Abstract
Introduction
Similar to other Southern African countries, Swaziland has been severely affected by HIV, with over a quarter of its reproductive-age adults estimated to be living with the virus, equating to an estimate of 170,000 people living with HIV. The last several years have witnessed an increase in the understanding of the potential vulnerabilities among men who have sex with men (MSM) in neighbouring countries with similarly widespread HIV epidemics. To date, there are no data characterizing the burden of HIV and the HIV prevention, treatment and care needs of MSM in Swaziland.
Methods
In 2011, 324 men who reported sex with another man in the last 12 months were accrued using respondent-driven sampling (RDS). Participants completed HIV testing using Swazi national guidelines as well as structured survey instruments administered by trained staff, including modules on demographics, individual-level behavioural and biological risk factors, social and structural characteristics and uptake of HIV services. Population and individual weights were computed separately for each variable with a data-smoothing algorithm. The weights were used to estimate RDS-adjusted univariate estimates with 95% bootstrapped confidence intervals (BCIs). Crude and RDS-adjusted bivariate and multivariate analyses were completed with HIV as the dependent variable.
Results
Overall, HIV prevalence was 17.6% (n=50/284), although it was strongly correlated with age in bivariate- [odds ratio (OR) 1.2, 95% BCI 1.15–1.21] and multivariate-adjusted analyses (adjusted OR 1.24, 95% BCI 1.14–1.35) for each additional year of age. Nearly, 70.8% (n=34/48) were unaware of their status of living with HIV. Condom use with all sexual partners and condom-compatible-lubricant use with men were reported by 1.3% (95% CI 0.0–9.7).
Conclusions
Although the epidemic in Swaziland is driven by high-risk heterosexual transmission, the burden of HIV and the HIV prevention, treatment and care needs of MSM have been understudied. The data presented here suggest that these men have specific HIV acquisition and transmission risks that differ from those of other reproductive-age adults. The scale-up in HIV services over the past decade has likely had limited benefit for MSM, potentially resulting in a scenario where epidemics of HIV among MSM expand in the context of slowing epidemics in the general population, a reality observed in most of the world.
Keywords: public health, men who have sex with men (MSM), Africa, HIV, Swaziland, epidemiology
Introduction
Swaziland is a small, land-locked, lower-middle-income country that is surrounded by South Africa and Mozambique; it has a population of approximately 1.1 million people and a life expectancy at birth of approximately 48 years [1]. Similar to other Southern African countries, Swaziland has been severely affected by HIV, with over a quarter of its reproductive-age adults (15–49) estimated to be living with the virus, equating to an estimate of 170,000 people living with HIV [2]. Moreover, the incidence of HIV appears to have peaked in 1998–1999 at 4.6% [95% confidence interval (CI) 4.27–4.95], according to estimates by the Joint United Nations Programme on HIV/AIDS (UNAIDS), while in 2009 it was estimated to be 2.7% (95% CI 2.2–3.1%) [3–6]. There appear to have been further declines in incidence according to 6054 person-years of follow-up data from 18,154 people followed from December 2010 to June 2011 as part of the Swaziland HIV Incidence Measurement Survey (SHIMS) longitudinal cohort. Overall incidence was approximately 2.4% (95% CI 2.1–2.7%), with incidence estimated to be 3.1% (95% CI 2.6–3.7) among women as compared to 1.7% (95% CI 1.3–2.1) among men [7]. Indeed, women and girls have been more burdened with HIV than men throughout the history of the HIV epidemic in Swaziland, with the HIV prevalence among women 15–24 in 2006 being estimated to be 22.6% compared to 5.9% among age-matched men and boys [5].
The 2009 Swaziland Modes of Transmission study characterized major drivers of incident HIV infections to be multiple concurrent partnerships before and during marriage as well as low levels of male circumcision [8]. These risk factors were confirmed in the SHIMS study, with risk factors for incident HIV infections among both men and women including not being married or living alone, having higher numbers of sex partners and having serodiscordant or unknown HIV status partners [7]. There are no known HIV prevalence estimates for key populations in Swaziland, including female sex workers (FSW) or men who have sex with men (MSM) [9,10]. The 2009 Swazi Modes of Transmission Study indicates that both sex work and male–male sexual practices are reportedly infrequent and assumed to be minor drivers of HIV risks in the setting of a broadly generalized HIV epidemic. However, the prevalence of these risk factors has not been measured in the HIV surveillance systems that are used to inform the Modes of Transmission Surveys [11]. The last several years have witnessed an increase in the understanding of the potential vulnerabilities among these same key populations through targeted studies including MSM in neighbouring countries with similarly widespread HIV epidemics [12,13].
The largest body of data is available from South Africa, where the first study completed in 1983 of 250 MSM demonstrated a high prevalence of HIV, syphilis and hepatitis B virus [14]. More recently, a study of rural South African men found that approximately 3.6% of men studied (n=46) reported a history of having sex with another man [15]. Among these men, HIV prevalence was 3.6 times higher than among men not reporting male partners (95% CI 1.0–13.0, p=0.05) [16]. There have also been several targeted studies of MSM in urban centres across South Africa that consistently highlight a population of men who have specific risk factors for HIV acquisition and transmission and limited engagement in the continuum of HIV care [17–19]. Relatively recent studies from other countries, including Lesotho, Malawi, Namibia and Botswana, have shown similar diverse populations of MSM [16,20,21]. Diversity among populations of MSM across Southern Africa manifests through diverse sexual orientations and practices ranging from those who are gay identified, with primarily male sexual partners, to those who are straight identified, with both male and female sexual partners [22]. Diversity has also been measured in the range of HIV-related risk practices among MSM, including understanding of the HIV acquisition and transmission risks associated with unprotected anal intercourse and of the levels of use of condoms and condom-compatible lubricants (CCLs) [23].
To better characterize vulnerabilities and HIV prevention, treatment and care needs among MSM in Swaziland, a cross-sectional assessment was completed to provide an unbiased estimate of the prevalence of HIV and syphilis among adult MSM in Swaziland. This study was completed in equal collaboration with the Swaziland National AIDS Program (SNAP) in the Ministry of Health. This study further sought to describe the significant correlates of prevalent infections, including individual behavioural characteristics, and describe social and structural HIV-related factors and risks for HIV infection among MSM.
Methods
Sampling
MSM in Swaziland were recruited via respondent-driven sampling (RDS), a peer referral sampling method designed for data collection among hard-to-reach populations [24]. Potential participants were required to be at least 18 years of age, report anal sex with another man in the previous 12 months, be able to provide informed consent in either English or siSwati, be willing to undergo HIV and syphilis testing and possess a valid recruitment coupon.
