Abstract
The use of gonadotropins to trigger oocyte maturation both in vivo and in vitro has provided precious and powerful knowledge that has significantly increased our understanding of the ovarian function. Moreover, the efficacy of most assisted reproductive technologies (ART) used in both humans and livestock species relies on gonadotropin input, mainly FSH and LH. Despite the significant progress achieved and the huge impact of gonadotropins, the exact molecular pathways of the two pituitary hormones, FSH and LH, still remain poorly understood. Moreover, these pathways may not be the same when moving from the in vivo to the in vitro context. This misunderstanding of the intricate synergy between these two hormones leads to a lack of consensus about their use mainly in vitro or in ovulation induction schedules in vivo. In order to optimize their use, additional work is thus required with a special focus on comparing the in vitro versus the in vivo effects. In this context, this overview will briefly summarize the downstream gene expression pathways induced by both FSH in vitro and LH in vivo in the cumulus compartment. Based on recent microarray comparative analysis, we are reporting that in vitro FSH stimulation on cumulus cells appears to achieve at least part of the gene expression activity after in vivo LH stimulation. We are then proposing that the in vitro FSH-response of cumulus cells have similitudes with the in vivo LH-response.
Keywords: Transcriptomic overlap, Genomic substitution, Gonadotropin molecular signalling
Introduction
Mammalian female reproductive function is finely regulated by a set of coordinated endocrine signals that allow successful events of oocyte developmental competence, granulosa cells differentiation, extracellular matrix (ECM) production, ovulation, fertilization, and early embryonic development. Gonadotropins (mainly FSH and LH) are the main extra-ovarian endocrine factors involved in the control of these ovarian functions [1-6]. The release of these two anterior-pituitary hormones is governed by the hypothalamus via the GnRH (gonadotropin-releasing hormone) and modulated by other ovarian factors such as activin and inhibin [7]. Following a gonadotropin-independent phase, the mammalian follicular growth first becomes FSH-dependent at the secondary stage and then LH-dependent prior to ovulation [8-12]. While FSH is mainly involved in follicular growth, cellular proliferation and oestrogen production (aromatase activity), LH induces androgen biosynthesis, final maturation of the oocyte and ovulation [13-19]. To achieve their functions, FSH and LH trigger multiple downstream cascades of intra-ovarian pathways that are necessary for proper female fertility [13,14,17,20-23]. In addition, it has been shown that the efficiency of most assisted reproductive technologies (ART) used in both humans and livestock, including ovarian stimulation and oocyte in vitro maturation (IVM), relies on gonadotropin input [24-31].
Interestingly, successful gonadotropin-induced maturation of oocyte was shown to require de novo mRNA synthesis in follicular somatic cells. This gene expression activity aims at supplying the oocyte and follicular cells with crucial factors to achieve subsequent events of maturation and ovulation [32-35].
In vitro, it has been demonstrated that FSH improves oocyte maturation (both nuclear and cytoplasmic), cumulus cell (CCs) expansion, in vitro fertilization (IVF) and early embryo development in several mammalian species including cattle [30,36,37], mouse [38], pig [39-41] and human [22,42,43]. Since FSH has improved significantly the oocyte maturation and developmental competence, its receptor, FSHR, was assumed to be expressed in mural granulosa cells (MGC) and CCs starting at the secondary follicular stage in most mammals, including mice, pigs, sheep, cows and humans [13,40,44-47]. FSH is also used to trigger the follicular growth during the preovulatory stage. Once they reach the fully grown stage, the superovulation is thereafter induced by LH in livestock animals and human [25,27,28,48-50]. Assuming that functional LH receptors are absent in CCs in the in vitro context [37,51,52], FSH has been the major gonadotropin used in IVM to trigger oocyte maturation fulfillment [16,30,53].
To be effective, LH signaling thus depends on the expression of functional luteinizing hormone/choriogonadotropin receptor (LHCGR) in the follicle. LHCGR expression was reported in theca and granulosa cells [54-56] but was absent in both oocytes and CCs [37,51,52]. Therefore, the meiotic induction effect of LH on CCs was recently proposed to be indirectly mediated through the EGF-like factors [18,20,56,57]. The addition of LH into the IVM media might therefore not be needed in vitro [30,47,58]. However, Peng et al. [59] have reported the expression of LHCGR in rat CCs after PMSG stimulation. Similar findings confirmed this LHCGR expression in cumulus cells downstream the FSH pathway in human [60] and pig [31,61] both in vivo and vitro, raising therefore a controversy that needs further exploration.
In this review, we attempt to briefly address the general pathways of FSH and LH in follicular cells (mainly in CCs) that result in downstream transcriptional activity. Special focus will be given to the common features between the transcriptionally upregulated genes through FSH in vitro versus their LH counterparts in vivo. Based on common structural and functional features between FSH and LH, the hypothesis of partial replacement or “compensation” of LH action by FSH in vitro is explored. Using several findings reported in previous studies and microarray data in our laboratory, we propose herein an interesting aspect of the gonadotropin actions that may increase our understanding of their molecular pathways as well as their intricate synergy.
Gonadotropin-mediated gene expression and oocyte developmental competence
In view of the gonadotropins’ beneficial effects, they are used both in vitro and in vivo to improve oocyte developmental competence. Although their molecular mechanism of action remains ill-defined, we supposed that their genomic effects in vitro could be different from those in vivo, where they act in synergy and where both granulosa and theca cells are present. This hypothesis emerged from the difference in blastocyst rates between in vivo and in vitro oocyte maturation. For example, in cows, if the follicular development is supported by FSH, the rate of oocytes with successful developmental competence in vivo is between 60 and 80% [27,28]. In contrast, if oocytes are recovered from unstimulated antral follicles (slaughterhouse), this percentage drops to an average of 25 or 45% in ideal IVM conditions (IVM Schedule: 6h with FSH + 16 to 18h without any hormone supplement) [30,62]. It is clear that the in vivo context, which includes the sequential effects of gonadotropins (FSH & LH) and the presence of other intrafollicular factors, is far more suitable to oocyte competence acquisition. Interestingly, data in our laboratory and elsewhere showed that adding FSH to IVM media allowed an increase in blastocyst rate equivalent to half of the in vivo maturation success rates [45,63]. To explain this increased development in absence of an LH surge but in presence of FSH (using recombinant human FSH (rhFSH) without contamination risk), we assume that FSH is able to accomplish its own biological function and to substitute at least part of the effects of LH, resulting in developmental competence of some oocytes. In the absence of LH, FSH appears to be able to exert key functions normally achieved by LH. Our preliminary data comparing the transcriptomic effects of FSH in vitro versus LH in vivo highlight the necessity of further investigation to demystify the molecular overlap between FSH and LH. Concerning the cell signaling and although, the high doses of FSH in vitro will increase significantly the cAMP in mammalian cumulus cells and downstream pathways [64-67] (which may mimic the LH preovulatory effect), this second messenger rise is not enough to explain this important FSH functional substitution of the LH effect.
This possible compensation/substitution of the in vivo effects of LH by FSH in vitro could be explored at many levels (metabolic, physiological, morphological, transcriptomic, etc.). The present work is an overview of possible transcriptomic compensation of LH by FSH in vitro and briefly reviews their respective signaling pathways that may induce gene expression, followed by a case report of genomic effect comparison of FSH in vitro versus LH in vivo in bovine CCs.
Main signaling pathways of FSH in vitro
It has been shown that FSH is a key regulator of ovarian function, in particular follicular growth and granulosa cell differentiation [11,68]. FSHβ-deficient mice were unable to develop past the preantral stage [11]. These observations confirm that folliculogenesis is gonadotropin-dependent starting at the antral stage. The main functions triggered by FSH in the mammalian ovary are cell proliferation, apoptosis prevention, estradiol production, cell secretion, and regulation of several other genes [5,16,38,61,69-71]. Additionally, high doses of FSH are an essential ingredient in IVM media and was shown to efficiently promote full oocyte maturation in several mammalian species in vitro including cow [30,33], mouse [72] and pig [4,73]. This in vitro FSH effect is initiated in cumulus-oocyte complex (COCs) via its receptor (FSHR) on cumulus cells (CCs). It has been known for decades that FSH has no effect on denuded oocytes in vitro. FSHR is a GPCR (G-protein-coupled receptor) with a specific seven-transmembrane domain that was shown to activate the classical FSHR/AC/cAMP/PKA pathway. Among the two activated isoforms of PKA, only PKAII was shown to be involved in the transcriptional events in CCs required for meiosis resumption (GVBD) [74,75]. This de novo gene expression is indispensable for gonadotropin-induced oocyte maturation in murine and feline species [75,76] and was shown to involve the MAPK downstream of the cAMP-dependent PKA pathway in most mammals including mouse [77,78], rat [79] and cow [80]. In fact, it was shown that PKA phosphorylation and, EGF-like factors overexpression and secretion are both required to the FSH activation of ERK1/2 in pig and mice cumulus cells [61,81-83]. Additional signaling cross-talk events between the cAMP/PKA and the EGFR-MAPKs cascades were shown to up-regulate the PKA pathway via the synthesis of PGE2 [84-86]. Additionally, the inhibition of the MAPK pathway in CCs (or COCs) impaired gonadotropin-induced oocyte maturation and prevented the over-expression of crucial genes, such as PTSG2 and HAS2, required for oocyte maturation fulfillment, CC expansion, and steroidogenesis [87-89]. Interestingly, this MAPK effect is dependent on the PKA pathway but also on some oocyte paracrine factors reported to induce the EGF-like factors in CCs [90]. Additional PKA gene expression activity was associated with its two catalytic subunits that were able to transit to the CC nucleus. Several key genes were reported to be expressed downstream of this pathway, including HAS2, TNFAIP6, PTGS2, CYP19A1 and EGF-like factors ([75,91-96], recently reviewed in [20,21]). This transcriptional activity was mediated mainly – but not exclusively – through phosphorylation of CREB (CRE-binding protein) and therefore its binding to the CRE (cAMP-responsive-element) region in the promoter [97]. Additional transcription factors including AP1, SP1 and C/EBP family were also reported to contribute in the transcriptomics action of FSH [16,98,99]. FSH-induced PGE2 secretion is also an additional indirect effect that maintains the cAMP levels and stimulates the overexpression of the EGF-like factors [81].
FSH-mediated gene expression activity also occurs in a PKA-independent manner. In fact, it was demonstrated that FSH phosphorylates PKB/Akt and SGK1 via the PI3K (phosphatidylinositol-dependent kinase)/PDK1 (phosphoinositide-dependent kinase1) pathway in rat granulosa cells [100], mouse CCs [101,102] and porcine CCs [103], to support oocyte maturation in vitro. Interestingly, the PI3K/PKB pathway downstream of FSH was shown to induce cell survival and progesterone production in porcine CCs [31,103].
