Abstract
Catalase (CAT) is a peroxisomal antioxidant enzyme that is up-regulated upon oxidative stress. Previous studies have found associations between some single nucleotide polymorphisms (SNPs) located in the CAT promoter region in a variety of metabolic diseases. This is the first study that analyzes the association between erythrocyte CAT activity and candidate CAT SNPs with childhood obesity. The association study showed a significant positive association of the promoter variant −844A/G with childhood obesity and biomarkers of obesity such as weight, body mass index (BMI), BMI Z-Score, and adipocyte fatty acid-binding protein, along with a tendency toward significance with insulin resistance biomarkers. In addition, CAT erythrocyte activity was found to be significantly lower in obese children, and it was significantly correlated with obesity and insulin resistance biomarkers. No association was found between erythrocyte CAT activity and the SNP −844A/G. However, further in vitro and in vivo studies are needed to fully understand the role of CAT activity and SNPs in the development of insulin resistance in the setting of obesity. We hypothesize that CAT plays a role in early metabolic complications of obesity. Antioxid. Redox Signal. 19, 1970–1975.
Introduction
Obesity is a complex multifactorial disease that results from the interaction between an individual's environment and genetic background. Childhood obesity is becoming a major health concern in first-world countries and is nowadays a major risk factor for cardiovascular disease, type 2 diabetes, and metabolic syndrome in adulthood.
In recent years, oxidative stress has gained importance in the setting of obesity. Markers of oxidative stress have been associated with adult obesity, and it is known that oxidative damage and inflammation are present in children at the onset of obesity (2). The oxidative stress is also due to the alteration of the antioxidant defence system, which includes vitamins and antioxidant enzymes. One of the most important endogenous antioxidant enzymes is catalase (CAT), a peroxisomal antioxidant enzyme that degrades hydrogen peroxide into oxygen and water, which is known to be up-regulated upon oxidative stress. CAT activity has been found to be lower in nonobese children presenting insulin resistance defined as high values of homeostasis model assessment for insulin resistance (HOMA-IR) (8).
Innovation.
Previous studies have shown catalase (CAT) to be involved in metabolic diseases, including glucose disorders. The present study investigated the role of CAT genotypes and activity in prepubertal childhood obesity for the first time. Erythrocyte CAT activity was decreased in obese children and significantly correlated with insulin resistance biomarkers. Moreover, the single nucleotide polymorphism (SNP) −844A/G in the promoter of the CAT gene was associated with childhood obesity. Although the SNP −844A/G was not associated with CAT activity, it was associated with adipocyte fatty acid-binding protein levels, which could indicate a role of CAT in adipose tissue dysfunction present in obesity.
The study of single nucleotide polymorphisms (SNPs) could be an additional approach in the study of oxidative stress implications in obesity. Genes traditionally linked with obesity have been the most widely studied in the search for common genetic variations that may be implicated in the development of this disease. However, the studies including genes of antioxidant enzymes have been fewer. None of the previous studies has observed the association between antioxidant enzymes SNPs and obesity.
The CAT gene is located on chromosome 11p13 and contains 13 exons. To this moment, CAT SNPs have been related to a variety of diseases and conditions other than obesity; however, it is clear that they could play a role in glucose disorders (5). Interestingly, the A allele of the −844A/G polymorphism (rs769214) was associated with lower renutrition efficiency in the elderly, possibly through the creation of a PAX6-binding site on the CAT promoter that impacts its transcription, and subsequently, the processing of proglucagon and proinsulin (4). It has also been reported that the haplotype −844G; −89A; −20T in the 5′UTR region of the CAT gene is associated with a lower expression rate under oxidative stress damage as seen in a luciferase assay conducted in four human cell lines (9). These previous studies have proved that SNPs in the CAT gene may be a risk factor of metabolic diseases, but no studies have been conducted in order to study their association with the presence of obesity or its biomarkers.
Therefore, the aim of this study was to analyze the association of these known CAT SNPs along with representative SNPs from the whole CAT gene with childhood obesity in a cohort of prepubertal Spanish children. In addition, we wanted to analyze the main biomarkers related to obesity, insulin resistance, and oxidative stress and to study their association with erythrocyte CAT activity and SNPs in the setting of childhood obesity.
