Abstract
Background
Immune thrombocytopenic purpura (ITP) is an autoimmune disease characterized by platelet destruction resulting from autoantibodies against platelet proteins, particularly platelet glycoprotein IIb/IIIa. Heat shock proteins (Hsp) have been shown to be major antigenic determinants in some autoimmune diseases. Antibodies to Hsps have also been reported to be associated with a number of pathological states.
Methods
Using western blot, we measured the levels of the 60 kDa heat shock protein (Hsp60) and of the inducible 71 kDa member of the Hsp70 family (Hsp71) in lymphocytes and the presence of antibodies against these hsps in plasma of 29 pediatric patients with ITP before the treatment and in 6 other patients before and after treatment.
Results
Interestingly only one out of 29 patients showed detectable Hsp60 in lymphocytes while this heat shock protein was detected in the 30 control children. Hsp71 levels were slightly lower in lymphocytes of patients with ITP than in controls (1567.8 ± 753.2 via 1763.2 ± 641.8 integrated optical density (IOD) units). There was a small increase of Hsp71 after recovery from ITP. The titers of plasma antibodies against Hsp60 and Hsp71 were also examined. Antibodies against Hsp71 were more common in ITP patients (15/29) than in control children (5/30). The titer of anti-Hsp71 was also higher in children patients with ITP. The prevalence of ITP children with antibodies against Hsp71 (51.7%) was as high as those with antibodies against platelet membrane glycoproteins (58.3%).
Conclusions
In summary, pediatric patients with ITP showed no detectable expression of Hsp60 in lymphocytes and a high prevalence of antibody against Hsp71 in plasma. These changes add to our understanding of the pathogenesis of ITP and may be important for the diagnosis, prognosis and treatment of ITP.
Background
Immune thrombocytopenic purpura (ITP) is an autoimmune disease characterized by a low platelet count secondary to accelerated platelet destruction by antiplatelet antibodies that generally recognize platelet membrane glycoproteins (GPs) [1]. The triggering or immunogenic stimulus involved and the role of antiplatelet antibodies in the disease remain unclear. There is an unbalanced immune response due to inflammation (i.e., viral infection, autoimmunity), or to exposure to environmental agents (i.e., drug, H2O2) [2-6]. Whether the stimulus is endogenous, i.e. truly "self" or exogenous ("non-self") is unknown. Some recent evidence argues for an immune-mediated mechanism in ITP-increased HLA-DR expression, defects in cellular and humoral immunity, and specific autoantibody production. There are several forms of management of ITP ranging from drugs such as corticosteroids, a variety of immunosuppressants and immunoglobulins to splenectomy [2,6,7]. However some patients may be receiving unnecessary treatment especially in the case of pediatric patients [8] as the cause and etiopathogenesis of ITP remain unknown.
Heat shock proteins (Hsp) are highly conserved proteins found in prokaryotes and eukaryotes. Induction of Hsps can be triggered by many stresses. These include exposure to supraoptimal temperatures (heat shock) and to various chemicals (xenobiotics, drugs, growth factors, hormones). Pathological states incurring during viral, bacterial or parasitic infections, fever, inflammation, malignancy or autoimmunity, can also induce an increase in synthesis of Hsps [9-12]. Many Hsps are also expressed at low levels under normal physiological conditions. Hsps are usually grouped into several families (Hsp110, Hsp90, Hsp/Hsc70 (Hsc: Heat shock cognate), Hsp60, Hsp47 and the small Hsps (Hsp10-30) on the basis of their apparent molecular masses after sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). Hsps can act as intracellular chaperones of naive, aberrantly folded or mutated proteins, as stimulators of cytokine signal transduction cascades, as well as in cytoprotection against the stress stimuli described above [11,13-15]. A role of Hsps in the inflammatory response is suggested by different findings. Hsps participate in cytokine signal transduction and in the control of cytokine gene expression [16]. Hsps enhance antigen presentation to T lymphocytes, can be displayed on the surface of cells and may be important in targeting cytotoxic cells [16,17]. Moreover, recent investigations also indicate that Hsps have the ability to modulate the cellular immune response since as cellular chaperones, they participate in transport through the various cellular compartments. Thus Hsps may act in transfer of peptides between cellular compartments, and in binding endogenous antigenic peptides and transporting them to the major histocompatibility complexes [18-20]. Hsps have also been reported to be involved in tumorogenesis and in inflammation [21-23].
Many investigations have shown that Hsps and autoantibodies to these proteins play a role in the pathogenesis and/or prognosis of certain diseases [24-31]. Since an important component of ITP is mediated by autoantibodies, we decided to examine if antibodies against Hsps could be detected in pediatric patients with ITP. We also checked if ITP was accompanied by fluctuations in the levels of Hsp60, of the inducible member of the Hsp70 family referred to here as Hsp71 and if the expression of these Hsps changed after recovery from ITP. The data show that Hsp60 is not detectable in lymphocytes of most pediatric ITP patients. Whether the expression of Hsp60 was much lower or totally absent in the ITP children is unknown at the moment. Finally these patients show a high prevalence of antibody against Hsp71 that may be of clinical significance.
