidate predictors were identified by bivariate analysis and included in the multivariable model if the P- value was <.1. (Appendix A) Backward step-wise selection was applied to generate odds ratios (OR) and corresponding 95% confidence intervals (CI). The multivariable models were adjusted for age and gender. Predicted probabilities for adverse outcome were calculated based on the final models. P-values <.05 were considered significant. SPSS version 19.0 statistical software (IBM Corp. SPSS Statistics, Armonk, NY) was used for statistical analyses.
RESULTS
Redo-CEA versus CAS after prior CEA
Patient characteristics
Among patients who underwent re-intervention after prior ipsilateral CEA, preoperative characteristics were comparable between redo-CEA and CAS. (Table I) The mean age was 69 years in both groups; 58.5% were men in the CEA group and 63.2% in the CAS group. 36% of patients were symptomatic undergoing redo-CEA versus 31% undergoing CAS (P = 0.3). All symptomatic patients had ≥50% stenosis, while in asymptomatic patients, 93% of patients undergoing redo-CEA and 95% of CAS patients had high-grade ≥70% stenosis. Chronic obstructive pulmonary disease (COPD) was more common in the CEA group (32.5% vs 20% CAS, P <.01). A greater proportion of patients in the CEA group were on preoperative antiplatelet therapy (aspirin or clopidogrel). Time from initial CEA to re-intervention was available for 52 patients (26 CAS and 26 CEA). Median time-interval to CEA was 36 months compared to 17.5 months to CAS (P=0.08).
Table I.
Demographics and patient characteristics of patients undergoing redo-CEA or CAS after prior ipsilateral CEA in the VSGNE
| Redo-CEA n = 212 |
CAS after prior CEA n =220 |
||||
|---|---|---|---|---|---|
| n | % | n | % | P-value | |
| Age, yr (mean ± SD) | 68.8 ± 9.2 | 68.9 ± 8.5 | 0.4 | ||
| Age >80 yr | 26 | 12.3 | 25 | 11.4 | 0.9 |
| Gender | 0.3 | ||||
| Male | 124 | 58.5 | 139 | 63.2 | |
| Female | 88 | 41.5 | 80 | 36.4 | |
| Race (non-white) | 2 | 0.9 | 3 | 1.4 | 1 |
| Ipsilateral symptoms | 76 | 35.9 | 69 | 31.4 | 0.3 |
| TIA | 54 | 25.5 | 55 | 25.0 | |
| Stroke | 22 | 10.4 | 14 | 6.4 | |
| Ipsilateral degree of ICA stenosis | 0.5 | ||||
| <50% | 7 | 3.3 | 3 | 1.4 | |
| 50 – 59% | 5 | 2.4 | 5 | 2.3 | |
| 60 – 69% | 7 | 3.3 | 6 | 2.7 | |
| 70 – 79% | 37 | 17.5 | 29 | 13.2 | |
| ≥ 80% | 151 | 71.2 | 174 | 79.1 | |
| Occluded | 3 | 1.4 | 3 | 1.4 | |
| Symptomatic patients ≥ 50% stenosis | 212 | 100 | 220 | 100 | 1 |
| Asymptomatic patients ≥ 70% stenosis | 197 | 93.0 | 209 | 95.0 | 0.4 |
| Any Smoke (prior or current) | 191 | 90.1 | 188 | 85.5 | 0.2 |
| Hypertension (≥140/90 or history) | 189 | 89.2 | 207 | 94.1 | 0.1 |
| Diabetes (on medication) | 70 | 33.0 | 70 | 31.8 | 0.8 |
| Coronary artery disease | 82 | 38.7 | 78 | 35.5 | 0.6 |
| CABG/PCI | 81 | 38.2 | 79 | 35.9 | 0.5 |
| Congestive heart failure | 19 | 9.0 | 22 | 10.0 | 0.7 |
| COPD | 69 | 32.6 | 44 | 20.0 | <.01 |
| Antiplatelet therapy | 192 | 90.6 | 213 | 96.8 | <.01 |
| Statin | 175 | 82.5 | 183 | 83.2 | 0.9 |
| Stress test abnormal (MI or ischemia) | 18 | 8.5 | 18 | 8.2 | 0.9 |
| On dialysis | 1 | 0.5 | 2 | 0.9 | 1 |
| Creatinine (>1.78 mg/dL) | 12 | 5.7 | 13 | 5.9 | 0.3 |
| ASA 3 and 4 | 93 | 43.9 | 101 | 45.9 | 0.6 |
| Contralateral occlusion | 24 | 12.1 | 24 | 11.5 | 1 |
| Urgent procedures | 27 | 12.7 | 21 | 9.5 | 0.4 |
| Prior radiation | 107 | 1.2 | |||
| Eversion CEA | 8 | 3.8 | |||