Survey administration and HIV testing
All participants completed face-to-face surveys and received HIV and syphilis tests on site. Surveys were administered by trained members of the research staff and lasted approximately one hour. The study was completely anonymous and did not collect any identifiable information; we used verbal rather than signed consent to further ensure anonymity. Questions on socio-demographics (e.g., age, marital status and education), behavioural HIV-related risk factors (e.g., HIV-related knowledge, attitudes and risk behaviours) and structural factors (e.g., stigma, discrimination and social cohesion) were included [25]. HIV and syphilis tests were conducted by trained phlebotomists or nurses, according to official Swazi guidelines. Test results, counselling and any necessary treatment (for syphilis) and/or referrals (for HIV) were provided on site. Participant surveys and test results were linked using reproducible, yet anonymous, 10-digit codes.
Analytical methods
Population and individual weights were computed separately for each variable by the data-smoothing algorithm using RDS for Stata [26]. The weights were used to estimate RDS-adjusted univariate estimates with 95% bootstrapped confidence intervals (BCIs). Crude bivariate regression analyses were also conducted to assess the association of HIV status with demographic variables as well as a selection of variables either expected or shown to be associated with HIV status in the literature. All demographic variables were then included in the initial multivariate logistic regression model regardless of the estimated strength of their crude bivariate association with HIV status. Non-demographic variables were included in the initial multivariate model if the chi-square p value of association with HIV status was ≤0.25 in the bivariate analyses. Most of the demographics variables, however, dropped out of the final model after controlling for other independent variables.
Because regression analyses of RDS data using sample weights are complicated due to the fact that weights are variable-specific [27], RDS-adjusted bivariate and multivariate analyses were conducted using individualized weights that were specific to the outcome variable (i.e., HIV status) [27]. The adjusted odds ratio (aOR) estimates were not statistically different from the unadjusted estimates in the bivariate analyses, although some slight differences were observed in the multivariate analyses. Thus, only the unadjusted odds ratios (ORs) are reported for bivariate analyses, while both are presented in Table 1 for multivariate analyses. All data processing and analyses were conducted using Stata 12.1 [28].
Table 1.
Sociodemographic characteristics of a sample of men who have sex with men in Swaziland in 2011
| Variable | Categories | N | Crude percentage | RDS-adjusted percentage | 95% confidence interval | Homophily (−1 to +1) | |
|---|---|---|---|---|---|---|---|
| Age in years | Under 21 | 94 | 30.0 | 36.3 | 27.4 | 45.2 | 0.199 |
| 21–25 | 142 | 45.4 | 45.1 | 36.3 | 53.8 | 0.143 | |
| 26–30 | 56 | 17.9 | 12.0 | 7.2 | 16.7 | 0.148 | |
| 31 and older | 21 | 6.7 | 6.7 | 2.9 | 10.4 | 0.026 | |
| Some secondary, high school or lower | 108 | 34.5 | 44.8 | 35.6 | 53.9 | 0.104 | |
| Education level | Completed secondary or high school | 133 | 42.5 | 40.4 | 32.4 | 48.4 | 0.119 |
| Post-high-school vocational training or higher | 72 | 23.0 | 14.8 | 9.4 | 20.2 | 0.180 | |
| Unemployed | 97 | 32.3 | 30.7 | 22.5 | 38.9 | 0.189 | |
| Employment status | Employed | 101 | 33.7 | 27.5 | 19.5 | 35.5 | 0.203 |
| Student | 102 | 34.0 | 41.8 | 32.6 | 51.0 | −0.001 | |
| Marital status with a woman | Married or cohabitating | 13 | 4.2 | 1.8 | 0.1 | 3.5 | −0.018 |
| Single, never married | 298 | 95.8 | 98.2 | 96.5 | 99.9 | −1.423 | |
| Current housing tenure | Renting place | 92 | 29.4 | 27.7 | 20.6 | 34.9 | 0.046 |
| Own place | 51 | 16.3 | 18.3 | 12.0 | 24.6 | −0.126 | |
| Staying with someone | 101 | 32.3 | 34.8 | 27.1 | 42.5 | 0.119 | |
| Family | 42 | 13.4 | 10.6 | 5.6 | 15.5 | 0.201 | |
| Other | 27 | 8.6 | 8.6 | 4.5 | 12.8 | −0.095 | |
| Urban or rural origin | Urban | 192 | 61.5 | 61.0 | 52.6 | 69.5 | 0.101 |
| Rural | 120 | 38.5 | 39.0 | 30.5 | 47.4 | 0.172 | |
| Number of children | Zero | 274 | 87.8 | 89.5 | 84.7 | 94.2 | −0.174 |
| One or more | 38 | 12.2 | 10.5 | 5.8 | 15.3 | 0.115 | |
| Gender Identification | Man | 225 | 72.6 | 82.5 | 76.9 | 88.1 | −0.29 |
| Woman | 79 | 25.5 | 15.7 | 10.4 | 20.9 | 0.17 | |
| Both | 6 | 1.9 | 1.8 | 0.0 | 3.9 | −0.018 | |
| Sexual orientation identification | Gay or homosexual | 198 | 63.5 | 56.3 | 48.0 | 64.6 | 0.242 |
| Bisexual | 109 | 34.9 | 40.5 | 32.3 | 48.6 | 0.062 | |
| Heterosexual or straight | 5 | 1.6 | 3.2 | 0.0 | 7.4 | 0.096 | |
| Age at first sex with a man | Under 21 years | 238 | 77.0 | 77.6 | 70.7 | 84.6 | 0.110 |
| 21 and above | 71 | 23.0 | 22.4 | 15.4 | 29.3 | 0.083 | |
| Ever been to jail or prison? | No | 276 | 88.2 | 86.8 | 81.4 | 92.1 | 0.216 |
| Yes | 37 | 11.8 | 13.2 | 7.9 | 18.6 | 0.157 | |
Missing data
Eleven out of the 324 participants were excluded from this analysis due to missing data on key RDS-related variables. There were 29 out of 313 participants with missing data on at least one variable used in the multivariate analyses. Only two variables had data missing for more than three participants: age at first sex with another man (n missing=4) and knowledge about the type of anal sex position that puts you most at risk of HIV infection (n missing=6). Two of the 29 participants with missing data were living with HIV; thus, the effective crude HIV prevalence used in the multivariate model was 17.6% (50/284) versus 16.6% (52/313) without missing data, and RDS-adjusted 13.4% (95% BCI: 7.9–19.7; homophily: HIV−=−0.0991, HIV+=0.134) versus 12.7% (BCI: 7.3–18.1; homophily: HIV−=−0.0899, HIV+=0.1358) Although the total number of cases with missing data is not very small (9.3%: 29/313), the number missing by variable is very small. Due to the small change in HIV prevalence in the analysis sample compared to the complete sample as shown in this article, no effort was made to impute missing data. The 29 cases were excluded in the multivariate regression models.