PKC was also reported to mediate the effects of FSH in CCs by activation of MAPK. This PKC action upstream of the MAPK pathway (and possibly through other pathways) induced the expression of key factors (de novo proteins) required for meiotic maturation of the oocyte, including the EGF-like factors in most mammalian species [45,104-106]. Similar effects induced by PMA (phorbol 12-myristate13-acetate), which is a PKC activator, were shown in CCs. Moreover, induction of the PKC pathway by FSH was associated with the mobilization of intracellular calcium that is assumed to be favorable to oocyte maturation and subsequent fertilization [23,104,107-110].
In vivo, the EGF like factors overexpression in CCs occurred following the action of LH-induced PGE2 secreted from granulosa cells through the PKA/CREB/MAPK pathway. This aforementioned pathway raised PGE2 production also in CCs which in turn amplify the local expression of EGF like factors amplification [18,20,56,57]. In vitro, EGF like factors and in presence of FSH (or forskolin) in vitro were reported to play a crucial role in IVM. Taken together, the EGF like factors are key players involved in gonadotropin-induced maturation in mammalian COCs [81,82,105,111,112] and probably further fertilization [113]. In fact, the EGF-like factors were shown to act in a positive regulation loop to overexpress PGE2 in porcine cumulus cells [81]. The PGE2 will thereafter by autocrine effect increase the EGF-like peptides that triggered gene expression in CCs through the extracellular signal-regulated kinases (ERK1/2/3) and PGE2 pathways [89,91,114,115].
FSH was also able to rapidly (within 1 hour) activate the MEK/MAPK pathway in mouse CCs to allow oocyte maturation [77]. The most studied MAPK are ERK1/2, JNK/SAPK (c-junterminal kinase/stress-activated protein kinases) and p38MAPK. Several transcription factors were reported to act downstream of the MAPK and ERK including AP1 and ATF2, CMYC [115,116]. In this context, P38MAPK was also phosphorylated by FSH through the cAMP/Epac(exchange protein activated by cAMP)/Rap (Ras-like related proteins)/Raf pathway, which is PKA-independent [100]. ERK1/2 was also involved in mural granulosa cells and CC steroidogenesis (progesterone and estradiol) induced by FSH [117,118]. Once produced, these steroids, mainly progesterone, were shown to promote gene expression and contribute to oocyte competence and CC expansion [119-121].
Several studies were performed to assess the gene expression patterns in follicular cells induced by FSH in vitro. These sets of genes induce numerous biological and molecular functions associated with cell signaling, CC expansion, steroidogenesis, gene expression, etc. [23,34,61,91,98,122-125]. Analysis of these gene expression patterns has yielded insights into the molecular involvement of FSH in CC function leading to oocyte developmental competence acquisition.
Do cumulus cells express LHCGR?
Before reviewing the LH pathways, in particular those leading to gene expression in CCs, it is important to discuss available data about the possible expression of LHCGR in this compartment. Some studies have reported the absence of LHCGR in CCs [37,51,52]. The addition of LH in FSH-based media for COC maturation in vitro therefore does not improve oocyte developmental competence [30,37,126]. In contrast, other studies documented LHCGR expression in CCs, suggesting that LH might have a direct effect [59,127]. Additional evidence seem to confirm these aforementioned findings in CCs of several mammalian species including pig [31,61], mouse [128,129], rat [130], cow (isoform E) [55] and human [131]. Beneficial effects of LH on in vitro embryo yields were even shown but the amount of LH used (1ug per ml) was likely contaminated by enough FSH (1%) to questions the conclusion [132,133]. These opposite findings may be due to differences in several parameters such as the COCs’ follicular stage, the tissue type (granulosa or cumulus), the gonadotropins’ origin (recombinant versus purified) and the detection technique and its sensitivity. To resolve this issue, the analysis of the protein functionality is required as several isoforms of the LH receptors are present in granulosa and cumulus cells [55,60,134,135]. It is also possible that the appearance of such receptor the cumulus is follicle size dependant or follicle differentiation dependant [60,134] creating an ambiguous response when pools are used. In the same way, the expression variation of particular LHCGR isoforms in CCs according to the follicular stage could also be the cause of this discrepancy. The analysis of a limited isoform’s population may be insufficient to confirm the absence of these receptors in CCs. In the mouse, the oocyte is believed to control the mRNA stability for the LH receptor. Sufficient data about the differential expression of LHCGR according to both the cell subtype (theca, granulosa or cumulus) and the follicular stage is still lacking. Possible reconciliation that reinforces our hypothesis was reported recently by the sequential culture system (FSH followed by LH) suggested by Kawashima et al. [61]. In this study and elsewhere, FSH was shown to trigger the expression of functional LHCGR that could respond to the subsequent action of LH and result in greater developmental competence until the blastocyst stage both in vivo and in vitro culture [129].
Main gene pathways induced by LH in vivo
Similarly to FSH, the contribution of LH in follicle dominant selection, oocyte final maturation, ovulation and subsequent fertilization was studied. In fact, LH is necessary in the selection of the dominant follicle in cattle and horse ([136] for review). This dominance is marked in cattle by an increasing dependence of the follicle on LH, mainly at the signalling and transcription levels [137,138]. While only FSH was able to induce CC expansion in vitro, LH and hCG were able to promote this mucification in vivo through the Ras/Raf/MAPK pathway (downstream of cAMP) as well as oocyte maturation [1,31,78,139,140]. Dr. Richards’ group has recently shown in vitro that the LH-induced transcriptional events are required for oocyte maturation, CC mucification, ovulation and luteinization are induced through activation of some downstream transcription effectors such as C/EBPβ (CCAAT/Enhancer-binding protein-beta) via the ERK1/2 pathway [115], reviewed in [21,125,141,142].
Like FSH in vitro, LH was also shown to activate the PKAII isoform which triggers gene expression events required for oocyte maturation fulfillment [143-145]. Additionally, LH mediates the overexpression of the EGF-like factors mainly EREG, AREG (through the p38 MAPK), BTC and NRG1 (via the C/EBP). These growth factors propagate and amplify the LH signal in CCs as previously suggested ([56,89], reviewed in [114,146]). Other key genes were also induced by LH, notably those involved in CC expansion and prostaglandin synthesis such as HAS2, TNFAIP6, PTX3, CSPG2, PTSG2, etc. [147]. Knockout of these crucial genes in mouse causes severe defects in the animal reproductive phenotype and subsequent fertility (reviewed in [21].
In porcine and bovine CCs, LH was also shown to induce steroidogenesis, mainly progesterone and estradiol [15,148]. LH receptor null mice were infertile with defective steroid production [149,150]. Moreover, the gene expression patterns in CCs were deeply affected in PGR null mice supporting a key transcriptional role of progesterone in oocyte maturation and subsequent ovulation and fertilization. Moreover, PGR is a nuclear receptor that acts as a transcription factor to mediate the LH ovulatory response by the expression of key genes such as ADAMTS1 and Catepsin L [151] for reviews [87,125,152-154]. The inhibition of PGR or progesterone action prevents meiosis resumption and ovulation in pig [155,156]. Moreover the PRKO mice are unable to spontaneously ovulate, but when the oocytes are punctured and collected prior to ovulation, they are able to reach the blastocyst stage [154,157,158].
In addition to the PGR, LH surge also induces various other transcription factors leading to diverse transcriptional effects and physiological responses [152]. The PKC pathway was suggested as a possible transduction mode of this LH stimulation [159]. PKC epsilon was furthermore shown to induce a survival (anti-apoptotic) effect on human CCs downstream of the PI3K/Akt pathway [160]. This PKC action is possibly associated to the reported LH-induced intracellular rise in calcium in follicular cells [161,162].
Concerning oocyte competence, the presence of LH receptor is associated with the dominant status in bovine and other mono-ovulating species [9,136,163]. The dominant follicle in bovine occurs at the average diameter size of 8.5 mm and is associated with an increase capacity to generate competent oocyte. In contrast, the follicles used for this study and in almost all study using in vitro maturation of bovine oocytes are much smaller, normally from 3-5mm size. In these follicles the cumulus has not been in contact with granulosa cells responding to any LH signalling although theca cells do have LH receptors at the 3mm size stage [164,165]. The oocytes from such small tertiary follicles have a limited capacity to reach the blastocyst stage in vitro after IVM-IVF and culture. The addition of FSH in vitro at low doses in a two step IVM culture system (6 h with rhFSH (0.1 μg/ml) + 18 h without hormones) in order to mimic the preovulatory intra-follicle conditions before the LH surge allowed an increase of the oocyte competence from around 20% to close to 45% [45].
Therefore the FSH effect in vitro on cumulus cells looks to accomplish its role and mimics the in vivo LH-ovulatory effect especially at higher doses. The rise in developmental rate in vitro is similar between oocytes from follicles with or without LH [30]. No report has been published yet on the comparison of cumulus transcriptome from dominant follicles compared to smaller ones as used in our study.
Human chorionic gonadotropin (hCG) is another gonadotropin that has high affinity for the LH receptor, named for this reason the LHCGR. In addition to the same α-subunit shared between LH and hCG, this affinity is primarily due to high similarities between the two β-subunits. Interestingly, hCG is able to trigger most of the LH effects for longer periods due to its greater half-life [166]. This property is desired in the ovarian stimulation drugs since it allows more time, flexibility and management possibilities during the ovarian stimulation programs, particularly in human IVF. For these reasons, hCG has often been used instead of LH due to its LH-like effect (reviewed in [167]).
Analogous to the FSH effect, the LH activation of several signal transduction pathways in CCs leads to diverse but well organized in vivo transcriptional responses that contribute to suitable oocyte competence acquisition, subsequent ovulation and fertilization and early embryo development. These beneficial effects were confirmed both in vivo and in vitro[18,28,56,168] and reviewed by [17,26].
Comparative analysis of FSH and LH pathways
Despite their specific biological functions, FSH and LH share some interesting similarities. In fact, both are pituitary-derived glycoproteins composed of a heterodimer of two subunits (α and β). These two subunits are linked by non-covalent bonds. While the α-subunit is common between all pituitary gonadotrophins, the β-subunit is specific, and, importantly, binds to the receptor, thus it exerts the biological effects [14]. Expression of these subunits is differentially induced by the pulsatile gonadotropin-releasing hormone (GnRH) via the PKC/MAPK signaling pathway [169]. Interestingly, both FSH and LH exert their stimulatory effects through a seven transmembrane receptor (7TMR). These receptors are members of the G protein-coupled receptors (GPCR) family that stimulate several signaling pathways mainly through G proteins [170-172]. Moreover and as discussed before, the two gonadotropins are able to induce gene expression events by targeting numerous transcription factors downstream of key signaling pathways, such as PKA, PKC, PKB/Akt, MAPK and PI3K. These transcriptional activities of FSH in vitro or LH in vivo are essential to achieve successful oocyte developmental competence. These similarities in molecular structure, the specific receptor, and the transcriptional pathways and gene targets lend support to the hypothesis of possible overlapping genomic roles between these two gonadotropins.