Anthropometric and biochemical characteristics of the study population
Characteristics of the study population are shown in Table 1. As expected, weight, body mass index (BMI), BMI Z-score, and waist circumference (WC) were significantly higher in obese children compared with the normal-weight controls.
Table 1.
Anthropometric and Biochemical Characteristics of the Children
| Variable | Normal-weight | Obese | p |
|---|---|---|---|
| Sex (male/female) | 110/81 | 105/89 | |
| Age (years) | 8.9±0.1 | 8.7±0.1 | 0.127 |
| Weight (kg) | 29.2±0.4 | 51.3±0.9 | <0.001 |
| BMI (kg/m2) | 16.60±0.11 | 26.94±0.26 | <0.001 |
| BMI Z-score | −0.22±0.04 | 3.57±0.11 | <0.001 |
| WC (cm) | 58.3±0.5 | 81.3±0.9 | <0.001 |
| SBP (mmHg) | 95±1 | 110±1 | <0.001 |
| DBP (mmHg) | 58±1 | 69±1 | <0.001 |
| Glucose (mg/dl) | 83±1 | 84±1 | 0.846 |
| Insulin (mU/L) | 5.2±0.2 | 10.3±0.5 | <0.001 |
| QUICKI | 0.390±0.002 | 0.351±0.002 | <0.001 |
| HOMA-IR | 1.08±0.04 | 2.14±0.11 | <0.001 |
| Adiponectin (mg/L) | 27.87±0.81 | 22.41±0.79 | <0.001 |
| Leptin (μg/L) | 4.13±0.29 | 22.69±0.98 | <0.001 |
| TAG (mg/dl) | 54±1 | 75±3 | <0.001 |
| Total cholesterol (mg/dl) | 172±2 | 165±2 | 0.017 |
| HDL-C (mg/dl) | 65±1 | 52±1 | <0.001 |
| LDL-C (mg/dl) | 94±2 | 96±2 | 0.422 |
| ApoA1 (mg/dl) | 151±2 | 133±2 | <0.001 |
| ApoB (mg/dl) | 67±1 | 72±1 | 0.006 |
| A-FABP (ng/ml) | 9.60±0.59 | 27.14±1.45 | <0.001 |
| ox-LDL (mg/L) | 2.073±0.152 | 1.912±0.136 | 0.429 |
| Catalase (KHB) | 104±3 | 90±2 | <0.001 |
| GR (μmol/g Hb/min) | 3.624±0.195 | 3.472±0.123 | 0.507 |
| GPX (mol/g Hb/min) | 0.018±0.001 | 0.019±0.001 | 0.575 |
| TAC (mM Trolox) | 2.18±0.05 | 1.76±0.06 | <0.001 |
| Retinol (μg/ml) | 0.247±0.005 | 0.256±0.005 | 0.158 |
| α-Tocopherol (μg/mL mg TAG) | 0.162±0.005 | 0.124±0.004 | <0.001 |
| β-carotene (μg/L mg TAG) | 3.044±0.192 | 1.358±0.078 | <0.001 |
| hsCRP (mg/L) | 1.004±0.283 | 3.546±0.279 | <0.001 |
Data shown as mean±SEM.
p, Student's t-test p-value.
A-FABP, adipocyte fatty acid-binding protein; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; BMI, body mass index; DBP, diastolic blood pressure; GPX, glutathione peroxidase; GR, glutathione reductase; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment for insulin resistance; hsCRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; ox-LDL, oxidized LDL; QUICKI, quantitative insulin sensitivity check index; SBP, systolic blood pressure; SEM, standard error of the means; TAC, total antioxidant capacity; TAG, triglycerides; WC, waist circumference.
The antioxidant defence status was affected in obese children, as CAT activity in erythrocytes as well as plasma total antioxidant capacity (TAC) were found to be significantly reduced in obese children. In addition, serum vitamin concentrations of α-tocopherol and β-carotene were lower in obese children; whereas no changes were observed in retinol. The oxidative stress biomarker oxidized low-density lipoprotein (ox-LDL) showed no differences between the two groups.
Insulin resistance and metabolic syndrome features showed the expected differences between obese and normal-weight children. Plasma adiponectin concentrations were significantly lower in obese children when compared with the normal-weight children, and oppositely leptin concentrations were higher.