| One or more medical high risk factor(s) | 58 | 26.4 | |||
| One or more anatomical high risk factor(s) | 141 | 64.1 | |||
| Refused surgery | 38 | 17.3 | |||
TIA, transient ischemic attack, ICA, internal carotid artery, CABG, coronary artery bypass grafting, PCI, percutaneous coronary intervention, COPD, chronic obstructive pulmonary disease, MI, myocardial infarction, ASA, American Society of Anesthesiology. Bold: P-value <.05
Outcomes
Among symptomatic patients, outcome after CEA vs CAS did not differ significantly; 30-day stroke and stroke/death rate were 3.9% vs 4.4% (P = 1.0) and stroke/death/MI rate was 6.6% vs 5.8% (P = 1.0). (Table II) For asymptomatic patients, outcome after CEA vs CAS was also statistically similar: 30-day stroke and stroke/death were 2.9% vs 2.0% (P = 0.7) and stroke/death/MI rate was 4.4% vs 3.3% (P = 0.8). Length of stay after CEA was 2.2 days, compared to 1.9 days after CAS (P = 0.4). During follow-up, rate of restenosis ≥70% was 5.2% after CEA and 3.0% after CAS (P = 0.5, OR 0.6, 95% CI 0.2–2.0). Only one symptomatic lesion (ipsilateral stroke at 13 months) was identified in a patient who underwent CAS.
Table II.
Thirty-day outcome of patients undergoing redo-CEA and CAS after prior ipsilateral CEA in the VSGNE
| Redo-CEA n = 76 |
CAS after prior CEA n = 69 |
|||||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | P-value | OR | 95% CI | ||
| Stroke | 3 | 3.9 | 3 | 4.4 | 1.0 | 1.1 | 0.2–5.7 | |
| Symptomatic | Stroke/Death | 3 | 3.9 | 3 | 4.4 | 1.0 | 1.1 | 0.2 – 5.7 |
| Stroke/Death/MI | 5 | 6.6 | 4 | 5.8 | 1.0 | 0.9 | 0.2 – 3.4 | |
| Redo-CEA n = 136 |
CAS after prior CEA n = 151 |
|||||||
| Stroke | 4 | 2.9 | 3 | 2.0 | 0.7 | 0.7 | 0.2 – 3.0 | |
| Asymptomatic | Stroke/Death | 4 | 2.9 | 3 | 2.0 | 0.7 | 0.7 | 0.2 – 3.0 |
| Stroke/Death/MI | 6 | 4.4 | 5 | 3.3 | 0.8 | 0.7 | 0.2 – 2.5 | |
OR, odds ratio, CI, confidence interval, MI, myocardial infarction
Redo-CEA vs primary CEA
Patient characteristics
Comparison of demographics and patient characteristics showed that COPD, smoking (current or prior), contralateral occlusion and previous CABG or percutaneous coronary intervention were more common in patients undergoing redo-CEA compared to primary CEA. Eversion CEA was more frequently used in primary procedures (9.8% vs 3.8% redo-CEA, P < .01). Patching was more common with redo-CEA (96% vs 87% primary CEA, P<.01). (Appendix B, online)
Outcomes
Among symptomatic patients undergoing redo-CEA vs primary CEA, 30-day stroke, stroke/death, and stroke/death/MI rates were higher after redo-CEA, but not statistically different (stroke: 4.0% vs 1.5%, P = 0.1, stroke/death: 4.0% vs 1.8%, P = 0.2 and stroke/death/MI: 6.6% s 2.8%, P = 0.07). (Table III) Asymptomatic patients undergoing redo-CEA compared to those undergoing primary CEA had significantly higher rates for stroke (2.9% vs 0.8%, P = 0.03), stroke/death (2.9% vs 0.9%, P = 0.04) and stroke/death/MI (4.4% vs 1.7%, P = 0.03). CNI at discharge was similar after primary CEA (5.1%, n = 470) and redo-CEA (6.1%, n = 13, P = 0.8, OR 1.2, 95% CI 0.7 – 2.1). One wound infection (0.5%) was seen after redo-CEA versus 7 (0.1%) after primary procedure (P = 0.2). 1.4% (n = 3) had bleeding complications after redo- CEA versus 1.0% (n = 90) after primary CEA (P = 1.0). Restenosis ≥70% was statistically similar in patients undergoing primary CEA compared to redo-CEA (2.8% vs 5.2%, P = 0.2, OR 1.7, 95% CI 0.9 – 4.2).
