Abstract
Background
Disease activity is a major factor in menstrual disorders in systemic lupus erythematosus (SLE) patients not receiving alkylating therapy. However, the ovarian reserve of SLE women with normal menstruation is still unclear.
Methods
Twenty-three SLE patients naïve to cytotoxic agents (SLE group) and nineteen SLE patients receiving current or previous cyclophosphamide (CTX) therapy (without other cytotoxic agents; SLE-CTX group) were enrolled. Twenty-one age-matched healthy women served as controls. All patients and controls had a regular menstrual cycle. Basal hormone levels, including follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), and anti-Müllerian hormone (AMH), and antral follicle count (AFC) were analyzed in the two study groups and compared with the control group.
Results
No significant differences were found between the SLE, SLE-CTX, and control groups in age, body mass index (BMI), and basal FSH and LH levels. The E2 (P=0.023) levels were high and the AMH (P=0.000) values and AFC (P=0.001) were significantly lower in the SLE and SLE-CTX groups compared to control. However, these values were similar between the SLE and SLE-CTX groups.
Conclusion
SLE patients not receiving alkylating therapy who had normal menstruation and short illness duration still had an impaired ovarian reserve.
Introduction
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease that is characterized by the production of non-organ-specific autoantibodies. It predominantly affects young adults (median age at diagnosis <40 years), and women with SLE have a higher risk of developing menstrual irregularities compared with a healthy population.1–3
In patients with irregular cycles, the mean SLE disease activity index (SLEDAI) levels and the frequency of patients with SLEDAI ≥8 were found to be significantly higher2 than in patients with regular cycles, and the disease activity was shown to be a major factor associated with menstrual cycle disorders in SLE patients before treatment with alkylating agents,3 so the high SLEDAI levels were associated with impaired ovarian function. There was also a higher risk of patients having an irregular cycle after cyclophosphamide (CTX) therapy, as this therapy is known to damage ovarian follicles.3,4 However, it is currently unknown whether SLE women with or without alkylating therapy who have normal menstruation would have impaired ovarian reserve compared to healthy women. Therefore, in this study we assessed ovarian reserve in SLE patients with a normal menstrual cycle.
Anti-Müllerian hormone (AMH) has been shown to have great promise as a possible marker of ovarian function. The hormone is expressed in granulosa cells of growing follicles and reflects the size of the primordial follicle pool.5 An association between the number of ovarian follicles and AMH levels has been observed in women,6–8 and therefore it has been used to predict ovarian responsiveness to controlled ovarian stimulation during assisted reproduction.9,10 Some studies have suggested that AMH may be an early indicator of waning ovarian function in chemotherapy patients, and may be superior to current markers of ovarian reserve.11 Antral follicle count (AFC) also has a good correlation with ovarian response during assisted reproduction.12
In this study, we examined follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), AMH, and AFC in an effort to assess ovarian reserve in SLE patients with normal menstruation regardless of previous alkylating therapy. The objective of this study was to determine whether these SLE women have impaired ovarian function compared to healthy women.
Materials and Methods
Subjects
This study was approved by the Institutional Review Board of SunYat-sen University, and informed written consent was obtained from all participants. Between April 2010 and May 2011, 42 SLE female patients were followed at our University Hospital and were divided into two groups based on their used of alkylating therapy: SLE patients using CTX (SLE-CTX) (n=19), and SLE patients not using CTX (n=23). Twenty-one healthy patients were also recruited for the study with a mean age that matched the experimental groups. All patients and control group volunteers had a normal menstrual cycle, which was defined as flow duration of 3–7 days and cycle duration of 25–35 days. None of the patients were diagnosed with endometriosis or polycystic ovarian syndrome, or had a history of ovarian surgery or oral contraceptives usage within the previous 3 months. Age of onset, duration of illness, SLEDAI, current medications, body mass index (BMI), age at menarche, and previous treatment were recorded for all patients.
