Abstract
Primary objective
The aim of this study was to investigate the cost-benefits of a residential post-acute neurobehavioural rehabilitation programme and its effects on care needs and social participation of adults with acquired brain injury.
Research design
Retrospective multi-centre design.
Methods and procedures
Data on occupation, adaptability and level of support required were collected at admission, discharge and 6-months follow-up. Cost analysis was performed on cost estimates based on level of support.
Main outcomes and results
Significant gains were observed in all areas of functioning, with individuals progressing towards higher levels of independence and more participation in society upon discharge.
Conclusions
Cost-benefits of up to £1.13 million were demonstrated for individuals admitted to rehabilitation within a year of sustaining a brain injury and of up to £0.86 million for those admitted more than 1 year after injury. Functional gains and reductions in levels of care required upon discharge were maintained 6 months later. These results demonstrate that post-acute neurobehavioural rehabilitation can have a positive impact on the lives of individuals with brain injury and that the associated costs are off-set by significant savings in the longer-term.
Keywords: Acquired brain injury, economic appraisal, functional ability, healthcare, social care
Introduction
It is estimated that ∼760 000 people in the UK are living with the long-term effects of head injury [1] and that a further 372 000–702 000 live with stroke-associated disability [2]. However, studies on the incidence of other forms of acquired brain injury (ABI) are limited, with no reports of prevalence available in Europe, including the UK [3]. Continual advances in medical techniques mean that more people suffering brain injuries are surviving, generally with little reduction in life expectancy [4], but often suffering long-term disability. Many brain injury survivors will never be able to work again and may require full-time care for the rest of their lives. The economic burden of ABI is, therefore, substantial.
It has been recognized that the greatest barrier to social recovery and community reintegration following ABI is persisting neurobehavioural disability, which causes problems with regulation of mood, behaviour and executive function [5]. Research to date has demonstrated improved social functioning in those living with a brain injury following a period of neurorehabilitation [6–11]. However, while the evidence in support of the clinical effectiveness of rehabilitation following ABI is abundant, there is a relative scarcity of evidence relating to its cost-benefits [12, 13].
It seems reasonable to expect that improvements in social independence that have been found after rehabilitation should translate into reduced costs in caring for those individuals post-discharge, but few studies to date have investigated this issue. Turner-Stokes et al. [14, 15] found that an investment in extended-stay rehabilitation for patients with complex neurological needs at an early stage could be offset in a relatively short period by long-term savings in the costs of care. Wood et al. [5] demonstrated the cost-benefits of a post-acute neurobehavioural rehabilitation programme, aimed at improving community integration and managing behavioural problems, even for individuals admitted several years post-injury. Worthington et al. [16] conducted a comprehensive cost-benefit analysis of neurobehavioural rehabilitation in a post-acute setting and showed that the initial costs of rehabilitation were generally recovered within 2 years, even when accounting for the costs of ongoing direct care and for future inflation. More recently, Van Heugten et al. [17] evaluated the impact of a residential community reintegration programme on societal costs (including healthcare, informal care and productivity losses) before and after treatment and concluded that the significant reduction of ∼18% in societal costs per patient achieved, justified investment in the programme.
Evidence demonstrating the cost-benefits of post-acute rehabilitation programmes is growing. However, the current economic climate has placed constraints on healthcare funding and has raised increasing concerns about how to support the costs of long-term care among individual patients and carers around the world. While this further emphasizes the importance of demonstrating the cost effectiveness of post-acute rehabilitation for those with acquired brain injuries of various aetiologies, it has also had implications for the characteristics of the individuals who use these services. The hospital-based lengths of stay have tended to decrease [18–20], resulting in an increase in rehabilitation needs of those who receive post-acute rehabilitation.
This paper aims to report an up-to-date and contextualized cost-benefit analysis of post-acute rehabilitation services in a large cohort comprising over 200 individuals, as well as examining social, functional and behavioural outcomes following neurobehavioural rehabilitation. Costs of direct care and a broad range of outcome measures, including accommodation and occupational status, independent living ability and social roles were assessed at admission, discharge and 6-months follow-up. The relationship between outcome and time since injury was also examined. It was hypothesized that, after post-acute neurobehavioural rehabilitation, (i) there would be significant improvements in functional ability, accommodation and occupational status, towards greater independent living ability which (ii) would be apparent in all individuals, albeit time since injury could influence the extent of positive outcomes achieved (with greater gains for those admitted within 1-year than for those admitted more than 1 year after sustaining a brain injury) and (iii) that these gains would translate to a significant reduction in direct care costs over a lifetime.
Method
The study employed a retrospective design. Outcome data were collected from all individuals admitted to the residential rehabilitation centres operated by the Brain Injury Rehabilitation Trust (BIRT) as they progressed through the services. The 10 sites included in this study were dispersed throughout the UK (Southwest; Southeast; Midlands; North of England and Scotland). Details of the rehabilitation programme have been described in Wood and Worthington [21]. The programme is based on an assessment of the individual’s neurological and neuropsychological deficits. These deficits are taken into account when designing person-centred rehabilitation programmes. Behavioural psychology underpins the management of behaviour that presents a challenge. Goal setting is used and compensatory strategies are developed with the individual. The programme is run by a psychology-led, interdisciplinary team. The approach is holistic and the overarching goal is to enable the person to return to as independent and participatory life as possible. Ethical approval was obtained from the Disabilities Trust Research Ethics Committee. All participants included in this study gave informed consent upon admission, authorizing their anonymized data to be used for the purposes of evaluation of rehabilitation.