Sample size calculation
The sample size was calculated based on the ability to detect significant differences in condom use among MSM living with HIV and those not living with HIV. There were no known estimates of condom use among MSM in Swaziland, but previous studies of MSM from nearby countries estimated that consistent condom use during anal sex with other men among MSM is approximately 50% [19]. In addition, a systematic review and meta-analysis of the literature on behavioural interventions targeting MSM have demonstrated that behavioural interventions can increase reported condom use by approximately 16.5% in all risk categories of MSM [29,30]. Thus, this study was powered on the assumption that those who have received information about preventing HIV infection from other men would have a 16.5% increase in reported consistent condom use. A power analysis demonstrated that with 80% power, we would require 160 participants. Estimates of appropriate design effects for RDS have varied in the literature, and we used a design effect of 2, planning for the accrual of 324 MSM [31]. This sample size facilitates the detection of significant differences in HIV-related protective practices, such as consistent condom use, and targeted HIV-prevention measures, and is sufficient for key social factors such as experiences with stigma and discrimination.
Ethics
The study received approval for research on human participants from both the National Ethics Committee of Swaziland as well as the Institutional Review Board of the Johns Hopkins Bloomberg School of Public Health.
Results
Three hundred and twenty-four men were accrued from six seeds over a range of between 1 and 14 waves of accrual, with the largest recruitment chain including 123 participants. As shown in Table 2, the majority of men sampled were under 30 years of age, with a mean age of 23.1 and a mode of 22. The crude sample was relatively educated, although highly educated men were oversampled in this study when comparing the crude results to RDS-adjusted results (unadjusted 23.0% and adjusted 14.8%). Most of the study participants had never been married (98.2%, 95% CI 96.5–99.9), with only 13 men reporting either cohabitating with a woman or being married to a woman. Similarly, only about one in 10 men reported having children (10.5%, 95% CI 5.8–15.3). Notably, the majority of the sample of participants did not self-identify as straight or heterosexual, with approximately two-thirds reporting being gay and one-third reporting being bisexual. When asked about gender identification, nearly a quarter of the sample reported identifying as a woman, although the adjusted proportion was 15.7% (95% CI 10.4–20.9). More than one-tenth of men reported having been to jail or prison (13.2%, 95% CI 7.9–18.6). Among 71 men aged 18–19, the HIV prevalence was 0%, compared to 8.8% (n=6/68) among participants aged 20–21, 15% (n=9/60) among participants aged 22–23, 21.4% (n=12/56) among participants aged 24–26, and finally 43.1% (n=25/58) among participants aged 27–43 (data not shown). In total, 29.2% (n=14/48) of participants living with HIV reported previously being told that they had HIV, although four participants not found to be living with HIV reported being given this diagnosis.
Table 2.
HIV-related sexual and drug risk factors among MSM in Swaziland
| Variable | Categories | N | Crude percentage | RDS-adjusted percentage | 95% CI | |
|---|---|---|---|---|---|---|
| Number of male sexual partners in the past 12 months | 1 | 103 | 33.0 | 42.1 | 34.0 | 50.2 |
| 2 | 68 | 21.8 | 20.3 | 14.7 | 26.0 | |
| 3 | 70 | 22.4 | 20.3 | 14.2 | 26.5 | |
| 4+ | 71 | 22.8 | 17.2 | 11.7 | 22.7 | |
| Number of main male partners in the past 12 months | 1 | 37 | 11.8 | 17.1 | 10.7 | 23.6 |
| 2 | 183 | 58.5 | 57.2 | 49.7 | 64.7 | |
| 3 | 61 | 19.5 | 18.5 | 13.0 | 24.1 | |
| 4+ | 32 | 10.2 | 7.1 | 3.9 | 10.4 | |
| Number of male casual sexual partners in the past 12 months | None | 132 | 42.4 | 46.6 | 39.0 | 54.2 |
| 1–2 | 127 | 40.8 | 41.1 | 33.8 | 48.5 | |
| 3+ | 52 | 16.7 | 12.3 | 7.7 | 16.8 | |
| Number of female sexual partners in the past 12 months | None | 198 | 64.3 | 53.6 | 44.9 | 62.4 |
| 1 | 52 | 16.9 | 19.5 | 12.5 | 26.5 | |
| 2 | 29 | 9.4 | 15.1 | 8.7 | 21.6 | |
| 3+ | 29 | 9.4 | 11.8 | 6.2 | 17.4 | |
| Number of both male and female sex partners in the past 12 months | Only male | 221 | 70.6 | 64.3 | 56.4 | 72.3 |
| Male and female | 92 | 29.4 | 35.7 | 27.7 | 43.6 | |
| In general, how often have you used a condom in the past six months? | Never or almost never | 30 | 9.7 | 11.5 | 6.0 | 17.0 |
| Sometimes | 79 | 25.6 | 27.0 | 19.7 | 34.4 | |
| Almost always | 58 | 18.8 | 18.9 | 12.9 | 24.9 | |
| Always | 141 | 45.8 | 42.5 | 34.7 | 50.3 | |
| Had unprotected insertive anal sex in the past 12 months | No | 190 | 60.9 | 55.5 | 46.8 | 64.2 |
| Yes | 122 | 39.1 | 44.5 | 35.8 | 53.2 | |
| Had unprotected receptive anal sex in the past 12 months | No | 211 | 68.7 | 69.8 | 62.7 | 76.8 |
| Yes | 96 | 31.3 | 30.2 | 23.2 | 37.3 | |
| Condom use with main male partners in the past 12 months | Not always | 137 | 47.2 | 51.9 | 41.8 | 62.0 |
| Always | 153 | 52.8 | 48.1 | 38.0 | 58.2 | |
| Condom use with casual male partners in the past 12 months | Not always | 54 | 17.3 | 16.6 | 10.2 | 23.0 |
| Always | 150 | 47.9 | 46.1 | 38.6 | 53.6 | |
| No casual partner | 109 | 34.8 | 37.3 | 29.8 | 44.9 | |
| Condom use with regular female partners in the past 12 months | Not always | 47 | 49.5 | 61.8 | 41.3 | 82.3 |
| Always | 48 | 50.5 | 38.2 | 17.7 | 58.7 | |
| Condom use with casual female partners in the past 12 months | Not always | 31 | 45.6 | 55.6 | 25.2 | 86.0 |
| Always | 37 | 54.4 | 44.4 | 14.0 | 74.8 | |
| Used water-based lubricant (WBL) in the past 12 months | No | 203 | 64.9 | 76.3 | 69.8 | 82.8 |
| Uses WBL | 110 | 35.1 | 23.7 | 17.2 | 30.2 | |
| Safe sex with men (condoms and water-based lubricant) in the past 12 months | Does not | 257 | 82.1 | 87.4 | 82.4 | 92.4 |
| Does | 56 | 17.9 | 12.6 | 7.6 | 17.6 | |
| Safe sex with women (condoms) in the past 12 months | Does not | 66 | 60.0 | 75.3 | 60.5 | 90.0 |
| Does | 44 | 40.0 | 24.7 | 10.0 | 39.5 | |
| Safe sex with both men and women in the past 12 months | Does not | 104 | 95.7 | 98.7 | 90.3 | 100.0 |
| Does | 6 | 4.3 | 1.3 | 0.0 | 9.7 | |
| Injected illicit drugs in the past 12 months | No | 304 | 97.1 | 97.7 | 96.1 | 99.3 |
| Yes | 9 | 2.9 | 2.3 | 0.7 | 3.9 | |