Methods
Subjects and groups
The present study comprised two different groups. The first ITP group consisted of 29 ITP pediatric patients aged from 7 to 10 years (male: 18, female: 11) and was conducted from March to November of 2000 in Wuhan. The second group consisted of 6 different ITP pediatric patients aged from 7 to 10 years and was conducted in March and April of 2001. All patients were diagnosed according to criteria and principles of management of ITP defined by the Chinese Association of Hematology. This diagnosis is mainly based on the medical history, symptoms and signs, physical examination, peripheral blood smear, and bone marrow aspiration. Patients who were referred with bleeding complaints (easy bruising, purpura, mucosal bleeding) and had isolated thrombocytopenia with no history of other clinical conditions that can cause thrombocytopenia were included in our investigation. At the time of the ITP diagnosis, patients were excluded from further study if they had a history or clinical findings of antiphospholipid syndrome, systemic lupus erythematosus, other autoimmune disorders, acquired immunodeficiency syndrome, or malignancies. The control group comprised 30 children (15 males, 15 females) aged from 7 to 8 years (mean age: 7.5) who had no diseases after physical examination and had no history of respiratory and virus infection in the preceding two weeks. We also measured changes of Hsp71 levels in lymphocytes of the second group of 6 patients with ITP at admission and after recovery. Treatments consisted of dexamethasone administration followed by prednisone. None of the patients received blood transfusion.
Evaluation of health status
Health status was evaluated in all patients and controls using a questionnaire and by clinical examination and laboratory determinations. The questionnaire designed according to diagnostic criteria and principles of ITP, with emphasis on disease history including family history, symptoms, use of drug and food, was answered by the child and/or parent. The clinical examination included a general examination (signs), weight, height, pulse, oral temperature, electrocardiography and chest x-ray. Laboratory tests included the regular analyses of blood and urine (haemoglobin, blood typing, platelet and white blood cell count) and, hepatic functional tests (GPT, GOT, hepatitis B antigens and antibodies).
Detection of Hsp60 and Hsp71 in lymphocytes
Blood samples were collected from all pediatric ITP patients before any treatment was given. Additional blood samples were collected from the second group of 6 patients after treatment. About 5 ml of venous blood were collected and divided into two aliquots, one to separate plasma and lymphocytes for Hsp analysis and the other aliquot for the routine laboratory tests mentioned above. Plasma from heparinized whole blood was collected, sedimented cells suspended in 3.0 ml of normal saline and lymphocytes were isolated using Ficoll-Hypaque (Biochemical Reagent Co., Shanghai, China). The collected lymphocytes were washed twice with PBS, counted and the concentrations of lymphocytes adjusted to 10,000 per ul with PBS. 10 ul of lymphocyte suspension was centrifuged and cells lysed in SDS-sample buffer by boiling at 100°C for 5 min, and proteins loaded on SDS-PAGE gels as described previously [28-30]. After electrophoresis, proteins were transferred electrophoretically to nitrocellulose membranes [28-30,32]. Transfer was monitored by staining with Ponceau Red. The membrane was saturated with blocking buffer (PBS containing 5% skim milk powder) for 1 h at 37°C with gentle agitation and washed with PBS-0.05% Tween 80 for 5 min. It was then incubated at 37°C for 1 h with gentle agitation with rabbit anti-human Hsp60 or anti-human Hsp71 antibodies [see [32]] diluted 1:1000 in PBS containing 5% skim milk powder. After washing the membranes six times (6 × 10 min) with 10 ml PBS-0.05% Tween 80, horseradish peroxydase (HRP) conjugated goat anti-rabbit IgG in blocking buffer (1:1000) was added and the membranes further incubated at 37°C for 1 h. Membranes were finally washed four times with 10 ml PBS-0.05% Tween 80. The presence of Hsp60 and Hsp71 was revealed using DAB (3,3-diaminobenzidine tetrahydrochloride) buffer for 3–5 min [29,30,32]. Hsp60 and Hsp71 were quantified using an imaging densitometer (Shimadzu CS-930, Shimadzu, Japan) and integrated optical density (IOD) was used to represent the relative levels of these two proteins.
Determination of antibodies to Hsp60 and Hsp71 in plasma of subjects
Recombinant human Hsp60 was obtained through the expression of human Hsp60 cDNA in Escherichia coli BL2 (DE3) cells using a pET vector. Recombinant inducible human Hsp71 was obtained through the expression of the human cDNA coding for the inducible Hsp71 in NaCl-induced E. coli GJ1168 cells using pET30 as expression vector [32,33]. Approximately 10–15 μg of recombinant human Hsp60 or Hsp71 was loaded on each SDS-PAGE gel without combs and transferred electrophoretically to nitrocellulose membranes [28]. The band containing Hsp60 or Hsp71 was cut into 2 mm × 3 mm pieces and marked with a small red dot on the protein side of the membrane. The pieces were placed in individual wells of an ELISA plate, rinsed with PBS, and saturated with 100 μl of blocking buffer (PBS containing 5% skim milk powder) for 1 h at 37°C with gentle agitation and washed with PBS-0.05% Tween 80 for 5 min. The plasma diluted 1:10, 1:20, 1:40 or 1:80 in 100 μl PBS containing 5% skim milk powder was incubated with the nitrocellulose membrane pieces at 37°C for 2 h with gentle agitation. After washing the membranes six times (10 min each) with 200 μl PBS-0.05% Tween 80, 100 μl of HRP-conjugated goat anti-human IgG (Sigma) in blocking buffer (1:2500) was added and the incubation continued at 37°C for 1 h. Membranes were washed six times (10 min) with 200 μl PBS-0.05% Tween 80. The presence of antibodies to Hsp60 or Hsp71 was revealed using DAB (3,3-diaminobenzidine tetrahydrochloride) buffer for 3–5 min. A brown band on the membrane piece is regarded as positive and no colour as negative [30].