Participants
BIRT admit individuals who fulfil the following admission criteria: they must be over 18 years-old; have had an acquired non-progressive brain injury of any aetiology; be post-acute or medically stable; have primarily cognitive problems and/or challenging behaviour and require specialist and/or lifelong support. A total of 359 individuals, who had been discharged from BIRT services over the course of 27 months, were assessed. Of those, 71 individuals who had been prematurely discharged (15 transferred due to additional health needs; 28 transferred to a more appropriate service; 26 self-discharged; two deaths) were excluded from the study, as well as those who did not have a diagnosis of ABI (n = 4) and those who were admitted to the services more than once (n = 10). The final sample included 274 individuals (196 males) at the point of admission and 89 individuals at follow-up. The two groups did not differ in gender, diagnosis, age at injury, injury severity, time since injury and length of stay. Furthermore, cases that had questionnaires with more than 25% of the items missing were excluded from the analyses. The final numbers are reported in detail in each table.
Measures
Outcome measures were selected to reflect the aims of the rehabilitation programme. These were to increase independence, psychological and emotional adjustment to brain injury and participation. Measures were also selected for their strong psychometric properties and their widespread acceptance and use. The outcome measures described below were collected at three time points: within the first month of admission, assessing the needs and functioning observed before rehabilitation, at discharge and 6-months after discharge. At admission and discharge, each service user was rated by a member of the clinical team (usually a psychologist). At 6-month follow-up, the outcome measures were completed by the service user or a carer and submitted to the respective service by post or they were completed over the phone with a member of the clinical team. While comparing reports by staff with those provided by the service user or their carer is not ideal, other studies have found that the two sources of information correlate strongly and significantly [22, 23]. Using the same medium of administration of measures at admission, discharge and follow-up would have been preferable. However, imposing such methodological restrictions would have greatly limited the feasibility of the present study. Furthermore, research has shown that postal and telephone surveys are useful alternatives for data collection in outcomes research [24].
Supervision Rating Scale (SRS)
The SRS is a 14-point rating scale that has been specifically developed to assess support needs of those living with a brain injury [25, 26]. The scale comprises five levels of supervision ranging from independent, overnight supervision, part-time supervision, full-time indirect supervision and full-time direct supervision. These ratings were used as the basis for estimating the number of hours of care required by each individual (see Costs).
Mayo Portland Adaptability Inventory-4 (MPAI-4)
The MPAI-4 [22] was specifically designed for the post-acute assessment of people with ABI and evaluation of rehabilitation programmes. The 29 core items of the inventory are reported in this study. These are grouped into three sub-scales. The Ability Scale comprises items focusing on mobility, use of hands, vision, hearing, dizziness, motor speech, verbal and non-verbal communication, attention and concentration, memory, fund of information (including semantic and autobiographical memory; problem-solving and visuo-spatial abilities). The Adjustment scale includes items that measure psychological adjustment following the injury (anxiety, depression, irritability, anger and aggression, pain and headache, sensitivity to mild problems, inappropriate social interaction, impaired self-awareness, etc.). The Participation scale comprises items that evaluate the roles in society, including the degree of social contact, initiation and money management ability. MPAI-4 standardized scores between 50–60 suggest moderate-to-severe limitations, when compared with other people with ABI, and scores between 30–40 suggest mild limitations.
Community Disposition Ratings—Accommodation (CDR-A) [unpublished]
Place of residence is frequently used as a proxy measure for independent living ability and social recovery. The CDR-A comprises 10 mutually exclusive categories, in ascending order from more independent to more dependent living arrangements (i.e. lower scores correspond to more independent living ability). Unpublished data indicate an excellent inter-rater reliability (0.94) and satisfactory concurrent validity (0.44–0.54).
Community Disposition Ratings—Occupation (CDR-O) [unpublished]
Levels of engagement in a constructive occupation have been used as a sensitive measure of activity and participation. The scale includes eight mutually exclusive categories, ranging from academic or competitive employment to absence of productive activity. In a severely injured population such as those included in this study, solely focusing on remunerative employment or education would not provide sufficient discrimination. Unpublished studies on the CDR-O demonstrated adequate inter-rater reliability (0.63) and good concurrent validity (0.60–0.63).
Data analyses
Non-parametric tests were used to investigate whether the observed differences between admission, discharge and follow-up were statistically significant. A Bonferroni adjusted p-value of 0.017 was used throughout to control for familywise error. As costs were estimated directly from scores on the SRS (see Economic Appraisal), statistical analyses were not reported again for this set of data. All analyses were conducted with IBM SPSS Statistics for Windows, Version 19.0. Missing data were handled using list-wise deletion (i.e. cases with missing data were excluded from the analyses).