Treatment with the following drugs was allowed in patients of the SLE group: glucocorticosteroids, azathioprine, hydroxychloroquine, cyclosporin A, methotrexate, mycophenolate mofetil, leflunomide, intravenous immunoglobulins, and rituximab. Patients of the SLE-CTX group received current or previous CTX therapy and/or one of drugs listed above, but did not receive other cytotoxic agents. Complete ovarian function was concomitantly assessed by measuring hormone serum levels during the follicular phase (second to fifth day) of the menstrual cycle and evaluated AFC (approximate diameter: 2–9 mm) by vaginal ultrasound.
Hormone assays
FSH, LH, and E2 measurements were obtained using a microparticle enzyme immunoassay (Abbott ARCHITECT I System, Abbott Diagnostics). Intra- and inter-assay coefficients of variation were limited to 3.3% and 6.2%, respectively. The AMH serum level was measured using a repeated enzyme-linked immunosorbent assay (AMH Gen II Elisa Test, Beckman Coulter, Diagnostic Systems Laboratories; normal range 1–8 ng/mL). Values <1 ng/mL were regarded as reduced and values <0.4 were regarded as strongly reduced ovarian reserve. All serum samples were tested at the same time to minimize day-to-day assay variation.
Statistical analysis
Statistical analyses were performed using the statistical package for the social sciences (SPSS) software package (ver. 13.0; SPSS Inc.). Normality was tested using the Kolmogorov-Smirnov test. Differences in data between groups were determined using one-way analysis of variance. Correlation between AMH and duration of illness was analyzed using Pearson's correlation coefficient. For all analyses, P<0.05 was defined as statistically significant.
Results
The mean duration of illness in the SLE group was 3.04 years (range: 0.5–12 years) and 2.27 years (range: 0.5–6 years) in the SLE-CTX group. The median CTX dosage used was 4.05 g (range: 0.70–20.90 g). The average age [30.00±4.37 (range: 20–40) and 28.87±5.27 (range: 19–37) vs. 28.95±6.03 (range: 23–37) years, respectively; P=0.747], age at menarche (P=0.392), and body mass index (BMI) (P=0.114) were similar between the two study groups and the control group. All patients in the SLE-CTX and SLE groups experienced menarche before disease onset, and only one patient in the SLE-CTX group received CTX at 36 years of age. All other patients received CTX before the age of 35.
The mean SLEDAI (5.10±3.59 vs. 2.09±1.81, P=0.039) and the dose of prednisone currently used (16.26±12.84 vs. 8.70±6.94 mg/d, P=0.020) in the SLE-CTX patients were significantly higher than the SLE group, respectively. The basal average levels of serum FSH (P=0.207) and LH (P=0.518) were comparable between the three groups, but a trend of higher FSH levels was observed in the SLE-CTX group. Significantly higher basal estradiol values (53.27±30.01 and 44.20±14.22 vs. 36.10±10.48 pg/mL; P=0.007), lower AMH levels (1.15±1.28 and 1.71±1.29 vs. 3.33±1.76 ng/mL; P=0.000) and less AFC (7.80±4.54 and 10.90±3.00 vs. 14.61±4.93; P=0.001) were observed in the SLE-CTX and SLE groups compared to the control group, respectively, but were similar between the SLE-CTX and SLE groups (Table 1). In addition, we divided the SLE-CTX patients into two groups based on if they were currently or had previously received CTX and did not find any significant difference in AMH levels (1.06±1.10 vs. 1.18±1.37 ng/mL, respectively; P=0.619) (Table 2).
Table 1.