| Used non-injection illicit drugs in the past 12 months | No | 203 | 65.1 | 66.4 | 58.5 | 74.3 |
| Yes | 109 | 34.9 | 33.6 | 25.7 | 41.5 | |
| Used alcohol in the last month | None | 121 | 39.0 | 36.1 | 28.7 | 43.5 |
| At least one day | 189 | 61.0 | 63.9 | 56.5 | 71.3 | |
| Which is the safest lubricant to use during anal sex? | Non-WBL | 128 | 49.8 | 63.5 | 53.6 | 73.4 |
| WBL | 129 | 50.2 | 36.5 | 26.6 | 46.4 | |
| Can you get HIV from sharing a needle to inject illegal drugs? | No | 3 | 1.0 | 1.0 | −0.2 | 2.2 |
| Yes | 303 | 99.0 | 99.0 | 97.8 | 100.2 | |
| What type of sex puts you most at risk for HIV infection? | Vaginal | 110 | 35.1 | 43.7 | 36.1 | 51.2 |
| Anal | 75 | 24.0 | 18.2 | 12.4 | 24.0 | |
| Oral | 25 | 8.0 | 8.6 | 3.8 | 13.5 | |
| Which type of anal sex position puts you most at risk for HIV infection? | Insertive (top) | 63 | 20.5 | 24.0 | 17.1 | 30.9 |
| Receptive (bottom) | 95 | 30.9 | 31.1 | 23.8 | 38.4 | |
| Insertive and receptive anal sex carry equal risk | 149 | 48.5 | 44.9 | 36.6 | 53.2 | |
| Answered all of the above correctly | No | 278 | 88.8 | 90.9 | 87.0 | 94.8 |
| Yes | 35 | 11.2 | 9.1 | 5.2 | 13.0 | |
The majority of men had multiple male sexual partners over the past 12 months (57.9%, 95% CI 49.8–66.0). Moreover, most study participants had multiple main sexual partners, or boyfriends, over the past 12 months (82.9%, 95% CI 76.4–89.3) (Table 3). About one-third of participants reported having had both male and female sexual partners in the previous 12 months (35.7%, 95% CI 27.7–43.6). Approximately one-half of the participants reported always using condoms during sex, although significant numbers of men reported both unprotected insertive and receptive anal intercourse in the past 12 months. Condom use was not significantly different between main and casual male or female partners. Overall, safe sex with other men, defined as always using condoms and water-based lubricants over the last 12 months, was not common, with 12.6% (95% CI 7.6–12.6) measured to report this behaviour. Safe sex, defined as condom use with all sexual partners over the last 12 months, was significantly higher with female partners (at 40.0% in the crude assessment) than with male partners (p<0.05). Overall, safe sex with all sexual partners was uncommon and was reported by 4.3% (RDS-adjusted 1.3%, 95% CI 0.0–9.7). Knowledge of basic questions related to safe sex for MSM, including sexual positioning, type of sexual act and lubricant use, was low, with 11.2% (RDS-adjusted 9.1%, 95% CI 5.2–13.0) of participants providing correct answers.
Table 3.
Service uptake and structural HIV risks among MSM in Swaziland
| Variable | Categories | N | Crude percentage | RDS-adjusted percentage | 95% CI | |
|---|---|---|---|---|---|---|
| Participated in any meetings related to HIV/AIDS in the past 12 months | No | 175 | 55.9 | 58.5 | 51.1 | 65.8 |
| Yes | 138 | 44.1 | 41.5 | 34.2 | 48.9 | |
| Participated in any meetings related to HIV/AIDS in the past 12 months related to MSM | No | 243 | 78.4 | 83.5 | 78.1 | 88.8 |
| Yes | 67 | 21.6 | 16.5 | 11.2 | 21.9 | |
| Received information about preventing HIV from sex with women in last 12 months | No | 60 | 19.4 | 20.9 | 14.5 | 27.2 |
| Yes | 250 | 80.6 | 79.1 | 72.8 | 85.5 | |
| Received information about preventing HIV from sex with other men in last 12 months | No | 226 | 72.4 | 78.5 | 72.9 | 84.1 |
| Yes | 86 | 27.6 | 21.5 | 15.9 | 27.1 | |
| Level of concern related to HIV in the last 12 months | Not worried | 86 | 27.6 | 31.8 | 24.9 | 38.8 |
| Not very worried | 61 | 19.6 | 18.2 | 12.1 | 24.2 | |
| Somewhat worried | 52 | 16.7 | 16.8 | 10.0 | 23.6 | |
| Very worried | 113 | 36.2 | 33.2 | 26.0 | 40.3 | |
| Access to condoms: do you have them when you need them? | No access | 3 | 1.0 | 1.0 | -0.4 | 2.3 |
| Difficult or little access | 58 | 18.6 | 16.8 | 11.2 | 22.4 | |
| Some access | 36 | 11.6 | 12.6 | 7.2 | 18.0 | |
| Very easy access | 214 | 68.8 | 69.6 | 61.9 | 77.4 | |
| Symptoms of sexually transmitted infection (STI) in the past 12 months | No | 247 | 79.2 | 78.5 | 72.4 | 84.6 |
| Yes | 65 | 20.8 | 21.5 | 15.4 | 27.6 | |
| Tested for STI in the past 12 months | No | 266 | 87.2 | 86.1 | 80.5 | 91.7 |
| Yes | 39 | 12.8 | 13.9 | 8.3 | 19.5 | |
| Diagnosis of STI in the past 12 months | No | 287 | 92.6 | 92.2 | 88.3 | 96.1 |
| Yes | 23 | 7.4 | 7.8 | 3.9 | 11.7 | |
| Been tested for HIV in the past 12 months | No | 144 | 46.0 | 49.3 | 41.8 | 56.8 |
| Yes, once | 94 | 30.0 | 31.2 | 24.2 | 38.2 | |
| Yes, >1 | 75 | 24.0 | 19.5 | 13.5 | 25.4 | |
| Ever been told that you have HIV? | No | 284 | 94.0 | 95.7 | 92.5 | 98.9 |
| Yes | 18 | 6.0 | 4.3 | 1.1 | 7.5 | |
| Perceived human rights violations | No | 63 | 20.1 | 20.4 | 14.5 | 26.3 |
| Yes | 250 | 79.9 | 79.6 | 73.7 | 85.5 | |
| Experienced human rights violations | No | 152 | 48.6 | 48.9 | 40.5 | 57.2 |
| Yes | 161 | 51.4 | 51.1 | 42.8 | 59.5 | |
| Disclosure to healthcare workers | No | 218 | 69.6 | 75.0 | 69.0 | 81.0 |
| Yes | 95 | 30.4 | 25.0 | 19.0 | 31.0 | |
| Disclosure to family | No | 146 | 46.6 | 56.0 | 48.3 | 63.6 |
| Yes | 167 | 53.4 | 44.0 | 36.4 | 51.7 | |
Table 4 demonstrates levels of service uptake, with evidence of statistically significantly lower levels of access to targeted services focused on preventing HIV transmission via sex between men as compared to sex between men and women (p<0.05 for both). Notably, only about half of the sample was somewhat or very worried about HIV. Just under half of the men who had symptoms of a sexually transmitted infection (STI) were tested in the previous 12 months, with 7.8% (95% CI 3.9–11.7) diagnosed in this same time frame. About half of the sample had been tested for HIV in the previous 12 months (50.7%, 95% CI 43.2–59.2), including some who were tested more than one time. Reports of any experienced rights violations related to sexual practices, including denial of care, police-mediated violence and physical or verbal harassment, were reported by about half of the sample, although perceived rights violations related to sexual orientation (fear of seeking healthcare and fear of walking in the community) were more common, with 79.6% (95% CI 73.7–85.5) calculated to report this. Disclosure of sexual practices to healthcare workers was reported by one-quarter of the sample (25.0%, 95% CI 19.0–31.0), whereas about half of the participants (44.0%, 95% CI 36.4–51.7) had reported disclosure of sexual practices to a family member.