Table III.
Thirty-day outcome of patients undergoing primary CEA versus redo-CEA in the VSGNE
| Primary CEA n = 3033 |
Redo-CEA n = 76 |
|||||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | P-value | OR | 95% CI | ||
| Stroke | 46 | 1.5 | 3 | 3.9 | 0.12 | 2.6 | 0.8 – 8.6 | |
| Symptomatic | Stroke/Death | 53 | 1.7 | 3 | 3.9 | 0.16 | 2.3 | 0.7 – 7.5 |
| Stroke/Death/MI | 71 | 2.3 | 5 | 6.6 | 0.05 | 2.4 | 0.96 – 6.1 | |
| Primary CEA n = 6059 |
Redo-CEA n = 136 |
|||||||
| Stroke | 49 | 0.8 | 4 | 2.9 | 0.03 | 3.7 | 1.3 – 10.5 | |
| Asymptomatic | Stroke/Death | 54 | 0.9 | 4 | 2.9 | 0.04 | 3.4 | 1.2 – 9.4 |
| Stroke/Death/MI | 105 | 1.7 | 6 | 4.4 | 0.04 | 2.6 | 1.1 – 6.1 | |
OR, odds ratio, CI, confidence interval, MI, myocardial infarction
CAS after prior CEA vs primary CAS
Patients who underwent primary CAS had more medical comorbidities than patients undergoing CAS after prior CEA, such as coronary artery disease, congestive heart failure, COPD and an abnormal stress test. (Data not shown) No significant difference in stroke or death rate was identified for both symptomatic (4.4% vs 7.6% primary CAS, P=0.6) and asymptomatic (2.0% vs 0.7% primary CAS, P = 0.4) patients. Technical failure (2.3% vs 1.8% primary CAS, P=NS) and access site complications (8.6% vs 5.9% primary CAS, P=NS) were statistically similar, while significantly more patients required treatment for brady-arrhythmias during primary CAS compared to patients undergoing CAS after prior CEA (27.4% [n = 121] vs 12.8% [n = 28], P < .01).
Multivariable analyses
Among all patients undergoing CEA (symptomatic and asymptomatic), redo-CEA was an independent predictor for 30-day stroke/death (OR 2.6, 95% CI 1.4 – `4.7, P = .002) and stroke/death/MI (OR 2.1, 95% CI 1.3 – 3.5, P = .002). (Table IV) Other predictive factors for stroke/death were age > 80 years, symptomatic status, hypertension, contralateral occlusion and urgent procedures. Preoperative antiplatelet therapy proved to be protective. Other predictors for stroke/death/MI were female gender, symptomatic status, hypertension, congestive heart failure, contralateral occlusion and urgent procedures (<24 hours of admission). Patients undergoing redo-CEA vs primary CEA had a significantly higher predicted adverse outcome, reflecting they are a higher risk population in the redo-group (Figure 2).
Table IV.
Multivariable model for adverse outcome among symptomatic and asymptomatic patients undergoing CEA (n = 9305)
| Stroke/Death | Stroke/Death/MI | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age >80 yr | 1.8 | 1.2 – 2.7 | .004 | - | - | - |
| Female gender | - | - | - | 1.4 | 1.1 – 1.9 | .013 |
| Ipsilateral symptoms | 2.1 | 1.4 – 3.0 | <.001 | 1.7 | 1.2 – 2.2 | .001 |
| Prior ipsilateral CEA | 2.6 | 1.4 – 4.7 | .002 | 2.1 | 1.3 – 3.5 | .002 |
| Hypertension | 2.5 | 1.2 – 5.4 | .020 | 1.8 | 1.0 – 3.0 | .036 |
| Congestive heart failure | - | - | - | 2.0 | 1.4 – 2.9 | <.001 |
| Antiplatelet therapy | 0.5 | 0.3 – 0.9 | .009 | - | - | - |
| Contralateral occlusion | 2.4 | 1.4 – 4.0 | .001 | 1.9 | 1.2 – 3.0 | .003 |
| Urgency | 1.8 | 1.2 – 2.8 | .008 | 1.6 | 1.1 – 2.2 | .014 |
MI, myocardial infarction, OR, odds ratio, CI, confidence interval
Figure 2. Predicted and observed stroke or death rate of patients undergoing primary CEA and redo-CEA.