Correlation of erythrocyte CAT activity with obesity, insulin resistance, and oxidative stress biomarkers in plasma
CAT activity in erythrocytes was found to be negatively correlated with obesity biomarkers as weight (r=−0.120, p=0.019), BMI (r=−0.160, p=0.002), and BMI Z-Score (r=−0.168, p=0.001). In addition, CAT activity was correlated with insulin resistance biomarkers by showing a negative correlation with insulin (r=−0.112, p=0.031) and HOMA-IR (r=−0.104, p=0.046), and a positive correlation with quantitative insulin sensitivity check index (QUICKI) (r=0.110, p=0.035). Moreover, CAT activity was negatively correlated with plasma leptin (r=−0.221, p<0.0001), a biomarker that is intimately related with obesity and insulin resistance. With regard to oxidative stress biomarkers, CAT activity was found to be positively correlated with plasma β-carotene values (r=0.112, p=0.030), but not with retinol, α-tocopherol, or TAC. No significant correlation was found between erythrocyte CAT activity and plasma adipocyte fatty acid-binding protein (A-FABP).
These significant correlations between erythrocyte CAT activity and obesity, insulin resistance, and oxidative stress biomarkers show that an altered antioxidant defence status, including diminished CAT activity, could be involved in obesity and its complications as early as in infancy. Moreover, previous findings of reduced CAT activity in children with insulin resistance (8) are in line with these results, further supporting a possible role of oxidative stress in the development of insulin resistance associated with obesity.
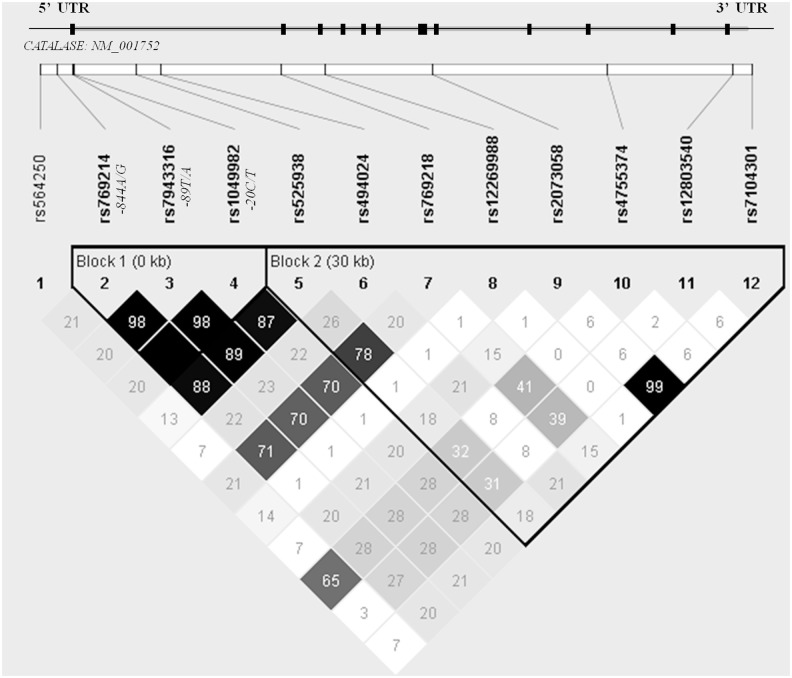
Association of CAT SNPs with childhood obesity
We found the SNP −844G (rs769214) that was previously associated with higher renutrition efficiency in the elderly (4) to be associated with a higher risk of childhood obesity in our study population. In addition, two neighbour polymorphisms, −89A (rs7943316) and −20T (rs1049982), which form a haplotype with −844G, showed a highly similar association due to strong linkage disequilibrium (LD) between the variants (D′=1.00; r2=1.00) (Fig. 1). Population stratification was discarded by conducting a meta-analysis with the individual association results from each of the studied populations (Córdoba and Santiago de Compostela) (I2=0.00). The results from the logistic regression analysis, adjusted for sex and age, as well as SNPs' allele frequencies and Hardy–Weinberg (HW) p-values are shown in Table 2. The following analyses were conducted considering the SNP −844A/G and not the haplotype, because the individual association results were stronger than the haplotype association results (data not shown).
FIG. 1.
Linkage disequilibrium diagram of the studied variants with the exon–intron scheme of the catalase gene. The r2 values between the genotyped single nucleotide polymorphisms are shown in each cell, black cells correspond to r2=1.00. Block definition followed the four gamete rule.