Costs
Only direct costs were included in the analysis. SRS scores were used to estimate the number of daily hours of care required at admission, discharge and follow-up. The costs of care were calculated on the basis of current hourly rates of care for support staff. These are in accordance with the National Joint Salaries Agreement for Local Authorities for 2010. Rehabilitation costs were based on the weekly rate for placement in the respective rehabilitation centre and the length of stay for each individual. Cost savings were defined as the difference in support costs before rehabilitation and at follow-up once the cost of rehabilitation was taken into account [15]. As no person included in the analysis had multiple admissions for treatment within the 6-months follow-up period, lifetime cost savings were calculated on the basis of the difference in costs of support at admission and at follow-up over a hypothetical life expectancy for the group and discounted to present value according to standard procedures [15, 27]. A sensitivity analysis was also conducted to determine cost-benefits for a range of year-on-year discount rates, calculated by using a discounting formula [16] (see Appendix).
Results
The aetiology and characteristics of the patient cohort are displayed in Tables I and II. Participants displayed moderate levels of neurobehavioural disorder at admission, based on the scores on the Adjustment scale of the MPAI-4 (M = 51.63, SD = 9.06, Range = 10–80). The majority of service users had sustained a traumatic brain injury (TBI). The severity of the injury was measured through lowest recorded Glasgow Coma Scale (GCS) score. However, this information was only available for 22% (61) of the sample for two main reasons. First, there are no widely established measures of injury severity for those with a non-TBI. Second, it is often difficult to access hospital records at the post-acute stage of rehabilitation. The available data show that 71% (43/61) of the study participants had a GCS score of 8 or less, indicating that they had suffered a severe brain injury. This is consistent with the information from the functional measures, which indicate moderate-to-severe disability and a high level of dependency in the majority of the sample.
Table I.
Aetiology of brain injury.
| Type of brain injury | n | % |
|---|---|---|
| TBI | 152 | 55.5 |
| Intracranial haemorrhage/CVA | 58 | 21.2 |
| Hypoxia | 29 | 10.6 |
| Encephalitis | 15 | 5.4 |
| Neoplasm | 6 | 2.2 |
| Other | 14 | 5.1 |
Table II.
Characteristics of the patient cohort.
| n | Median | Mean | SD | Range | |
|---|---|---|---|---|---|
| Age at injury (years) | 272 | 41.5 | 40.93 | 16.49 | 3–76 |
| Lowest GCS | 61 | 6 | 6.69 | 3.73 | 3–15 |
| Time since injury (weeks) | 273 | 20 | 102.57 | 271.91 | 1–2325 |
| Length of stay (weeks) | 274 | 21 | 25.64 | 27.18 | 0–223 |
| TSD-FU (weeks) | 80 | 28.5 | 33.88 | 13.17 | 23–78 |
TSD-FU, Time since Discharge to Follow-up; Lowest GCS, Lowest Glasgow Coma Scale score.
Clinical outcome was measured on the basis of accommodation and occupational status and scores on the MPAI-4. Projected savings in costs with hours of care was the economic measure.
The main outcome variables and Age At Admission were not significantly correlated, therefore this variable was not considered in further analyses. In this sample, the relationship between Time Since Injury and Length Of Stay was moderate (Spearman’s Rho (n = 273) = 0.401, p < 0.01), indicating that individuals who had been admitted to rehabilitation longer after injury tended to have longer lengths of stay than those admitted earlier. The sample was, therefore, partitioned into two groups (up to 1 year and more than 1 year post-injury) to investigate differences in outcomes associated with later admission to rehabilitation.
Support needs
Table III shows the changes in support needs before rehabilitation, at discharge and at 6-months follow-up. Most of the individuals in the sample needed full-time indirect or full-time direct supervision at the time they were admitted into rehabilitation. At discharge, there was a significant shift towards lower levels of supervision (z = 10.82, p < 0.001). This shift was apparent even for those who had been admitted more than 1-year post-injury (z = 4.35, p < 0.001).
Table III.
Changes in rankings on Supervision Rating Scale over time.
| Admission (n = 267) | Discharge (n = 249) | Follow-up (n = 85) | |
|---|---|---|---|
| Level 1. Independent | 16 | 98 | 39 |
| Level 2. Overnight | 3 | 13 | 3 |
| Level 3. Part-time supervision | 45 | 75 | 26 |
| Level 4. Full-time indirect supervision | 88 | 42 | 11 |
| Level 5. Full-time direct supervision | 115 | 21 | 6 |
Independent living ability
The ability to live independently and engage in social roles was assessed with the Participation scale of the MPAI-4 (Table IV). The results show an improvement (decrease in total scores) from Admission to Discharge (t(189) = 8.95, p < 0.01), from Discharge to Follow-up (t(67) = 3.42, p < 0.01) and from Admission to Follow-up (t(57) = 6.96, p < 0.01), indicating that individuals had progressively become more independent, displaying fewer difficulties in initiating various activities and socializing with others.
Table IV.