Evidence of genomic effects’ similitude between LH in vivo and FSH in vitro
In addition to their structural and functional (receptor and downstream pathways) similarities, the goal here is to look for common genes that are transcriptionally upregulated (directly or indirectly) by the two gonado-tropins and that could support our hypothesis of an LH-like effect of FSH in vitro. These transcriptomic similitudes between the action of LH in vivo and FSH in vitro. To this end, we compared the FSH-induced genes in CCs in conditions associated with an increase of oocyte competence in vitro[23] to the in vivo context 6 hours after the LH surge. Because finding a timeline to compare cumulus cell status and gene expression patterns in vivo versus in vitro can be difficult, we used the meiotic status of the oocyte as a suitable reference. Thus, our analysis focused on the comparison of CCs gene expression patterns in vitro at 6 hours of IVM (oocyte entering GVBD stage) [23] versus the overexpressed genes in CCs at 6 hours after the LH surge in vivo (when the oocyte is again entering the GVBD stage) [173].
For the in vitro study, the focus was on the definition of the FSH-induced gene expression effect in bovine CCs (around GVBD) associated with oocyte competence in vitro[23]. Although the whole molecular pathway of FSH action in CCs is not fully defined, we supposed that this genomic effect in vitro (Figure 1B) could be different from its counterpart in vivo, where FSH acts in synergy with LH (Figure 1A). The in vitro versus in vivo differences in blastocyst outcome support this assumption. Concerning the subsequent in vivo study, we have analyzed the LH-induced gene expression effect in vivo (close to the GVBD) again on bovine CCs [174]. This latter in vivo context should better reflect the real mechanism of CC contribution to oocyte competence acquisition. In fact, LH was reported to induce final maturation of the oocyte in vivo by acting on CCs which express and deliver competence inducers to the oocyte [28,59]. Keeping all these considerations in mind, our analysis focused on the comparison of the genomic action of FSH in vitro versus LH in vivo. A non-exhaustive list of common molecular genes between LH and FSH, expressed in CCs, and associated with oocyte final maturation is provided (Table 1). Among the 133 significant candidates induced by FSH in vitro, 22 genes were also induced by LH in vivo. This means that in vivo, LH is able to induce the transcription of around 16.5% (22/133) of all the genes overexpressed by FSH in vitro. Strikingly, these common candidates correspond to almost 32% (22/69) of the genes overexpressed via LH in vivo (Figure 2).
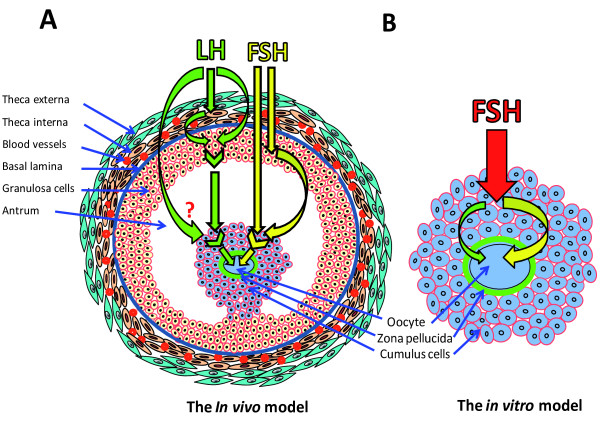
Figure 1.
Model of gonadotropin-mediated induction of oocyte competence in vivo versus in vitro: In addition to its own effects, could FSH in vitro substitute for some of the effects of LH? FSH: follicle stimulating hormone; LH: luteinizing hormone.
Table 1.
Common overexpressed genes in bovine CCs around the GVBD between FSH in vitro versus LH in vivo
| No. | Gene name | Gene/protein full name (if available) | Accession no. |
|---|---|---|---|
| 1 |
ATP1B4 |
Bos taurus ATPase, (Na+)/K + transporting, beta 4 polypeptide |
NM_001101919 |
| 2 |
ATP6V1C1 |
Bos taurus ATPase, H + transporting, lysosomal 42kDa, V1 subunit C1 |
NM_176676 |
| 3 |
BAMBI |
Bos taurus BMP and activin membrane-bound inhibitor homolog |
NM_001046309 |
| 4 |
HSPA8 |
Bos taurus heat shock 70 kDa protein 8 |
NM_174345 |
| 5 |
INHBA* |
Bos taurus inhibin, beta A |
NM_174363 |
| 6 |
PAPD1 |
Bos taurus PAP associated domain containing 1 |
BC104501 |
| 7 |
PSMA2 |
Bos taurus proteasome (prosome, macropain) subunit, alpha type, 2 |
BC102206 |
| 8 |
RHOA |
Bos taurus ras homolog gene family, member A |
NM_176645 |
| 9 |
RPL3 |
Bos taurus ribosomal protein L3 |
BT021012 |
| 10 |
SELK |
Bos taurus similar to selenoprotein K |
BC108150 |
| 11 |
SLC25A5 |
Bos taurus solute carrier family 25 member 5 |
BC102950 |
| 12 |
TNFAIP6* |
Bos taurus tumor necrosis factor, alpha-induced protein 6 |
NM_001007813 |
| 13 |
UBA6 |
Bos taurus ubiquitin-like modifier activating enzyme 6 |
NM_001083438 |
| 14 |
CHSY1 |
Homo sapiens carbohydrate (chondroitin) synthase 1 |
NM_014918 |
| 15 |
EREG● |
Homo sapiens epiregulin |
NM_001432 |
| 16 |
FOXO3A |
Homo sapiens forkhead box O3 (FOXO3), transcript variant 2, mRNA |
NM_201559 |
| 17 |
PGR* |
Bos taurus progesterone receptor |
NC_007313.4 |
| 18 |
NEAT1 |
Homo sapiens nuclear enriched abundant transcript 1 |
EF177379 |
| 19 |
SGMS2 |
Homo sapiens sphingomyelin synthase 2 (SGMS2), transcript variant |
NM_001136258 |
| 20 |
AGPAT9 |
PREDICTED: Bos taurus similar to 1-acyl-sn-glycerol-3-phosphate O-acyltransferase 9 |
XM_597964 |
| 21 |
RBMX |
PREDICTED: Bos taurus similar to Heterogeneous nuclear ribonucleoprotein G |
XM_875611 |
| 22 | SLC39A8 | PREDICTED: Bos taurus similar to Solute carrier family 39 (zinc transporter), member 8 | XM_584935 |
Real-time PCR validation of overexpression following FSH in vitro(*) or LH in vivo (●); α = 0.05%.
Figure 2.
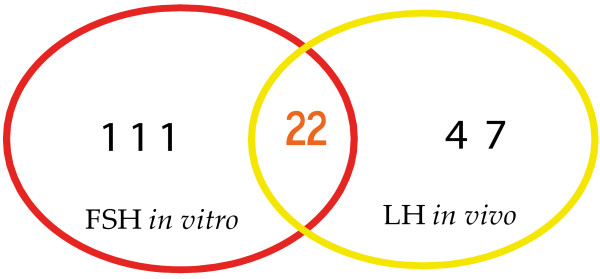
Common genes overexpressed by FSH in vitro versus LH in vivo in bovine CCs around the GVBD, as revealed by microarray.FSH: follicle stimulating hormone; LH: luteinizing hormone.
These findings mean that about one third of the genes induced by LH in vivo could be induced by FSH in vitro. FSH action in vitro therefore seems to act for both its own and LH’s in vivo functions [16]. Using its common downstream pathways of gene expression with LH, FSH in vitro appears to reproduce its in vivo function and substitute at least partially for the in vivo activity of LH (Figure 1B).
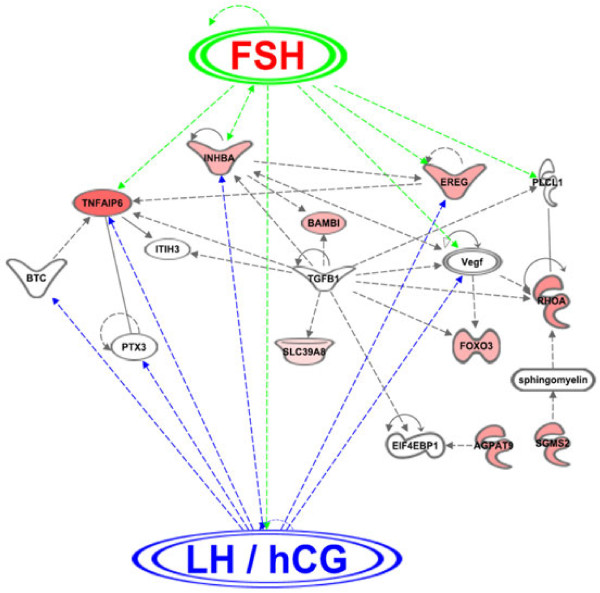
Analysis of the gene networks of the 22 common genes, using the gene Ingenuity Pathway Analysis (IPA) software (Ingenuity Systems, http://www.ingenuity.com/products/ipa; [175], confirms the high overlap between FSH and LH at the transcriptional level. Figure 3 illustrates the gene network with highest score following the IPA analysis. In this network, several key gene pathways involved in oocyte competence, steroidogenesis, CC differentiation and mucification, ovulation and luteinization were activated by both FSH and LH. Surprisingly, most of these common target genes (and therefore their functions) are influenced by the TGFbeta factors (Figure 3). These growth factors in the follicular context may correspond to the oocyte-secreted factors (mainly GDF9 and BMP15) reported as crucial factors in the oocyte-follicular cells dialog [176-178].
Figure 3.
A summary of a gene network including common gene candidates induced by both FSH in vitro and LH/hCG in vivo as revealed by the IPA software. FSH: follicle-stimulating hormone; LH/hCG: luteinizing hormone/human chorionic gonadotropin; EREG: epiregulin; TNFAIP6: tumor necrosis factor, alpha-induced protein 6; INHBA: inhibin, beta A; BAMBI: BMP and activin membrane-bound inhibitor homolog (Xenopus laevis); FOXO3: forkhead box O3; RHOA: ras homolog gene family, member A; TGFB1: transforming growth factor, beta 1; PTX3: pentraxin-related gene, rapidly induced by IL-1 beta; BTC: betacellulin; ITIH3: inter-alpha (globulin) inhibitor H3; SLC39A8: solute carrier family 39 (zinc transporter), member 8; VEGF family: vascular endothelial growth factor; PLCL1: phospholipase C-like 1; SGMS2: sphingomyelin synthase 2; AGPAT9: 1-acylglycerol-3-phosphate O-acyltransferase 9; EIF4EBP1: eukaryotic translation initiation factor 4E binding protein 1.