Table 2.
Logistic Regression Analysis of CAT Single Nucleotide Polymorphisms with Obesity Under the Additive Model of Inheritance
| |
|
|
Obese |
Normal-weight |
|
MAF |
|
|
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP ID | Usual designation | Allele 1/Allele 2 | 11 | 12 | 22 | 11 | 12 | 22 | Risk allele | Obese | Normal-weight | HW p | OR (95% CI) | p |
| rs564250 | G/A | 117 | 53 | 7 | 117 | 59 | 5 | A | 0.299 | 0.326 | 0.864 | 1.00 (0.68, 1.46) | 0.987 | |
| rs769214 | −844A/G | A/G | 65 | 89 | 23 | 84 | 81 | 16 | G | 0.503 | 0.448 | 0.414 | 1.42 (1.03, 1.96) | 0.035 |
| rs7943316 | −89T/A | T/A | 65 | 89 | 23 | 82 | 83 | 16 | A | 0.503 | 0.459 | 0.298 | 1.38 (1.00, 1.91) | 0.050 |
| rs1049982 | −20C/T | C/T | 65 | 89 | 23 | 83 | 81 | 16 | T | 0.503 | 0.450 | 0.414 | 1.41 (1.02, 1.95) | 0.039 |
| rs525938 | A/G | 73 | 84 | 20 | 86 | 81 | 14 | G | 0.475 | 0.448 | 0.400 | 1.29 (0.93, 1.79) | 0.127 | |
| rs494024 | G/A | 87 | 70 | 20 | 68 | 89 | 24 | A | 0.396 | 0.492 | 0.727 | 0.73 (0.54, 1.00) | 0.051 | |
| rs769218 | G/A | 86 | 77 | 14 | 102 | 68 | 11 | A | 0.435 | 0.376 | 0.790 | 1.30 (0.93, 1.83) | 0.126 | |
| rs12269988 | A/G | 166 | 11 | 0 | 167 | 14 | 0 | G | 0.062 | 0.077 | 1.000 | 0.77 (0.34, 1.77) | 0.542 | |
| rs2073058 | A/G | 85 | 78 | 14 | 91 | 79 | 11 | G | 0.441 | 0.437 | 0.354 | 1.11 (0.80, 1.56) | 0.529 | |
| rs4755374 | A/C | 132 | 43 | 2 | 137 | 41 | 3 | C | 0.243 | 0.227 | 0.816 | 1.03 (0.66, 1.61) | 0.892 | |
| rs12803540 | A/G | 124 | 46 | 5 | 139 | 36 | 4 | G | 0.263 | 0.201 | 0.381 | 1.35 (0.89, 2.07) | 0.160 | |
| rs7104301 | +33078A/G | A/G | 84 | 79 | 14 | 91 | 79 | 11 | G | 0.446 | 0.437 | 0.202 | 1.13 (0.81, 1.59) | 0.467 |
Values of the statistically significant associations are shown in bold.
Allele 1/2, major/minor allele; CI, confidence interval; HW, Hardy–Weinberg; MAF, minor allele frequency; OR, odds ratio (adjusted for sex and age); p, p-value of the logistic regression analysis; SNP, single nucleotide polymorphism.
Association of the SNP −844A/G with obesity and insulin resistance biomarkers
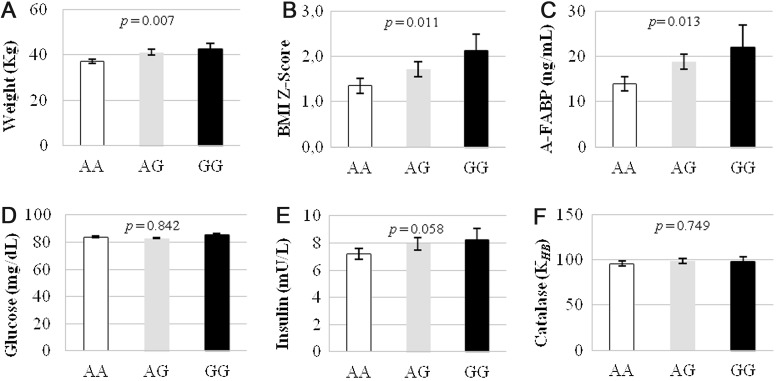
The association of the SNP −844A/G with some of the analyzed biomarkers is shown in Figure 2. The presence of one risk allele was linked to an increase of 2.9 kg in weight or 0.43 U of BMI Z-Score in children. Insulin resistance markers showed a tendency toward significance, individuals with one risk allele had higher insulin (0.83 mU/L per risk allele, p=0.058) and HOMA-IR values (0.17 U per risk allele, p=0.085); whereas lower QUICKI (−0.01 units per risk allele, p=0.073). This finding could indicate a role of CAT in the progress of insulin resistance and obesity in children.