Mean (SD) general and independent living ability (as estimated from the Abilities and Participation scales of the MPAI-4).
| Admission (n = 211) | Discharge (n = 209) | Follow-up (n = 86) | |
|---|---|---|---|
| Participation | 53.60 (11.21) | 48.06 (11.33) | 41.77 (12.62) |
| Abilities | 48.97 (10.23) | 43.14 (11.61) | 38.92 (15.61) |
These changes parallelled those observed on the Ability scale of the MPAI-4, which showed a significant improvement after rehabilitation that was maintained 6 months later (t(58) = 4.02, p < 0.01).
Accommodation
Changes in type of residence before rehabilitation, at discharge and at 6-months follow-up are displayed in Table V. Wilcoxon analysis of paired rankings showed that the changes in accommodation status from Admission to Discharge (z = 11.08, p < 0.001), Admission to Follow-up (z = 7.24, p < 0.001) and Discharge to Follow-up (z = 2.92, p < 0.01) were significant.
Table V.
Changes in CDR accommodation over time.
| Admission (n = 267) | Discharge (n = 251) | Follow-up (n = 88) | |
|---|---|---|---|
| Alone with partner or friend independently | 17 | 71 | 41 |
| With parents independently (as before injury) | 10 | 18 | 9 |
| With parents independently (only since injury) | 1 | 12 | 2 |
| Supported living in own house | 7 | 68 | 13 |
| Supported living in shared house | 4 | 22 | 7 |
| Supported living in parents’ house | 11 | 12 | 2 |
| Transitional living (short-stay) | 5 | 5 | 3 |
| Residential facility (medium-long stay)/hospital | 181 | 36 | 10 |
| Psychiatric hospital/residential unit | 26 | 5 | 0 |
| Behaviour disorders unit | 5 | 2 | 1 |
Occupation
The measure of occupation showed significant changes in productive use of time following rehabilitation (Table VI, z = 9.07, p < 0.001), which was maintained at Follow-up. The proportion of individuals with no productive activity was greatly reduced from 69% at Admission to 36% at Follow-up. Only a few individuals progressed to higher levels of occupation (academic or competitive paid employment; vocational training); however, this is expected in a group with this degree of injury severity.
Table VI.
Changes in occupational status over time.
| Admission (n = 267) | Discharge (n = 251) | Follow-up (n = 87) | |
|---|---|---|---|
| Academic environment or competitive paid employment (full-time) | 3 | 15 | 7 |
| Vocational training | 2 | 6 | 0 |
| Supported employment (paid/unpaid) | 1 | 15 | 7 |
| Sheltered employment (paid/unpaid) | 2 | 6 | 0 |
| Independent home maker | 4 | 9 | 6 |
| Volunteer | 0 | 17 | 6 |
| Recreational or day activity programme | 70 | 129 | 30 |
| No productive activity | 185 | 54 | 31 |
Economic appraisal
The scores on the SRS were used to estimate the number of hours of care required at the three time points (Table VII). This was done by assigning an average number of hours of carer input required to maintain each of the 14 degrees of supervision, ranging from 0–24 hours. (The conversion of SRS ratings into average daily hours of support can be provided upon request to the authors.) The annual costs were then calculated by multiplying the number of hours of care required by each individual by the respective hourly care costs (£8.66) and by the number of days in a year (365). Savings were computed by calculating the difference between annual costs of care at admission and annual costs of care at follow-up, using the same procedure and formula as Worthington et al. [16]. Based on current life expectancies of 78.5 years for males and 82.4 for females [28], while adjusting for the 2.5:1 male bias in the present sample and given a mean age of 43.44 (SD = 15.23) years at discharge in this cohort, the value that was used as the basis for cost saving projections was 36 years, corresponding to a mean life span of ∼79.6 years. However, recent studies have suggested that life expectancies among those who have suffered a brain injury may be shorter than in the general population [29, 30]. For this reason, the declining exponential approximation of life expectancy method [31] was used to compute life expectancy adjustments based on mortality rates for TBI injury survivors whose demographic characteristics overlap with the majority of individuals in the present sample (i.e. 40.15 per 1000 people for brain injury survivors with TBI aetiology, residing in the UK, aged between 15–54 and within 1-year post-injury). The cost-saving projections that reflect these adjustments are also reported. Using life expectancies specific to individuals who have sustained a severe brain injury takes into account the health issues associated with the condition, which may affect life expectancy in the longer term. These are, therefore, more conservative estimates than the ones extrapolated from life expectancies for the general population.
Table VII.
Median hours of support per day (as estimated from the Supervision Rating Scale scores) by time post-injury.
| 0–12 months | >1 year | |
|---|---|---|
| Admission | 24 | 17 |
| Discharge | 5 | 11 |
| Follow-up | 1 | 11 |
| CS (Ad-FU) | 23 | 6 |
CS (Ad-FU), Median saving in care hours at follow-up compared to admission.