Demographic Features, Ovarian Reserve Hormonal Profile, and Antral Follicle Count in SLE Patients and Controls
| SLE-CTX group (n=19) | SLE group (n=23) | Control group (n=21) | P | |
|---|---|---|---|---|
| Mean age (years) | 28.87±5.27 | 30.00±4.37 | 28.95±6.03 | 0.747 |
| Mean age at menarche (years) | 13.90±1.52 | 13.20±1.47 | 13.46±0.83 | 0.392 |
| Body mass index (kg/m2) | 21.60±1.20 | 20.30±2.29 | 20.54±1.86 | 0.114 |
| Mean duration of disease (years) | 2.27±1.69 (0.5–6) | 3.04±2.79 (0.5–12) | – | 0.299 |
| Mean SLEDAI | 5.10±3.59 (0–15) | 2.09±1.81 (0–6) | – | 0.039 |
| Median dosage of CTX (g) | 4.05 (0.70–20.90) | – | – | – |
| Median dosage of prednisone (mg/d) | 20 (0–40) | 10 (0–20) | – | – |
| Mean dosage of prednisone (mg/d) | 16.26±12.84 | 8.70±6.94 | – | 0.020 |
| FSH (IU/L) | 7.34±5.34 | 5.18±1.52 | 4.85±1.00 | 0.207 |
| LH (IU/L) | 3.51±1.71 | 3.79±2.08 | 3.14±1.47 | 0.518 |
| E2 (pg/mL) | 53.27±30.01a | 44.20±14.22a | 36.10±10.48b | 0.007 |
| AMH (ng/mL) | 1.15±1.28a | 1.71±1.29a | 3.33±1.76b | 0.000 |
| AFC | 7.80±4.54a | 10.90±3.00a | 14.61±4.93b | 0.001 |
Values expressed in mean±SD or median (range). a vs. b, P<0.05.
AMH, anti-Müllerian hormone; AFC, antral follicle count; CTX cyclophosphamide; E2, estradiol; FSH, follicle stimulating hormone; LH, luteinizing hormone; SLE, systemic lupus erythematosus; SLEDAI, SLE disease activity index.
Table 2.
Anti-Müllerian Hormone Levels in SLE-CTX Patients Who Were Currently or Had Previously Taken CTX
| Past (n=14) | Current (n=5) | P | |
|---|---|---|---|
| Mean age (years) | 29.36±6.30 | 27.80±5.72 | 0.868 |
| AMH (ng/mL) | 1.18±1.37 | 1.06±1.10 | 0.619 |
Values expressed in mean±SD.
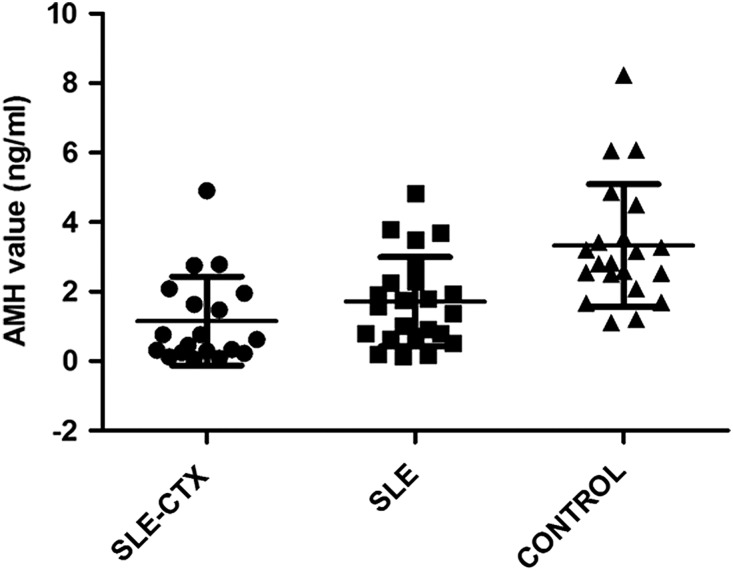
A strongly reduced ovarian reserve (AMH level below 0.4 ng/mL) was found in seven patients (33.3%) in the SLE-CTX group, two subjects (9.1%) in the SLE group, and no patients in the control group (Fig. 1). In addition, there was no correlation between the AMH values of the lupus patients and duration of illness (P=0.841).