Statistical analyses
Results were expressed as the mean ± standard deviation (SD) of n samples. Where appropriate, the probability of significant differences between 2 groups for quantity was determined by the unpaired Student t test. Comparison between groups for frequency of an event was done by the Chi-square test/Fisher exact test. P < 0.05 was considered significant. All data were analysed using Excel 2000 (Microsoft Corporation, Seattle, USA) and the Statistical Package for Social Sciences (SPSS Inc, Chicago, USA) software.
Results
Clinical features of ITP pediatric patients and treatment
A blood routine was performed on all pediatric patients with ITP, As expected, the number of white blood cells (7.4 ± 3.3 × 109/L) and the concentration of haemoglobin (107 ± 16 g/L, range 93–138) while a bit low in some patients with ITP, did not differ significantly from that of controls (range 112–140 g/l) and were within normal values (4 to 10 × 109/L and 110 to 160 g/L respectively). However the mean number of platelets (32 ± 26, range 1.0 to 82.0 × 109/L) was significantly lower than its normal value (100 to 300 × 109/L) and is in the abnormal range. There was no difference in the number of platelets between male and female patients with ITP. Table 1 summarizes the clinical information on the 29 individual pediatric patients. Most patients (23/29) presented with acute ITP. Treatment was initiated with dexamethasone followed by prednisone in all cases. Intravenous gamma globulin (IVIG) was only given in very severe cases. Vitamin C was also provided depending on the condition of the patient (data not shown).
Table 1.
Summary of data obtained for individual pediatric patients with ITP
| NO | Gender | Age | Diagnosis | BPC | Hsp71 | Hsp60 | Anti-Hsp71 | Anti-Hsp60 |
| 1 | F | 7 | acute | 76 | 900 | ND | 80 | - |
| 2 | M | 7 | acute | 38 | 2120 | ND | 20 | - |
| 3 | F | 7 | acute | 5 | 1410 | ND | - | - |
| 4 | F | 8 | acute | 43 | 860 | ND | - | - |
| 5 | M | 8 | acute | 2 | 990 | ND | 20 | - |
| 6 | M | 8 | acute | 33 | 700 | ND | 80 | - |
| 7 | M | 8 | acute | 2 | 1200 | ND | 80 | - |
| 8 | M | 8 | acute | 1 | 1580 | ND | - | - |
| 9 | F | 8 | acute | 9 | 2490 | ND | - | - |
| 10 | F | 8 | acute | 46 | 1910 | ND | - | - |
| 11 | M | 8 | acute | 6 | 1250 | ND | 80 | - |
| 12 | M | 8 | acute | 56 | 1570 | ND | 80 | - |
| 13 | M | 9 | acute | 50 | 1020 | ND | - | - |
| 14 | M | 9 | acute | 82 | 900 | ND | 80 | - |
| 15 | F | 9 | acute | 19 | 1000 | ND | - | - |
| 16 | M | 9 | acute | 18 | 1600 | ND | 40 | 80 |
| 17 | M | 9 | acute | 64 | 2590 | DET | - | - |
| 18 | M | 10 | acute | 23 | 1790 | ND | 20 | - |
| 19 | F | 10 | acute | 67 | 880 | ND | - | - |
| 20 | F | 10 | acute | 26 | 4000 | ND | 80 | - |
| 21 | M | 10 | acute | 44 | 1850 | ND | - | 40 |
| 22 | F | 10 | acute | 73 | 2210 | ND | - | - |
| 23 | M | 10 | acute | 11 | 2600 | ND | 20 | 10 |
| 24 | M | 7 | chronic | 4 | 1230 | ND | - | - |
| 25 | M | 8 | chronic | 4 | 880 | ND | 80 | - |
| 26 | M | 8 | chronic | 25 | 1700 | ND | - | - |
| 27 | F | 9 | chronic | 73 | 1030 | ND | - | - |
| 28 | M | 10 | chronic | 5 | 1300 | ND | 80 | - |
| 29 | F | 10 | chronic | 4 | 1900 | ND | 20 | - |
M: Male; F: Female; BPC: blood platelets counts; ND: not detected; DET: detected; -: negative. All blood samples were collected before medication was started.