The progesterone receptor (PGR) is another interesting candidate commonly expressed in response to FSH and LH (Table 1). These findings are in line with recent reports in bovine cumulus in vitro confirming gonadotropin induction of PGR expression [23,151,179]. This receptor is also essential in reproduction and particularly in the ovulatory process through stimulation of the expression of enzymes crucial to ovulation such as ADAMTS1 and CTSL (cathepsin L), and the inhibition of follicular cell apoptosis [179,180]. Moreover, PGR is involved in intracellular signaling and kinase activities required for oocyte maturation and subsequent ovulation [87,121,181]. This nuclear receptor has a transcriptional role in mediating gonadotropin stimulation by downstream expression of several key genes. These observations were confirmed by important alterations of CC gene expression patterns and crucial signaling pathways in PGR null mice [89,153,158,182].
To our knowledge, this is the first time that the mimicking of LH action in vivo by FSH in vitro is highlighted at the transcriptomic level. It is important to note that this analysis was made using a custom-made microarray [23]. It is expected that the number of common candidates between the two gonadotropins may increase if commercial whole genome microarrays were used.
Concluding remarks
These similarities between FSH effect in vitro and LH in vivo, although still preliminary, support our hypothesis of potential functional substitution between FSH and LH. They are also consistent with previous results where the addition of LH to FSH-based IVM media did not result in any additive effect either in cumulus expansion or in oocyte competence as measured by the blastocyst yield. Moreover, this probable functional mimetic action of LH function by FSH in vitro, should help in improving in vitro culture systems and ovulation induction programs through a better understanding of the FSH/LH synergy in vivo. Furthermore, these common candidates will serve as a precious preliminary tool to monitor such mimetic action and should advance our understanding of the molecular pathways that lead to successful oocyte maturation, cumulus cells differentiation, ovulation and subsequent fertilization.
However, additional studies are required to confirm our results including the overexpressed and underexpressed genes, and to investigate the FSH/LH synergy. Studying the gene expression patterns induced by FSH, LH and (FSH + LH) in sequential culture system could be an interesting way to validate these findings.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All this study was achieved at Laval University. MAS designed the study. MA contributed in the study design, analyzed the data and drafted the manuscript. Both MAS and FJR were involved in data interpretation, performed a critical revision of the manuscript and gave their final approval for publication. All authors read and approved the final manuscript.
Contributor Information
Mourad Assidi, Email: mourad.assidi@gmail.com.
François J Richard, Email: Francois.Richard@fsaa.ulaval.ca.
Marc-André Sirard, Email: Marc-Andre.Sirard@fsaa.ulaval.ca.
Acknowledgements
This study was supported by The Natural Sciences and Engineering Research Council of Canada (NSERC), Canada.
References
- Eppig JJ. Regulation of cumulus oophorus expansion by gonadotropins in vivo and in vitro. Biol Reprod. 1980;23:545–552. doi: 10.1095/biolreprod23.3.545. [DOI] [PubMed] [Google Scholar]
- Dekel N, Hillensjo T, Kraicer PF. Maturational effects of gonadotropins on the cumulus-oocyte complex of the rat. Biol Reprod. 1979;20:191–197. doi: 10.1095/biolreprod20.2.191. [DOI] [PubMed] [Google Scholar]
- Espey LL, Richards JS. Temporal and spatial patterns of ovarian gene transcription following an ovulatory dose of gonadotropin in the rat. Biol Reprod. 2002;67:1662–1670. doi: 10.1095/biolreprod.102.005173. [DOI] [PubMed] [Google Scholar]
- Sha W, Xu BZ, Li M, Liu D, Feng HL, Sun QY. Effect of gonadotropins on oocyte maturation in vitro: an animal model. Fertil Steril. 2010;93:1650–1661. doi: 10.1016/j.fertnstert.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev. 1994;15:725–751. doi: 10.1210/edrv-15-6-725. [DOI] [PubMed] [Google Scholar]
- Hillier SG. Current concepts of the roles of follicle stimulating hormone and luteinizing hormone in folliculogenesis. Hum Reprod. 1994;9:188–191. doi: 10.1093/oxfordjournals.humrep.a138480. [DOI] [PubMed] [Google Scholar]
- Gregory SJ, Kaiser UB. Regulation of gonadotropins by inhibin and activin. Semin Reprod Med. 2004;22:253–267. doi: 10.1055/s-2004-831901. [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ. The physiology of follicle selection. Reprod Biol Endocrinol. 2004;2:31. doi: 10.1186/1477-7827-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginther OJ, Bergfelt DR, Kulick LJ, Kot K. Selection of the dominant follicle in cattle: role of two-way functional coupling between follicle-stimulating hormone and the follicles. Biol Reprod. 2000;62:920–927. doi: 10.1095/biolreprod62.4.920. [DOI] [PubMed] [Google Scholar]
- Hunter RHF. In: Physiology of the Graafian Follicle and Ovulation. Cambridge University Press. Hunter RHF, editor. Cambridge: Cambridge University Press; 2003. Formation and structure of ovaries: elaboration of follicular compartments; pp. 24–377. [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nature genetics. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the 'two-cell, two-gonadotrophin' model revisited. Mol Cell Endocrinol. 1994;100:51–54. doi: 10.1016/0303-7207(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Accardo C, Dattena M, Pilichi S, Mara L, Chessa B, Cappai P. Effect of recombinant human FSH and LH on in vitro maturation of sheep oocytes; embryo development and viability. Anim Reprod Sci. 2004;81:77–86. doi: 10.1016/j.anireprosci.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Howles CM. Role of LH and FSH in ovarian function. Mol Cell Endocrinol. 2000;161:25–30. doi: 10.1016/s0303-7207(99)00219-1. [DOI] [PubMed] [Google Scholar]
- Shimada M, Terada T. FSH and LH induce progesterone production and progesterone receptor synthesis in cumulus cells: a requirement for meiotic resumption in porcine oocytes. Mol Hum Reprod. 2002;8:612–618. doi: 10.1093/molehr/8.7.612. [DOI] [PubMed] [Google Scholar]
- Sirard MA, Desrosier S, Assidi M. In vivo and in vitro effects of FSH on oocyte maturation and developmental competence. Theriogenology. 2007;68(Suppl 1):S71–76. doi: 10.1016/j.theriogenology.2007.05.053. [DOI] [PubMed] [Google Scholar]
- Mattioli M, Barboni B. Signal transduction mechanism for LH in the cumulus-oocyte complex. Mol Cell Endocrinol. 2000;161:19–23. doi: 10.1016/s0303-7207(99)00218-x. [DOI] [PubMed] [Google Scholar]
- Panigone S, Hsieh M, Fu M, Persani L, Conti M. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol. 2008;22:924–936. doi: 10.1210/me.2007-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise T, Suss U, Stranzinger G, Wuthrich K, Maurer RR. Cumulus and oocyte maturation and in vitro and in vivo fertilization of oocytes in relation to follicular steroids, prolactin, and glycosaminoglycans throughout the estrous period in superovulated heifers with a normal LH surge, no detectable LH surge, and progestin inhibition of LH surge. Domest Anim Endocrinol. 1994;11:59–86. doi: 10.1016/0739-7240(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Zhang M, Ouyang H, Xia G. The signal pathway of gonadotrophins-induced mammalian oocyte meiotic resumption. Mol Hum Reprod. 2009;15:399–409. doi: 10.1093/molehr/gap031. [DOI] [PubMed] [Google Scholar]
- Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier SG. Paracrine support of ovarian stimulation. Mol Hum Reprod. 2009;15:843–850. doi: 10.1093/molehr/gap086. [DOI] [PubMed] [Google Scholar]
- Assidi M, Dufort I, Ali A, Hamel M, Algriany O, Dielemann S, Sirard MA. Identification of potential markers of oocyte competence expressed in bovine cumulus cells matured with follicle-stimulating hormone and/or phorbol myristate acetate in vitro. Biol Reprod. 2008;79:209–222. doi: 10.1095/biolreprod.108.067686. [DOI] [PubMed] [Google Scholar]
- Adriaenssens T, Wathlet S, Segers I, Verheyen G, De Vos A, Van der Elst J, Coucke W, Devroey P, Smitz J. Cumulus cell gene expression is associated with oocyte developmental quality and influenced by patient and treatment characteristics. Hum Reprod. 2010;25:1259–1270. doi: 10.1093/humrep/deq049. [DOI] [PubMed] [Google Scholar]
- Baerwald AR, Walker RA, Pierson RA. Growth rates of ovarian follicles during natural menstrual cycles, oral contraception cycles, and ovarian stimulation cycles. Fertil Steril. 2009;91:440–449. doi: 10.1016/j.fertnstert.2007.11.054. [DOI] [PubMed] [Google Scholar]
- Weghofer A, Schnepf S, Barad D, Gleicher N. The impact of luteinizing hormone in assisted reproduction: a review. Curr Opin Obstet Gynecol. 2007;19:253–257. doi: 10.1097/GCO.0b013e3280bad843. [DOI] [PubMed] [Google Scholar]
- Blondin P, Bousquet D, Twagiramungu H, Barnes F, Sirard MA. Manipulation of follicular development to produce developmentally competent bovine oocytes. Biol Reprod. 2002;66:38–43. doi: 10.1095/biolreprod66.1.38. [DOI] [PubMed] [Google Scholar]
- Dieleman SJ, Hendriksen PJ, Viuff D, Thomsen PD, Hyttel P, Knijn HM, Wrenzycki C, Kruip TA, Niemann H, Gadella BM. et al. Effects of in vivo prematuration and in vivo final maturation on developmental capacity and quality of pre-implantation embryos. Theriogenology. 2002;57:5–20. doi: 10.1016/s0093-691x(01)00655-0. [DOI] [PubMed] [Google Scholar]
- Zuelke KA, Brackett BG. Luteinizing hormone-enhanced in vitro maturation of bovine oocytes with and without protein supplementation. Biol Reprod. 1990;43:784–787. doi: 10.1095/biolreprod43.5.784. [DOI] [PubMed] [Google Scholar]