FIG. 2.
Values of weight (A), body mass index (BMI) Z-Score (B), adipocyte fatty acid-binding protein (A-FABP) (C), glucose (D), insulin (E), and erythrocyte catalase (CAT) activity (F) of each genotype of the CAT gene variant −844A/G: AA (open), AG (gray), GG (solid). p, p-values from the linear regression association analysis adjusted for sex and age.
Individuals carrying the variant −844G showed significantly higher plasma A-FABP levels, with an increase of 4.84 ng/ml per risk allele (p=0.013). We hypothesize that this association could be due to the effect of this variant in adipose tissue. The haplotype −844G; −89A; −20T that we describe in the present study was previously shown to be associated to lower CAT expression in cell lines under high oxidative stress (9). As already described by previous studies (2), obese individuals show an enhanced oxidative stress in their adipose tissue. This stress could be observed as leading to a decrease of CAT expression in adipose tissue of the individuals carrying the risk haplotype in the present study. In addition, it is known that oxidative stress enhances adipocyte differentiation in adipose tissue, and that in this process the expression of A-FABP, a well-known adipose tissue marker, is enhanced (6). In this way, the decrease of CAT activity in adipose tissue would further increase the oxidative stress already present in obesity and alter the expression of A-FABP. The relationship between CAT genetic variations and circulating A-FABP further supports the link between the adipose tissue alterations and the development of obesity in the presence of an imbalanced redox state. Furthermore, the fact that the correlation between −844A/G and plasma A-FABP is not found to be paralleled by the correlation between erythrocyte CAT activity and A-FABP could be due to a depot-specific effect of the genotype at the initial stages of adipose tissue dysfunction in obesity.
No association was found between any of the studied SNPs and the antioxidant enzymes' activities (CAT, glutathione reductase [GR] and glutathione peroxidase [GPX]). Likewise, no association was found between the SNPs and the analyzed vitamins or TAC. The absence of association between −844A/G and CAT activity in erythrocytes does not differ from the previous studies conducted on this genetic variant, which found no association of erythrocyte CAT activity with the variant (4).
Conclusion
The importance of studying CAT genetic variants in metabolic diseases other than obesity has been highlighted in previous studies (5). In the present study, we show that CAT activity and its SNPs seem to play a role in various aspects of the metabolic alterations which take place in obesity. Here, we show for the first time that the previously studied CAT SNP −844A/G is associated with a higher childhood obesity risk along with increased weight, BMI, BMI Z-Score, and A-FABP values. In addition, in this study, we show for the first time that CAT activity in erythrocytes is decreased in obese children and it is significantly correlated with obesity, insulin resistance, and oxidative stress phenotypes.
Despite the previous associations found between SNP −844A/G and other disease states or phenotypes, the associations that we describe here need to be validated in larger cohorts of obese children. Furthermore, studies with human adipocyte models will be crucial to better understand the association of CAT polymorphisms with the development of insulin resistance and the possible alterations of adipose tissue, including a higher expression of markers such as A-FABP.