Levels of Hsp60 and Hsp71 in lymphocytes
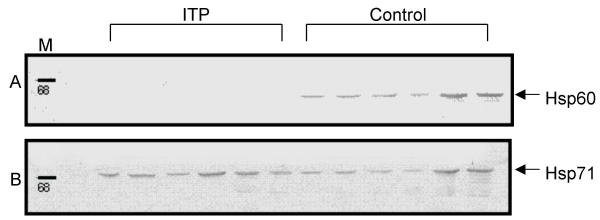
Aberrant expression of Hsps has been associated with many disease states [24,25]. We therefore determined the levels of Hsp60 and the inducible Hsp71 in lymphocytes of patients with ITP by Western blot in order to find out if there was any association between Hsp expression and ITP. Figure 1 shows examples of Western blots for Hsp60 and Hsp71 in lymphocytes of pediatric patients with ITP and in controls. As can be seen, Hsp60 was not detected in lymphocytes of pediatric patients with ITP while there was a variable but detectable expression of this Hsp in all controls (Fig. 1A). Throughout the present investigation, we found only one patient out of 29 (Patient no 17, Table 1) who had a detectable level of Hsp60, while this Hsp was detected in all 30 individuals from the control group. The expression of Hsp60 is significantly different (P < 0.0001) between ITP and control groups. Figure 1B shows the expression level of Hsp71 in the same patients with ITP and in controls. The relative values of Hsp71 were quantified using an imaging densitometer and are given in IOD units in Table 1. Patients with ITP tended to have a slightly lower level of Hsp71 (1567.8 ± 753.2 IOD units) but this difference with controls (1763.2 ± 641.8 IOD units) was not statistically significant. Patients with the acute form had a higher mean value (1627 ± 786 IOD units, range 700–4000) than those with the chronic form (1340 ± 391 IOD units, range 880–1900). This difference was not significant (P = 0.398).
Figure 1.
Detection of Hsp60 and Hsp71 in lymphocytes of patients with ITP and in controls by Western blot A: Examples of detection of Hsp60. Lane 1:Protein marker; Lane 2–8: Patients with ITP; Lane 9–14: controls. B: Examples of detection of Hsp71. Lane 1:Protein marker; Lane 2–8: Patients with ITP; Lane 9–14: controls.
Changes of Hsp60 and Hsp71 in lymphocytes before and after recovery
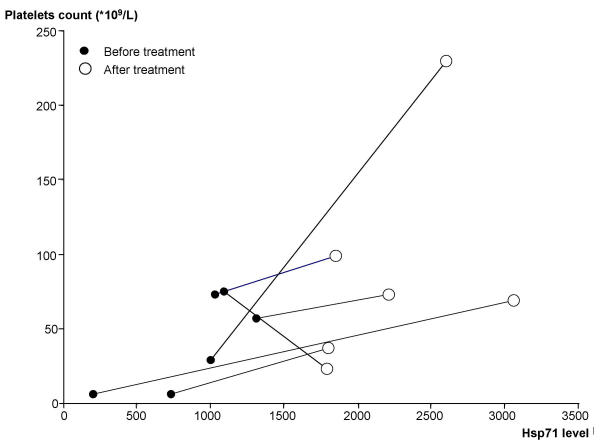
To find out if changes in Hsp levels were related to the state of the disease, we compared Hsp60 and Hsp71 levels in lymphocytes of a second group of six pediatric patients with ITP at admission to the hospital (before) and upon release after treatment. These six patients were hospitalized for approximately 3 weeks and were given treatments similar to the other ITP patients. None of these 6 patients showed expression of Hsp60 either before or after treatment. In the case of Hsp71, there were changes and the level of Hsp71 was higher after three weeks of treatment for ITP (1951 ± 753 IOD units) than before (1160 ± 727 IOD units) (P = 0.094) (Table 2). This increase was accompanied by an increase of platelet count (from 35.0 ± 30.8 to 94.5 ± 69.5 × 109/L, P = 0.084) (Table 2). We also analysed the changes in the Hsp71 levels and platelet counts in these patients. As illustrated in Figure 2, five of the six patients showed an increase in the expression levels of Hsp71 and in platelet counts after treatment while a decrease of platelet counts was noted in one patient.
Table 2.
Changes of blood platelets counts and Hsp71 levels in pediatric patients with ITP at admission and after treatment
| NO | Gender | Age | Diagnosis | At admission | After treatment | ||
| BPC | Hsp71 | BPC | Hsp71 | ||||
| 1 | F | 10 | acute | 73 | 1030 | 99 | 1850 |
| 2 | M | 10 | acute | 6 | 200 | 69 | 3060 |
| 3 | F | 8 | acute | 29 | 1000 | 230 | 2600 |
| 4 | M | 10 | acute | 6 | 730 | 37 | 1800 |
| 5 | F | 10 | acute | 75 | 1090 | 23 | 1790 |
| 6 | M | 8 | acute | 57 | 1310 | 73 | 2210 |
M: Male; F: Female; BPC: blood platelets counts. Hsp71 values are given in integrated optical density units (see methods)
Figure 2.
Changes in the association of Hsp71 versus platelets count in patients with ITP before and after treatment.
Presence of plasma antibodies against Hsp60 and Hsp71 in patients and controls
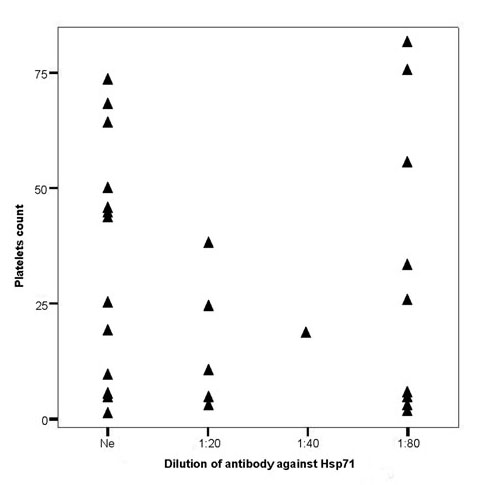
The presence of antibodies against Hsps has been reported in a number of diseases and it has been suggested that Hsps might be involved in the pathogenesis of the diseases and/or be of use for disease prognosis [28-31,34]. Since there were no available data for pediatric patients with ITP, we next determined if these patients had antibodies to the inducible Hsp71 and to Hsp60. As shown in Table 3 and 4, antibodies against Hsp60 and Hsp71 were found in both normal children and in ITP patients. Over 50% of ITP patients had a positive reaction to Hsp70 as compared to 16.7% of controls (P < 0.05) (Table 3). Moreover the titer of these antibodies was higher with more than half of the patients (9/15) showing a positive reaction at a dilution of 1:80. Figure 3 shows a comparison of platelet number in the 29 ITP patients with the dilution of antibodies against Hsp71. Interestingly, the platelet counts of ITP patients showing no antibody against Hsp71 are significantly higher than those positive for antibody against this Hsp (42.8 ± 27.4 vs 21.4 ± 21.7 × 109/L, P < 0.05). Finally as shown in table 4, there are no differences in the incidence of antibodies against Hsp60 between patients with ITP and matched controls (P > 0.05).