- Ali A, Sirard MA. The effects of 17beta-estradiol and protein supplement on the response to purified and recombinant follicle stimulating hormone in bovine oocytes. Zygote. 2002a;10:65–71. doi: 10.1017/s0967199402002095. [DOI] [PubMed] [Google Scholar]
- Shimada M, Nishibori M, Isobe N, Kawano N, Terada T. Luteinizing hormone receptor formation in cumulus cells surrounding porcine oocytes and its role during meiotic maturation of porcine oocytes. Biol Reprod. 2003;68:1142–1149. doi: 10.1095/biolreprod.102.010082. [DOI] [PubMed] [Google Scholar]
- Meinecke B, Meinecke-Tillmann S. Effects of alpha-amanitin on nuclear maturation of porcine oocytes in vitro. J Reprod Fertil. 1993;98:195–201. doi: 10.1530/jrf.0.0980195. [DOI] [PubMed] [Google Scholar]
- Farin CE, Yang L. Inhibition of germinal vesicle breakdown in bovine oocytes by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole (DRB) Mol Reprod Dev. 1994;37:284–292. doi: 10.1002/mrd.1080370307. [DOI] [PubMed] [Google Scholar]
- Rodriguez KF, Farin CE. Gene transcription and regulation of oocyte maturation. Reprod Fertil Dev. 2004a;16:55–67. doi: 10.10371/RD03078. [DOI] [PubMed] [Google Scholar]
- Tatemoto H, Terada T. Time-dependent effects of cycloheximide and alpha-amanitin on meiotic resumption and progression in bovine follicular oocytes. Theriogenology. 1995;43:1107–1113. doi: 10.1016/0093-691x(95)00074-i. [DOI] [PubMed] [Google Scholar]
- Nivet AL, Bunel A, Labrecque R, Belanger J, Vigneault C, Blondin P, Sirard MA. FSH withdrawal improves developmental competence of oocytes in the bovine model. Reproduction. 2012;143:165–171. doi: 10.1530/REP-11-0391. [DOI] [PubMed] [Google Scholar]
- van Tol HT, van Eijk MJ, Mummery CL, van den Hurk R, Bevers MM. Influence of FSH and hCG on the resumption of meiosis of bovine oocytes surrounded by cumulus cells connected to membrana granulosa. Mol Reprod Dev. 1996;45:218–224. doi: 10.1002/(SICI)1098-2795(199610)45:2<218::AID-MRD15>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Adriaens I, Cortvrindt R, Smitz J. Differential FSH exposure in preantral follicle culture has marked effects on folliculogenesis and oocyte developmental competence. Hum Reprod. 2004;19:398–408. doi: 10.1093/humrep/deh074. [DOI] [PubMed] [Google Scholar]
- Sasseville M, Gagnon MC, Guillemette C, Sullivan R, Gilchrist RB, Richard FJ. Regulation of gap junctions in porcine cumulus-oocyte complexes: contributions of granulosa cell contact, gonadotropins, and lipid rafts. Mol Endocrinol. 2009;23:700–710. doi: 10.1210/me.2008-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Campbell KH, Craigon J, Luck MR. Dynamic changes in meiotic progression and improvement of developmental competence of pig oocytes in vitro by follicle-stimulating hormone and cycloheximide. Biol Reprod. 2005;72:399–406. doi: 10.1095/biolreprod.104.034553. [DOI] [PubMed] [Google Scholar]
- Wu J, Xu B, Wang W. Effects of luteinizing hormone and follicle stimulating hormone on the developmental competence of porcine preantral follicle oocytes grown in vitro. J Assist Reprod Genet. 2007;24:419–424. doi: 10.1007/s10815-007-9154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkkanoglu M, Isikoglu M, Aydin D, Ozgur K. Clinical effects of ovulation induction with recombinant follicle-stimulating hormone supplemented with recombinant luteinizing hormone or low-dose recombinant human chorionic gonadotropin in the midfollicular phase in microdose cycles in poor responders. Fertil Steril. 2007;88:665–669. doi: 10.1016/j.fertnstert.2006.11.150. [DOI] [PubMed] [Google Scholar]
- Yan J, Yang Y, Liying Y, Zichuan L, Ping L, Huailiang F, Qi Z, Jie Q. In vitro maturation of cumulus-partially enclosed immature human oocytes by priming with gonadotropin. Fertil Steril. 2011;96:629–634. doi: 10.1016/j.fertnstert.2011.06.054. e621. [DOI] [PubMed] [Google Scholar]
- Merriman JA, Whittingham DG, Carroll J. The effect of follicle stimulating hormone and epidermal growth factor on the developmental capacity of in-vitro matured mouse oocytes. Hum Reprod. 1998;13:690–695. doi: 10.1093/humrep/13.3.690. [DOI] [PubMed] [Google Scholar]
- Ali A, Sirard MA. Protein kinases influence bovine oocyte competence during short-term treatment with recombinant human follicle stimulating hormone. Reproduction. 2005;130:303–310. doi: 10.1530/rep.1.00387. [DOI] [PubMed] [Google Scholar]
- Salustri A, Yanagishita M, Hascall VC. Synthesis and accumulation of hyaluronic acid and proteoglycans in the mouse cumulus cell-oocyte complex during follicle-stimulating hormone-induced mucification. J Biol Chem. 1989;264:13840–13847. [PubMed] [Google Scholar]
- Anderiesz C, Ferraretti A, Magli C, Fiorentino A, Fortini D, Gianaroli L, Jones GM, Trounson AO. Effect of recombinant human gonadotrophins on human, bovine and murine oocyte meiosis, fertilization and embryonic development in vitro. Hum Reprod. 2000;15:1140–1148. doi: 10.1093/humrep/15.5.1140. [DOI] [PubMed] [Google Scholar]
- Zafeiriou S, Loutradis D, Michalas S. The role of gonadotropins in follicular development and their use in ovulation induction protocols for assisted reproduction. Eur J Contracept Reprod Health Care. 2000;5:157–167. doi: 10.1080/13625180008500389. [DOI] [PubMed] [Google Scholar]
- O'Herlihy C, Pepperell RJ, Evans JH. The significance of FSH elevation in young women with disorders of ovulation. Br Med J. 1980;281:1447–1450. doi: 10.1136/bmj.281.6253.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Temporal relationships between ovulation and defined changes in the concentration of plasma estradiol-17 beta, luteinizing hormone, follicle-stimulating hormone, and progesterone. I. Probit analysis. World Health Organization, Task Force on Methods for the Determination of the Fertile Period, Special Programme of Research, Development and Research Training in Human Reproduction. Am J Obstet Gynecol. 1980;138:383–390. [PubMed] [Google Scholar]
- Amsterdam A, Koch Y, Lieberman ME, Lindner HR. Distribution of binding sites for human chorionic gonadotropin in the preovulatory follicle of the rat. J Cell Biol. 1975;67:894–900. doi: 10.1083/jcb.67.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttinck F, Charpigny G, Mermillod P, Loosfelt H, Meduri G, Freret S, Grimard B, Heyman Y. Expression of components of the insulin-like growth factor system and gonadotropin receptors in bovine cumulus-oocyte complexes during oocyte maturation. Domest Anim Endocrinol. 2004;27:179–195. doi: 10.1016/j.domaniend.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Calder MD, Caveney AN, Smith LC, Watson AJ. Responsiveness of bovine cumulus-oocyte-complexes (COC) to porcine and recombinant human FSH, and the effect of COC quality on gonadotropin receptor and Cx43 marker gene mRNAs during maturation in vitro. Reprod Biol Endocrinol. 2003;1:14. doi: 10.1186/1477-7827-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp TA, Rahal JO, Mayo KE. Cellular localization and hormonal regulation of follicle-stimulating hormone and luteinizing hormone receptor messenger RNAs in the rat ovary. Mol Endocrinol. 1991;5:1405–1417. doi: 10.1210/mend-5-10-1405. [DOI] [PubMed] [Google Scholar]
- Robert C, Gagne D, Lussier JG, Bousquet D, Barnes FL, Sirard MA. Presence of LH receptor mRNA in granulosa cells as a potential marker of oocyte developmental competence and characterization of the bovine splicing isoforms. Reproduction. 2003;125:437–446. [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- Reizel Y, Elbaz J, Dekel N. Sustained activity of the EGF receptor is an absolute requisite for LH-induced oocyte maturation and cumulus expansion. Mol Endocrinol. 2010;24:402–411. doi: 10.1210/me.2009-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Carnevale EM, Seidel GE Jr, Squire EL. Effects of gonadotropins on bovine oocytes matured in TCM-199. Theriogenology. 2001;56:661–670. doi: 10.1016/s0093-691x(01)00597-0. [DOI] [PubMed] [Google Scholar]
- Peng XR, Hsueh AJ, LaPolt PS, Bjersing L, Ny T. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology. 1991;129:3200–3207. doi: 10.1210/endo-129-6-3200. [DOI] [PubMed] [Google Scholar]
- Jeppesen JV, Kristensen SG, Nielsen ME, Humaidan P, Dal Canto M, Fadini R, Schmidt KT, Ernst E, Yding Andersen C. LH-receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J Clin Endocrinol Metab. 2012;97:E1524–1531. doi: 10.1210/jc.2012-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima I, Okazaki T, Noma N, Nishibori M, Yamashita Y, Shimada M. Sequential exposure of porcine cumulus cells to FSH and/or LH is critical for appropriate expression of steroidogenic and ovulation-related genes that impact oocyte maturation in vivo and in vitro. Reproduction. 2008;136:9–21. doi: 10.1530/REP-08-0074. [DOI] [PubMed] [Google Scholar]
- van de Leemput EE, Vos PL, Zeinstra EC, Bevers MM, van der Weijden GC, Dieleman SJ. Improved in vitro embryo development using in vivo matured oocytes from heifers superovulated with a controlled preovulatory LH surge. Theriogenology. 1999;52:335–349. doi: 10.1016/s0093-691x(99)00133-8. [DOI] [PubMed] [Google Scholar]
- Rizos D, Ward F, Duffy P, Boland MP, Lonergan P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Mol Reprod Dev. 2002;61:234–248. doi: 10.1002/mrd.1153. [DOI] [PubMed] [Google Scholar]
- Guixue Z, Luciano AM, Coenen K, Gandolfi F, Sirard MA. The influence of cAMP before or during bovine oocyte maturation on embryonic developmental competence. Theriogenology. 2001;55:1733–1743. doi: 10.1016/s0093-691x(01)00516-7. [DOI] [PubMed] [Google Scholar]
- Hubbard CJ. The effects of forskolin and LH on cAMP changes and maturation in the follicle-enclosed oocytes of hamsters. Acta Endocrinol (Copenh) 1985;110:413–420. doi: 10.1530/acta.0.1100413. [DOI] [PubMed] [Google Scholar]