Notes
Study population
This is a case–control study in which we recruited 194 obese children (105 male and 89 female) and 191 normal-weight children (110 male and 81 female) of Caucasian ethnicity, aged 3–13, in two cities of Spain (Córdoba and Santiago de Compostela). Childhood obesity was defined according to Cole et al. (3). The inclusion criteria were prepubertal state, absence of disease related with nutritional status, and absence of endogenous obesity. The exclusion criteria were disease, under-nutrition, pubertal stage, and the use of medication that alters blood pressure or glucose or lipid metabolism. After initial assessments at schools or primary care centres, children fulfilling the inclusion criteria were invited for a clinical examination in the appropriate participating hospital. The parents or guardians were informed about the purpose and procedures of the study before written consent was obtained, and all children gave their assent. Sex hormones were measured to confirm the prepubertal stage (data not shown). The protocol was performed in accordance with the Declaration of Helsinki (Edinburgh 2000, revised) and following the recommendations of the Good Clinical Practice of the CEE (Document 111/3976/88 July 1990) and the legal in-force Spanish regulation that regulates Clinical Investigation in human beings (RD 223/04 about Clinical Assays) and was approved by the Ethics Committee on Human Research of the University of Granada, the Ethics Committee of the Reina Sofía University Hospital of Córdoba, and the Bioethics Committee of the University of Santiago de Compostela.
Anthropometric and biochemical measurements
Anthropometric measurements were taken by a single examiner with the children barefooted and in their underwear. Body weight (kg), height (cm), and WC (cm) were measured using standardized procedures, and BMI and BMI Z-Score were calculated. Obesity was defined according to BMI, using the age- and sex-specific cut-off points proposed by Cole et al. (linked to adult cut-offs of 25 and 30 kg/m2) (3). Blood pressure was measured thrice by the same examiner following international recommendations. Blood samples were drawn via the antecubital vein after the children had fasted overnight.
Biochemical analyses were performed at the participating University Hospital Laboratories following internationally accepted quality control protocols. Specific biomarkers were analyzed using LINCOplexTM kits with human monoclonal antibodies (Linco Research). Adiponectin was measured using the kit catalogue # HADK1-61K-A, and leptin was analyzed using the kit catalogue # HADK2-61K-B. ox-LDL was quantified by using an enzyme-linked immunosorbent assay (ELISA) kit (Biomedica Medizinprodukte GmbH & Co KG). High-sensitivity C-reactive protein was determined with a particle-enhanced turbidimetric immunoassay. Plasma TAC was assessed by using the spectrophotometric commercial antioxidant assay kit from Cayman (Cat. No. 709001; Cayman). A-FABP plasma concentrations were measured by ELISA (Cat. No. RD191036200R; BioVendor). Erythrocyte activities of the antioxidant enzymes CAT and GR were assayed spectrophotometrically. Erythrocyte GPX activity was determined spectrophotometrically by the coupled enzyme procedure with tert-butyl hydroperoxide as substrate. Hemoglobin concentration in the blood samples was determined spectrophotometrically by the colorimetric cyanmethemoglobin method, using Sigma Diagnostic reagents. QUICKI and HOMA-IR were calculated using plasma glucose and insulin values. Retinol and α-tocopherol were analyzed by high-pressure liquid chromatography (HPLC) coupled to an electrochemical detector after extraction with 1-propanol. β-Carotene was also determined after extraction with 1-propanol in an HPLC system attached to a multi-wavelength ultraviolet detector set at 450 nm. All compounds were identified by predetermining the retention times of individual standards.
DNA isolation and genotyping
Genomic DNA was extracted using the QIAamp Blood kit (Qiagen). A total of 13 SNPs in the CAT gene were selected from the HapMap and NCBI databases based on their location. In addition to previously known SNPs, each missense variation was selected and then, others located in the promoter, 3′UTR and 5′UTR regions, all with a minor allele frequency higher than 0.05 in the Caucasian population. Genotyping was performed with the Illumina GoldenGate (Illumina) protocols on 96-well format Sentrix® arrays. Two hundred fifty nanograms of sample DNA were used per assay. Typing of the 13 SNPs resulted in genotype success rates of >95%, except for SNP rs1001179 that was excluded from further analysis. HW equilibrium for each SNP was examined by the exact test using PLINK version 1.07 software (7). The HW equilibrium p-values were greater than 0.05 in both obese and normal-weight groups for all SNPs, and the allele frequencies of the SNPs observed in the current study were similar to those reported in HapMap for Caucasians (data not shown). LD was analyzed with Haploview 4.2 software (1).
Statistical analysis
All statistical analyses were performed using either PLINK or SPSS (version 15.0.1). Normal distribution of clinical parameter data was assessed. Mean comparisons between obese and normal-weight children for continuous variables were performed using the Student's t-test for unpaired samples. Correlations were tested using the Pearson's correlation coefficient test.