As in past research, sensitivity analyses were conducted based on standard discount rates of 3% and 5%. However, to account for current economic trends as forecast by the Bank of England [32], a lower discount rate of 1.5% was also used. These results, as well as non-discounted savings, are displayed in Table VIII. For individuals who were admitted for rehabilitation up to one year after the injury, it takes just over a year for cost savings to be apparent. Time in achieving cost off-set is considerably longer (4–5 years) for those who have sustained a brain injury one or more years before rehabilitation. However, savings of £0.19–0.86 million in life-time costs of care were still found in this group. Life-time cost savings among individuals who had been admitted to rehabilitation within a year of their brain injury ranged from £0.57–£1.13 million.
Table VIII.
Changes in care costs.
| 0–12 months | >1 year | ||
|---|---|---|---|
| Mean daily pre-rehabilitation care costs | £165.95 | £135.84 | |
| Mean daily discharge care costs | £69.90 | £84.37 | |
| Mean daily follow-up care costs | £51.67 | £85.60 | |
| Cost saving per day | £114.28 | £50.24 | |
| Cost saving per year | £41 712.20 | £18 337.60 | |
| Mean cost of rehabilitation | £54 080.76 | £85 810.71 | |
| Estimated lifetime care cost savings | |||
| Community life expectancy | Discounted at 1.5% | £1 134 799.42 | £858 056.63 |
| 36 years | Discounted at 3% | £891 682.70 | £653 101.87 |
| Discounted at 5% | £671 217.01 | £465 580.63 | |
| Head injury life expectancy | Discounted at 1.5% | £845 271.99 | £312 474.83 |
| 25 years | Discounted at 3% | £707 352.12 | £251 842.23 |
| Discounted at 5% | £568 900.85 | £190 976.01 | |
Discussion
This study examined the effectiveness of a neurobehavioural approach to rehabilitation in improving functional ability of individuals with an ABI and its potential for reducing dependency and, hence, the direct costs of long-term care. It was found that there was an improvement on all measures of social outcome at discharge which was maintained, or showed further gains, 6 months later.
More than 80% of the clients who were in hospital or living in a residential facility at the time of admission moved to accommodation in the community. This move was paralleled by significant reductions in supervision, where it was observed that 80% of individuals who had required full-time direct supervision at admission no longer did so at discharge. These gains in functional independence were accompanied by clinically significant gains in abilities, social participation and occupational status. However, it is possible that measures like the MPAI-4, which focus on a wide range of behaviours, under-estimate the changes that occur from admission to discharge, as clinical staff, the client and their family become progressively more aware of the person’s difficulties as they progress through rehabilitation. Most of the clients also made progress towards higher levels of productive activity. Admittedly, very few individuals were able to return to open, competitive environments such as paid employment or higher education upon discharge or even in the subsequent 6-months. However, this is to be expected in a sample with such severe injuries and past studies have shown a similar pattern of results [5, 15, 33].
Previous studies have investigated the effect of Time Since Injury on outcomes by partitioning the sample into groups. Wood et al. [5] focused on differences between those who had been admitted less than two years, 2–5 years and more than five years post-injury. Worthington et al. [16] investigated whether there were different outcomes within the 2-year window. The current study investigated differences in outcomes between people who were admitted to post-acute rehabilitation up to 1-year and more than 1-year post-injury. These time frames were considered more appropriate given the characteristics of the current cohort (skewed towards earlier admission to post-acute rehabilitation), while still enabling an examination of the clinical and cost-benefits of rehabilitation after a period of significant spontaneous recovery.
The cost-benefit of rehabilitation was examined and a 68% reduction in direct costs of care was found for individuals who had sustained their injury within one year of admission. As expected, the reduction in costs among those who had sustained their injury more than one year before admission was smaller (37%), but still statistically and, undoubtedly, economically significant. Overall, it was found that cost savings could range between £0.19–£1.13 million per individual lifetime, depending on the time lag between sustaining a brain injury and admission to rehabilitation, the level of inflation discounted and the notional life expectancy of the group.
It has been suggested that better results are achieved when rehabilitation begins within the first year after injury [15], although it is always difficult to separate the added value of rehabilitation from spontaneous recovery in the early stages. The present findings corroborate this view. However, they also suggest that the benefits achieved by individuals who begin rehabilitation more than one year after injury should not be ignored. It is likely that the savings in direct costs of care will add to savings in societal costs, as individuals who become more independent require less direct supervision, which in turn enables informal carers to return to work, resulting in an overall reduction of costs associated with loss of productivity. Van Heugten et al. [17] showed significant reductions in societal costs of brain injury in a sample studied much later after injury (6 years), suggesting that rehabilitation can have a significant impact on costs well-beyond the 2nd year post-injury. This is especially important when considering the rehabilitation of neurobehavioural disorders, as these are known to often arise for the first time late after injury [34, 35].