Table 3.
Presence of plasma antibodies against Hsp71 in patients with ITP and controls.
| Groups | Number of children | Dilution of antibodies against Hsp71 (n and Percentage) | |||||
| negative | positive | 1:10 | 1:20 | 1:40 | 1:80 | ||
| Control | 30 | 25 (83.3) | 5(16.7) | 0(0) | 3(10.0) | 0(0) | 2 (6.7) |
| ITP | 29 | 14(48.3) * | 15(51.7) * | 0(0) | 5(17.2) | 1(3.4) | 9 (31.0) * |
*P < 0.05, compared with control; n: represents the number of children at different dilutions of antibodies against Hsp71.
Table 4.
Presence of plasma antibodies against Hsp60 in patients with ITP and control.
| Groups | Number of children | Dilution of antibodies against Hsp60 (n and Percentage) | |||||
| negative | positive | 1:10 | 1:20 | 1:40 | 1:80 | ||
| Control | 30 | 28(93.3) | 2(6.7) | 0(0) | 0(0) | 1(3.3) | 1(3.3) |
| ITP | 29 | 26(89.7) | 3(10.3) | 1(3.4) | 0(0) | 1(3.4) | 1 (3.4) |
n: represents the number of children at different dilutions of antibodies against Hsp60.
Figure 3.
Distribution of platelet counts (×109/L) of the 29 ITP patients versus the titer of antibodies against Hsp71.
The presence of antibodies against Hsp71 did not seem to be related to the form of ITP. Three of the six patients with the chronic form showed antibodies to Hsp71 as compared to 13 out of the 23 patients presenting with the acute form. The majority of patients in both groups had titers of anti-Hsp71 of 1:80 (66% for chronic and 61% for acute) (see Table 1).
Discussion
Many investigations have shown that the aberrant expression of Hsps or the presence of antibodies against Hsps may be involved in the pathogenesis of many diseases and/or may be of use for prognosis and treatment [24,25,28-31,34]. ITP is an autoimmune diseases caused by platelet destruction resulting from autoantibodies against platelet surface proteins, particularly platelet glycoprotein IIb/IIIa. However, how antibodies against platelets are produced and how platelets are destroyded remains unknown. Hsps may be of importance in both immune and inflammatory responses. Whether patients with ITP exhibit different levels of Hsps and antibodies against Hsps has only now been examined.
Our data on a group of 29 pediatric patients with ITP show that there is a slightly lower level of Hsp71 (although not statistically significant) in lymphocytes of patients compared with controls (1567.8 ± 753.2 versus 1763.2 ± 641.8 IOD units). Interestingly, Hsp60 was only detected in 1 of the 29 pediatric patients with ITP in a western blot assay under conditions where this Hsp was detected in all controls. These data are likely to be of clinical interest for the following reasons. Firstly, Hsps can be induced by a wide variety of environmental stresses and pathological stimuli such as viral, bacterial or parasitic infections, fever, inflammation, malignancy and autoimmunity. Many of these conditions may contribute to the pathogenesis of ITP and some can also cause an imbalance of the immune response and autoimmune diseases [2-6]. Interestingly, we did not find any induction of Hsps in these patients. Secondly, induced Hsps can protect cells, organs and organisms against many damages caused by these stresses [35-44]. A low level or absence of Hsps might render cells more sensitive to such stresses.
Since Hsps also play an important role in the immune response, they could serve as an early warning to the host's immune response during the onset of disease [45]. Hsps require no adjuvant to confer immunogenicity to bound peptides as if they possessed an intrinsic "danger" signature [46]. This "danger signal" alerts the immune system to the death of a cell under stress and the role of Hsps as protein carriers allows the immune effector cells to survey the peptides released by the stressed cell and to activate against new or unrecognized peptides carried by Hsps [18]. Hsp60 and Hsp71 not only shuttle immunogenic peptides [47] but can also activate antigen-presenting cells [16,19]. These studies demonstrating the importance of Hsps in immune response, indicate that a low expression of Hsps (i.e absence of Hsp60 in lymphocytes of patients with ITP) may be related to the formation of autoantibodies against platelets. An emerging body of data supports a role for Hsps in the inflammatory response: Hsps participate in cytokine signal transduction and in the control of cytokine gene expression [16], enhance antigen presentation to T lymphocytes, and Hsps displayed on the surface of cells are important for targeting cytotoxic cells [17]. Vabulas et al. [46] reported that endocytosed human and chlamydial Hsp60 use toll-like receptor 2 and 4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. The present investigation shows a decrease of Hsp60 in patients with ITP. Whether the decrease in Hsp60 occurs before or during its pathogenesis and is an inducing factor or simply an effect is unknown.