Both predicted and observed rates were significantly different between primary CEA and Redo-CEA (P<.01).
DISCUSSION
In a large regional database, CAS and redo-CEA revealed equivalent perioperative and one year outcome in both asymptomatic and symptomatic restenosis after prior CEA. Adverse outcome of re-intervention was increased compared to primary CEA, regardless of symptom status.
The results of the current study indicate that patients with symptomatic or asymptomatic restenosis after prior CEA form a high-risk group for intervention, regardless of revascularization procedure or symptom status. Despite the increased risk compared to primary CEA, both CAS and CEA proved to be suitable options to treat symptomatic patients with restenosis after prior CEA. In asymptomatic patients, the benefit of intervention is less clear with stroke/death rate of 2.9% after CEA, which is the upper limit acceptable for asymptomatic lesions based on societal guidelines For these patients, a non-operative approach with medical treatment might be considered to achieve optimal long-term stroke prevention given that the natural history of asymptomatic lesions seems generally benign and some may regress over time.19 However, others have shown increased stroke risk in patients with severe stenosis (≥70%), indicating that a more aggressive approach may be warranted in this subset of patients.3,20
Few studies have reported an increased stroke risk after redo-CEA compared to primary 5 CEA.9,10 Aburahma et al.’s study yielded an ipsilateral stroke rate of 4.8% (6/124) after redo- CEA, compared to 0.8% (2/265) following primary intervention with five of six strokes in the redo group happening in symptomatic patients.9 In contrast, more recent studies did not detect a difference in stroke rate compared to primary surgery, and concluded that redo-CEA was as safe as primary CEA.21–23 However, small sample size limited the ability to detect statistical differences or to stratify patients by symptom status in most of these series. Others have reported on outcome after redo-CEA in single center cohorts without a control group.24–30 While most of these studies reported ‘acceptable’ perioperative stroke/death rates (0 – 4.6%, all patients), several groups have reported increased risk for local complications such as nerve injury (4.6% – 21%) and wound hematoma (4.2%)7,8,20,30,31. We did not identify an increased risk for CNI compared to primary CEA, nor did we note an increased risk for other local complications with redo-surgery in a much larger population. As illustrated by a greater predicted stroke or death rate than was actually observed in the redo-group, the increased risk for re-intervention was therefore indicative of a high-risk population rather than a high-risk procedure.
Under the assumption that surgical risk with redo-CEA was increased, CMS approved reimbursement for CAS in patients with symptomatic, severe (>70%) restenosis after CEA. This policy was mainly based on the results of the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial, designed to compare CAS vs CEA in a high-risk population.32,33 In SAPPHIRE, the 30-day stroke, death, MI rate in the CEA arm was as high as 9.8% (vs 4.8% CAS, P=.09). The MI rate of 6.6% strongly influenced this composite endpoint. Moreover, the generalizability of this cohort may be limited as approximately 70% of the study population was asymptomatic and the study design lacked stratification within the various high-risk groups (only 22% had recurrent stenosis after CEA). Despite controversy over the applicability of the SAPPHIRE results and the classification of ‘high-risk’7,34, CAS was increasingly performed and evaluated in patients with restenotic lesions.The SVS Vascular Registry (VR) data indicated a protective effect of CAS in restenotic lesions compared to primary CAS and this observation was supported by a sub-analysis in the current study.12,35 A combination of a higher risk population in the primary CAS group and a supposedly more stable plaque in restenotic lesions caused by intimal hyperplasia36 may explain these findings. This hypothesis is further supported by the lower risk of procedural bradycardia in CAS after prior CEA, which has also been previously shown.37 Our results suggested that patients undergoing CAS after redo-CEA were treated for intimal hyperplasia rather than ‘late’ restenosis (>24 Months) through progression of atherosclerotic restenosis. Yet, the reported risk for 30-day stroke/death/MI after CAS in restenotic lesions is still relatively high in both asymptomatic (SVS VR: 3.5% and VSGNE: 3.3% [current analysis]) and symptomatic patients (SVS VR: 6.7% and VSGNE: 5.8% [current analysis]) and not superior to redo-CEA.35 Long-term results after CAS have not been thoroughly discussed in the current literature.13,38, 10 Our findings indicate that rate of restenosis ≥70% after one year is similar after CAS and CEA (3.0% vs 5.2%, NS). The vast majority lesions remained asymptomatic without a need for re-intervention.