- Modina S, Luciano AM, Vassena R, Baraldi-Scesi L, Lauria A, Gandolfi F. Oocyte developmental competence after in vitro maturation depends on the persistence of cumulus-oocyte comunications which are linked to the intracellular concentration of cAMP. Ital J Anat Embryol. 2001;106:241–248. [PubMed] [Google Scholar]
- Webb RJ, Marshall F, Swann K, Carroll J. Follicle-stimulating hormone induces a gap junction-dependent dynamic change in [cAMP] and protein kinase a in mammalian oocytes. Dev Biol. 2002;246:441–454. doi: 10.1006/dbio.2002.0630. [DOI] [PubMed] [Google Scholar]
- Robker RL, Richards JS. Hormonal control of the cell cycle in ovarian cells: proliferation versus differentiation. Biol Reprod. 1998;59:476–482. doi: 10.1095/biolreprod59.3.476. [DOI] [PubMed] [Google Scholar]
- Kaipia A, Hsueh AJ. Regulation of ovarian follicle atresia. Annu Rev Physiol. 1997;59:349–363. doi: 10.1146/annurev.physiol.59.1.349. [DOI] [PubMed] [Google Scholar]
- Slomczynska M, Szoltys M, Duda M, Sikora K, Tabarowski Z. Androgens and FSH affect androgen receptor and aromatase distribution in the porcine ovary. Folia Biol (Krakow) 2003;51:63–68. [PubMed] [Google Scholar]
- Okazaki T, Nishibori M, Yamashita Y, Shimada M. LH reduces proliferative activity of cumulus cells and accelerates GVBD of porcine oocytes. Mol Cell Endocrinol. 2003;209:43–50. doi: 10.1016/j.mce.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Montgomery RR, Ward-Bailey PF, Eppig JJ. Regulation of oocyte maturation in the mouse: possible roles of intercellular communication, cAMP, and testosterone. Dev Biol. 1983;95:294–304. doi: 10.1016/0012-1606(83)90030-1. [DOI] [PubMed] [Google Scholar]
- Jin S, Zhang M, Lei L, Wang C, Fu M, Ning G, Xia G. Meiosis activating sterol (MAS) regulate FSH-induced meiotic resumption of cumulus cell-enclosed porcine oocytes via PKC pathway. Mol Cell Neurosci. 2006;249:64–70. doi: 10.1016/j.mce.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Rodriguez KF, Petters RM, Crosier AE, Farin CE. Roles of gene transcription and PKA subtype activation in maturation of murine oocytes. Reproduction. 2002;123:799–806. doi: 10.1530/rep.0.1230799. [DOI] [PubMed] [Google Scholar]
- Ning G, Ouyang H, Wang S, Chen X, Xu B, Yang J, Zhang H, Zhang M, Xia G. 3',5'-cyclic adenosine monophosphate response element binding protein up-regulated cytochrome P450 lanosterol 14alpha-demethylase expression involved in follicle-stimulating hormone-induced mouse oocyte maturation. Mol Endocrinol. 2008;22:1682–1694. doi: 10.1210/me.2007-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin CE, Rodriguez KF, Alexander JE, Hockney JE, Herrick JR, Kennedy-Stoskopf S. The role of transcription in EGF- and FSH-mediated oocyte maturation in vitro. Anim Reprod Sci. 2007;98:97–112. doi: 10.1016/j.anireprosci.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y-Q, Rubinstein S, Luria A, Lax Y, Breitbart H. Involvement of MEK-Mitogen-Activated Protein Kinase Pathway in Follicle-Stimulating Hormone-Induced but Not Spontaneous Meiotic Resumption of Mouse Oocytes. BiolReprod. 2001;65:358–365. doi: 10.1095/biolreprod65.2.358. [DOI] [PubMed] [Google Scholar]
- Su YQ, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology. 2002a;143:2221–2232. doi: 10.1210/endo.143.6.8845. [DOI] [PubMed] [Google Scholar]
- Maizels ET, Cottom J, Jones JC, Hunzicker-Dunn M. Follicle stimulating hormone (FSH) activates the p38 mitogen-activated protein kinase pathway, inducing small heat shock protein phosphorylation and cell rounding in immature rat ovarian granulosa cells. Endocrinology. 1998;139:3353–3356. doi: 10.1210/endo.139.7.6188. [DOI] [PubMed] [Google Scholar]
- Fissore RA, He CL, Vande Woude GF. Potential role of mitogen-activated protein kinase during meiosis resumption in bovine oocytes. Biology of reproduction. 1996;55:1261–1270. doi: 10.1095/biolreprod55.6.1261. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Okamoto M, Kawashima I, Okazaki T, Nishimura R, Gunji Y, Hishinuma M, Shimada M. Positive feedback loop between prostaglandin E2 and EGF-like factors is essential for sustainable activation of MAPK3/1 in cumulus cells during in vitro maturation of porcine cumulus oocyte complexes. Biol Reprod. 2011;85:1073–1082. doi: 10.1095/biolreprod.110.090092. [DOI] [PubMed] [Google Scholar]
- Downs SM, Chen J. EGF-like peptides mediate FSH-induced maturation of cumulus cell-enclosed mouse oocytes. Mol Reprod Dev. 2008;75:105–114. doi: 10.1002/mrd.20781. [DOI] [PubMed] [Google Scholar]
- Caixeta ES, Machado MF, Ripamonte P, Price C, Buratini J. Effects of FSH on the expression of receptors for oocyte-secreted factors and members of the EGF-like family during in vitro maturation in cattle. Reprod Fertil Dev. 2013;25:890–899. doi: 10.1071/RD12125. [DOI] [PubMed] [Google Scholar]
- Gerits N, Kostenko S, Shiryaev A, Johannessen M, Moens U. Relations between the mitogen-activated protein kinase and the cAMP-dependent protein kinase pathways: Comradeship and hostility. Cell Signal. 2008;20:1592–1607. doi: 10.1016/j.cellsig.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Hsieh M, Zamah AM, Conti M. Epidermal growth factor-like growth factors in the follicular fluid: role in oocyte development and maturation. Semin Reprod Med. 2009;27:052–061. doi: 10.1055/s-0028-1108010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y, Shimada M. The release of EGF domain from EGF-like factors by a specific cleavage enzyme activates the EGFR-MAPK3/1 pathway in both granulosa cells and cumulus cells during the ovulation process. J Reprod Dev. 2012;58:510–514. doi: 10.1262/jrd.2012-056. [DOI] [PubMed] [Google Scholar]
- Richards JS. Genetics of ovulation. Semin Reprod Med. 2007;25:235–242. doi: 10.1055/s-2007-980217. [DOI] [PubMed] [Google Scholar]
- Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci. 2007a;120:1330–1340. doi: 10.1242/jcs.000968. [DOI] [PubMed] [Google Scholar]
- Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20:1352–1365. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- Su YQ, Denegre JM, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ. Oocyte-dependent activation of mitogen-activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte-cumulus cell complex. Dev Biol. 2003;263:126–138. doi: 10.1016/s0012-1606(03)00437-8. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Hishinuma M, Shimada M. Activation of PKA, p38 MAPK and ERK1/2 by gonadotropins in cumulus cells is critical for induction of EGF-like factor and TACE/ADAM17 gene expression during in vitro maturation of porcine COCs. J ovarian res. 2009;2:20. doi: 10.1186/1757-2215-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner SA, Day AJ, Rugg MS, Breyer RM, Gomer RH, Richards JS. Disrupted function of tumor necrosis factor-alpha-stimulated gene 6 blocks cumulus cell-oocyte complex expansion. Endocrinology. 2003a;144:4376–4384. doi: 10.1210/en.2003-0487. [DOI] [PubMed] [Google Scholar]
- Tirone E, D'Alessandris C, Hascall VC, Siracusa G, Salustri A. Hyaluronan synthesis by mouse cumulus cells is regulated by interactions between follicle-stimulating hormone (or epidermal growth factor) and a soluble oocyte factor (or transforming growth factor beta1) J Biol Chem. 1997;272:4787–4794. doi: 10.1074/jbc.272.8.4787. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Robayna IJ, Alliston TN, Buse P, Firestone GL, Richards JS. Functional and subcellular changes in the A-kinase-signaling pathway: relation to aromatase and Sgk expression during the transition of granulosa cells to luteal cells. Mol Endocrinol. 1999;13:1318–1337. doi: 10.1210/mend.13.8.0334. [DOI] [PubMed] [Google Scholar]
- Nuttinck F, Reinaud P, Tricoire H, Vigneron C, Peynot N, Mialot JP, Mermillod P, Charpigny G. Cyclooxygenase-2 is expressed by cumulus cells during oocyte maturation in cattle. Mol Reprod Dev. 2002;61:93–101. doi: 10.1002/mrd.1135. [DOI] [PubMed] [Google Scholar]
- Luckenbach JA, Yamamoto Y, Guzman JM, Swanson P. Identification of ovarian genes regulated by follicle-stimulating hormone (Fsh) in vitro during early secondary oocyte growth in coho salmon. Mol Cell Endocrinol. 2013;366:38–52. doi: 10.1016/j.mce.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Johannessen M, Delghandi MP, Moens U. What turns CREB on? Cell Signal. 2004a;16:1211–1227. doi: 10.1016/j.cellsig.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Perlman S, Bouquin T, van den Hazel B, Jensen TH, Schambye HT, Knudsen S, Okkels JS. Transcriptome analysis of FSH and FSH variant stimulation in granulosa cells from IVM patients reveals novel regulated genes. Mol Hum Reprod. 2006;12:135–144. doi: 10.1093/molehr/gah247. [DOI] [PubMed] [Google Scholar]
- Labrecque R, Vigneault C, Blondin P, Sirard MA. Gene expression analysis of bovine oocytes with high developmental competence obtained from FSH-stimulated animals. Mol Reprod Dev. 2013;80:428–440. doi: 10.1002/mrd.22177. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Robayna IJ, Falender AE, Ochsner S, Firestone GL, Richards JS. Follicle-Stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-lnduced kinase (Sgk): evidence for A kinase-independent signaling by FSH in granulosa cells. Mol Endocrinol. 2000;14:1283–1300. doi: 10.1210/mend.14.8.0500. [DOI] [PubMed] [Google Scholar]
- Kalous J, Solc P, Baran V, Kubelka M, Schultz RM, Motlik J. PKB/AKT is involved in resumption of meiosis in mouse oocytes. Biology of the cell/under the auspices of the European Cell Biology Organization. 2006;98:111–123. doi: 10.1042/BC20050020. [DOI] [PubMed] [Google Scholar]