The genotypic relative risk was assessed by comparing the obese group with the normal-weight group and calculating the odds ratio and the 95% confidence interval using logistic regression analysis under an additive model adjusted for age and sex. A meta-analysis was performed to discard population stratification from the two cities of recruitment. The association of the SNPs with obesity, insulin resistance, metabolic syndrome, and oxidative stress biomarkers was analyzed using a linear regression model adjusted for age and sex.
Abbreviations Used
- A-FABP
adipocyte fatty acid binding protein
- ApoA1
apolipoprotein A1
- ApoB
apolipoprotein B
- BMI
body mass index
- CAT
catalase
- CI
confidence interval
- DBP
diastolic blood pressure
- ELISA
enzyme-linked immunosorbent assay
- GPX
glutathione peroxidise
- GR
glutathione reductase
- HDL-C
high-density lipoprotein cholesterol
- HOMA-IR
homeostasis model assessment for insulin resistance
- HPLC
high-pressure liquid chromatography
- hsCRP
high-sensitivity C-reactive protein
- HW
Hardy-Weinberg
- LD
linkage disequilibrium
- LDL-C
low-density lipoprotein cholesterol
- MAF
minor allele frequency
- OR
odds ratio
- ox-LDL
oxidized LDL
- QUICKI
quantitative insulin sensitivity check index
- SBP
systolic blood pressure
- SEM
standard error of the means
- SNP
single nucleotide polymorphism
- TAC
total antioxidant capacity
- TAG
triglycerides
- WC
waist circumference
Acknowledgments
The authors would like to thank the children and parents who participated in the study. This study was funded by the Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica (I+D+I), Instituto de Salud Carlos III-Fondo de Investigación Sanitaria [PI 020826, PI051968], the Consejería de Innovación y Ciencia, Junta de Andalucía [P06-CTS 2203] and the Consejería de Salud, Junta de Andalucía [0098/2005], the Ministerio de Universidades y Tecnología, Instituto de Salud Carlos III, Fondo de Investigaciones Sanitarias, Redes temáticas de investigación cooperativa RETIC [Red SAMID RD08/0072/0028], Ministerio de Ciencia e Innovación, and Campus de Excelencia Internacional de Granada GREIB-CTS461.
References
- 1.Barrett JC. Fry B. Maller J. Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 2.Codoñer-Franch P. Valls-Bellés V. Arilla-Codoñer A. Alonso-Iglesias E. Oxidant mechanisms in childhood obesity: the link between inflammation and oxidative stress. Transl Res. 2011;158:369–384. doi: 10.1016/j.trsl.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Cole TJ. Bellizzi MC. Flegal KM. Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hebert-Schuster M. Cottart CH. Laguillier-Morizot C. Raynaud-Simon A. Golmard JL. Cynober L. Beaudeux JL. Fabre EE. Nivet-Antoine V. Catalase rs769214 SNP in elderly malnutrition and during renutrition: is glucagon to blame? Free Radic Biol Med. 2011;51:1583–1588. doi: 10.1016/j.freeradbiomed.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Hebert-Schuster M. Fabre EE. Nivet-Antoine V. Catalase polymorphisms and metabolic diseases. Curr Opin Clin Nutr Metab Care. 2012;15:397–402. doi: 10.1097/MCO.0b013e328354a326. [DOI] [PubMed] [Google Scholar]
- 6.Higuchi M. Dusting GJ. Peshavariya H. Jiang F. Hsiao ST. Chan EC. Liu GS. Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and forkhead box o1 mediated upregulation of antioxidant enzymes. Stem Cells Dev. 2013;22:878–888. doi: 10.1089/scd.2012.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purcell S. Neale B. Todd-Brown K. Thomas L. Ferreira MA. Bender D. Maller J. Sklar P. de Bakker PI. Daly MJ. Sham PC. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin MJ. Park E. Contribution of insulin resistance to reduced antioxidant enzymes and vitamins in nonobese Korean children. Clin Chim Acta. 2006;365:200–205. doi: 10.1016/j.cca.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z. Li Y. Wang B. He Y. Wang Y. Xi H. Li Y. Wang Y. Zhu D. Jin J. Huang W. Jin L. A haplotype of the catalase gene confers an increased risk of essential hypertension in Chinese Han. Hum Mutat. 2010;31:272–278. doi: 10.1002/humu.21185. [DOI] [PubMed] [Google Scholar]