Few other groups have attempted to compare CAS and CEA directly in patients with restenosis after prior CEA. In a series of 83 patients, Aburahma et al.31 reported increased 30-day stroke rates after CAS compared to CEA (16% vs 2.4%) and >50% in stent restenosis at 6 months, as defined by duplex ultrasound. In a later report comprising 192 patients (72 redo-CEA and 120 CAS), the same group did not detect any differences in 30-day stroke rate between redo- CEA and CAS (3% vs 1%, P=0.6), while the increased risk for restenosis after CAS (mean time of follow-up 2 yr) persisted.15 Several studies have however shown elevated sonographic velocities after stenting in the absence of angiographically proven restenosis, which might have caused increased rates of restenosis greater than 50% after CAS.39 Two other groups showed equivalent outcome between CAS and CEA albeit with smaller numbers.14,16 Nolan et al. using VSGNE data sought to compare real world outcomes of CAS and CEA and found that a history of prior ipsilateral CEA was an independent risk factor for stroke or death in a model including all patients undergoing CAS and CEA.17 This observation prompted us to further stratify this cohort using a larger number of patients. While primary CEA in symptomatic patients has proven to be beneficial over CAS17, patients with symptomatic recurrent stenosis do equally well with CAS. Similar predictors for adverse outcome were previously shown in the SVGNE.17,40 While age >80 year was associated with stroke and death, female gender and congestive heart failure were predictive for stroke/death/MI. Preoperative antiplatelet therapy was protective for stroke and death, but was not associated with stroke/death/MI.
The results of this study must be interpreted in the context of its design including the limitations of the dataset. The VSGNE does not record the duration from primary CEA to secondary intervention, however, we were able to identify this time interval for several patients who also underwent their primary CEA procedure in the VSGNE. We are also not aware of the reasons for intervention in patients with asymptomatic lesions <70%. Reporting bias is inherent to any registry-based study and potentially leads to under-reporting of events. The low stroke rate in the VSGNE compared to RCTs such as CREST is likely in part caused by the absence of a routine postoperative evaluation by a neurologist. However, it seems unlikely that there was bias in the reporting of events between CAS and CEA, patients with and without prior CEA or symptomatic and asymptomatic patients. Furthermore, we used the Social Security Death Index to ensure that all deaths were captured in our dataset. The lack of a standard protocol to identify postoperative MI might have lead to lower rates compared to the randomized controlled trials. Furthermore, the relatively low event rate after revascularization procedures, particularly in the re-intervention groups, may have resulted in a type II error limiting our ability to identify significant differences. However, this is the largest comparison to date of CAS versus redo-CEA in patients with restenosis after prior CEA, and we were able to quantify the potential effect size and direction among these patients, stratified for symptom status. Also, follow-up length was limited at a median of one year. Lastly, the duplex criteria were determined at each individual center and are thus not uniform across the VSGNE. Nonetheless, all the vascular laboratories in the VSGNE centers are certified by the Intersocietal Commission for the Accreditation of Vascular Laboratories.1 These factors should be considered while interpreting our results on restenosis.
In conclusion, we found that in a large regional quality improvement registry reflecting real world outcome, patients undergoing re-intervention after prior CEA are at increased risk for adverse events, regardless of procedure. For patients presenting with symptomatic recurrent carotid artery stenosis, both CAS and CEA are suitable options. For asymptomatic patients, the risk and benefits of intervention should be carefully weighed for individual patients. Future work should focus on identifying those asymptomatic lesions that will eventually become symptomatic, and which asymptomatic patients have increased risk for perioperative adverse outcome.