Given the presence of significant differences in Hsp levels and since the immune response to Hsps can be modulated, modifying the immune response to Hsps may be a potential new treatment option for patients with ITP. However at this stage the possible roles of Hsps in the etiopathogenesis or treatment of ITP remain to be further investigated.
The present study also shows that antibodies against the inducible Hsp71 are particularly prevalent in pediatric patients with ITP. 51.7% of patients had antibodies to Hsp71 and more than half of those were still positive at dilutions of 1:80. Berchtold et al. [1] reported that rates of autoantibodies against platelet membrane glycoproteins in children with acute and chronic ITP were 26.7%, 58.3% respectively. Thus the high prevalence of antibodies to Hsp71 is significant and merits further analysis considering that the diagnosis for ITP is basically based on exclusion [8]. Some possible reasons for the production of autoantibodies against Hsps are: 1) genetic factors; 2) viral infection; 3) denaturation and release of Hsps as a result of cell damage and 4) the presence of anti-specific lymphocytes. No obvious genetic diseases were documented in the pediatric patients with ITP. However, there were frequent histories of viral or bacterial infection and treatment with some drugs in pediatric patients before the occurrence of ITP.
Conclusions
Many investigations suggest that autoantibodies against Hsps are important in the generation, formation and prognosis of diseases [28-31,34,48]. Our previous results show that the high prevalence of antibodies against Hsp71 in benzene-poisoned workers was associated with a decrease in white blood cells and with an increase in activities of serum superoxide dismutase and lymphocyte DNA damage [28]. There is an immunological cross-reaction between microorganisms and autoantigens of human as Hsps show a high degree of amino acid sequence homology between different species from prokaryotes to humans [48]. Gross et al. [49] showed that a 66 kDa protein purified from rabbit reticulocyte lysate, which seems to be involved in platelet destruction, could promote the recycling of Hsp70. However, the mode of action of autoantibody is complex, involving modulation of the expression and function of Fc receptors, interference with the activation of complement and the cytokine network, provision of antiidiotypic antibody, and effects on the activation, differentiation, and effector functions of T and B cells [50]. Therefore it will be important to understand the mechanisms involved in the generation of antibodies against both Hsp71 and platelets, and to understand their role in the pathogenesis of ITP, and whether they can be used for diagnosis, prognosis and/or treatment of ITP.
Competing interests
We declare that there is no competing interest in this work.
Authors' contributions
CX performed the immuno assays, SC the Western blots together with DY in addition to participating to discussion with MY and FD. MY designed the experiments and was responsible of patient diagnosis with FD. JL carried out the sampling with RW in addition to performing some laboratory analysis. RMT and TW initiated the project, designed the experiments and wrote the manuscript together with DY.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
We are particularly grateful to all individuals who voluntarily accepted to participate in the study and to the patients and their parents for their understanding and support for this investigation. We also thank many members of the medical personnel of Wuhan Children Hospital for their generous help in the sampling and examination of subjects. We thank Prof RW Currie (Dalhousie University, Halifax, Canada) for editing the English. This work is supported by grants from the National Natural Science Foundation of China (TW) and the Canadian Institutes of Health Research (RMT). TW held a Teaching and Research Award Program for Outstanding Young Teachers in Higher Education Institution of the Ministry of Education, P.R.C.
Contributor Information
Chengfeng Xiao, Email: xiaoc@biology.queensu.ca.
Sheng Chen, Email: sheng@utsc.utoronto.ca.
Mingchun Yuan, Email: wut@mails.tjmu.edu.cn.
Fuyue Ding, Email: wut@mails.tjmu.edu.cn.
Dongliang Yang, Email: dlyang@tjh.thmu.edu.cn.
Ruibo Wang, Email: rwang@up.uc.hc.edu.
Jianxin Li, Email: wut@mails.tjmu.edu.cn.
Robert M Tanguay, Email: robert.tanguay@rsvs.ulaval.ca.
Tangchun Wu, Email: wut@mails.tjmu.edu.cn.