FIG. 1.
Anti-Müllerian hormone (AMH) levels (mean±standard deviation) in the systemic lupus erythematosus cyclophosphamide patients who were currently or had previously taken (SLE-CTX), patients naïve to cytotoxic agents (SLE), and control groups. The mean AMH level was significantly higher in the control group than in the SLE-CTX and SLE groups.
Discussion
This study has demonstrated that SLE patients with normal menstruation and short illness duration still had impaired ovarian reserve regardless of CTX therapy (without other cytotoxic agents). To the best of our knowledge, this is the first study that has performed a complete hormonal profile concomitantly with ovarian ultrasound in SLE patients with normal menstruation and identified a subclinical impairment in ovarian reserve.
Here we found similar LH and FSH levels, significantly higher estradiol values, notably lower AMH levels, and less AFC in SLE-CTX and SLE groups compared with the healthy control group. In addition, higher estradiol values, lower AMH levels and less AFC were demonstrated in the SLE-CTX group compared to the SLE group, but these differences did not reach statistical significance. The marked increase in estradiol levels during the early follicular phase demonstrating the low ovarian reserve is due to luteal out-of-phase events and appears to be triggered by prolonged high FSH levels during the follicular phase.13
The disease activity was a major factor associated with menstrual cycle disorders in SLE patients before treatment with alkylating agents. The high SLE disease activity was associated with impaired ovarian function.3 In our study, all patients had a regular flow cycle, the SLEDAI was low (2.09±1.8, range: 0–6), and although the mean illness duration was short (mean: 3.04 years; range: 0.5–12 years) in the SLE group, the ovarian reserve of the patients was still significantly impaired.
It is well-known that CTX is the major cause of early menopause in SLE patients rather than the disease itself or glucocorticoid treatment, and therefore our finding that the SLE-CTX and SLE groups had no significant differences in ovarian reserve may be due to the use of CTX by patients before 35 years of age (except one patient) and a low cumulative dose (median CTX dosage: 4.05 g) in the SLE-CTX patients. A previous study showed that SLE patients have a higher risk of developing menstrual irregularities when on CTX therapy, especially for those taking a cumulative dose of more than 10 g3. For the SLE-CTX patients in our study, only two patients had a cumulative dose greater than 10 g (17.5 and 20.9 g, respectively). In addition, the results could be due to the significantly high dose of prednisone administered, as it is known that glucocorticoids have a suppressive effect on the hypothalamic-pituitary-ovarian axis, thereby inducing a reduction in LH and FSH serum levels 14,15. We also did not find a difference in AMH levels between the patients who were currently using CTX and those who had used it in the past. This could be due to the small number of patients in our study using CTX (n=5) and therefore will require further investigation.
In the SLE-CTX group, the mean illness duration was 2.27 years (range: 0.5–6 years), and although these patients had a low cumulative CTX dosage and regular menstruation, their ovarian reserve was still lower compared to patients in the SLE group. In addition, a strongly reduced ovarian reserve (AMH<0.4 ng/mL) was found in seven lupus patients (33.3%) in the SLE-CTX group compared with only two patients (9.1%) in the SLE group and no patients in the control group. Taken together, these results demonstrate that CTX therapy impairs ovarian reserve in the context of SLE, which has direct impact on ovarian function.