Acknowledgements
Suzanne E. Dahlberg PhD
Support: This work was supported by the NIH T32 Harvard-Longwood Research Training in Vascular Surgery grant HL007734.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This manuscript was presented at the 41st Annual Symposium of the Society for Clinical Vascular Surgery, March 12-16, 2013 Miami; plenary session
Author Disclosures: Marc L. Schermerhorn is a consultant for Endologix, Medtronic, and Boston Scientific. Frans L. Moll is a consultant for Best Doctors and for Medtronic. For all the other authors none were disclosed.
References
- 1.Goodney PP, Nolan BW, Eldrup-Jorgensen J, Likosky DS, Cronenwett JL. Restenosis after carotid endarterectomy in a multicenter regional registry. J Vasc Surg. 2010;52:897–904. doi: 10.1016/j.jvs.2010.05.005. 5 e1–2; discussion −5. [DOI] [PubMed] [Google Scholar]
- 2.van Lammeren GW, Peeters W, de Vries JP, de Kleijn DP, De Borst GJ, Pasterkamp G, et al. Restenosis after carotid surgery: the importance of clinical presentation and preoperative timing. Stroke. 2011;42:965–971. doi: 10.1161/STROKEAHA.110.603746. [DOI] [PubMed] [Google Scholar]
- 3.Lal BK, Beach KW, Roubin GS, Lutsep HL, Moore WS, Malas MB, et al. Restenosisafter carotid artery stenting endarterectomy: aanalysis of CRESTa randomised controlled trial. Lancet Neurol. 2012;11:755–763. doi: 10.1016/S1474-4422(12)70159-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS Guideline on the Management of Patients With Extracranial Carotid and Vertebral Artery Disease A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery Developed in Collaboration With the American Academy of Neurology and Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2011;57:e16–e94. doi: 10.1016/j.jacc.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Vidale S. Restenosis after carotid endarterectomy and stenting. Lancet Neurol. 2012 doi: 10.1016/S1474-4422(12)70261-2. [DOI] [PubMed] [Google Scholar]
- 6.Lal BK. Recurrent carotid stenosis after CEA and CAS: diagnosis and management. Semin Vasc Surg. 2007;20:259–266. doi: 10.1053/j.semvascsurg.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Mozes G, Sullivan TM, Torres-Russotto DR, Bower TC, Hoskin TL, Sampaio SM, et al. Carotid endarterectomy in SAPPHIRE-eligible high-risk patients: implications for selecting patients for carotid angioplasty and stenting. J Vasc Surg. 2004;39:958–965. doi: 10.1016/j.jvs.2003.12.037. discussion 65–6. [DOI] [PubMed] [Google Scholar]
- 8.AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. J Vasc Surg. 2000;32:649–654. doi: 10.1067/mva.2000.109751. [DOI] [PubMed] [Google Scholar]
- 9.AbuRahma AF, Jennings TG, Wulu JT, Tarakji L, Robinson PA. Redo carotid endarterectomy versus primary carotid endarterectomy. Stroke. 2001;32:2787–2792. doi: 10.1161/hs1201.099649. [DOI] [PubMed] [Google Scholar]
- 10.Meyer FB, Piepgras DG, Fode NC. Surgical treatment of recurrent carotid artery stenosis. J Neurosurg. 1994;80:781–787. doi: 10.3171/jns.1994.80.5.0781. [DOI] [PubMed] [Google Scholar]
- 11.Hobson RW, 2nd, Lal BK, Chakhtoura E, Goldstein J, Haser PB, Kubicka R, et al. Carotid artery stenting: analysis of data for 105 patients at high risk. J Vasc Surg. 2003;37:1234–1239. doi: 10.1016/s0741-5214(02)75448-7. [DOI] [PubMed] [Google Scholar]
- 12.White RA, Sicard GA, Zwolak RM, Sidawy AN, Schermerhorn ML, Shackelton RJ, et al. Society of Vascular Surgery Vascular Registry® comparison of carotid artery stenting outcomes for atherosclerotic vs nonatherosclerotic carotid artery disease. J Vasc Surg. 51:1116–1123. doi: 10.1016/j.jvs.2009.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leger AR, Neale M, Harris JP. Poor durability of carotid angioplasty and stenting for treatment of recurrent artery stenosis after carotid endarterectomy: an institutional experience. J Vasc Surg. 2001;33:1008–1014. doi: 10.1067/mva.2001.113485. [DOI] [PubMed] [Google Scholar]