- Zhang L, Liang Y, Liu Y, Xiong CL. The role of brain-derived neurotrophic factor in mouse oocyte maturation in vitro involves activation of protein kinase B. Theriogenology. 2010;73:1096–1103. doi: 10.1016/j.theriogenology.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Shimada M, Ito J, Yamashita Y, Okazaki T, Isobe N. Phosphatidylinositol 3-kinase in cumulus cells is responsible for both suppression of spontaneous maturation and induction of gonadotropin-stimulated maturation of porcine oocytes. J Endocrinol. 2003a;179:25–34. doi: 10.1677/joe.0.1790025. [DOI] [PubMed] [Google Scholar]
- Fan HY, Huo LJ, Chen DY, Schatten H, Sun QY. Protein kinase C and mitogen-activated protein kinase cascade in mouse cumulus cells: cross talk and effect on meiotic resumption of oocyte. Biol Reprod. 2004;70:1178–1187. doi: 10.1095/biolreprod.103.024737. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhou B, Yan J, Xu B, Tai P, Li J, Peng S, Zhang M, Xia G. Epidermal growth factor receptor activation by protein kinase C is necessary for FSH-induced meiotic resumption in porcine cumulus-oocyte complexes. J Endocrinol. 2008;197:409–419. doi: 10.1677/JOE-07-0592. [DOI] [PubMed] [Google Scholar]
- Freimann S, Ben-Ami I, Dantes A, Ron-El R, Amsterdam A. EGF-like factor epiregulin and amphiregulin expression is regulated by gonadotropins/cAMP in human ovarian follicular cells. Biochem Biophys Res Commun. 2004;324:829–834. doi: 10.1016/j.bbrc.2004.09.129. [DOI] [PubMed] [Google Scholar]
- Su YQ, Xia GL, Byskov AG, Fu GD, Yang CR. Protein kinase C and intracellular calcium are involved in follicle-stimulating hormone-mediated meiotic resumption of cumulus cell-enclosed porcine oocytes in hypoxanthine-supplemented medium. Mol Reprod Dev. 1999;53:51–58. doi: 10.1002/(SICI)1098-2795(199905)53:1<51::AID-MRD6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Baldi E, Luconi M, Muratori M, Marchiani S, Tamburrino L, Forti G. Nongenomic activation of spermatozoa by steroid hormones: facts and fictions. Mol Cell Endocrinol. 2009;308:39–46. doi: 10.1016/j.mce.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Teves ME, Guidobaldi HA, Unates DR, Sanchez R, Miska W, Publicover SJ, Morales Garcia AA, Giojalas LC. Molecular mechanism for human sperm chemotaxis mediated by progesterone. PloS one. 2009;4:e8211. doi: 10.1371/journal.pone.0008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima I, Liu Z, Mullany LK, Mihara T, Richards JS, Shimada M. EGF-like factors induce expansion of the cumulus cell-oocyte complexes by activating calpain-mediated cell movement. Endocrinology. 2012;153:3949–3959. doi: 10.1210/en.2012-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, McKenzie LJ, Matzuk MM. Revisiting oocyte-somatic cell interactions: in search of novel intrafollicular predictors and regulators of oocyte developmental competence. Mol Hum Reprod. 2008;14:673–678. doi: 10.1093/molehr/gan064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130:791–799. doi: 10.1530/rep.1.00793. [DOI] [PubMed] [Google Scholar]
- Halet G. PKC signaling at fertilization in mammalian eggs. Biochim Biophys Acta. 2004;1742:185–189. doi: 10.1016/j.bbamcr.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Assidi M, Sirard M-A. In: Cumulus Cell Gene Expression as a Marker of Oocyte Quality. Coticchio G, Albertini DF, De Santis L, editor. London: Springer; 2013. Oogenesis; pp. 231–252. [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KC, Auersperg N, Leung PC. Mitogen-activated protein kinases in normal and (pre)neoplastic ovarian surface epithelium. Reprod Biol Endocrinol. 2003;1:71. doi: 10.1186/1477-7827-1-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillensjo T, Magnusson C, Svensson U, Thelander H. Effect of luteinizing hormone and follicle-stimulating hormone on progesterone synthesis by cultured rat cumulus cells. Endocrinology. 1981;108:1920–1924. doi: 10.1210/endo-108-5-1920. [DOI] [PubMed] [Google Scholar]
- Moore RK, Otsuka F, Shimasaki S. Role of ERK1/2 in the differential synthesis of progesterone and estradiol by granulosa cells. Biochem Biophys Res Commun. 2001;289:796–800. doi: 10.1006/bbrc.2001.6052. [DOI] [PubMed] [Google Scholar]
- Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci USA. 2000;97:4689–4694. doi: 10.1073/pnas.080073497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Hsieh M, Doyle KH, Falender AE, Lo YK, Sharma SC. Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog Horm Res. 2002a;57:195–220. doi: 10.1210/rp.57.1.195. [DOI] [PubMed] [Google Scholar]
- Kim J, Bagchi IC, Bagchi MK. Control of ovulation in mice by progesterone receptor-regulated gene networks. Mol Hum Reprod. 2009;15:821–828. doi: 10.1093/molehr/gap082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salustri A. Paracrine actions of oocytes in the mouse pre-ovulatory follicle. Int J Dev Biol. 2000;44:591–597. [PubMed] [Google Scholar]
- Paradis F, Moore HS, Pasternak JA, Novak S, Dyck MK, Dixon WT, Foxcroft GR. Pig preovulatory oocytes modulate cumulus cell protein and gene expression in vitro. Mol Cell Endocrinol. 2010;320:87–96. doi: 10.1016/j.mce.2010.01.034. [DOI] [PubMed] [Google Scholar]
- Caixeta ES, Ripamonte P, Franco MM, Junior JB, Dode MA. Effect of follicle size on mRNA expression in cumulus cells and oocytes of Bos indicus: an approach to identify marker genes for developmental competence. Reprod Fertil Dev. 2009;21:655–664. doi: 10.1071/RD08201. [DOI] [PubMed] [Google Scholar]
- Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010;120:963–972. doi: 10.1172/JCI41350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadyar F, Zeinstra E, Colenbrander B, Vanderstichele HM, Bevers MM. In vitro maturation of bovine oocytes in the presence of bovine activin A does not affect the number of embryos. Anim Reprod Sci. 1996;45:37–45. doi: 10.1016/s0378-4320(96)01574-6. [DOI] [PubMed] [Google Scholar]
- Chen L, Russell PT, Larsen WJ. Sequential effects of follicle-stimulating hormone and luteinizing hormone on mouse cumulus expansion in vitro. Biol Reprod. 1994;51:290–295. doi: 10.1095/biolreprod51.2.290. [DOI] [PubMed] [Google Scholar]
- Fu M, Chen X, Yan J, Lei L, Jin S, Yang J, Song X, Zhang M, Xia G. Luteinizing hormone receptors expression in cumulus cells closely related to mouse oocyte meiotic maturation. Front Biosci. 2007;12:1804–1813. doi: 10.2741/2189. [DOI] [PubMed] [Google Scholar]
- Sanchez F, Romero S, Albuz FK, Smitz J. In vitro follicle growth under non-attachment conditions and decreased FSH levels reduces Lhcgr expression in cumulus cells and promotes oocyte developmental competence. J Assist Reprod Genet. 2012;29:141–152. doi: 10.1007/s10815-011-9690-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukovsky A, Chen TT, Wimalasena J, Caudle MR. Cellular localization of luteinizing hormone receptor immunoreactivity in the ovaries of immature, gonadotropin-primed and normal cycling rats. Biol Reprod. 1993;48:1367–1382. doi: 10.1095/biolreprod48.6.1367. [DOI] [PubMed] [Google Scholar]
- Yang SH, Son WY, Yoon SH, Ko Y, Lim JH. Correlation between in vitro maturation and expression of LH receptor in cumulus cells of the oocytes collected from PCOS patients in HCG-primed IVM cycles. Hum Reprod. 2005;20:2097–2103. doi: 10.1093/humrep/dei045. [DOI] [PubMed] [Google Scholar]
- Younis AI, Brackett BG, Fayrer-Hosken RA. Influence of serum and hormones on bovine oocyte maturation and fertilization in vitro. Gamete Res. 1989;23:189–201. doi: 10.1002/mrd.1120230206. [DOI] [PubMed] [Google Scholar]
- Zuelke KA, Brackett BG. Increased glutamine metabolism in bovine cumulus cell-enclosed and denuded oocytes after in vitro maturation with luteinizing hormone. Biol Reprod. 1993;48:815–820. doi: 10.1095/biolreprod48.4.815. [DOI] [PubMed] [Google Scholar]
- Nogueira MF, Buratini J Jr, Price CA, Castilho AC, Pinto MG, Barros CM. Expression of LH receptor mRNA splice variants in bovine granulosa cells: changes with follicle size and regulation by FSH in vitro. Mol Reprod Dev. 2007;74:680–686. doi: 10.1002/mrd.20656. [DOI] [PubMed] [Google Scholar]
- Calder MD, Caveney AN, Sirard MA, Watson AJ. Effect of serum and cumulus cell expansion on marker gene transcripts in bovine cumulus-oocyte complexes during maturation in vitro. Fertil Steril. 2005;83(Suppl 1):1077–1085. doi: 10.1016/j.fertnstert.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Ginther OJ. Selection of the dominant follicle in cattle and horses. Anim Reprod Sci. 2000;60–61:61–79. doi: 10.1016/s0378-4320(00)00083-x. [DOI] [PubMed] [Google Scholar]
- Mihm M, Baker PJ, Ireland JL, Smith GW, Coussens PM, Evans AC, Ireland JJ. Molecular evidence that growth of dominant follicles involves a reduction in follicle-stimulating hormone dependence and an increase in luteinizing hormone dependence in cattle. Biol Reprod. 2006;74:1051–1059. doi: 10.1095/biolreprod.105.045799. [DOI] [PubMed] [Google Scholar]
- Fayad T, Levesque V, Sirois J, Silversides DW, Lussier JG. Gene expression profiling of differentially expressed genes in granulosa cells of bovine dominant follicles using suppression subtractive hybridization. Biol Reprod. 2004;70:523–533. doi: 10.1095/biolreprod.103.021709. [DOI] [PubMed] [Google Scholar]
- Liang CG, Huo LJ, Zhong ZS, Chen DY, Schatten H, Sun QY. Cyclic adenosine 3′,5′-monophosphate-dependent activation of mitogen-activated protein kinase in cumulus cells is essential for germinal vesicle breakdown of porcine cumulus-enclosed oocytes. Endocrinology. 2005;146:4437–4444. doi: 10.1210/en.2005-0309. [DOI] [PubMed] [Google Scholar]
- Sela-Abramovich S, Chorev E, Galiani D, Dekel N. Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology. 2005;146:1236–1244. doi: 10.1210/en.2004-1006. [DOI] [PubMed] [Google Scholar]
- Sun QY, Miao YL, Schatten H. Towards a new understanding on the regulation of mammalian oocyte meiosis resumption. Cell Cycle. 2009;8:2741–2747. doi: 10.4161/cc.8.17.9471. [DOI] [PubMed] [Google Scholar]