One of the motives for reporting this update of cost-efficiency data with post-acute residential rehabilitation programmes was to examine how changes in policies associated with the current economic climate have influenced the characteristics of the populations who are now admitted into post-acute rehabilitation. In comparison with studies published in the last 13 years [5], this study found evidence of a decrease in lengths of stay in rehabilitation, as well as a decrease in time from injury to admission to post-acute rehabilitation. More importantly, evidence was found that those admitted to post-acute rehabilitation over the last six years showed greater rehabilitation needs than those admitted in the past. There was a larger percentage of direct admissions from an acute hospital or residential facility than in the sample reported by Worthington et al. [16] (77% vs 52%), as well as a larger percentage of individuals in need of full-time direct supervision (43% vs 28%) at the point of admission to rehabilitation. Nevertheless, the savings demonstrated in this study for those admitted for rehabilitation within 12 months of injury are comparable to those reported in earlier studies on samples of individuals with less complex needs [15] and the gains in terms of independent living ability are even greater. Forty-six per cent of the present sample were living in the community without support at 6-months follow-up, a percentage greater than reported in earlier studies (Wood et al. [5], 21%; Worthington et al. [16], 23%). However, it should be pointed out that the follow-up timings (6-months) reported here are much shorter than the average 17.44 and 33.15 months from discharge to follow-up reported by Worthington et al. [16] and Wood et al. [5], respectively. Furthermore, there have been changes in the thresholds for assigning personal support in the past years, which may have had an influence on the number of people who qualify for this kind of support upon discharge.
One of the strengths of the present study was the inclusion of a wide variety of internationally recognized measures of outcome, along with the cost analysis of post-acute rehabilitation of a reasonably large sample. This procedure should enable more reliable comparisons across studies and cohorts in future research concerning the clinical and economic benefits of post-acute neurorehabilitation. Direct cost savings were calculated while adjusting for the cost of rehabilitation, future inflation, as well as notional life-expectancies for the general population and the brain injured population. Therefore, the estimates of savings reported are likely to prove fairly conservative. However, an assumption is made that individuals who benefitted from rehabilitation would either maintain or reduce their care needs observed at follow-up. This may not be the case for a small percentage of individuals who are discharged but later re-admitted to rehabilitation centres.
This study focused only on the costs associated with the provision of direct care. It is impossible to ascertain whether the behavioural and social benefits resulting from rehabilitation reported here would necessarily lead to a decrease in societal costs of care. Using a methodology that includes indirect costs, such as that employed by van Heugten et al. [17], could have been informative. However, the authors concur with these authors that this type of analysis is more reliable when investigating individuals who have been living in the community for longer periods, as this would allow for the full benefits of reintegration to arise. The present study excluded individuals who had been prematurely discharged for different reasons. Therefore, it is unclear whether the benefits reported herein only apply to the ‘typical’ service user of a neurobehavioural rehabilitation service or whether some benefits would also be apparent among those who were discharged early. The lack of equivalence in the demographic characteristics of those who did and who did not complete the rehabilitation programme in the current cohort, as well as the small number of follow-up data sets among those who were prematurely discharged (n = 8) precludes a comparison of outcomes and savings between these two groups. Should it be the case that these individuals do not benefit from residential rehabilitation, future research should endeavour to understand the factors that predict whether or not specific clients are likely to engage with the rehabilitation process and aim to find the best fit between individual and service at the point of first referral.
As this study did not include a control group, it is impossible to fully account for the influence of natural recovery on the observed improvements. However, it is reasonable to assume that clinical benefits and cost-benefits are, to some extent, the direct result of rehabilitation, as these were observed among brain injury survivors admitted to rehabilitation more than 1-year post-injury as well as those admitted within 1-year. Finding a no-treatment or even an alternative treatment control group is extremely problematic for brain injury rehabilitation. First, there are ethical issues in not offering any intervention to a person who has suffered a brain injury. Additionally, although various approaches to brain injury rehabilitation exist, these are not so much alternative treatments, but rather different ways of managing different problems or different stages of the recovery process. It must also be recognized that part of such rehabilitation (as for any other medical treatment) is to provide the safety, care and support required to enable the person to recover spontaneously. For those with severe cognitive and behavioural changes this is not easy for families to provide without some guidance from a rehabilitation centre. It is also important to note that many of the groups at risk of an ABI (e.g. drug and alcohol abusers; the homeless) typically have little family support available.
Conclusions
An analysis of the clinical and cost-benefits of post-acute neurobehavioural rehabilitation demonstrated that this approach leads to gains in functional ability and independence and to significant reductions in costs of direct care over a lifetime. These benefits remained apparent 6-months post-discharge. Further studies investigating the benefits of neurorehabilitation in individuals who have been discharged to the community for longer periods of time would clarify whether these benefits are maintained over longer time spans and whether further savings can be achieved.
Acknowledgements
We would like to thank Ms. Lorraine Haye for her help in collating the data for this study as well as those too numerous to mention who assisted in the collection of these data.
Appendix
The following equation was used to calculate year-on-year discount rates for cost savings:
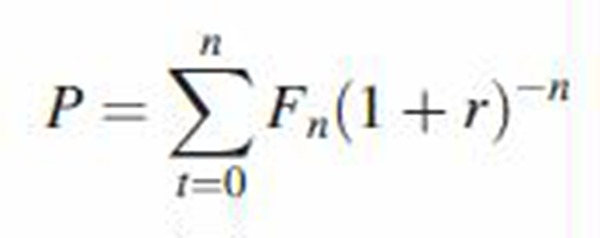 |
where P = present value, t = time, Fn = future costs, r = annual interest (discount) rate. The discount factor is (1 + r)–n.