SLE is a chronic autoimmune rheumatic disease characterized by the potential involvement of virtually every organ and system of the body. Most aspects of the immune response are altered in SLE patients, and particularly in the increased production of multiple autoantibodies.16 The prevalence of a variety of autoantibodies, including rheumatoid factor as well as antibodies to DNA, steroid cells, islet cells, parietal cells, acetyl-choline receptors, and granulosa cell growth antibodies have been described in premature ovarian failure (POF) patients.17 SLE patients also have organ-specific antibodies directed against ovaries,18 which have been shown to be associated with POF in females with SLE.19 In addition, there are numerous reports describing the association of premature menopause ovarian autoantibodies and other organ- and non-organ-specific autoantibodies.20,21
The presence of antiphospholipid antibodies (aPLs) in SLE patients were found to be associated with compromised ovarian reserve and POF.22 Moreover, the presence of aPLs is correlated with a primary autoimmune process, and therefore the autoimmune defeat of the ovary could be the primary cause of decreased ovarian reserve and POF. Cross-reactivity between aPLs and other proteins and cell components has been widely documented, which supports the systemic characteristic of SLE.23–25 Furthermore, the interaction between aPLs and lipoprotein components is associated with atherosclerosis in SLE patients.26 Atherosclerosis in the ovarian artery may cause reduced ovarian volume in SLE patients, which correlates with decreased ovarian reserve.27
The levels of the soluble form of the Fas apoptosis antigen (sFas) were significantly higher in SLE patients than in healthy controls, and sFas levels correlated with organ damage, including ovary damage.28
Regular menstrual periods are an easily ascertainable external parameter of intact ovarian function; however, the presence of regular bleeding is not synonymous with non-impaired ovarian reserve. Lawrenz et al.29 found that in premenopausal SLE patients not receiving alkylating therapy, the AMH values were significantly lower compared to the control group. However, the patient population of that study consisted of those with regular and irregular bleeding. Our study is the first to show that even with regular menstrual flow, short illness duration, and no alkylating therapy, SLE patients still had significantly impaired ovarian reserve.
Although the SLEDAI score in the SLE-CTX group was significant higher than in SLE patients, we did not find a significant difference in ovarian reserve between the two groups. The could be because the SLEDAI score of all patients was lower than the threshold that leads to a more significant impairment in ovarian reserve Thus, prospective studies are warranted to evaluate ovarian reserve in different phases of the disease.
In conclusion, although our study only assessed 23 patients not receiving alkylating treatment, it showed that SLE patients still had significantly lower ovarian reserve despite normal menstruation and short illness duration. Although limited data on the influence of medication other than CTX/other cytotoxic agents, our results suggest that the disease itself has a negative influence on ovarian reserve. Moreover, in 19 patients receiving CTX therapy (without other cytotoxic agents), we illustrated that even with low cumulative CTX doses and normal menstrual flow cycle, the patients still had impaired ovarian function based on SLE itself.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant no. 30973202), the Guangdong Provincial Fund of Industry, Education, and Academy (grant no. 2008B090500194), and the Doctoral Fund of Ministry of Education of China (grant no. 20090171110059).