Table 4.
Bivariate and multivariate associations with HIV status among men who have sex with men (MSM) in Swaziland
| Bivariate | Multivariate – crude | Multivariate – RDS weighted | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Variable | Categories | Estimate | [95% CI] | Estimate | [95% CI] | Weighted estimate | Weighted estimate 95% CI |
| Current age | Years | 1.23 | [1.15–1.31] | 1.24*** | [1.14–1.35] | 1.28*** | [1.15–1.43] |
| Man | 1 | 1 | 1 | ||||
| Gender | Woman | 2.14 | [0.90–5.05] | 3.96** | [1.66–9.43] | 3.23* | [1.07–9.71] |
| Both | – | – | – | ||||
| Education level | Some secondary, high school or lower | 1 | 1 | 1 | |||
| Completed secondary or high school | 1.06 | [0.44–2.56] | 1.32 | [0.54–3.18] | 1.51 | [0.46–5.00] | |
| Post-high-school vocational training or higher | 1.34 | [0.47–3.77] | 0.56 | [0.20–1.57] | 0.62 | [0.18–2.16] | |
| Age at first sex with another man | Under 21 years | 1 | 1 | 1 | |||
| 21 and above | 2.38 | [0.99–5.72] | 1.24 | [0.49–3.14] | 0.71 | [0.18–2.75] | |
| Urban or rural origin | Urban | 1 | 1 | 1 | |||
| Rural | 1.99 | [0.91–4.35] | 0.79 | [0.34–1.79] | 1.33 | [0.45–3.93] | |
| Ever been to jail or prison? | No | 1 | 1 | 1 | |||
| Yes | 2.75* | [1.08–7.00] | 3.00* | [1.01–8.85] | 4.37* | [1.38–13.84] | |
| Diagnosis with an STI other than HIV in last 12 months | No | 1 | 1 | 1 | |||
| Yes | 1.57 | [0.49–5.07] | 6.26** | [1.68–23.39] | 4.30* | [1.04–17.72] | |
| Number of casual male partners in the last 12 months | None | 1 | 1 | 1 | |||
| 1–2 | 0.51 | [0.20–1.26] | 0.33* | [0.13–0.85] | 0.26* | [0.08–0.85] | |
| 3+ | 1.12 | [0.42–2.98] | 1.04 | [0.37–2.95] | 0.50 | [0.13–1.97] | |
| Which type of anal sex position puts you most at risk for HIV infection? | Insertive (top) | 1 | 1 | 1 | |||
| Receptive (bottom) | 0.91 | [0.33–2.54] | 0.49 | [0.17–1.42] | 0.53 | [0.14–2.08] | |
| Insertive and receptive anal sex carry equal risk | 0.96 | [0.37–2.54] | 0.39 | [0.14–1.06] | 1.43 | [0.32–6.41] | |
| In the past 12 months, have you used any non-injectable drug that was not prescribed? | No | 1 | 1 | 1 | |||
| Yes | 0.84 | [0.375–1.865] | 0.356* | [0.136–0.935] | 0.366 | [0.12–1.11] | |
| What kind of access to condoms do you have when you need them? | No access | 1 | 1 | 1 | |||
| Difficult or little access | 0.13 | [0.007–2.380] | 0.008** | [0.000–0.224] | 0.031 | [0.001–1.23] | |
| Some access | 0.36 | [0.021–6.115] | 0.043 | [0.002–1.022] | 0.170 | [0.01–5.35] | |
| Very easy access | 0.49 | [0.034–7.054] | 0.043* | [0.002–0.893] | 0.264 | [0.007–10.020] | |
| In the past 30 days, how many days did you drink at least one drink of alcohol? | Zero | 1 | 1 | 1 | |||
| At least one day | 1.30 | [0.55–3.07] | 1.81 | [0.74–4.41] | 2.19 | [0.60–7.96] | |
| Analysis sample | 284 | 284 | |||||
Exponentiated coefficients; 95% CI=95% confidence intervals
p<0.05
p<0.01
p<0.001.
HIV prevalence was strongly correlated with age in both bivariate analyses (OR 1.23, 95% BCI 1.15–1.21) for each year of age and multivariate-adjusted analyses (aOR 1.24, 95% BCI 1.14–1.35) (Table 1). Other statistically significant associations with HIV in adjusted analyses included identifying as the female gender, having ever been to jail or prison, having lower numbers of casual partners, being diagnosed with an STI in the last 12 months and having easier access to condoms.