- 14.Attigah N, Kulkens S, Deyle C, Ringleb P, Hartmann M, Geisbusch P, et al. Redo surgery or carotid stenting for restenosis after carotid endarterectomy: results of two different treatment strategies. Ann Vasc Surg. 2010;24:190–195. doi: 10.1016/j.avsg.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 15.AbuRahma AF, Abu-Halimah S, Hass SM, Nanjundappa A, Stone PA, Mousa A, et al. Carotid artery stenting outcomes are equivalent to carotid endarterectomy outcomes for patients with post-carotid endarterectomy stenosis. J Vasc Surg. 2010;52:1180–1187. doi: 10.1016/j.jvs.2010.06.074. [DOI] [PubMed] [Google Scholar]
- 16.Hobson RW, 2nd, Goldstein JE, Jamil Z, Lee BC, Padberg FT, Jr., Hanna AK, et al. Carotid restenosis: operative and endovascular management. J Vasc Surg. (2nd) 1999;29:228–235. doi: 10.1016/s0741-5214(99)70376-9. discussion 35–8. [DOI] [PubMed] [Google Scholar]
- 17.Nolan BW, De Martino RR, Goodney PP, Schanzer A, Stone DH, Butzel D, et al. Comparison of carotid endarterectomy and stenting in real world practice using a regional quality improvement registry. J Vasc Surg. 2012;56:990–996. doi: 10.1016/j.jvs.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE) J Vasc Surg. 2007;46:1093–1101. doi: 10.1016/j.jvs.2007.08.012. discussion 101–2. [DOI] [PubMed] [Google Scholar]
- 19.Ricotta JJ, O’Brien MS, DeWeese JA. Natural history of recurrent and residual stenosis after carotid endarterectomy: implications for postoperative surveillance and surgical management. Surgery. 1992;112:656–661. discussion 62–3. [PubMed] [Google Scholar]
- 20.O’Donnell TF, Jr., Rodriguez AA, Fortunato JE, Welch HJ, Mackey WC., Jr Management of recurrent carotid stenosis: should asymptomatic lesions be treated surgically? J Vasc Surg. 1996;24:207–212. doi: 10.1016/s0741-5214(96)70095-2. [DOI] [PubMed] [Google Scholar]
- 21.Coyle KA, Smith RB, 3rd, Gray BC, Salam AA, Dodson TF, Chaikof EL, et al. Treatment of recurrent cerebrovascular disease. Review of a 10-year experience. Ann Surg. 1995;221:517–521. doi: 10.1097/00000658-199505000-00009. discussion 21–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Domenig C, Hamdan AD, Belfield AK, Campbell DR, Skillman JJ, LoGerfo FW, et al. Recurrent stenosis and contralateral occlusion: high-risk situations in carotid endarterectomy? Ann Vasc Surg. 2003;17:622–628. doi: 10.1007/s10016-003-0068-0. [DOI] [PubMed] [Google Scholar]
- 23.Hill BB, Olcott Ct, Dalman RL, Harris EJ, Jr., Zarins CK. Reoperation for carotid stenosis is as safe as primary carotid endarterectomy. J Vasc Surg. 1999;30:26–35. doi: 10.1016/s0741-5214(99)70173-4. [DOI] [PubMed] [Google Scholar]
- 24.de Borst GJ, Zanen P, de Vries JP, van de Pavoordt ED, Ackerstaff RG, Moll FL. Durability of surgery for restenosis after carotid endarterectomy. J Vasc Surg. 2008;47:363–371. doi: 10.1016/j.jvs.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 25.O’Hara PJ, Hertzer NR, Karafa MT, Mascha EJ, Krajewski LP, Beven EG. Reoperation for recurrent carotid stenosis: early results and late outcome in 199 patients. J Vasc Surg. 2001;34:5–12. doi: 10.1067/mva.2001.115601. [DOI] [PubMed] [Google Scholar]