Disclosure Statement
No competing financial interests exist.
References
- 1.Shabanova SS. Ananieva LP. Alekberova ZS. Guzov II. Ovarian function and disease activity in patients with systemic lupus erythematosus. Clin Exp Rheumatol. 2008;26:436–441. [PubMed] [Google Scholar]
- 2.Pasoto SG. Mendonça BB. Bonfá E. Menstrual disturbances in patients with systemic lupus erythematosus without alkylating therapy: Clinical, hormonal and therapeutic associations. Lupus. 2002;11:175–180. doi: 10.1191/0961203302lu163oa. [DOI] [PubMed] [Google Scholar]
- 3.Fatnoon NN. Azarisman SM. Zainal D. Prevalence and risk factors for menstrual disorders among systemic lupus erythematosus patients. Singapore Med J. 2008;49:413–418. [PubMed] [Google Scholar]
- 4.Gonzalez-Crespo MR. Gomez-Reino JJ. Merino R, et al. Menstrual disorders in girls with systemic lupus erythematosus treated with cyclophosphamide. Br J Rheumatol. 1995;34:737–741. doi: 10.1093/rheumatology/34.8.737. [DOI] [PubMed] [Google Scholar]
- 5.van Beek R. van den Heuvel-Eibrink M. Laven J, et al. Anti-Mullerian hormone is a sensitive serum marker for gonadal function in women treated for Hodgkin's lymphoma during childhood. J Clin Endocrinol Metab. 2007;92:3869–3874. doi: 10.1210/jc.2006-2374. [DOI] [PubMed] [Google Scholar]
- 6.van Rooij I. Tonkelaar I. Broekmans F, et al. Anti-mullerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11:601–606. doi: 10.1097/01.gme.0000123642.76105.6e. [DOI] [PubMed] [Google Scholar]
- 7.Fanchin R. Schonauer L. Righini C. Guibourdenche J. Frydman R. Taieb J. Serum anti-Mullerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH, and LH on day 3. Hum Reprod. 2003;18:323–327. doi: 10.1093/humrep/deg042. [DOI] [PubMed] [Google Scholar]
- 8.Seifer D. MacLaughlin D. Christian B. Feng B. Shelden R. Early follicular serum mullerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77:468–471. doi: 10.1016/s0015-0282(01)03201-0. [DOI] [PubMed] [Google Scholar]
- 9.Muttukrishna S. McGarrigle H. Wakim R. Khadum I. Ranieri D. Serhal P. Antral follicle count, anti-mullerian hormone and inhibin B: Predictors of ovarian response in assisted reproductive technology? Br J Obstet Gynecol. 2005;112:1384–1390. doi: 10.1111/j.1471-0528.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 10.Ficicioglu C. Kutlu T. Baglam E. Bakacak Z. Early follicular antimullerian hormone as an indicator of ovarian reserve. Fertil Steril. 2006;85:592–596. doi: 10.1016/j.fertnstert.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Anderson R. Themmen A. Al-Qahtani A. Groome N. Cameron D. The effects of chemotherapy and long-term gonadotropin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod. 2006;21:2583–2592. doi: 10.1093/humrep/del201. [DOI] [PubMed] [Google Scholar]
- 12.Kwee J. Elting ME. Schats R. McDonnell J. Lambalk CB. Ovarian volume and antral follicle count for the prediction of low and hyper responders with in vitro fertilization. Reprod Biol Endocrinol. 2007;15:5–9. doi: 10.1186/1477-7827-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hale GE. Hughes CL. Burger HG. Robertson DM. Fraser IS. Atypical estradiol secretion and ovulation patterns caused by luteal out-of-phase (LOOP) events underlying irregular ovulatory menstrual cycles in the menopausal transition. Menopause. 2009;16:50–59. doi: 10.1097/GME.0b013e31817ee0c2. [DOI] [PubMed] [Google Scholar]
- 14.Saketos M. Sharma N. Santoro NF. Suppression of the hypothalamic-pituitary- ovarian axis in normal women by glucocorticoids. Biol Reprod. 1993;49:1270–1276. doi: 10.1095/biolreprod49.6.1270. [DOI] [PubMed] [Google Scholar]
- 15.Gallant C. Kenny P. Oral glucocorticoids and their complications. A review. J Am Acad Dermatol. 1986;14:161–177. doi: 10.1016/s0190-9622(86)70018-2. [DOI] [PubMed] [Google Scholar]