Declaration of interest
The authors work for the Brain Injury Rehabilitation Trust, a not-for-profit organization that offers post-acute neurobehavioural rehabilitation. The authors alone are responsible for the content and writing of the paper.
References
- 1.Tennant A. Admission to hospital following head injury in England: Incidence and socio-economic associations. BMC Public Health. 2005;5:21. doi: 10.1186/1471-2458-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Mahony PG, Thompson RG, Dobson R, Rodgers H, James OFW. The prevalence of stroke and associated disability. Journal of Public Health Medicine. 1999;21:166–171. doi: 10.1093/pubmed/21.2.166. [DOI] [PubMed] [Google Scholar]
- 3.Tagliaferri F, Compagnone C, Korsic M, Servadei F, Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochirurgica. 2006;148:255–268. doi: 10.1007/s00701-005-0651-y. [DOI] [PubMed] [Google Scholar]
- 4.Shavelle RM, Strauss DJ, Day SM, Ojdana KA. Life expectancy. In: Zasler ND, Katz DI, Zafonte RD, editors. Brain injury medicine: Principles and practice. New York: Demos Medical Publishing; 2007. pp. 247–261. [Google Scholar]
- 5.Wood RLl, McCrea JD, Wood LM, Merriman RN. Clinical and cost effectiveness of post-acute neurobehavioural rehabilitation. Brain Injury. 1999;13:69–88. doi: 10.1080/026990599121746. [DOI] [PubMed] [Google Scholar]
- 6.Eames PG, Cotterill G, Kneale TA, Storrar AL, Yeomans P. Outcome of intensive rehabilitation after severe brain injury: A long-term follow-up study. Brain Injury. 1995;10:631–650. doi: 10.1080/026990596124061. [DOI] [PubMed] [Google Scholar]
- 7.Eames PG, Wood RLI. Rehabilitation after severe brain injury: A follow-up study of a behaviour modification approach. Journal of Neurology, Neurosurgery and Psychiatry. 1985;48:613–619. doi: 10.1136/jnnp.48.7.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke WH, Wesolowski MD, Guth ML. Comprehensive head injury rehabilitation: An outcome evaluation. Brain Injury. 1988;2:313–322. doi: 10.3109/02699058809150902. [DOI] [PubMed] [Google Scholar]
- 9.Cope DN. Psychopharmacology for behavioural deficits: Disorders of cognition and affect. In: Wood RLl., editor. Neurobehavioural sequelae of traumatic brain injury. Hove: Lawrence Erlbaum Associates; 1990. pp. 250–273. [Google Scholar]
- 10.Cope DN, Cole JR, Hali KM, Barkan H. Brain injury: Analysis of outcome in a post-acute rehabilitation system. Part 1: General analysis. Brain Injury. 1991;5:111–125. doi: 10.3109/02699059109008083. [DOI] [PubMed] [Google Scholar]
- 11.Malec JF, Smigielski JS, DePompolo RW, Thompson JM. Outcome evaluation and prediction in a comprehensive integrated post-acute outpatient brain injury rehabilitation programme. Brain Injury. 1993;7:15–29. doi: 10.3109/02699059309008153. [DOI] [PubMed] [Google Scholar]
- 12.McGregor K, Pentland B. Head injury rehabilitation in the UK: An economic perspective. Social Science & Medicine. 1997;45:295–303. doi: 10.1016/s0277-9536(96)00345-0. [DOI] [PubMed] [Google Scholar]
- 13.Cooney F. The importance of health economics in rehabilitation medicine. Journal of Rehabilitation Medicine. 2010;42:284–285. doi: 10.2340/16501977-0532. [DOI] [PubMed] [Google Scholar]
- 14.Turner-Stokes L, Paul S, Williams H. Efficiency of specialist rehabilitation in reducing dependency and costs of continuing care for adults with complex acquired brain injuries. Journal of Neurology, Neurosurgery and Psychiatry. 2006;77:634–639. doi: 10.1136/jnnp.2005.073411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner-Stokes L. Cost-efficiency of longer-stay rehabilitation programmes: Can they provide value for money? Brain Injury. 2007;21:1015–1021. doi: 10.1080/02699050701591445. [DOI] [PubMed] [Google Scholar]
- 16.Worthington AD, Matthews S, Melia Y, Oddy M. Cost-benefits associated with social outcome from neurobehavioural rehabilitation. Brain Injury. 2006;20:947–957. doi: 10.1080/02699050600888314. [DOI] [PubMed] [Google Scholar]
- 17.Van Heugten CM, Geurtsen GJ, Derksen RE, Martina JD, Geurts ACH, Evers SMAA. Intervention and societal costs of residential community integration for patients with acquired brain injury: A cost-analysis of the brain integration programme. Journal of Rehabilitation Medicine. 2011;43:647–652. doi: 10.2340/16501977-0818. [DOI] [PubMed] [Google Scholar]