Discussion
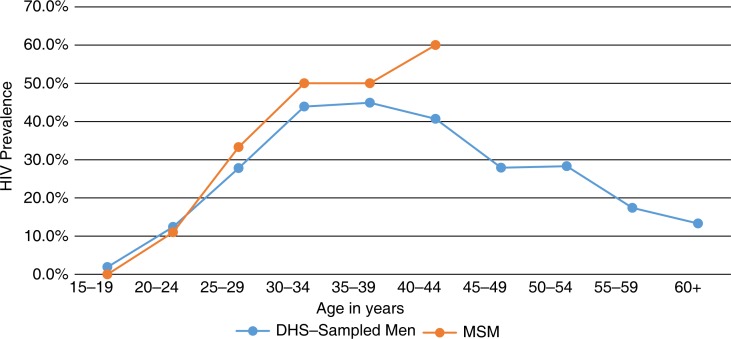
In the country with the highest HIV prevalence in the world, this study describes the burden of HIV and associated characteristics among MSM who were accrued using RDS. Interpreting the prevalence of HIV among MSM and its relationship with the widespread and generalized female-predominant epidemic in Swaziland is challenging on a number of levels. The significant association between HIV and age suggests that the expanding epidemic among MSM in Swaziland is not new and represents cumulative HIV acquisition risk exposures. The burden of HIV among all men aged 15–19 is approximately 2% in Swaziland, increasing to 12.4% among those aged 20–24 and up to 44.9% among those aged 35–39. While the participants in our study were relatively young, the HIV prevalence was consistent with that of general reproductive-age men until age 24–26, when the prevalence of HIV among age-matched MSM appears to be higher than that of other men sampled as part of the Swazi DHS study (Figure 1) [2]. Given that relatively few men in our sample reported female sexual partners, their HIV acquisition and transmission risks are likely different from those of other men in Swaziland and potentially more related to anal intercourse. Conversely, Swaziland may be among a small number of countries where even the low acquisition risks associated with insertive penile-vaginal intercourse is counterbalanced by the significantly higher HIV prevalence among women, resulting in significant acquisition risks associated with sex with women. However, the idea that acquisition risk for MSM primarily related to sex with other men is reinforced by the results that condom use was lower with male sexual partners than with female sexual partners. Condoms being used more frequently during sex with women as compared to sex with other men have been observed in other studies of MSM across Sub-Saharan Africa and provide an argument against MSM being a population that bridges the HIV epidemic from within their sexual networks to lower risk heterosexual networks [19,20,32,33]. However, to answer this question, phylogenetic studies and the characterization of sexual networks are needed to better describe patterns of HIV transmission.
Figure 1.
Prevalence of HIV by age among Swazi men who have sex with men, 2011.
Participants were far more likely to have received information about preventing HIV infection during sex with women as compared to sex with other men. This lack of access to or uptake of information, education and communication services has resulted in participants in this study having a limited knowledge base of the sexual risks associated with same-sex practices. Primarily, participants incorrectly believed that unprotected penile-vaginal intercourse was associated with the highest risk of HIV transmission, consistent with earlier studies of MSM across Sub-Saharan Africa. Numerous studies have shown the opposite: HIV is far more efficiently transmitted during anal intercourse as compared to vaginal intercourse [13,34]. There was also limited knowledge related to the importance of water-based lubricants being CCLs, which is especially important during anal intercourse given the absence of physiological lubrication in the anal canal. The importance of CCL was underscored as ultimately being the determining factor in just six study participants reporting safe sex with all partners in this study. Thus, while there is significant provision of general HIV-prevention messaging across Swaziland, there has been limited information focused on educating MSM on how to prevent HIV acquisition and transmission during sex with other men. Data suggest that starting with simple and proven approaches, including peer education programmes, is necessary to educate these men about their risks and protective behavioural strategies [35]. However, these approaches will likely not be sufficient to change the trajectory of HIV epidemics given the high risk of infection associated with unprotected anal intercourse with non-virally suppressed HIV serodiscordant partners. Thus, moving forward necessitates assessing the feasibility of combination approaches that integrate advances such as antiretroviral-mediated pre-exposure prophylaxis and universal access to antiretroviral therapy for people living with HIV [13]. However, the success or failure in achieving coverage with these HIV prevention, treatment and care approaches among MSM will, in part, be determined by the level of stigma affecting MSM.
It is now broadly accepted that addressing the needs of people living with HIV is vital to protect their own health as well as prevent onward transmission of HIV [36]. In addition, mean and total viral loads in a population have been linked to population-level transmission rates of HIV [37]. Only a quarter of the men living with HIV in this study were aware of their diagnosis, demonstrating the need to increase HIV testing, linkage to CD4 testing, and antiretroviral treatment and adherence support for those who are eligible. A recent systematic review and meta-analysis of self-testing for HIV in both low- and high-risk populations demonstrated that self-testing was both appropriate and associated with increased uptake of HIV tests [38]. This may be especially relevant in the Swazi context, where fear of seeking healthcare was prevalent, suggesting the need to study new strategies to overcome barriers to HIV testing among MSM in Swaziland, including leveraging community networks and potentially self-testing [39]. In this study, being a person living with HIV was associated with lower numbers of casual male partners in the last 12 months. This relationship appeared to be stronger among those who were aware of their status, although it was not statistically significant because of limited numbers. In addition, these data are consistent with earlier research findings that simply being made aware of one's status of living with HIV can change one's sexual practices to decrease onward transmission [40]. This further argues for implementation science research focused on optimal strategies to scale-up HIV testing for MSM in Swaziland [41].
Over one-quarter of participants in this study self-identified as women, and this was independently associated with living with HIV. There is nearly a complete dearth of information related to HIV among transgender people across Sub-Saharan Africa [42,43]. However, where transgender people have been studied, they have been found to be the most vulnerable to HIV acquisition because of increased structural barriers to HIV prevention, treatment and care services and because of increased sexual risks, including unprotected receptive anal intercourse [43]. Given the limited information available about transgender people, transgender was assessed in this study as both a sexual orientation and a gender identity. There was a significant disconnect between these two as no participants self-identified as being transgender. Ultimately, further ethnographic research is needed to better understand the HIV-prevention needs of transgender people in Swaziland.
Having been to jail was also independently associated with living with HIV among MSM in this study. Globally, incarceration has been shown to be an important risk factor for HIV, given the limited access to HIV-prevention services such as condoms and CCLs, the interruption of HIV treatment as well as exposure to higher risk sexual partners [44–47]. While further research is needed on same-sex practices within jails, there is likely a need to provide HIV-prevention services for men in Swazi prison settings [47].
The methods employed in this study have several limitations. While RDS is an effective approach to characterize asymptotically unbiased estimates intended to approximate population-based estimates of characteristics in the absence of a meaningful sampling frame, there are still several uncertainties in the most appropriate tools for interpretation of these data [48]. Moreover, the sample of men accrued here was relatively young, consistent with recruitment challenges observed in other studies of MSM across sub-Saharan Africa. While we conducted significant engagement with older MSM, fear associated with inadvertent disclosure limited their participation in the study. Only with improved social environments will more information about the needs of older MSM become available in difficult contexts [49]. In addition, while RDS was used to accrue a diverse sample, all of the seeds were connected with Rock of Hope, a newly registered organization serving the needs of lesbian, gay, bisexual and transgender populations in Swaziland. We thus may have overestimated actual service uptake among MSM in Swaziland.
Conclusions
The implementation of the research project was guided by recent guidelines to inform HIV-related research with MSM in rights-constrained environments [50]. While these men had not been previously engaged in research on HIV prevention, treatment and care, the success of this study highlights the fact that accrual of this population is both feasible and informative for the HIV response in Swaziland. Moreover, the interconnected social and sexual networks leveraged for accrual can likely serve to disseminate HIV-prevention approaches via MSM throughout the country. While the epidemic in Swaziland is one driven by heterosexual transmission, the burden of HIV and the HIV prevention, treatment and care needs of MSM have been understudied, and these men have been underserved in the context of large-scale programmes [51]. The data presented here suggest that these men have specific HIV acquisition and transmission risks that differ from those of other reproductive-age adults. Encouragingly, Swaziland has seen declines in the rate of new HIV infections over the last seven years, and these declines are related to HIV testing and treatment scale-up [5]. However, the increase in HIV services likely has had limited benefit for MSM, which may result in a scenario where epidemics of MSM expand in the context of slowing epidemics in the general population – a reality observed in most of the world [13].