- Duggavathi R, Murphy BD. Development. Ovulation signals. Science. 2009;324:890–891. doi: 10.1126/science.1174130. [DOI] [PubMed] [Google Scholar]
- Newhall KJ, Criniti AR, Cheah CS, Smith KC, Kafer KE, Burkart AD, McKnight GS. Dynamic anchoring of PKA is essential during oocyte maturation. Curr Biol. 2006;16:321–327. doi: 10.1016/j.cub.2005.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez KF, Farin CE. Developmental capacity of bovine cumulus oocyte complexes after transcriptional inhibition of germinal vesicle breakdown. Theriogenology. 2004b;61:1499–1511. doi: 10.1016/j.theriogenology.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Downs SM, Hunzicker-Dunn M. Differential regulation of oocyte maturation and cumulus expansion in the mouse oocyte-cumulus cell complex by site-selective analogs of cyclic adenosine monophosphate. Dev Biol. 1995;172:72–85. doi: 10.1006/dbio.1995.0006. [DOI] [PubMed] [Google Scholar]
- Liang CG, Su YQ, Fan HY, Schatten H, Sun QY. Mechanisms regulating oocyte meiotic resumption: roles of mitogen-activated protein kinase. Mol Endocrinol. 2007;21:2037–2055. doi: 10.1210/me.2006-0408. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, Wayne CM, Ochsner SA, White L, Richards JS. Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Mol Endocrinol. 2006;20:1300–1321. doi: 10.1210/me.2005-0420. [DOI] [PubMed] [Google Scholar]
- Dieleman SJ, Kruip TA, Fontijne P, de Jong WH, van der Weyden GC. Changes in oestradiol, progesterone and testosterone concentrations in follicular fluid and in the micromorphology of preovulatory bovine follicles relative to the peak of luteinizing hormone. J Endocrinol. 1983;97:31–42. doi: 10.1677/joe.0.0970031. [DOI] [PubMed] [Google Scholar]
- Lei ZM, Mishra S, Zou W, Xu B, Foltz M, Li X, Rao CV. Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol Endocrinol. 2001;15:184–200. doi: 10.1210/mend.15.1.0586. [DOI] [PubMed] [Google Scholar]
- Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15:172–183. doi: 10.1210/mend.15.1.0582. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol. 2002;64:69–92. doi: 10.1146/annurev.physiol.64.081501.131029. [DOI] [PubMed] [Google Scholar]
- Richards JS. Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol. 2005;234:75–79. doi: 10.1016/j.mce.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Akison LK, Robker RL. The critical roles of progesterone receptor (PGR) in ovulation, oocyte developmental competence and oviductal transport in mammalian reproduction. Reprod Domest Anim. 2012;4(47 Suppl):288–296. doi: 10.1111/j.1439-0531.2012.02088.x. [DOI] [PubMed] [Google Scholar]
- Mathew D, Sellner E, Okamura C, Geisert R, Anderson L, Lucy M. Effect of progesterone antagonist RU486 on uterine progesterone receptor mRNA expression, embryonic development and ovarian function during early pregnancy in pigs. Soc Reprod Fertil Suppl. 2009;66:333–334. [PubMed] [Google Scholar]
- Shimada M, Yamashita Y, Ito J, Okazaki T, Kawahata K, Nishibori M. Expression of two progesterone receptor isoforms in cumulus cells and their roles during meiotic resumption of porcine oocytes. J Mol Endocrinol. 2004;33:209–225. doi: 10.1677/jme.0.0330209. [DOI] [PubMed] [Google Scholar]
- Fernandez-Valdivia R, Jeong J, Mukherjee A, Soyal SM, Li J, Ying Y, DeMayo FJ, Lydon JP. A mouse model to dissect progesterone signaling in the female reproductive tract and mammary gland. genesis. 2010;48:106–113. doi: 10.1002/dvg.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DL, Doyle KM, Ochsner SA, Sandy JD, Richards JS. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem. 2003;278:42330–42339. doi: 10.1074/jbc.M300519200. [DOI] [PubMed] [Google Scholar]
- Park JI, Kim SG, Chun JS, Seo YM, Jeon MJ, Ohba M, Kim HJ, Chun SY. Activation of protein kinase Czeta mediates luteinizing hormone- or forskolin-induced NGFI-B expression in preovulatory granulosa cells of rat ovary. Mol Cell Endocrinol. 2007;270:79–86. doi: 10.1016/j.mce.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Maraldi T, Riccio M, Sena P, Marzona L, Nicoli A, La Marca A, Marmiroli S, Bertacchini J, La Sala G, De Pol A. MATER protein as substrate of PKCepsilon in human cumulus cells. Mol Hum Reprod. 2009;15:499–506. doi: 10.1093/molehr/gap048. [DOI] [PubMed] [Google Scholar]
- Davis JS, Weakland LL, Farese RV, West LA. Luteinizing hormone increases inositol trisphosphate and cytosolic free Ca2+ in isolated bovine luteal cells. J Biol Chem. 1987;262:8515–8521. [PubMed] [Google Scholar]
- Mattioli M, Gioia L, Barboni B. Calcium elevation in sheep cumulus-oocyte complexes after luteinising hormone stimulation. Mol Reprod Dev. 1998;50:361–369. doi: 10.1002/(SICI)1098-2795(199807)50:3<361::AID-MRD13>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Ginther OJ, Gastal EL, Gastal MO, Bergfelt DR, Baerwald AR, Pierson RA. Comparative study of the dynamics of follicular waves in mares and women. Biol Reprod. 2004;71:1195–1201. doi: 10.1095/biolreprod.104.031054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braw-Tal R, Roth Z. Gene expression for LH receptor, 17 alpha-hydroxylase and StAR in the theca interna of preantral and early antral follicles in the bovine ovary. Reproduction. 2005;129:453–461. doi: 10.1530/rep.1.00464. [DOI] [PubMed] [Google Scholar]
- Xu Z, Garverick HA, Smith GW, Smith MF, Hamilton SA, Youngquist RS. Expression of follicle-stimulating hormone and luteinizing hormone receptor messenger ribonucleic acids in bovine follicles during the first follicular wave. Biology of reproduction. 1995;53:951–957. doi: 10.1095/biolreprod53.4.951. [DOI] [PubMed] [Google Scholar]
- Yen SSC, Llerena O, Little B, Pearson OH. Disappearance rates of endogenous luteinizing hormone and chorionic gonadotropin in man. J Clin Endocrinol Metab. 1968;28:1763–1767. doi: 10.1210/jcem-28-12-1763. [DOI] [PubMed] [Google Scholar]
- De Rensis F, Lopez-Gatius F, Garcia-Ispierto I, Techakumpu M. Clinical use of human chorionic gonadotropin in dairy cows: an update. Theriogenology. 2010;73:1001–1008. doi: 10.1016/j.theriogenology.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Li Q, Wigglesworth K, Matzuk MM, Eppig JJ. Mouse oocytes enable LH-induced maturation of the cumulus-oocyte complex via promoting EGF receptor-dependent signaling. Mol Endocrinol. 2010;24:1230–1239. doi: 10.1210/me.2009-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone NA, Kaiser UB. The biology of gonadotroph regulation. Curr Opin Endocrinol Diabetes Obes. 2009;16:321–327. doi: 10.1097/MED.0b013e32832d88fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli M, Puett D. In: Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. 6. Strauss JF, Barbieri RL, editor. Philadelphia: Saunders, Elsevier; 2009. The gonadotropin hormones and their receptors; pp. 35–55. [Google Scholar]
- Hai MV, De Roux N, Ghinea N, Beau I, Loosfelt H, Vannier B, Meduri G, Misrahi M, Milgrom E. Gonadotropin receptors. Ann Endocrinol (Paris) 1999;60:89–92. [PubMed] [Google Scholar]
- Menon KM, Menon B. Structure, function and regulation of gonadotropin receptors - a perspective. Mol Cell Endocrinol. 2012;356:88–97. doi: 10.1016/j.mce.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyttel P, Callesen H, Greve T. Ultrastructural features of preovulatory oocyte maturation in superovulated cattle. J Reprod Fertil. 1986;76:645–656. doi: 10.1530/jrf.0.0760645. [DOI] [PubMed] [Google Scholar]
- Assidi M, Dieleman SJ, Sirard MA. Cumulus cell gene expression following the LH surge in bovine preovulatory follicles: potential early markers of oocyte competence. Reproduction. 2010;140:835–852. doi: 10.1530/REP-10-0248. [DOI] [PubMed] [Google Scholar]
- IPA. Ingenuity Pathways Analysis, IPA, v8.0. 2010. Ingenuity® Systems, http://www.ingenuity.com.
- Drummond AE. TGFbeta signalling in the development of ovarian function. Cell Tissue Res. 2005;322:107–115. doi: 10.1007/s00441-005-1153-1. [DOI] [PubMed] [Google Scholar]
- Ingman WV, Robertson SA. The essential roles of TGFB1 in reproduction. Cytokine Growth Factor Rev. 2009;20:233–239. doi: 10.1016/j.cytogfr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Su YQ, Wu X, O'Brien MJ, Pendola FL, Denegre JN, Matzuk MM, Eppig JJ. Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: genetic evidence for an oocyte-granulosa cell regulatory loop. Dev Biol. 2004;276:64–73. doi: 10.1016/j.ydbio.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Salhab M, Tosca L, Cabau C, Papillier P, Perreau C, Dupont J, Mermillod P, Uzbekova S. Kinetics of gene expression and signaling in bovine cumulus cells throughout IVM in different mediums in relation to oocyte developmental competence, cumulus apoptosis and progesterone secretion. Theriogenology. 2011;75:90–104. doi: 10.1016/j.theriogenology.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Espey LL, Yoshioka S, Russell DL, Robker RL, Fujii S, Richards JS. Ovarian expression of a disintegrin and metalloproteinase with thrombospondin motifs during ovulation in the gonadotropin-primed immature rat. Biol Reprod. 2000;62:1090–1095. doi: 10.1095/biolreprod62.4.1090. [DOI] [PubMed] [Google Scholar]
- Palanisamy GS, Cheon YP, Kim J, Kannan A, Li Q, Sato M, Mantena SR, Sitruk-Ware RL, Bagchi MK, Bagchi IC. A novel pathway involving progesterone receptor, endothelin-2, and endothelin receptor B controls ovulation in mice. Mol Endocrinol. 2006;20:2784–2795. doi: 10.1210/me.2006-0093. [DOI] [PubMed] [Google Scholar]
- Ye Y, Cong P, Yu P, Qi M, Jin F. Preimplantation and prenatal genetic diagnosis for androgen insensitivity syndrome resulting from a novel deletion/insertion mutation. Clin Genet. 2012;82:295–296. doi: 10.1111/j.1399-0004.2012.01847.x. [DOI] [PubMed] [Google Scholar]