References
- Berchtold P, McMillan R, Tani P, Sommerville-Niesen S, Blanchette VS. Autoantibodies against platelet membrane glycoproteins in children with acute and chronic immune thrombocytopenic purpura. Blood. 1989;74:1600–1602. [PubMed] [Google Scholar]
- Bussel JB, Kunicki TJ, Michelson AD. Platelets: New understanding of platelet glycoproteins and their role in disease. Hematology (AM Soc Homatol Educ Program) 2000;2000:222–240. doi: 10.1182/asheducation-2000.1.222. [DOI] [PubMed] [Google Scholar]
- Nardi M, Tomlinson S, Greco MA, Karpatkin S. Complement-independent, peroxide-induced antibody lysis of platelets in HIV-1-related immune thrombocytopenia. Cell. 2001;106:551–561. doi: 10.1016/S0092-8674(01)00477-9. [DOI] [PubMed] [Google Scholar]
- Aster RH. Drug-induced immune throbocytopenia: an overview of pathogenesis. Semin Hematol. 1999;36:2–6. [PubMed] [Google Scholar]
- Moller E. Mechanisms for induction of autoimmunity in humans. Acta Pediatr (Suppl) 1998;424:16–20. doi: 10.1080/080352598750030690. [DOI] [PubMed] [Google Scholar]
- Imbach PA, Kuhne , Hollander G. Immunologic aspects in the pathogenesis and treatment of immune thrombocytopenic purpura in children. Curr Opin Pediatr. 1997;9:35–40. doi: 10.1097/00008480-199702000-00009. [DOI] [PubMed] [Google Scholar]
- Fujisawa K, Tani P, Piro L, McMillan R. The effect of therapy on platelet-associated autoantibody in chronic immune thrombocytopenic purpura. Blood. 1993;81:2872–2877. [PubMed] [Google Scholar]
- Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995–1008. doi: 10.1056/NEJMra010501. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat shock proteins. Ann Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Tissières A, Georgopoulos C. The stress response, function of the proteins, and perspectives. In: Morimoto RI, Tissieres A, Georgopoulos C, editor. In Stress proteins in Biology and Medicine. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY; 1990. pp. 1–36. [Google Scholar]
- Morimoto RI, Tissieres A, Georgopoulos C. Progress and perspectives in the biology of heat shock proteins and molecular chaperones. In: Morimoto RI, Tissières A, Georgopoulos C, editor. In The biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY; 1994. pp. 1–30. [Google Scholar]
- Michaud S, Marin R, Tanguay RM. Regulation of heat shock gene induction and expression during Drosophila development. Cell Mol Life Sci. 1997;53:104–113. doi: 10.1007/PL00000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower LE. Heat shock, stress protein, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Gething MJ. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Muchowski PJ, Schaffar G, Sittler A, Wanker EE, Hayer-Hartl MK, Hartl FU. Hsp70 and hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc Natl Acad Sci USA. 2000;97:7841–7846. doi: 10.1073/pnas.140202897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones E, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Moseley PL. Heat shock proteins and inflammatory response. Ann NY Acad Sci. 1998;856:206–213. doi: 10.1111/j.1749-6632.1998.tb08327.x. [DOI] [PubMed] [Google Scholar]
- Moseley PL. Stress proteins and the immune response. Immunopharmacology. 2000;48:299–302. doi: 10.1016/S0162-3109(00)00227-7. [DOI] [PubMed] [Google Scholar]
- Singh-Jasuja H, Hilf N, Arnold-Schild D, Schild H. The role of heat shock proteins and their receptors in activation of immune system. Biol Chem. 2001;382:629–636. doi: 10.1515/BC.2001.074. [DOI] [PubMed] [Google Scholar]
- Sato K, Torimoto Y, Tamura Y, Shindo M, Shinzaki H, Hirai K, Kohgo Y. Immunotherapy using heat-shock protein preparations of leukemia cells after syngeneic bone marrow transplantation in mice. Blood. 2001;98:1852–1857. doi: 10.1182/blood.V98.6.1852. [DOI] [PubMed] [Google Scholar]
- Wells AD, Malkovsky M. Heat shock proteins, tumor immunogenicity and antigen presentation: an integrated view. Immunol Today. 2000;21:129–132. doi: 10.1016/S0167-5699(99)01558-3. [DOI] [PubMed] [Google Scholar]
- Van Eden I. Stress proteins as targets for anti-inflammatory therapies. Drug Disc or Today. 2000;5:115–120. doi: 10.1016/S1359-6446(99)01464-6. [DOI] [PubMed] [Google Scholar]
- Blachere NE, Srivastava PK. Heat shock protein-based cancer vaccines and related thoughts on immunogenicity of human tumors. Semin Cancer Biol. 1995;6:349–355. doi: 10.1016/1044-579x(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- Minowada G, Welch WJ. Clinical implications of the stress response. J Clin Invest. 1995;95:3–12. doi: 10.1172/JCI117655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffmann SHE, Schooel B. Heat shock proteins as antigens in immunity against infection and self. In: Morimoto RI, Tissières A, Georgopoulos C, editor. In The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor Laboratory Press, NY; 1994. pp. 495–531. [Google Scholar]
- Frosttegard J, Lemmne C, Andersson B, Zee RVD, Liessling R, Faire U. Association of serum antibodies to heat shock protein 65 with borderline hypertension. Hypertension. 1997;29:40–44. doi: 10.1161/01.hyp.29.1.40. [DOI] [PubMed] [Google Scholar]