Acknowledgements
Primarily, we would like to acknowledge the study participants, who completed this study with little personal benefit and risk of inadvertent disclosure of sexual orientation. We want to especially acknowledge the team from PSI Swaziland, including Babazile Dlamini and Edward Okoth. In addition, the Swaziland Most-at-Risk Populations (MARPS) technical working group provided significant technical support, as did multiple agencies within the Swazi government. We want to thank all members of the Rock of Hope organization, who provided significant community support for this study that made it possible. From USAID Swaziland, Jennifer Albertini and Natalie Kruse-Levy are acknowledged for consistent support throughout the project; and from USAID Washington, Alison Cheng and Cameron Wolf provided technical support. We would like to acknowledge Andrea Vazzano for careful review of the manuscript.
Competing interests
The authors have no competing interests to declare.
Authors' contributions
SDB, ZM, JLG, CEK, DLK and DA conceptualized and designed the study. Implementation was led by ZM, DA and XM, with significant support by SM and BS. SDB, AG, JLG, SK and CEK developed the analytic strategy and completed data analysis. SDB, CEK and DA drafted the manuscript, with all authors providing critical inputs for the interpretation of the results. All authors have read and approved the final manuscript.
Funding
This work was supported by USAID∣Project SEARCH, Task Order No. 2, funded by the US Agency for International Development under Contract No. GHH-I-00-07-00032-00, beginning 30 September 2008, and supported by the President's Emergency Plan for AIDS Relief.
References
- 1.World B. Atlas method and PPP. Geneva: World Bank; 2009. Gross national income per capita 2008. [Google Scholar]
- 2.Macro I, Swaziland Central Statistics Office . Mbabane, Swaziland: USAID; 2008. Swaziland: demographic and health survey 2006–2007. [Google Scholar]
- 3.UNAIDS. Geneva: UNAIDS; 2009. AIDS epidemic update 2009. [Google Scholar]
- 4.UNAIDS. Geneva: United Nations; 2012. Report on the global AIDS epidemic. [Google Scholar]
- 5.UNAIDS, NERCHA, Ministry of Health S, UNICED. Mbabane, Swaziland: UNGASS; 2010. Monitoring the declaration of the commitment on HIV and AIDS (UNGASS): Swaziland country report. [Google Scholar]
- 6.Ghys PD, Saidel T, Vu HT, Savtchenko I, Erasilova I, Mashologu YS, et al. Growing in silence: selected regions and countries with expanding HIV/AIDS epidemics. AIDS. 2003;17(Suppl 4):S45–50. doi: 10.1097/00002030-200317004-00005. [DOI] [PubMed] [Google Scholar]
- 7.Reed JB, Justman J, Bicego G, Donnell D, Bock N, Ginindza H, et al., editors. Estimating national HIV incidence from directly observed seroconversions in the Swaziland HIV Incidence Measurement Survey (SHIMS) longitudinal cohort (FRLBX02). XIX International AIDS Conference; 2012 July 24; Washington, DC: International AIDS Society; 2012. [Google Scholar]
- 8.Swaziland National Emergency Response Council on HIV, AID. Mbabane, Swaziland: World Bank; 2009. Swaziland: HIV prevention response and modes of transmission analysis. [Google Scholar]
- 9.UNFPA, UNAIDS, Swaziland M, Swaziland National Emergency Response Council on HIV, AIDS. Situation analysis on commercial sex work in Swaziland; November–December 2007; Mbabane, Swaziland: UNFPA; 2007. [Google Scholar]
- 10.Baral S, Sifakis F, Cleghorn F, Beyrer C. Elevated risk for HIV infection among men who have sex with men in low- and middle-income countries 2000–2006: a systematic review. PLoS Med. 2007;4(12):e339. doi: 10.1371/journal.pmed.0040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baral S, Phaswana-Mafuya N. Rewriting the narrative of the epidemiology of HIV in sub-Saharan Africa. Sahara J. 2012;9(3):127–30. doi: 10.1080/17290376.2012.743787. [DOI] [PubMed] [Google Scholar]
- 12.Baral S, Beyrer C, Muessig K, Poteat T, Wirtz AL, Decker MR, et al. Burden of HIV among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(7):538–49. doi: 10.1016/S1473-3099(12)70066-X. [DOI] [PubMed] [Google Scholar]
- 13.Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380(9839):367–77. doi: 10.1016/S0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sher R. HIV infection in South Africa, 1982–1988 – a review. S Afr Med J. 1989;76(7):314–18. [PubMed] [Google Scholar]
- 15.Jewkes R, Dunkle K, Nduna M, Levin J, Jama N, Khuzwayo N, et al. Factors associated with HIV sero-positivity in young, rural South African men. Int J Epidemiol. 2006;35(6):1455–60. doi: 10.1093/ije/dyl217. [DOI] [PubMed] [Google Scholar]
- 16.Baral S, Adams D, Lebona J, Kaibe B, Letsie P, Tshehlo R, et al. A cross-sectional assessment of population demographics, HIV risks and human rights contexts among men who have sex with men in Lesotho. J Int AIDS Soc. 2011;14:36. doi: 10.1186/1758-2652-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baral S, Burrell E, Scheibe A, Brown B, Beyrer C, Bekker LG. HIV risk and associations of HIV infection among men who have sex with men in peri-urban Cape Town, South Africa. BMC Public Health. 2011;11:766. doi: 10.1186/1471-2458-11-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane T, Raymond HF, Dladla S, Rasethe J, Struthers H, McFarland W, et al. High HIV prevalence among men who have sex with men in Soweto, South Africa: results from the Soweto Men's study. AIDS Behav. 2011;15:626–34. doi: 10.1007/s10461-009-9598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rispel LC, Metcalf CA. Breaking the silence: South African HIV policies and the needs of men who have sex with men. Reprod Health Matters. 2009;17(33):133–42. doi: 10.1016/S0968-8080(09)33442-4. [DOI] [PubMed] [Google Scholar]
- 20.Beyrer C, Trapence G, Motimedi F, Umar E, Iipinge S, Dausab F, et al. Bisexual concurrency, bisexual partnerships, and HIV among Southern African men who have sex with men. Sex Transm Infect. 2010;86(4):323–7. doi: 10.1136/sti.2009.040162. [DOI] [PubMed] [Google Scholar]
- 21.Baral S, Trapence G, Motimedi F, Umar E, Iipinge S, Dausab F, et al. HIV prevalence, risks for HIV infection, and human rights among men who have sex with men (MSM) in Malawi, Namibia, and Botswana. PLoS One. 2009;4(3):e4997. doi: 10.1371/journal.pone.0004997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy V, Sandfort T, Rispel L. Johannesburg, South Africa: HSRC Press; 2009. From social silence to social science: same-sex sexuality, HIV & AIDS and gender in South Africa. [Google Scholar]