- 16.Morrow J. Nelson L. Watts R. Isenberg D. Autoimmune rheumatic disease. Oxford: Oxford University Press; 1999. Systemic lupus erythematosus; pp. 56–103. [Google Scholar]
- 17.Mignot MH. Schoemaker J. Kleingeld M. Rao BR. Drexhage HA. Premature ovarian failure. The association with autoimmunity. Eur J Obstet Reprod Biol. 1989;30:59–65. doi: 10.1016/0028-2243(89)90094-4. [DOI] [PubMed] [Google Scholar]
- 18.Moncayo-Naveda H. Moncayo R. Benz R. Wolf A. Lauritzen C. Organ-specific antibodies against ovary in patients with systemic lupus erythematosus. Am J Obstet Gynecol. 1989;160:1227–1229. doi: 10.1016/0002-9378(89)90200-7. [DOI] [PubMed] [Google Scholar]
- 19.Wheatcroft NJ. Salt C. Milford-Ward A. Cooke ID. Weetman AP. Identification of ovarian antibodies by immunofluorescence, enzyme-linked immunosorbent assay or immunoblotting in premature ovarian failure. Hum Reprod. 1997;12:2617–2622. doi: 10.1093/humrep/12.12.2617. [DOI] [PubMed] [Google Scholar]
- 20.Lubovsky J. Visintin J. Boyers S. Asari T. Caldwell B. deCheney A. Ovarian antibodies detected by immobilized antigen immunoassay in patients with premature ovarian failure. J Clin Endocrinol Metab. 1990;70:69–74. doi: 10.1210/jcem-70-1-69. [DOI] [PubMed] [Google Scholar]
- 21.Lubovsky J. Llanes B. Davies S. Binor Z. Radwanska E. Pong R. Ovarian autoimmunity: Greater frequency of autoantibodies in premature menopause and unexplained infertility than in general population. Clin Immunol. 1999;90:368–374. doi: 10.1006/clim.1998.4661. [DOI] [PubMed] [Google Scholar]
- 22.Chernyshov VP. Radysh TV. Gura IV. Tatarchuk TP. Khominskaya ZB. Immune disorders in women with premature ovarian failure in initial period. Am J Reprod Immunol. 2001;46:220–225. doi: 10.1034/j.1600-0897.2001.d01-5.x. [DOI] [PubMed] [Google Scholar]
- 23.Roeiy A. Valesini G. Friberg J, et al. Autoantibodies and common idiotypes in men and women with sperm antibodies. Am J Obstet Gynecol. 1988;158:596–603. doi: 10.1016/0002-9378(88)90037-3. [DOI] [PubMed] [Google Scholar]
- 24.Lanir N. Zilberman M. Yron I. Tennenbaum G. Shechter Y. Brenner B. Reactivity patterns of antiphospholipid antibodies and endothelial cells: effect of antiendothelial antibodies on cell migration. J Lab Clin Med. 1998;131:548–556. doi: 10.1016/s0022-2143(98)90063-4. [DOI] [PubMed] [Google Scholar]
- 25.Mizutani H. Kurata Y. Kosugi S, et al. Monoclonal anticardiolipin autoantibodies established from the (New Zealand white3BXSB)F1 mouse model of antiphospholipid syndrome cross-react with oxidized low-density lipoprotein. Arthritis Rheum. 1995;38:1382–1388. doi: 10.1002/art.1780381005. [DOI] [PubMed] [Google Scholar]
- 26.Delgado Alves J. Kumar S. Isenberg DA. Cross-reactivity between anti-cardiolipin, anti-high-density lipoprotein and anti-apolipoprotein A-I IgG antibodies in patients with systemic lupus erythematosus and primary antiphospholipid syndrome. Rheumatology (Oxford) 2003;42:893–899. doi: 10.1093/rheumatology/keg248. [DOI] [PubMed] [Google Scholar]
- 27.Aikawa NE. Sallum AM. Pereira RM, et al. Subclinical impairment of ovarian reserve in juvenile systemic lupus erythematosus after cyclophosphamide therapy. Clin Exp Rheumatol. 2012;30:445–449. [PubMed] [Google Scholar]
- 28.Al-Maini MH. Mountz JD. Al-Mohri HA, et al. Serum levels of soluble Fas correlate with indices of organ damage in systemic lupus erythematosus. Lupus. 2000;9:132–139. doi: 10.1191/096120300678828145. [DOI] [PubMed] [Google Scholar]
- 29.Lawrenz B. Henes J. Henes M, et al. Impact of systemic lupus erythematosus on ovarian reserve in premenopausal women: evaluation by using anti-Muellerian hormone. Lupus. 2011;20:1193–1197. doi: 10.1177/0961203311409272. [DOI] [PubMed] [Google Scholar]