- 18.Edwards SG, Thompson AJ, Losseff N, Playford ED. Shortening inpatient stay for stroke. Lancet. 2002;359:2205. doi: 10.1016/s0140-6736(02)09082-7. [DOI] [PubMed] [Google Scholar]
- 19.Kreutzer JS, Kolakowsky-Hayner SA, Cifu DX, Rosenthal M, Bushnik T, Zafonte R, Englander J, High W. Charges and lengths of stay for acute and inpatient rehabilitation treatment of traumatic brain injury 1990-1996. Brain Injury. 2001;15:763–774. doi: 10.1080/02699050010025786. [DOI] [PubMed] [Google Scholar]
- 20.Ottenbacher KJ, Smith PM, Illig SB, Fiedler RC, Granger CV. Length of stay and hospital readmission for persons with disabilities. American Journal of Public Health. 2000;90:1920–1923. doi: 10.2105/ajph.90.12.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood RLl, Worthington AD. Neurobehavioural rehabilitation in practice. In: Wood RLl, McMillan TM., editors. Neurobehavioural disability and social handicap after traumatic brain injury. Hove: Psychology Press; 2001. pp. 133–155. [Google Scholar]
- 22. Malec JF, Lezak MD. Manual for the Mayo-Portland Adaptability Inventory (MPAI-4) [internet]. San Jose, CA: Santa Clara Valley Medical Centre. [cited 2012 Dec 4]; Available from http://www.tbims.org/combi/mpai.
- 23.Malec JF. Comparability of Mayo-Portland Adaptability Inventory ratings by staff, significant others and people with acquired brain injury. Brain Injury. 2004;18:563–576. doi: 10.1080/02699050310001646134. [DOI] [PubMed] [Google Scholar]
- 24.Parker C, Dewey M. Assessing research outcomes by postal questionnaire with telephone follow-up. TOTAL Study Group. Trial of Occupational Therapy and Leisure. International Journal of Epidemiology. 2000;29:1065–1069. doi: 10.1093/ije/29.6.1065. [DOI] [PubMed] [Google Scholar]
- 25.Boake C. Supervision rating scale: A measure of functional outcome from brain injury. Archives of Physical Medicine. 1996;77:765–772. doi: 10.1016/s0003-9993(96)90254-3. [DOI] [PubMed] [Google Scholar]
- 26.Reed K, Boake C, Caroselli JS. The supervision rating scale (SRS): A program evaluation tool for post-acute brain injury rehabilitation. Archives of Clinical Neuropsychology. 1999;14:765–772. [Google Scholar]
- 27.Smith D, Gravelle H. The practice of discounting economic evaluation of health care interventions. International Journal of Technology Assessment in Health Care. 2001;17:236–243. doi: 10.1017/s0266462300105094. [DOI] [PubMed] [Google Scholar]
- 28. Office of National Statistics. 2010 based Period and Cohort Life Expectancy Tables [internet]. Newport, UK: Office of National Statistics. [cited 2012 Dec 4]; Available from http://www.ons.gov.uk/ons/rel/lifetables/period-and-cohort-life-expectancy-tables/2010-based/p-and-c-le.html.
- 29.Lee HY, Hwang JS, Jeng JS, Wang JD. Quality-adjusted life expectancy (QALE) and loss of QALE for patients with ischemic stroke and intracerebral hemorrhage: A 13-year follow-up. Stroke. 2010;41:739–744. doi: 10.1161/STROKEAHA.109.573543. [DOI] [PubMed] [Google Scholar]
- 30. McMillan TM, Teasdale GM, Weir CJ, Stewart E. Death after head injury: The 13 year outcome of a case control study. Journal of Neurology, Neurosurgery and Psychiatry 2011;82:931--935. [DOI] [PubMed]
- 31.Beck JR, Pauker SG, Gottlieb JE, Klein K, Kassirer JP. A convenient approximation of life expectancy (The ‘DEALE’). II. Use in medical decision making. American Journal of Medicine. 1982;73:889–897. doi: 10.1016/0002-9343(82)90787-2. [DOI] [PubMed] [Google Scholar]
- 32. Bank of England. Inflation Report November 2012 [internet]. London, UK: Bank of England 2012 [cited 2013 Jul 3] Available from: http://www.bankofengland.co.uk/publications/Documents/inflationreport/ir12feb.pdf.
- 33.Brooks N, McKinlay W, Symington C, Beattie A, Campsie L. Return to work within the first seven years of severe head injury. Brain Injury. 1987;1:5–19. doi: 10.3109/02699058709034439. [DOI] [PubMed] [Google Scholar]
- 34.Gualtari CT, Cox DR. The delayed neurobehavioural sequalae of traumatic brain injury. Brain Injury. 1991;5:219–232. doi: 10.3109/02699059109008093. [DOI] [PubMed] [Google Scholar]
- 35.Johnson R, Balleny H. Behaviour problems after brain injury: Incidence and need for treatment. Clinical Rehabilitation. 1996;10:173–181. [Google Scholar]