- 23.Baral S, Scheibe A, Sullivan P, Trapence G, Lambert A, Bekker LG, et al. Assessing priorities for combination HIV prevention research for men who have sex with men (MSM) in Africa. AIDS Behav. 2013;17:S60–9. doi: 10.1007/s10461-012-0202-5. [DOI] [PubMed] [Google Scholar]
- 24.Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Probl. 1997;44(2):174–99. [Google Scholar]
- 25.Baral S, Logie CH, Grosso A, Wirtz AL, Beyrer C. Modified social ecological model: a tool to guide the assessment of the risks and risk contexts of HIV epidemics. BMC Public Health. 2013;13(1):482. doi: 10.1186/1471-2458-13-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schonlau M, Liebau E. Respondent-driven sampling. Stata J. 2012;12(1):21. [Google Scholar]
- 27.Johnston L, O'Bra H, Chopra M, Mathews C, Townsend L, Sabin K, et al. The associations of voluntary counseling and testing acceptance and the perceived likelihood of being HIV-infected among men with multiple sex partners in a South African township. AIDS Behav. 2010;14(4):922–31. doi: 10.1007/s10461-008-9362-8. [DOI] [PubMed] [Google Scholar]
- 28.StataCorp. TX: StataCorp LP; 2010. Stata statistical software: release 11.1. College Station. [Google Scholar]
- 29.Beyrer C, Wirtz A, Walker D, Johns B, Sifakis F, Baral S. The global HIV epidemics among men who have sex with men (MSM): epidemiology, prevention, access to care, costs, and human rights. In: Bank W, editor. Washington, DC: World Bank; 2011. [Google Scholar]
- 30.Johnson WD, Diaz RM, Flanders WD, Goodman M, Hill AN, Holtgrave D, et al. Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men. Cochrane Database Syst Rev. 2008;3:CD001230. doi: 10.1002/14651858.CD001230.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Salganik MJ. Variance estimation, design effects, and sample size calculations for respondent-driven sampling. J Urban Health. 2006;83(6 Suppl):98–112. doi: 10.1007/s11524-006-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onyango-Ouma W, Birungi H, Geibel S. Nairobi: Population Council; 2005. Understanding the HIV/STI risks and prevention needs of men who have sex with men in Nairobi, Kenya. [Google Scholar]
- 33.Onyango-Ouma W, Birungi H, Geibel S. Engaging men who have sex with men in operations research in Kenya. Cult Health Sex. 2009;11:827–39. doi: 10.1080/13691050902844853. [DOI] [PubMed] [Google Scholar]
- 34.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010;39:1048–63. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sullivan PS, Carballo-Dieguez A, Coates T, Goodreau SM, McGowan I, Sanders EJ, et al. Successes and challenges of HIV prevention in men who have sex with men. Lancet. 2012;380(9839):388–99. doi: 10.1016/S0140-6736(12)60955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gardner EM, McLees MP, Steiner JF, del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das M, Chu PL, Santos GM, Scheer S, Vittinghoff E, McFarland W, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5(6):e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pant Pai N, Sharma J, Shivkumar S, Pillay S, Vadnais C, Joseph L, et al. Supervised and unsupervised self-testing for HIV in high- and low-risk populations: a systematic review. PLoS Med. 2013;10(4):e1001414. doi: 10.1371/journal.pmed.1001414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trapence G, Collins C, Avrett S, Carr R, Sanchez H, Ayala G, et al. From personal survival to public health: community leadership by men who have sex with men in the response to HIV. Lancet. 2012;380(9839):400–10. doi: 10.1016/S0140-6736(12)60834-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39(4):446–53. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 41.Padian NS, Holmes CB, McCoy SI, Lyerla R, Bouey PD, Goosby EP. Implementation science for the US President's Emergency Plan for AIDS Relief (PEPFAR) J Acquir Immune Defic Syndr. 2011;56(3):199–203. doi: 10.1097/QAI.0b013e31820bb448. [DOI] [PubMed] [Google Scholar]
- 42.Jobson G. Transgender in Africa: invisible, inaccessible, or ignored? SAHARA J. 2012;9(3):160–3. doi: 10.1080/17290376.2012.743829. [DOI] [PubMed] [Google Scholar]
- 43.Baral SD, Poteat T, Stromdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. 2012;13(3):214–22. doi: 10.1016/S1473-3099(12)70315-8. [DOI] [PubMed] [Google Scholar]
- 44.Wolfe MI, Xu F, Patel P, O'Cain M, Schillinger JA, St Louis ME, et al. An outbreak of syphilis in Alabama prisons: correctional health policy and communicable disease control. Am J Public Health. 2001;91(8):1220–5. doi: 10.2105/ajph.91.8.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beyrer C, Jittiwutikarn J, Teokul W, Razak MH, Suriyanon V, Srirak N, et al. Drug use, increasing incarceration rates, and prison-associated HIV risks in Thailand. AIDS Behav. 2003;7(2):153–61. doi: 10.1023/a:1023946324822. [DOI] [PubMed] [Google Scholar]
- 46.Harris RM, Sharps PW, Allen K, Anderson EH, Soeken K, Rohatas A. The interrelationship between violence, HIV/AIDS, and drug use in incarcerated women. J Assoc Nurses AIDS Care. 2003;14(1):27–40. doi: 10.1177/1055329002239188. [DOI] [PubMed] [Google Scholar]
- 47.Kyomya M, Todyrs KW, Amon JJ. Laws against sodomy and the HIV epidemic in African prisons. Lancet. 2012;380(9839):310–12. doi: 10.1016/S0140-6736(12)60682-5. [DOI] [PubMed] [Google Scholar]
- 48.Salganik MJ. Commentary: respondent-driven sampling in the real world. Epidemiology. 2012;23(1):148–50. doi: 10.1097/EDE.0b013e31823b6979. [DOI] [PubMed] [Google Scholar]
- 49.Semugoma P, Nemande S, Baral SD. The irony of homophobia in Africa. Lancet. 2012;380(9839):312–14. doi: 10.1016/S0140-6736(12)60901-5. [DOI] [PubMed] [Google Scholar]
- 50.AMFAR, Center for Public Health and Human Rights JHSoPH. New York: IAVI; 2011. Respect, protect, fulfill: best practices guidance in conducting HIV research with gay, bisexual, and other men who have sex with men (MSM) in rights-constrained environments. [Google Scholar]
- 51.AMFAR, Center for Public Health and Human Rights, JHSPH. Washington, DC: AMFAR; 2013. Achieving an AIDS-free generation for gay men and other MSM in Southern Africa. [Google Scholar]