- 26.Stoner MC, Cambria RP, Brewster DC, Juhola KL, Watkins MT, Kwolek CJ, et al. Safety and efficacy of reoperative carotid endarterectomy: a 14-year experience. J Vasc Surg. 2005;41:942–949. doi: 10.1016/j.jvs.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 27.Cho JS, Pandurangi K, Conrad MF, Shepard AS, Carr JA, Nypaver TJ, et al. Safety and durability of redo carotid operation: an 11-year experience. J Vasc Surg. 2004;39:155–161. doi: 10.1016/j.jvs.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Rockman CB, Riles TS, Landis R, Lamparello PJ, Giangola G, Adelman MA, et al. Redo carotid surgery: An analysis of materials and configurations used in carotid reoperations and their influence on perioperative stroke and subsequent recurrent stenosis. J Vasc Surg. 1999;29:72–80. doi: 10.1016/s0741-5214(99)70350-2. discussion −1. [DOI] [PubMed] [Google Scholar]
- 29.Coscas R, Rhissassi B, Gruet-Coquet N, Couture T, de Tymowski C, Chiche L, et al. Open surgery remains a valid option for the treatment of recurrent carotid stenosis. J Vasc Surg. 2010;51:1124–1132. doi: 10.1016/j.jvs.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Das MB, Hertzer NR, Ratliff NB, O’Hara PJ, Beven EG. Recurrent carotid stenosis. A five-year series of 65 reoperations. Ann Surg. 1985;202:28–35. doi: 10.1097/00000658-198507000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aburahma AF, Bates MC, Stone PA, Wulu JT. Comparative study of operative treatment and percutaneous transluminal angioplasty/stenting for recurrent carotid disease. J Vasc Surg. 2001;34:831–838. doi: 10.1067/mva.2001.118591. [DOI] [PubMed] [Google Scholar]
- 32.Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–1501. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 33.Gurm HS, Yadav JS, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, et al. Long-Term Results of Carotid Stenting versus Endarterectomy in High-Risk Patients. N Engl J Med. 2008;358:1572–1579. doi: 10.1056/NEJMoa0708028. [DOI] [PubMed] [Google Scholar]
- 34.Gasparis AP, Ricotta L, Cuadra SA, Char DJ, Purtill WA, Van Bemmelen PS, et al. High-risk carotid endarterectomy: fact or fiction. J Vasc Surg. 2003;37:40–46. doi: 10.1067/mva.2003.56. [DOI] [PubMed] [Google Scholar]
- 35.Schermerhorn ML, Fokkema M, Goodney P, Dillavou ED, Jim J, Kenwood CT, et al. The impact of Centers for Medicaid and Medicare Services high-risk criteria on outcome after carotid endarterectomy and carotid artery stenting in the SVS Vascular Registry. J Vasc Surg. 2013 doi: 10.1016/j.jvs.2012.10.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hellings WE, Moll FL, de Vries JP, de Bruin P, de Kleijn DP, Pasterkamp G. Histological characterization of restenotic carotid plaques in relation to recurrence interval and clinical presentation: a cohort study. Stroke. 2008;39:1029–1032. doi: 10.1161/STROKEAHA.107.496703. [DOI] [PubMed] [Google Scholar]
- 37.Mylonas SN, Moulakakis KG, Antonopoulos CN, Kakisis JD, Liapis CD. Carotid artery stenting-induced hemodynamic instability. J Endovasc Ther. 2013;20:48–60. doi: 10.1583/12-4015.1. [DOI] [PubMed] [Google Scholar]
- 38.AbuRahma AF, Abu-Halimah S, Bensenhaver J, Nanjundappa A, Stone PA, Dean LS, et al. Primary carotid artery stenting versus carotid artery stenting for postcarotid endarterectomy stenosis. J Vasc Surg. 2009;50:1031–1039. doi: 10.1016/j.jvs.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 39.Lal BK, HobsonIi RW, Tofighi B, Kapadia I, Cuadra S, Jamil Z. Duplex ultrasound velocity criteria for the stented carotid artery. J Vasc Surg. 2008;47:63–73. doi: 10.1016/j.jvs.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 40.Goodney PP, Likosky DS, Cronenwett JL. Factors associated with stroke or death after carotid endarterectomy in Northern New England. J Vasc Surg. 2008;48:1139–1145. doi: 10.1016/j.jvs.2008.05.013. [DOI] [PubMed] [Google Scholar]