- Wu T, Yuan Y, Wu Y, He H, Zhang G, Tanguay RM. Presence of antibodies to heat stress proteins in workers exposed to benzene and in patients with benzene poisoning. Cell Stress Chaperones. 1998;3:161–167. doi: 10.1379/1466-1268(1998)003<0161:POATHS>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Chen S, Xiao C, Wang C, Pan Q, Wang Z, Xie M, Mao Z, Wu Y, Tanguay RM. Presence of antibody against the inducible Hsp71 in patients with acute heat-induced illness. Cell Stress Chaperones. 2001;6:113–120. doi: 10.1379/1466-1268(2001)006<0113:POAATI>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Ma J, Chen S, Sun Y, Xiao C, Gao Y, Wang R, Poudrier J, Dargis M, Currie RW, Tanguay RM. Association of plasma antibodies against heat stress protein Hsp70 with hypertension and harsh working conditions. Cell Stress Chaperones. 2001;6:394–401. doi: 10.1379/1466-1268(2001)006<0394:AOPAAT>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Kiechl S, Mayr M, Metzler B, Egger G, Oberhollenzer F, Willeit J, Wick G. Association of serum antibodies to heat shock protein 65 with carotid atherosclerosis: clinical significance determined in a follow-up study. Circulation. 1999;100:1169–1174. doi: 10.1161/01.cir.100.11.1169. [DOI] [PubMed] [Google Scholar]
- Tanguay RM, Wu Y, Khandjian EW. Tissue-specific expression of heat shock stress proteins of the mouse in the absence of stress. Dev Genet. 1993;14:112–118. doi: 10.1002/dvg.1020140205. [DOI] [PubMed] [Google Scholar]
- Bhandari P, Gowrishnkar J. An Escherichia coli host strain useful for efficient overproduction of cloned gene products with NaCl as the inducer. J Bacteriol. 1997;179:4403–4406. doi: 10.1128/jb.179.13.4403-4406.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingai R, Maeda T, Onishi S, Yamamoto Y. Autoantibody against 70 kD heat-shock protein in patients with autoimmune liver diseases. J Hepatol. 1995;23:382–390. doi: 10.1016/0168-8278(95)80195-2. [DOI] [PubMed] [Google Scholar]
- Angelidis CE, Lazaridis I, Pagoulatos GN. Constitutive expression of heat shock protein 70 in mammalian cells confers thermotolerance. Eur J Biochem. 1991;199:35–39. doi: 10.1111/j.1432-1033.1991.tb16088.x. [DOI] [PubMed] [Google Scholar]
- Li GC, Li LY, Liu K, Mak JK, Chen L, Lee WMF. Thermal response of rat fibroblasts stably transfected with the human 70 kDa heat shock protein encoding gene. Proc Natl Acad Sci USA. 1991;88:1681–1685. doi: 10.1073/pnas.88.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollet E, Landary J, Lavoie J, Tanguay RM. Expression of Drosophila 27 kDa heat shock protein in rodent cell confers thermal resistance. Biochem Biophys Res Commun. 1992;185:116–120. doi: 10.1016/s0006-291x(05)80963-5. [DOI] [PubMed] [Google Scholar]
- Currie RW, Tanguay RM, Kingma JG. Heat-response and limitation of tissue necrosis during occusion/reperfusion in rabbit hearts. Circulation. 1993;87:963–971. doi: 10.1161/01.cir.87.3.963. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R, Nunnally I, Lindquist S, Taulien J, Perdrizet G, Karl I. Hyperthermia protects mice against the lethal effects of endotoxin. Am J Physiol. 1993;265:R1447–R1457. doi: 10.1152/ajpregu.1993.265.6.R1447. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Preville X, Chareyron P, Briolay J, Klemenz R, Arrigo P. Constitutive expression of human hsp27, Drosophila hsp27 and human alpha-B crystallin confers resistance to tumor necrosis factor alpha and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol. 1995;154:364–374. [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. Heat shock proteins and stress tolerance. In: Morimoto RI, Tissières A, Georgopoulos C, editor. In The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor Laboratory Press, NY; 1994. pp. 457–494. [Google Scholar]
- Marber MS, Mestril R, Chi S, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;96:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumier C, Krueger AM, Currie RW, Kontoyiannis D, Kollias G, Pagoulatos GN. Transgenic mice expressing the human inducible Hsp70 have hippocampal neurons resistant to ischemic injury. Cell Stress Chaperones. 1997;2:162–167. doi: 10.1379/1466-1268(1997)002<0162:TMETHI>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumier C, Ross BM, Currie RW, Angelidis CE, Kazlaris H, Kollias G, Pagoulatos GN. Transgenic mice expression of the human heat shock protein 70 have improved post-ischemic myocardial recovery. J Clin Invest. 1995;95:1854–1860. doi: 10.1172/JCI117865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNagel DC, Pierce SK. Heat shock proteins in immune response. Crit Rev Immunol. 1993;13:71–81. [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Hacker H, Wagner H. Endocytosed HSP60 use toll-like receptor 2(TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276:31332–31339. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- Srivastava PK, Menoret A, Basu S, Binder RJ, McQuade KL. Heat shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity. 1998;8:657–665. doi: 10.1016/s1074-7613(00)80570-1. [DOI] [PubMed] [Google Scholar]
- Mayr M, Metzler B, Kiechl S, Willeit J, Schett G, Xu Q, Wick G. Endothelial cytotoxicity mediated by serum antibodies to heat shock proteins of Escherichia coli and Chlamydia pneumoniae: immune reactions to heat shock proteins as a possible link between infection and atherosclerosis. Circulation. 1999;99:1560–1566. doi: 10.1161/01.cir.99.12.1560. [DOI] [PubMed] [Google Scholar]
- Gross M, Hessefort S. Purification and characterization of a 66-kDa protein from rabbit reticulocyte lysate which promotes the recycling of Hsp70. J Biol Chem. 1996;271:16833–16841. doi: 10.1074/jbc.271.28.16833. [DOI] [PubMed] [Google Scholar]
- Mackay IR, Rosen FS. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345:747–755. doi: 10.1056/NEJMra993360. [DOI] [PubMed] [Google Scholar]